Waterlogging tolerance and wood properties of transgenic Populus alba × glandulosa expressing Vitreoscilla hemoglobin gene ( Vgb)
Yiliang Li · Weixi Zhang · Wenxu Zhu · Bingyu Zhang · Qinjun Huang · Xiaohua Su
Abstract Because overexpression of Vitreoscilla hemoglobin gene ( Vgb) gene in plants can enhance tolerance to waterlogging, here Vgb was inserted into Populus alba × glandulosa to investigate its expression and effects on growth and physiological responses to waterlogging stress in the transgenic poplars. Southern blotting and RT-PCR analysis of Vgb-transgenic P. alba × glandulosa showed that the Vgb gene was integrated into the genome of the V13-81 and V13-85 transgenic lines and expressed. In greenhouse waterlogging stress tests, mortality of the transgenic poplar was significant lower than that of nontransgenic plants with increasing treatment time from 2 to 22 days. The transgenic plants had higher chlorophyll content and less chloroplast damage than in the control plants. Additionally, starch accumulation increased, and growth was enhanced in the transgenic plants, suggesting that the Vgb-expressing lines had improved energy reserves. Field trials of the transgenic poplar suggested that Vgb expression promotes growth and influences wood quality. Taken together, our results suggest that the expression of Vgb can increase the accumulation of chlorophyll and starch in the transgenic poplar, improve its ability to endure flooding, and improve growth and wood quality of the transgenic plants.
Keywords Populus alba × glandulosa · vgb gene · Waterlogging stress · Growth · Microscopy
Introduction
Water is the primary determinant of the productivity of a plant ecosystem, but too much water (waterlogging) or too little (drought) is harmful to plants. China suffers from serious flooding: 2/3 of the land area has different degrees of waterlogging. Frequent floods cause huge losses of human lives and property. Trees play a very important role in improving the environment; however, some of them lack high tolerance to waterlogging.
Oxygen is necessary for respiratory metabolism, and increasing plant cells’ oxygen content can promote plant growth. In waterlogged soil, the stress from decreased oxygen limits plant growth. The enzyme Vitreoscilla hemoglobin (VHb), produced by the aerobe Vitreoscilla during low oxygen stress (H?ggman et al. 2003), can increase intracellular oxygen levels and advance the activity of terminal oxidases when expressed in Escherichia coli and Populus × euramericana ‘Guariento’, under waterlogging stress (Tsai et al. 1995; Su et al. 2011). The Vhb gene from Vitreoscilla stercorariacodes encodes a hemoglobin-like protein which is proposed to have a substantial oxygen transfer function to its host under conditions of limited oxygen (Wakabayashi et al. 1986; Webster 1987). The overexpression of Vhb encoding VHb in hybrid aspen ( Populus tremula × tremuloides) seems to alter energy metabolism through increasing the relative volume of mitochondria, starch accumulation in chloroplasts, total flavonoid and certain individual flavonoids, but elongation growth was not generally improved in their greenhouse conditions (H?ggman et al. 2003). Other studies showed that overexpression of Vhb improves growth and dry mass of Nicotiana tabacum (Holmberg et al. 1997), Datura innoxia (Bülow et al. 1999) and Petunia hybrida Vilm (Mao et al. 2003); enhances the waterlogging tolerance of Populus × euramericana ‘Guariento’ (Su et al. 2011), and influences the metabolism of Vitis vinifera (Martinelli et al. 2013). On the other hand, some studies have shown that, hemoglobin genes exist in nearly all plant species (Hebelstrup et al. 2007), and overexpression of Nonsymbiotic hemoglobin (nsHb) in plants can enhance tolerance to salt stress of tobacco (Trevisan et al. 2011), stomatal conductance and transpiration of tomato when roots are exposed to waterlogging stress (Shi et al. 2012), seed yield of Arabidopsis (Thiel et al. 2011; Vigeolas et al. 2011), and possibly biomass by regulating the levels of nitric oxide (Hebelstrup et al. 2014; Misra et al. 2014). If overexpression of a hemoglobin gene in plant can enhance stress tolerance, growth, seed yield and other variables, then it could be a useful gene for breeding programs to improve tree. However, the cited studies were done in a greenhouse without any field tests, and wood properties of the transgenic trees were not compared with those of the wild-type lines.
