Ecological variations of woody species along an altitudinal gradient in the Qinling Mountains of Central China: area-based versus mass-based expression of leaf traits
Yongfu Chai · Hailin Shang · Xiaofei Zhang · Ruichang Zhang · Xiao Liu · Ming Yue
Abstract Leaf trait-based research has become the preferred method to understand the ecological strategies of plants. However, there is still a debate on whether area-based or mass-based traits provide different insights into environmental adaptations and responses. In this study, seven key leaf traits (maximum net photosynthetic rate, dark respiration rate, nitrogen content, photosynthetic nitrogen use effi ciency, leaf mass per area, leaf dry matter contents and leaf area) of 43 woody species were quantified on the basis of both area and mass along an altitudinal gradient (1100–2700 m) in the Qinling Mountains of China. Differences in leaf traits and bivariate correlations between the two expressions were compared. By considering different expressions, the strengths and directions of the responses of leaf traits to the altitudinal gradient were determined. Leaf traits showed large variations; interspecific variations contributed more to total variance than intraspecific variations. Bivariate correlations between photosynthetic traits and structural traits (mass per area, dry matter content, and area) were weaker on a mass basis than those on an area basis. Most traits exhibited quadratic trends along the altitudinal gradient, and these patterns were more noticeable for area-based than mass-based traits. Area-based traits were more sensitive to changes in temperature and precipitation associated with altitude. These results provide evidence that mass- versus area-based traits show different ecological responses to environmental conditions associated with altitude, even if they do not contain very broad spatial scales. Our results also indicate distinction of photosynthetic acclimation among the two expressions along an altitudinal gradient, reflecting trade-off s among leaf structure and physiological traits.
Keywords Altitude modulation · Bivariate relationship · Leaf trait · Qinling Mountains
Introduction
Understanding the mechanisms of plant adaptation to environmental changes is a primary goal of plant physiology and ecology (Wright et al. 2004a, 2005; Wang et al. 2018; Gong et al. 2019). The ecophysiological processes of plants are dominated by a suite of leaf traits that interact with each other, and the coordination pattern of these traits determines the interrelationship between plants and communities or ecosystem function (Poorter et al. 2014; Read et al. 2014; Vitória et al. 2019). In recent decades, especially with the global ‘leaf economics spectrum’ (LES) of plant traits described by Wright et al. ( 2004b), analyzing trait-based variations among organisms and exploring the bivariate relationships among leaf traits are increasingly used to predict plant resource-use strategies and functional responses to environmental change (Blonder et al. 2011; Dubuis et al. 2013; Funk and Cornwell 2013; Guo et al. 2018).
Leaf size varies by several orders of magnitude among species, and leaf traits are typically normalized by mass or area to study relationships among them (Bloomfi eld et al. 2018). Leaf traits on area- or mass-based expressions provide ecologically distinct insight into leaf functioning (Lloyd et al. 2013; Osnas et al. 2013; Rose et al. 2013; Poorter et al. 2014; Kenzo et al. 2015). Generally, leaf traits on a mass basis are crucial to leaf economy when revenues and expenditures per unit investment are counted, whereas leaf traits on an area basis are pivotal from a physiological standpoint because some physiological processes proceed as a fl ux per unit leaf area (Wright et al. 2004b ). Westoby et al. ( 2013) advocated for regarding leaf traits from both perspectives because each basis of expression has its merits and refl ects diff erent roles in resource-use strategies (Niinemets et al. 2014).
The environment acts as a selective fi lter on the distribution of plant species along abiotic gradients, and spatial variations in leaf traits across environmental gradients can potentially occur at diff erent scales (Wright et al. 2004a, 2005; Fujii et al. 2018). The global-scale leaf economics spectrum has described multivariate correlations among key leaf traits along a large climate gradient (Wright et al. 2004a), including maximum rate of net photosynthesis (Amax), dark respiration rate (Rdark), leaf nitrogen (N) and leaf phosphorus (P), leaf life span (LL), and leaf mass per area (LMA). The leaf economics spectrum (LES) confi nes leaf traits of species primarily to a single axis of variation if data are normalized by leaf mass. Nevertheless, trait correlations that defi ne the leaf economics spectrum are caused by basic physiological or morphological trade-off s experienced by both the role of mass- versus area-based traits (Saura-Mas et al. 2009). Osnas et al. ( 2013) statistically partitioned Amax, Rdark, N, and P from the Global Plant Trait Database into area- and mass- proportional components and found that correlations among these leaf traits, (primarily refl ecting interspecifi c variations), are much stronger when Amax, Rdark, N, and P are normalized by mass than by area. They argued that these traits were distributed approximately proportional to leaf area instead of mass, as expected for a light- and carbon dioxide–collecting organ. Moreover, much of the structure in the mass-normalized LES results from normalizing area-proportional traits by mass (Osnas et al. 2013). At a local scale, correlations among leaf traits have been regarded as an eff ective indicator of plant response to local microhabitats (Malhi et al. 2010; Almeida et al. 2013; Hu et al. 2014; Krix and Murray 2018). Although the local scale generally shows smaller subsets of species facing similar climate environments than regional or global scales (Kraft et al. 2008; Saura-Mas et al. 2009), variations in microhabitats likely lead to distinct ecological responses to a given abiotic gradient due to species turnover or intraspecifi c variation. However, it has not been clearly determined whether area-based and mass-based expressions of leaf traits show diff erent multivariate correlations and then lead to distinct responses to local abiotic gradients.
Altitude signifi cantly infl uences plant growth, structure, function, and metabolism (Berli et al. 2013; Bu et al. 2018) because environmental factors, such as water and heat fl uxes, light intensity, air temperatures and humidity, change signifi cantly with increasing altitude (Dunne et al. 2003; Guo et al. 2018). Altitudinal gradients also provide a unique, and sometimes the best, opportunity to study plant responses and adaptation under global climate change (Read et al. 2014), since climate, soil properties and vegetation can transform dramatically in short distances along an altitudinal gradient. In addition, although variations in leaf traits with elevation are universal, leaf trait-altitude relationships may differ among mountain ranges (Read et al. 2014; Vitória et al. 2019). Therefore, studies at a local scale on leaf traits and their relationships along an altitudinal gradient are signifi -cant for scaling up ecophysiological processes from the leaf to the ecosystem level (Chai et al. 2015). Comparing areabased versus mass-based expression of leaf trait relationships along an altitudinal gradient can not only provide more information about changes in leaf economics with environmental changes but also provide a deeper understanding of the mechanisms plants use to adapt to the environment.
In this study, we explored the patterns of variation in both mass- and area-based leaf traits and thus the bivariate relationships of 43 woody species spanning a 1600 m altitudinal gradient on the Qinling Mountains in Central China and used trait-based ecological strategies to explain vegetation responses to environmental change. Based on the global LES, seven key traits related to photosynthetic and structural characteristics of leaves were selected: maximum net photosynthetic rate (Amax), dark respiration rate (Rd), leaf nitrogen content (N), photosynthetic nitrogen use effi ciency (PNUE), leaf mass per area (LMA), leaf dry matter content (LDMC) and leaf area (LA). The fi rst three were based on mass and area (Amassand Aarea, Rdmassand Rdarea, Nmassand Narea), respectively. Based on these leaf trait measurements, we asked: (1) whether correlations among leaf traits across woody species are stronger if leaf traits are normalized by mass than if normalized by area? (2) Do the changes in altitude affect the bivariate relationships among leaf traits? and, (3) Are correlations between traits and climate factors related to altitude stronger if leaf traits are normalized by mass than normalized by area?
Materials and methods
Site selection and sampling
This study was conducted in the Taibai Mountain National Reserve (33°49′–34°05′ N, 107°22′–107°51′ E) on the northern slope of the Qinling Mountains. Taibai Mountain has a distinct vertical vegetation zone along an altitude from 780 to 3767 m a.s.l. Leaf traits were evaluated of species in the mountainous, deciduous broad-leaved forest zone that was divided into five forest subzones (Table 1).
Field work was conducted in July and August 2014. Five sites were chosen to represent each of the five forest types along altitudes from 1100 m to 2700 m a.s.l. at intervals of 400 m (Table 1). At each site, three 30 m × 40 m plots were established, all woody vascular species within each plot identified, and the abundance, coverage, and growth form (tree vs. shrub) documented. The location ofeach plot was randomly selected within each site, and topographic factors or species compositions were different for each plot in the 200 m range to avoid simple pseudoreplication and include more species (Yue and Zhou 1997). All the plots have the same aspect (south to east) and slope (approximately 30°). The soil type of this deciduous broad-leaved forest zone is mountain brown forest soil. Three mature and robust individuals ofevery dominant woody species in each plot were selected and leaf traits measured. The selected dominant species together represented more than 70% of the total vegetation cover. Altitude, latitude, and longitude ofeach plot were determined by GPS (Magellan GPS315, Magellan, San Dimas, CA, SA). All species and their mean trait values from different altitudes are listed in supplementary Table S1.
We focused on temperature and precipitation in this study because they are the most important ecological characteristics of mountains (Almeida et al. 2013; Tan and Wang 2016). The two meteorological parameters were calculated according to the data from the weather station nearest to the research site at each elevation based on the methods of Tang and Fang ( 2006). The related meteorology, geography and vegetation data are provided in Table 1.
Measurement of leaf traits
Photosynthesis was measured between 08:00 and 11:00 am to avoid the midday depression on clear days using a portable photosynthesis system (LI-6400, Li-Cor Inc., Lincoln, NE, USA) in the open mode. Photosynthetically active radiation (PAR) levels of 0, 20, 50, 100, 150, 200, 400, 600, 800, 1000, 1200, 1400, 1600, and 1800 μmol m?2s?1were provided by internal red-blue light sources. Measurements were taken on three fully expanded, undamaged young leaves from the tops of the different branches at mid-crown positions ofeach sample. All measurements were carried out in the field; sample twigs were beveled and immediately placed into distilled water to avoid water loss (Xu 2006). All photosynthetic data ofeach individual were used to fit a photosynthetic light-response curve, described as an empirical equation using nonlinear least squares regression (Bassman and Zwier 1991), and Amaxand Rdarkwere obtained from the light-response curves. The photosynthetic nitrogen use effi ciency (PNUE) was defined using Amaxdivided by Narea(Zheng and Shangguan 2007).
After the photosynthetic measurements, together with the nine leaves collected for the measurements, 30 fully expanded, mature leaves were immediately obtained from the same individuals. Fresh mass was determined using a high-precision electronic scale (± 0.001 g). The leaf area (LA) was determined by taking a digital photograph of a flattened fresh leaf to scale and analyzing its area via Motic Images Plus 2.0 (Motic (Xiamen) Electric Group Co., Ltd, Xiamen, China). Considering the evacuation of nonstructural carbohydrates, each photographed leaf was then immediatelyair-dried in the field. The leaves were oven-dried at 70 °C for
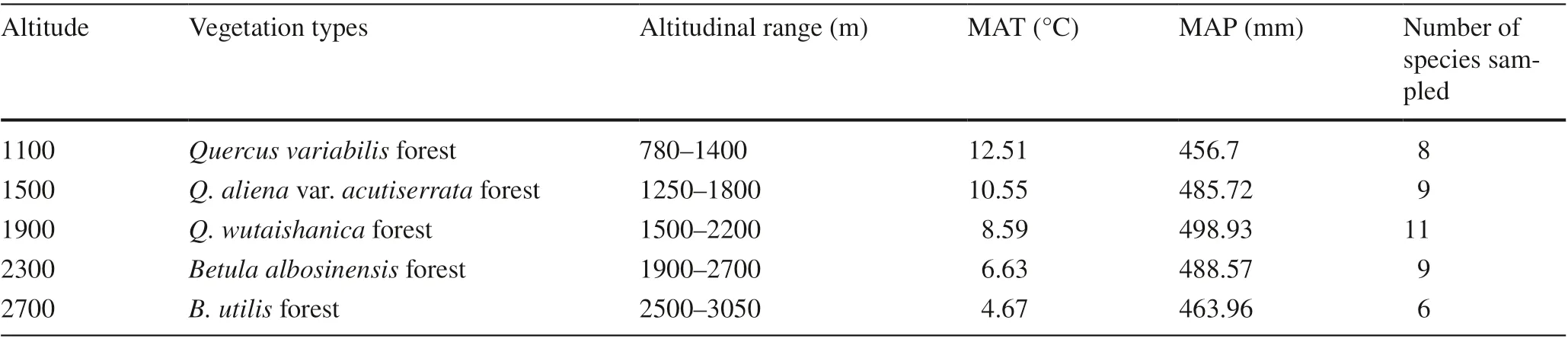
Table 1 Vegetation types and climate conditions of five sites along the altitudinal gradient
Results
48 h and dry weight measured on a semi-analytical balance. Leaf mass per area (LMA) was calculated as dry weight divided by fresh LA, and leaf dry matter content (LDMC) was the ratio of leaf dry weight to its fresh mass (Ryser et al. 2008). The dried leaves were pulverized using a ball mill and total nitrogen concentrations determined using an elemental analyzer (EuroEA3000, EuroVector S.p.A., Milan, Italy). Table 2 lists the abbreviations and units for all of the measured variables.
Statistical analyses
All leaf parameters were log10-transformed to acquire normalization. Mass- and area-based trait values can be interconverted by multiplying the mass-based values by the leaf mass per area. Two-tailed t-tests and one-way analysis of variance (ANOVA) were used to test significant differences of leaf traits among growth forms and elevations, respectively. Variance components were estimated with a linear random effects model to reveal whether variations in leaf traits between species were greater than within species.