Populus alba × glandulosa, introduced to China in 1984, is cultivated widely in North and Northwest China; however, the poor tolerance of poplar to waterlogging has resulted in huge losses of poplar production in South China because of heavy rainfall and flooding. Improving waterlogging tolerance would aid economic reconstruction and help conserve soil and water in the region. In this study, we inserted the Vhb gene V. stercorariacodes into P. alba × glandulosa and confirmed its integration and expression and analyzed the growth and physiological responses to waterlogging stress in the transgenic poplars (V13-81 and V13-85). The transgenic trees were also grown in field trials and their wood properties tested to evaluate the effects of the expression of the exogenous gene. This study provides a more in-depth understanding of the function of heterologous VHb and to advance molecular breeding programs aimed at improving waterlogging tolerance in plants.
Materials and methods
Ethics statement
All approvals for field trials of transgenic poplar were obtained from the State Forestry Administration, People’s Republic of China, under application number 2005-05.
Plant material and transgene
An interspecific hybrid of P. alba × P. glandulosa was used in this study. Briefly, Vgb was inserted into the poplar using Agrobacterium tumefaciens-mediated transformation. The transgenic poplar lines were produced as described in our previous study. The Vgb gene was under the control of the CaMV 35S promoter. Transgenic lines containing the exogenous gene were identified by PCR and Southern dot hybridization. Two Vgb-transgenic plants, V13-81 and V13-85, and one control (wild-type line, 13#) were chosen for further study in 2000 (Zhang et al. 2005).
Southern blotting
Genomic DNA from transgenic and control plants was extracted by the CTAB method (Lodhi et al. 1994) with slight modifications. Total DNA of both transgenic and control lines was digested with restriction enzyme HindIII, separated by electrophoresis in 0.8% agarose, then transferred to a Hybond-N + nylon membrane. The Vgb gene was labeled with an oligonucleotide random primer extension kit (Promega, China, Shanghai) for use as a probe for Southern blotting, which was done with the ECL direct nucleic acid labeling and detection system (Amersham, Buckinghamshire, UK), according to the manufacturer’s instructions.
Reverse transcription (RT) -PCR
An RNeasy Plant Mini Kit (Qiagen, China, Shanghai) was used to extract total RNA from the leaves of the transgenic plants. cDNA was synthesized from 1 μg total RNA using a RevertAid First Strand cDNA Synthesis Kit. The ubiquitin gene was used as an internal reference gene. The ubiquitin primers were P1 (5′-TGA GGC TTA GGG GAG GAA CT-3′) and P2 (5′-TGT AGT CGC GAG CTG TCT TG-3′). The primers for the Vgb gene were P1 (5′-GCG AAG CTT CCC TCA TGT TAG ACC AGC AAA -3′) and P2 (5′-GCG TCG ACG CGG CCT GAA ACT TTA TTCA-3′). The RT-PCR product for the ubiquitin gene was 620 bp and 440 bp for vgb. RNA from the wild-type hybrid poplars was used as the template for the negative control. PCR conditions for both genes were initial denaturation at 94 °C for 5 min; 30 cycles of 94 °C for 1 min, 60 °C for 1 min, 72 °C for 1 min; and a final extension at 72 °C for 10 min.
Greenhouse test of waterlogging tolerance of transgenic plants
In mid-April 2005, scions of transgenic and nontransgenic lines were inserted into plastic containers (30 cm tall, 24 cm diameter). The cutting medium comprised peat, perlite and sand (3:3:1), mixed evenly. Each container weighed 3.5 kg. The scions of different transgenic lines were pruned to be as consistent as possible in size and quality. Each container was used for one cutting and provided with normal water conditions. In early May, when the height of plants reached approximately 40 cm, 20 plants with similar growth were chosen from each line for the waterlogging stress experiment, with 10 replications. Waterlogging stress was provided by keeping the water level at 2 cm above the soil surface, and a plastic basin was placed under each container to keep the water in the soil in the containers. For the control (nonstressed), relative water content was kept at 60–80% by weighing container when watering every 5 days. Chlorophyll was extracted with 80% acetone from 0.2 g of fresh leaf tissue, then absorbance was measured at 663, 645, and 652 nm, and total chlorophyll was calculated as described previously (Inskeep and Bloom 1985). The amount of growth was determined by measuring the height and basal diameter of each plant after 22 days of waterlogging stress. At least three plants each from the transgenic and control lines from each treatment were sampled to measure biomass. Root, stem and leaves were oven-dried at 105 °C for 15 min. The plant tissue was left undisturbed for 60 h at 75 °C, and each sample was weighed separately.