Bivariate relationships were analyzed at the species level from all altitudinal gradients (general averages of the leaf traits for each species at all sites). The bivariate relationships were then analyzed across species from each altitudinal gradient and tested for whether the relationships significantly changed along the gradient. The leaf trait values are the averages for each species at each site. To summarize the variations in bivariate relationships across tree and shrub species, standardized major axis regressions (Model II) were performed in SMATR (Warton et al. 2006). Ordinary least squares (OLS) regression was carried out to quantify the relationships between leaf traits and temperature, precipitation and elevation. Quadratic polynomial regression was selected when a linear regression was insignificant. The coeffi cient of determination (R 2 ) was used to estimate the strength of the relationships. Statistical analyses were conducted in Statistica 6.0 (Statsoft, Tulsa, OK, USA), Origin Pro 7.5 (Origin Lab Corporation, Northampton, MA, USA) and SMATR ( https://githu b.com/dfals ter/smatr/).
Variations of area- and mass-based leaf traits across species
The coeffi cients of variation (CVs) of all leaf traits varied from 0.13 (Nmass) to 0.78 (LA) (Table 3). The CVs of Rdarkand N contents were higher on an area basis than those on a mass basis (Table 3, test statistic = 4.19 and 5.36, P < 0.05, based on an asymptotic test). The tree species had higher Aarea, Narea, LMA, LDMC and LA values than shrubs (Table 3). The variance decomposition showed that interspecific differences contribute on average 66.7% to total variance than intraspecific differences, on average 23.3% for all traits except for Rdmass(Table S2). The effects of altitude were significant for most traits (Table S3, P < 0.05).
For bivariate relationships, area-based correlations were usually stronger than those of mass-based correlations of photosynthetic traits (Fig.1), or between photosynthetic traits and structural traits (LMA, LDMC, and LA) (Figs. 2, 3, 4 ), except for correlations between LDMC and Amax(Fig.3), and LA and N contents (Fig.4). Bivariate relationships were significantly different among shrub and tree species between Nareaand Nmassand other traits (Figs. S1–S4). The three paired relationships, (Rdmassvs. LMA and LA vs. Narea, and Nareavs. Rdarea), significantly changed with altitude gradient (Fig.5). The strengths of the relationships of the first two declined significantly while that of Nareaversus Rdareaincreased significantly (Fig.5).
Altitudinal leaf trait variations and effects of precipitation and temperature
Aarea, Rdmass, Rdarea, Narea, PNUE, LMA and LDMC displayed significant quadratic patterns with increasing elevation, while LA decreased linearly (Fig.6). These patterns were consistent for all woody species. Regression analyses, especially between leaf traits and elevation, showed that the relationships were stronger for area-based values than that for mass-based values (Fig.6).
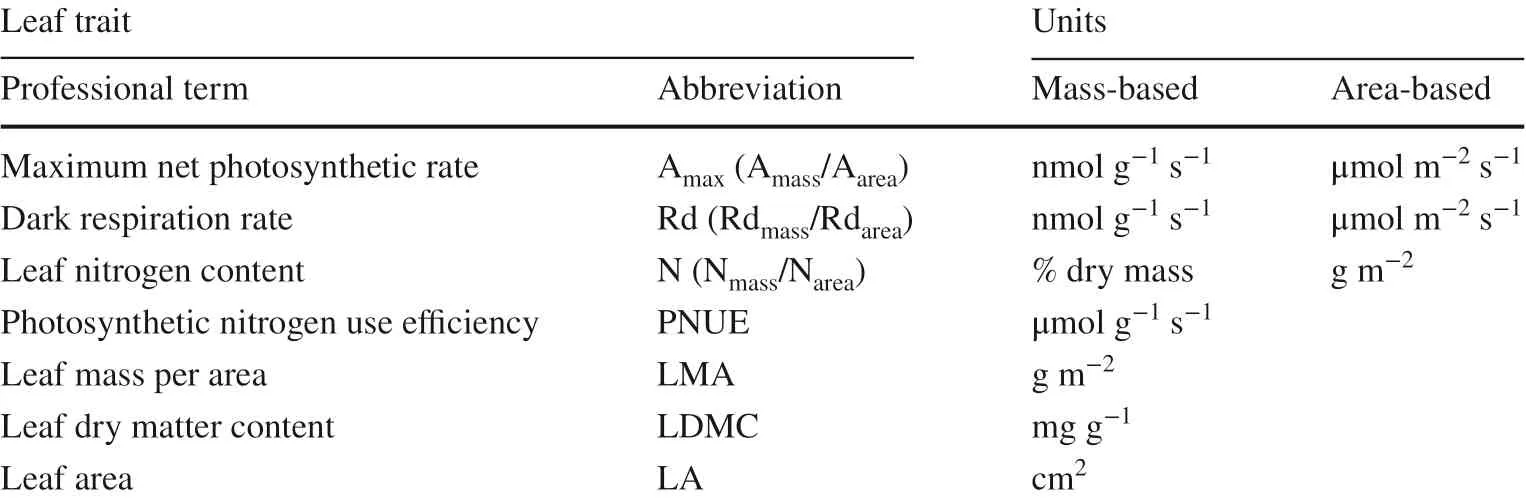
Table 2 Leaf traits, abbreviations and their units on a mass basis and/or an area basis
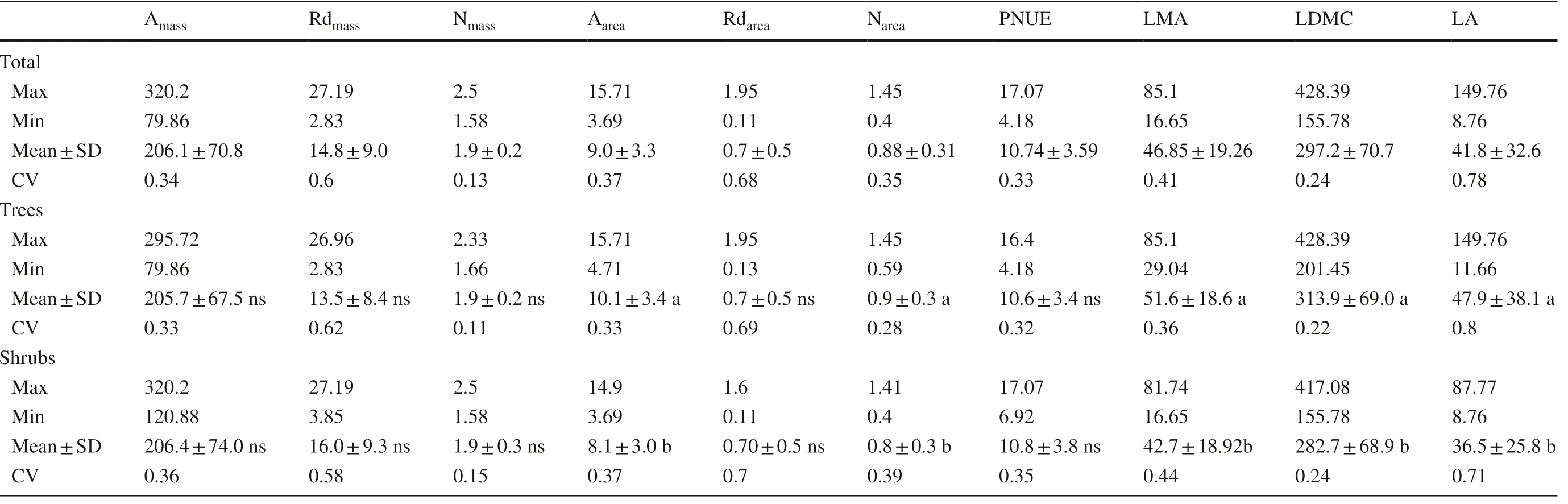
Table 3 Variation of leaf traits across all species (n = 43) and among diff erent life forms
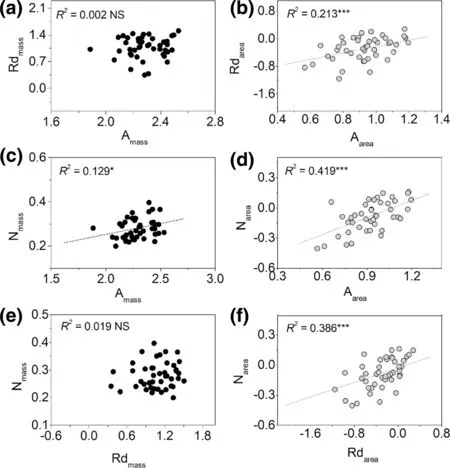
Fig.1 Patterns of bivariate relationships among Amax, Rd and leaf N across all species. All axes are log-scaled and based on either mass or area. The regression line is shown if the relationship was significant ( P < 0.05). * indicates significant effect. “NS” means no significant relationship ( P > 0.05)
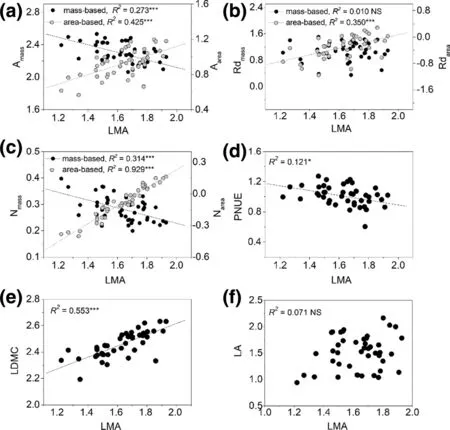
Fig.2 Bivariate relationships between LMA and other leaf traits across all species. All axes are log-scaled, and the values of A max , Rd and leaf N are presented based on both mass and area. The regression line is shown if the relationship was significant ( P < 0.05). * indicates significant effect. “NS” means no significant relationship ( P > 0.05)
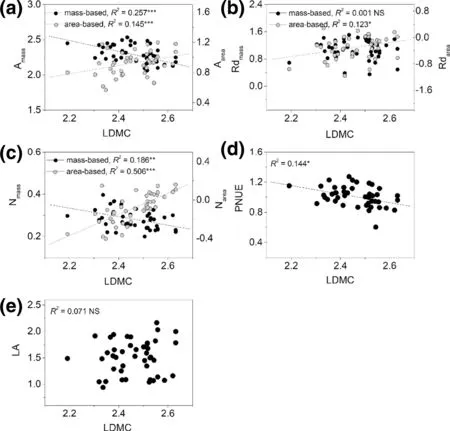
Fig.3 Bivariate relationships between LDMC and other leaf traits across all species. All axes are log-scaled, and the values of A max , Rd and leaf N are presented based on both mass and area. The regression line is shown if the relationship was significant ( P < 0.05). *indicates significant effect. “NS” means no significant relationship ( P > 0.05)
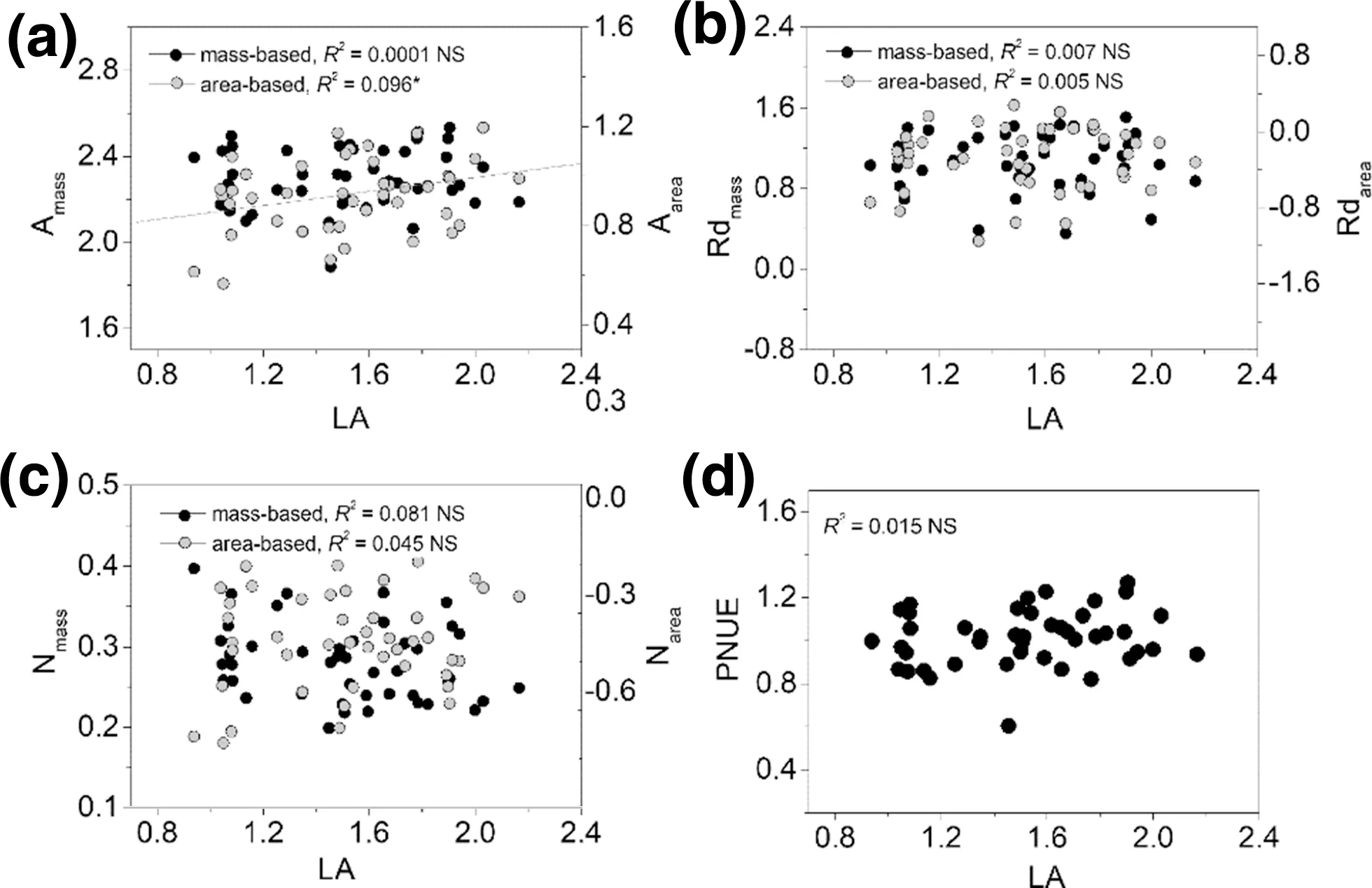
Fig.4 Bivariate relationships between LA and other leaf traits across all species. All axes are log-scaled, and the values of A max , Rd and leaf N are presented based on both mass and area. The regression line is shown if the relationship was significant ( P < 0.05). * indicates significant effect. “NS” means no significant relationship ( P > 0.05)
Temperature had significant effects on most traits except for Nmass, while these traits, except for leaf area (LA) showed similar quadratic response patterns to temperature as with altitude (Fig.S5). Leaf area increased linearly with increasing temperature (Fig.S5). The effects of precipitation on Aarea, Rdarea, Narea, LMA and LDMC were linear, while Rdmassand Nmassvaried nonlinearly with increasing precipitation (Fig.S6). Tree and shrub species showed similar response patterns for a specific trait along temperature and precipitation gradients.
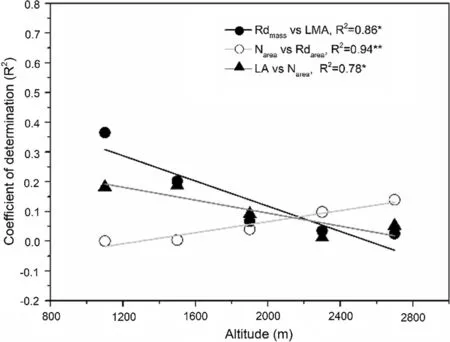
Fig.5 Altitudinal variation of bivariate relationships based on coefficient of determination. Only significant relationships are shown. * indicates significant effect
Discussion
Variations of leaf traits and bivariate relationships across species
The results indicate that there are large variations in leaf traits, (especially for leaf area) across woody species in the Qinling Mountains, and altitude and growth form have significant influences on the variations of most of these traits (Table 3, Table S3). These results indicate species adaptation to temperature and precipitation with altitude and the vertical changes in light conditions through the canopy (Santiago and Wright 2007). The effects of temperature and the associated climatic variables on variations in leaf traits with altitude have also been reported in previous studies (Poorter et al. 2009; van de Weg et al. 2009; Almeida et al. 2013; Read et al. 2014; Guo et al. 2018). Species turnover in particular facilitates variations in leaf traits with altitude because there is a greater contribution of interspecific variability than intraspecific variability to the overall functional variability for all traits except for mass-based dark respiration rates (Rdmass). Therefore, species-specific leaf traits can affect the adaptation of plants to microhabitats, resulting in species turnover and changes in ecological strategies along an altitudinal gradient.