Microscopy and morphometric analyses
For the light and electron microscopy, samples were cut from the middle of full-sized leaves outside the mid-vein and fixed for 4 h in 2.5% v/v glutaraldehyde in 0.1 M sodium phosphate buffer containing 1 mM MgCl2, pH 7.2. The cuttings were rinsed in the same buffer, post-fixed in 1% w/v OsO4in 0.1 M sodium phosphate buffer, and dehydrated in ethanol (modified from H?ggman et al. 2003 ). Sections of 60–80 nm in thickness were cut, stained with toluidine blue and photographed with a Leitz UC6 microscope. Ultrathin sections were cut in random orientations, stained with uranyl acetate and lead citrate, and imaged at 25,000 × with a Gatan (Pleasanton, CA, USA) 832 transmission electron microscope.
Field trials
For growth and wood quality analyses, cuttings of the transgenic lines V13-81 and V13-85 and the control line that were studied in the waterlogging stress experiments were planted in the nursery of the Chinese Academy of Forestry, Beijing, on April 2007. Sixteen plants ofeach line were planted in a square (four rows by four columns) with 40 cm × 50 cm spacing between trees. On October 2008, nine growing trees (2 years old) ofeach line were selected to measure growth and the physical and chemical characteristics of the wood.
Wood quality test
Wood quality was tested by the College of Material Science and Engineering, Northeastern Forestry University (Harbin). For physical properties, the drainage method was performed using the measured basic density; the conventional segregation method was performed using the measured fiber length, width, and microfibrillar angles, each line was measured in three plants as replicates. The GB2677.3-81, GB2677.5-81, GB2677.7-81, GB2677.8-81, GB2677.9-81 and GB2677.10-81 standards were used to measure the water, α-cellulose, holocellulose and total lignin content in the wood ofeach line; each line was measured in three plants as replicates.
Statistical analyses
One-way analysis of variance (ANOVA, α = 0.05) was used to analysis the differences between the transgenic and control lines for growth, physiological and wood quality properties, and multiple comparison of means was done using Duncan’s multiple range test. All analyses were done with Data Processing System (DPS) software (Tang and Feng 2002).
Results
Production of transgenic lines
Five transgenic lines were chosen randomly for Southern blot assays, four of the lines performed with confirmed to have Vgb integrated into the genome. A single-copy insertion of Vgb was found in these lines (Fig.1). The RT-PCR showed that Vgb was expressed in the V13-81 and V13-85 transgenic lines, but not in the non-transgenic control (Fig.2).
Evaluation of waterlogging tolerance
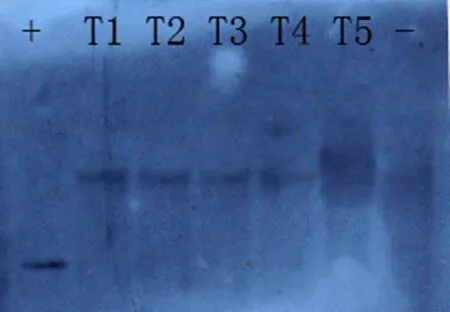
Fig.1 Southern blot analysis of Vitreoscilla hemoglobin ( Vgb) gene in transgenic and nontransgenic poplar. +, Positive control, T1–T4: transgenic lines (T2, V13-81; T3, V13-85); ?, nontransgenic plant
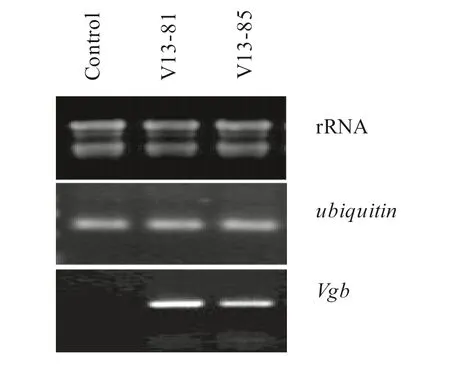
Fig.2 Reverse transcription (RT)-PCR analysis of Vgb expressed in transgenic poplars. Poplar ubiquitin gene was used as the reference gene
Waterlogging stress significantly inhibited the growth of transgenic poplars and control lines. After 2 days of waterlogging stress, some leaves of the control had yellowed and begun to wilt, and some leaves had dropped off by 4 days. Some control plants had died after 7 days, and by 12 days, only 50% of the control plants were alive. The survival rate was 33.33% after 17 days (Fig.3). Compared to the control, the leaves of transgenic lines showed little wilting at the early stage of waterlogging, and few lines had died by the end of the experiment (22 days). The survival rate was 71.43–75%, more than two times higher than that of control (Table 1).