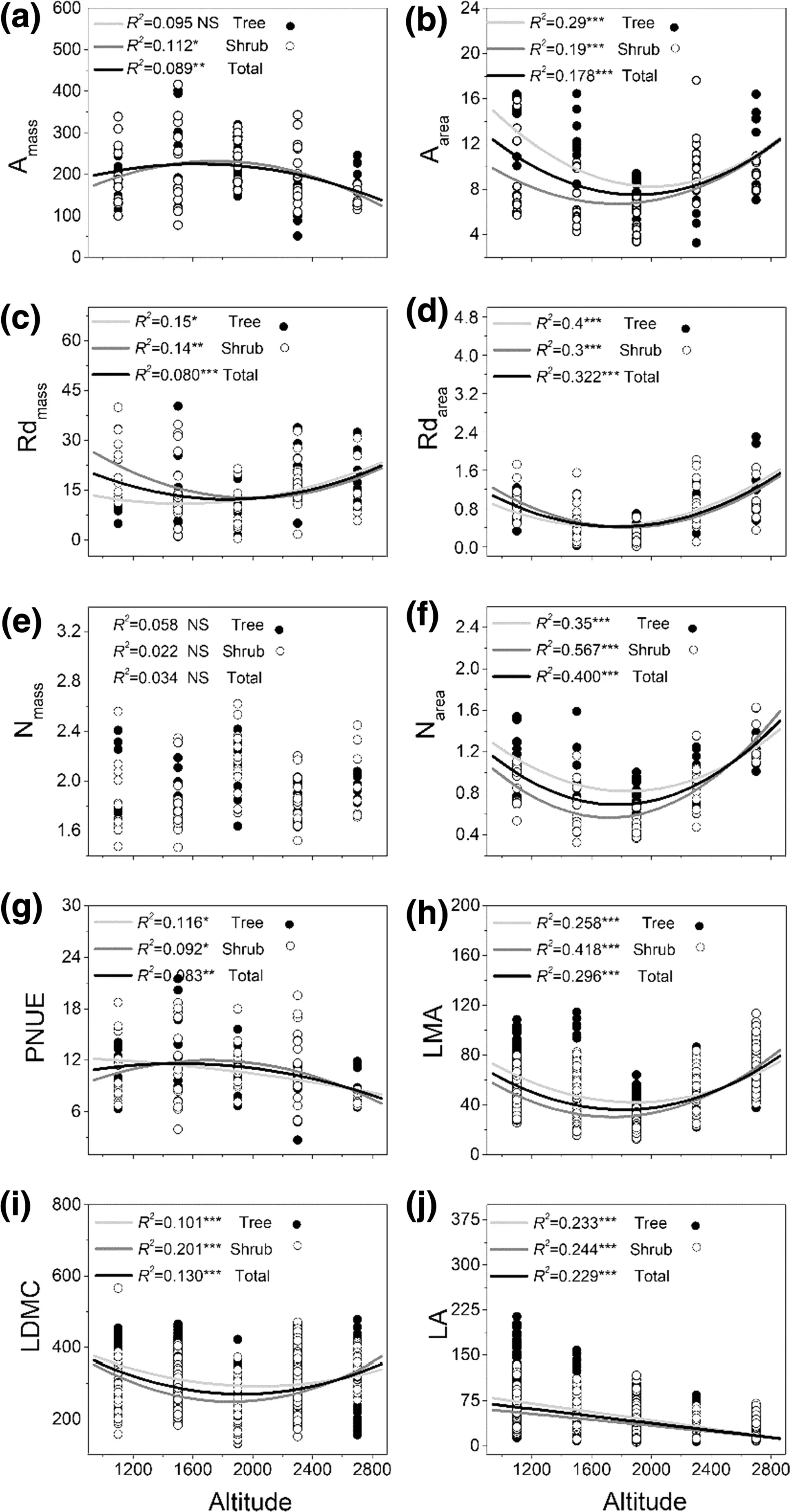
Fig.6 Altitudinal variation of leaf traits. Regression lines are displayed when the relationships that were significant. * indicates significant effect. “NS” means no significant relationship ( P > 0.05)
In recent years, there has been a debate over which traits provide a more informative insight into leaf functioning, with emphasis on the usefulness of area- versus mass-based expressions of photosynthetic capacity (Lloyd et al. 2013; Osnas et al. 2013; Niinemets et al. 2014). The trend of expressing leaf traits on a mass basis has been increasing because photosynthetic characteristics show stronger interrelations with each other and with other related parameters when they are considered on a mass rather than an area basis (Wright et al. 2004b; Funk and Cornwell 2013; Xiang et al. 2013; Sessa and Givnish 2014; Wang et al. 2016). The global leafeconomics spectrum suggests strong correlations among per-unit-mass traits. In contrast, in this study, bivariate correlations among most leaf traits were more strongly expressed on an area basis (Figs. 1, 2, 3, 4), indicating more pronounced variations per area than per mass in maximum rate of net photosynthesis (Amax), dark respiration rate (Rdark), and nitrogen content. A recent study examined the necessary conditions for the stronger correlation in the Amass–Nmassthan in the Aarea–Narearelationship (Hikosaka 2004): (1) a negative relationship between leaf mass per area (LMA) and photosynthetic nitrogen use effi ciency (PNUE) across species, since plants cannot maximize both PNUE and leaf toughness and need to allocate more biomass and nitrogen to create thick cell walls (Onoda et al. 2017). In fact, leaf mass per area is a key structural variable since variations in area-based photosynthetic rates (Aarea) and nitrogen contents can be traced back to variations in leaf mass per area (Rijkers et al. 2000); and, (2) Low Nmassbut similar Nareain low PNUE species (Hikosaka 2004). A negative correlation between photosynthetic nitrogen use effi ciency and leaf mass per area was confirmed in this study, which is in line with Rijkers et al. ( 2000), while neither Nmassnor Nareaare related to PNUE. This confirms that leaf nitrogen is allocated to physiological processes other than photosynthetic carbon acquisition (Lloyd et al. 2013). We focused along an altitude gradient and species at different altitudes respond to changes in the light environment through active modifications in area-based maximum net photosynthesis or passive acclimation of leaf structure (e.g., leaf mass per area) as a result of different nitrogen allocation (Hikosaka 2004). This observation has also been found in a study analyzing within-canopy variations in leaf structure and chemical and physiological traits, confirming strong correlations among area-based traits at a small- scale (Niinemets et al. 2014). In fact, compared with worldwide variations in leaf traits, variations at a small-scale are more compressed and likely to show opposite relationships. In the present study, there were significantly different bivariate relationships among shrub and tree species between Nareaor Nmassand other traits. This suggests that both forms of species have different nitrogen utilization strategies. Overall, these results emphasize that variations in photosynthetic acclimation may lead to different connections of leaf traits, even at a local scale, and provide a new understanding in the role of mass- versus area-based traits in vegetation acclimation.