After waterlogging treatment for 22 days, the heights and basal diameters were significantly greater in the transgenic lines relative to the control line. The heights of transgenic lines V13-81 and V13-85 lines were 9.66% (151.33 ± 1.86) and 11.11% (153.33 ± 0.67) significantly greater under normal conditions and 79.50% (119.67 ± 1.76) and 62.49% (108.33 ± 2.73) greater under waterlogging stress conditions compared with the control lines, respectively (138.00 ± 1.15, 66.67 ± 1.20). The transgenic lines also had a significantly larger basal diameter, 20.06–25% (0.82 ± 0.01, 0.85 ± 002) and 34–48% (0.67 ± 0.01b, 0.74 ± 0.01b) under normal and waterlogging stress, respectively, compared with the control line (Table 2).
The transgenic lines also showed a 53.51% (18.59 ± 0.63) and 67.96% (20.34 ± 0.25) significant increases of total biomass under normal conditions and835.29% (11.13 ± 0.20) and 733.61% (9.92 ± 0.14) under stress condition compared with the control plants, respectively. The biomasses of the roots, shoots and leaves of transgenic lines were also significantly higher than control both in normal and waterlogging condition (Table 3).
Chlorophyll assays
Leaf chlorophyll content was measured in fresh leaves of transgenic and control lines at the end of stress. The longer the waterlogging stress, the total chlorophyll content in the transgenic lines and control tended to decrease. After 7 days, the chlorophyll content of transgenic lines was significantly higher (11.76–14.71%) than in the control line. From 12 to 22 days, chlorophyll content in the transgenic and control lines remained relatively stable, with the content in the transgenic lines significantly higher (30.04–48.89%) than in the control plants (Fig.4). Data for chlorophyll content in individual plants of the transgenic and control lines are shown in Table 4.
Microscopy and morphometric analyses
Damage to leaves in transgenic lines and control after 22 days of waterlogging stress was ultrastructurally assessed using the 4–6th leaves. Mesophyll cells in leaves of transgenic lines remained long and columnar, the cell wall was intact, and the vacuole was clear, the chloroplast expands into hemisphere and the membrane of it was intact; The volume and number of starch granules in the matrix of chloroplasts was higher than in the wild type at 22 days, the mitochondria are abundant, and the matrix is dense (Fig.5 b). In the control cells, compared with the transgenic, cells were longer, and appeared to be plasmolyzed, the chloroplasts were longer, and the plasma membrane was blurred or degraded and had separated from the cell wall. The volume of the starch in the matrix was significantly smaller, the inner and outer membranes of the mitochondria were indistinct or broken, and small vacuoles had formed (Fig.5 a). All together, these data indicated that the leaves of control were more heavily damaged by waterlogging stress than those of the transgenic lines.
Field trial
Growth and wood quality of 2.5-year-old transgenic poplar trees in the field trial in Beijing were evaluated in September 2009. Height, basal diameter and biomass did notdiffer significantly between the transgenic and control lines. Biomass of the transgenic lines was 37.31–41.13% (fresh mass) and 19.46–25.13% (dry mass) greater than that of the control (Table 5), suggesting that the transgenic lines have the potential for better growth compared to control.
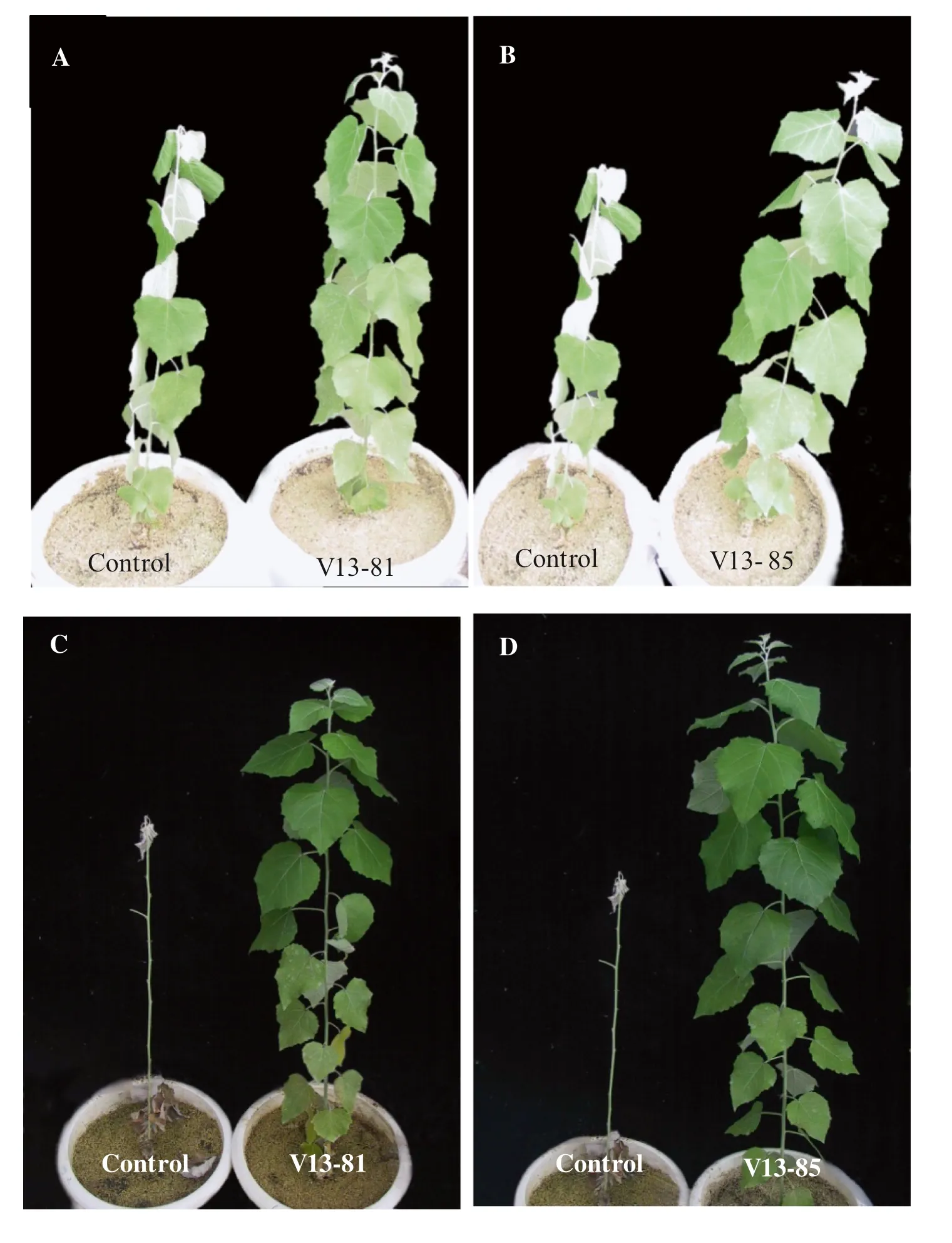
Fig.3 Effects of vgb expression on the growth of the wild type (control) and transgenic poplar lines V13-81 and V13-85 under waterlogging stress. a, b Four days of waterlogging stress. c, d Seventeen days of waterlogging stress. N = 9 plants
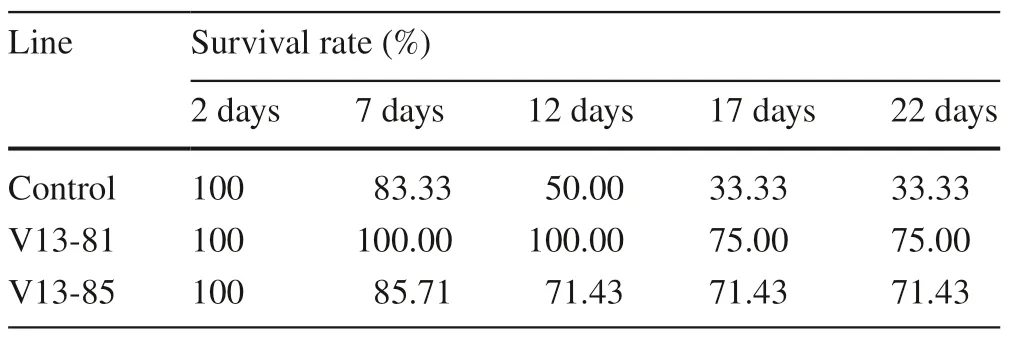
Table 1 Survival rate over time of transgenic and wild-type (control) lines under waterlogging stress
Wood property analysis
The wood specific gravity of the transgenic and control plants was low, between 0.3 and 0.4 g cm?3. Although fiber lengths and fiber widths of V13-85 were slightly larger than those of the control, slightly lower in V13-81 than in the control, the differences were significant. The microfiber angles of the Vgb-transgenic lines were larger, but not significantly, than those of the control (Table 6). Water and α-cellulose contents of V13-81 were significantly higher (8.18% and 4.35%, respectively) andholocellulose significantly lower (6.02%) than in the control. Water content in V13-85 was significantly higher (16.62%) and holocellulose and lignin were significantly lower (4.20% and 10.72%, respectively) than in the control (Table 7).