Altitudinal patterns of leaf traits on area- and mass-based expressions
Both quadratic and linear responses in leaf traits have been found along altitudinal gradients (van de Weg et al. 2009; Almeida et al. 2013; Read et al. 2014; Guo et al. 2018). In our dataset, most leaf trait values varied nonlinearly along the gradient except for leaf area and mass-based nitrogen contents. Species at mid altitudes showed high mass-based maximum net photosynthesis and photosynthetic nitrogen use effi ciencies, low dark respiration rates, area-based maximum net photosynthesis rates, area-based nitrogen contents, leaf dry matter contents, and leaf mass per area values. First of all, these patterns are related to changes in biotic and abiotic factors along an altitudinal gradient (van de Weg et al. 2009; Villar et al. 2013). Secondly, variations in photosynthetic net use effi ciency, area-based maximum net photosynthesis and nitrogen levels can be traced back to variations in leaf mass per area. In our study, mass-based leaf N contents were stable with altitude, while the leaf mass per area declined at mid altitude; both factors result in low area-based leaf nitrogen contents at mid altitudes. Area-based maximum net photosynthesis is strongly affected by various area-based nitrogen contents (Niinemets 2002; Niinemets et al. 2014), and therefore shows a similar pattern. Area-based leaf traits (e.g., maximum net photosynthesis, dark respiration rates, and nitrogen levels) were especially closely related to altitude than those of mass-based traits. Amassand Aareashowed contrasting patterns along the altitude gradient. This is partially in line with a study which found that many area-based physiological traits increased linearly with tree height, while mass-based traits only slightly increased (Kenzo et al. 2015). In this study, this weak relationship of leaf mass-based photosynthesis may be caused by compressed leaf traits at a local scale compared with worldwide variations in leaf traits (Niinemets et al. 2014). On the other hand, this confirms the appropriateness and utility of area-based expressions when considering small-scale variations in photosynthetic capacities (Westoby et al. 2013; Niinemets et al. 2014; Poorter et al. 2014). Further comprehensive studies of leaf traits (structural, chemical and allocational effi ciencies) with altitude should provide better understanding of the photosynthetic response of temperate forest species to altitude gradients. Another interesting result is that three paired relationships significantly changed with altitude. Declining coefficients of determination of Rdmassversus LMA and LA versus Nareaalong altitudinal gradients reveal that severe environments at high altitudes may decouple their functional links, while increasing R 2 of Nareaversus Rdareashow that functional links between nitrogen and respiration may be strengthen at high altitudes. This suggests that, for traits that link light interception to biomass invested in leaves, the roles of dark respiration, area-based nitrogen, and leaf area are very noticeable for their ecological variation across species along an altitudinal gradient.
Influences of precipitation and temperature changes to leaf traits with altitude
Maximum precipitation during the growing season and appropriate temperatures at mid-elevation allowed plants to invest more nitrogen in photosynthetic organs, resulting in minimum leaf mass per area, further bringing about increases in the light capture area and subsequently higher massed-based net photosynthesis and photosynthetic nitrogen use effi ciencies (Table 1). This is in agreement with the results of previous studies (Takashima et al. 2004; Onoda et al. 2017). However, these results also show that precipitation and temperature accounted for only 2.5–26.2% of the variations in leaf traits with altitude. Therefore, other factors such as light intensity and soil nutrition may also be important drivers of leaf traits variations with altitude (Zhang et al. 2005). According to previous research, soil organic carbon and total soil nitrogen displayed a risingfirst-and-declining-later trend with elevation in the Qinling Mountains, while total soil phosphorous varied much less spatially (Li et al. 2017). Moreover, temperature, water content and vegetation explain clearly variations ofecological stoichiometry of soil carbon, nitrogen and phosphorus. Therefore, the functional composition of a plant community is the result of a hierarchy of filters that select species from a local species pool (Adler et al. 2013), and the variations in leaf traits along an altitudinal gradient are influenced by multiple environmental factors rather than one single factor.
Conclusions
At a local scale in the Qinling Mountains, bivariate correlations among most leaf traits of woody species were stronger when expressed on a leaf-area basis rather than on a mass basis, which results from higher variations in leaf area. Most traits showed quadratic trends with altitude and area-based traits such as net photosynthesis, dark respiration rates and nitrogen levels were more closely related to altitude than mass-based values. Precipitation and temperature are important drivers influencing these variation patterns. The present study not only found dynamic co-variations between environment and plants in certain environmental gradients but further promotes understanding ofecological variations of plant leaf traits with altitude.
AcknowledgementsWe gratefully acknowledge the assistance of Pengcheng Wan and Mao Wang with field work and Jiangchao Guo with data processing.
References
Adler PB, Fajardo A, Kleinhesselink AR, Kraft NJB (2013) Trait-based tests of coexistence mechanisms. Ecol Lett 16:1294–1306
Almeida JP, Montúfar R, Anthelme F (2013) Patterns and origin of intraspecific functional variability in a tropical alpine species along an altitudinal gradient. Plant Ecol Divers 6:423–433
Bassman JH, Zwier JC (1991) Gas exchange characteristics of Populus trichocarpa, Populus deltoides and Populus trichocarpa × P. deltoides clones. Tree Physiol 8:145–159
Berli FJ, Alonso R, Bressan-Smith R, Bottini R (2013) UV-B impairs growth and gas exchange in grapevines grown in high altitude. Physiol Plantarum 149:127–140
Blonder B, Violle C, Bentley LP, Enquist BJ (2011) Venation networks and the origin of the leafeconomics spectrum. Ecol Lett 14:91–100
Bloomfield KJ, Cernusak LA, Eamus D, Ellsworth DS, Prentice IC, Wright IJ, Boer MM, Bradford MG, Cale P, Cleverly J, Egerton JJG, Evans BJ, Hayes LS, Hutchinson MF, Liddell MJ, Macfarlane C, Meyer WS, Prober SM, Togashi HF, Wardlaw T, Zhu L, Atkin OK (2018) A continental-scale assessment of variability in leaf traits: within species, across sites and between seasons. Funct Ecol. https://doi.org/10.1111/1365-2435.13097
Bu HY, Jia P, Qi W, Liu K, Xu DH, Ge WJ, Wang XJ (2018) The effects of phylogeny, life-history traits and altitude on the carbon, nitrogen, and phosphorus contents of seeds across 203 species from an alpine meadow. Plant Ecol 219:737–748
Chai YF, Zhang XF, Yue M, Liu X, Li Q, Shang HL, Meng QC, Zhang RC (2015) Leaf traits suggest different ecological strategies for two Quercus species along an altitudinal gradient in the Qinling Mountains. J Forest Res 20:501–513
Dubuis A, Rossier L, Pottier J, Pellissier L, Vittoz P, Guisan A (2013) Predicting current and future spatial community patterns of plant functional traits. Ecography 36:1158–1168
Dunne JA, Harte J, Taylor KJ (2003) Subalpine meadow flowering phenology responses to climate change: integrating experimental and gradient methods. Ecol Monogr 73:69–86
Fujii S, Cornelissen JHC, Berg MP, Mori AS (2018) Tree leaf and root traits mediate soil faunal contribution to litter decomposition across an elevational gradient. Funct Ecol 32:840–852
Funk JL, Cornwell WK (2013) Leaf traits within communities: context may affect the mapping of traits to function. Ecology 94:1893–1897
Gong Y, Ling H, Lv G, Chen Y, Guo Z, Cao J (2019) Disentangling the influence of aridity and salinity on community functional and phylogenetic diversity in local dryland vegetation. Sci Total Environ 653:409–422
Guo Z, Lin H, Chen S, Yang Q (2018) Altitudinal patterns of leaf traits and leaf allometry in bamboo Pleioblastus amarus. Front Plant Sci. https://doi.org/10.3389/fpls.2018.01110
Hikosaka K (2004) Interspecific difference in the photosynthesis–nitrogen relationship: patterns, physiological causes, and ecological importance. J Plant Res 117:481–494
Hu YB, Bellaloui N, Sun GY, Tigabu M, Wang JH (2014) Exogenous sodium sulfide improves morphological and physiological responses of a hybrid Populus species to nitrogen dioxide. J Plant Physiol 171(10):868–875
Kenzo T, Inoue Y, Yoshimura M, Yamashita M, Tanaka-Oda A, Ichie T (2015) Height-related changes in leaf photosynthetic traits in diverse Bornean tropical rain forest trees. Oecologia 177:191–202
Kraft NJB, Valencia R, Ackerly DD (2008) Functional traits and nichebased tree community assembly in an Amazonian forest. Science 322:580–582
Krix DW, Murray BR (2018) Landscape variation in plant leaf flammability is driven by leaf traits responding to environmental gradients. Ecosphere 9:e02093. https://doi.org/10.1002/ecs2.2093
Li DW, Wang ZQ, Tian HX, He WX, Geng ZC (2017) Carbon, nitrogen and phosphorus contents in soils on Taibai Mountain and their ecological stoichiometry relative to elevation. Acta Pedol Sin 54:160–170
Lloyd J, Bloomfield K, Domingues TF, Farquhar GD (2013) Photosynthetically relevant foliar traits correlating better on a mass versus an area basis: ofecophysiological relevance or just a case of mathematical imperatives and statistical quicksand? New Phytol 199:311–321
Malhi Y, Silman M, Salinas N, Bush M, Meir P, Saatchi S (2010) Introduction: elevation gradients in the tropics: laboratories for ecosystem ecology and global change research. Glob Change Biol 16:3171–3175
Niinemets ü (2002) Stomatal conductance alone does not explain the decline in foliar photosynthetic rate with increasing tree age and size in Picea abies and Pinus sylvestris. Tree Physiol 22:515–535
Niinemets ü, Keenan TF, Hallik L (2014) A worldwide analysis of within-canopy variations in leaf structural, chemical and physiological traits across plant functional types. New Phytol 205:973–993
Onoda Y, Wright IJ, Evans JR, Hikosaka K, Kitajima K, Niinemets ü (2017) Physiological and structural tradeoff s underlying the leaf economics spectrum. New Phytol 214:1447
Osnas JLD, Lichstein JW, Reich PB, Pacala SW (2013) Global leaf trait relationships: mass, area, and the leafeconomics spectrum. Science 340:741–744
Poorter HNU, Poorter L, Wright IJ, Villar R (2009) Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytol 182:565–588
Poorter H, Lambers H, Evans JR (2014) Trait correlation networks: a whole-plant perspective on the recently criticized leafeconomic spectrum. New Phytol 201:378–382
Read QD, Moorhead LC, Swenson NG, Bailey JK, Sanders NJ (2014) Convergent effects ofelevation on functional leaf traits within and among species. Funct Ecol 28:37–45
Rijkers T, Pons TL, Bongers F (2000) The effect of tree height and light availability on photosynthetic leaf traits of four neotropical species differing in shade tolerance. Funct Ecol 14:77–86
Rose L, Rubarth MC, Hertel D, Leuschner C (2013) Management alters interspecific leaf trait relationships and trait-based species rankings in permanent meadows. J Veg Sci 24:239–250
Ryser P, Bernardi J, Merla A (2008) Determination of leaf fresh mass after storage between moist paper towels: constraints and reliability of the method. J Exp Bot 59:2461–2467
Santiago LS, Wright SJ (2007) Leaf functional traits of tropical forest plants in relation to growth form. Funct Ecol 21:19–27
Saura-Mas S, Shipley B, Lioret F (2009) Relationship between postfire regeneration and leafeconomics spectrum in Mediterranean woody species. Funct Ecol 23:103–110
Sessa EB, Givnish TJ (2014) Leaf form and photosynthetic physiology of Dryopteris species distributed along light gradients in eastern North America. Funct Ecol 28:108–123
Takashima T, Hikosaka K, Hirose T (2004) Photosynthesis or persistence: nitrogen allocation in leaves ofevergreen and deciduous Quercus species. Plant, Cell Environ 27:1047–1054
Tan Q, Wang G (2016) Decoupling of nutrient element cycles in soil and plants across an altitude gradient. Sci Rep 6:34875
Tang ZY, Fang JY (2006) Temperature variation along the northern and southern slopes of Mt. Taibai, China. Agric For Meteorol 139:200–207
van de Weg MJ, Meir P, Grace J, Atkin OK (2009) Altitudinal variation in leaf mass per unit area, leaf tissue density and foliar nitrogen and phosphorus content along an Amazon-Andes gradient in Peru. Plant Ecol Divers 2:243–254
Villar R, Ruiz-Robleto J, Ubera JL, Poorter H (2013) Exploring variation in leaf mass per area (LMA) from leaf to cell: an anatomical analysis of 26 woody species. Am J Bot 100:1969–1980
Vitória AP, Alves LF, Santiago LS (2019) Atlantic forest and leaf traits: an overview. Trees. https://doi.org/10.1007/s0046 8-019-01864-z
Wang Z, Liu X, Bao WK (2016) Higher photosynthetic capacity and different functional trait scaling relationships in erect bryophytes compared with prostrate species. Oecologia 180:359–369
Wang X, Wiegand T, Anderson-Teixeira KJ, Bourg NA, Hao Z, Howe R, Jin G, Orwig DA, Spasojevic MJ, Wang S, Wolf A, Myers JA (2018) Ecological drivers of spatial community dissimilarity, species replacement and species nestedness across temperate forests. Glob Ecol Biogeogr 27:581–592
Warton DI, Wright IJ, Falster DS, Westoby M (2006) Bivariate linefitting methods for allometry. Biol Rev 81:259–291
Westoby M, Reich PB, Wright IJ (2013) Understanding ecological variation across species: area-based versus mass-based expression of leaf traits. New Phytol 199:322–323
Wright IJ, Groom PK, Lamont BB, Poot P, Prior LD, Reich PB, Schulze ED, Veneklaas EJ, Westoby M (2004a) Leaf traits relationships in Australian plant species. Func Plant Biol 31:551–558
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin FS, Cornelissen JHC, Diemer M, Flexas J, Garnier E, Groom PK, Gulias J, Hikosaka K, Lamont BB, Lee T, Lee W, Lusk C, Midgley JJ, Navas ML, Niinemets ü, Oleksyn J, Osada N, Poorter H, Poot P, Prior L, Pyankov VI, Roumet C, Thomas SC, Tjoelker MG, Veneklaas EJ, Villar R (2004b) The world-wide leafeconomics spectrum. Nature 428:821–827
Wright IJ, Reich PB, Cornelissen JHC, Falster DS, Groom PK, Hikosaka K, Lee W, Lusk CH, Niinemets ü, Oleksyn J, Osada N, Poorter H, Warton DI, Westoby M (2005) Modulation of leafeconomic traits and trait relationships by climate. Glob Ecol Biogeogr 14:411–421
Xiang S, Reich PB, Sun SC, Atkin OK (2013) Contrasting leaf trait scaling relationships in tropical and temperate wet forest species. Funct Ecol 27:522–534
Xu DQ (2006) Some noteworthy problems in measurement and investigation of photosynthesis. Plant Physiol Commun 42:1163–1167
Yue M, Zhou H (1997) Diversity of higher plants in deciduous broadleaved forests on the northern slope of Taibai Mountain. Acta Bot Yunnanica 19:171–176 (in Chinese with English abstract)
Zhang SB, Zhou ZK, Hu H, Xu K, Yan N, Li SY (2005) Photosynthetic performances of Quercus pannosa vary with altitude in the Hengduan Mountains, southwest China. Forest Ecol Manag 212:291–301
Zheng SX, Shangguan ZP (2007) Spatial patterns of photosynthetic characteristics and leaf physical traits of plants in the Loess Plateau of China. Plant Ecol 191:279–293
 Journal of Forestry Research2021年2期
Journal of Forestry Research2021年2期
- Journal of Forestry Research的其它文章
- Stem taper functions for Betula platyphylla in the Daxing’an Mountains, northeast China
- Leaf phenotypic variation of endangered plant Tetracentron sinense Oliv. and influence of geographical and climatic factors
- Variations in stem radii of Larix principis-rupprechtii to environmental factors at two slope locations in the Liupan Mountains, northwest China
- A review of ecological mechanisms for management practices of protective forests
- Variation of basic density, calorific value and volumetric shrinkage within tree height and tree age of Ugandan grown Eucalyptus grandis wood
- Natural variations in flavonoids and triterpenoids of Cyclocarya paliurus leaves