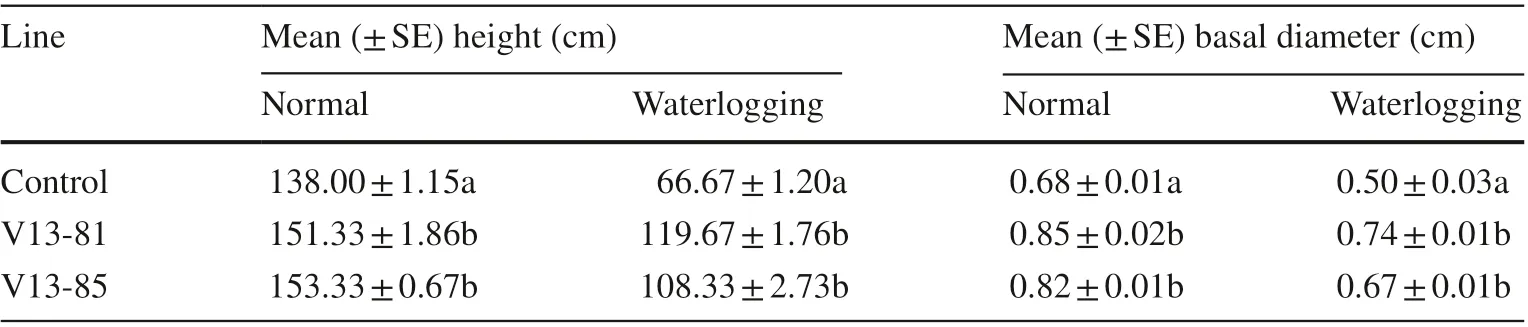
Table 2 Height and basal diameter of the wild type (control) and transgenic lines under normal watering and waterlogging stress
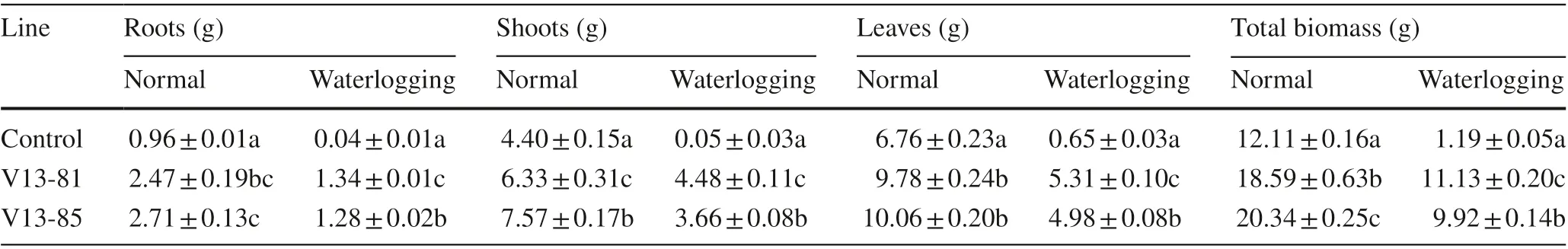
Table 3 Mean (± SE) biomass of the wild type (control) and transgenic lines under normal watering and waterlogging stress
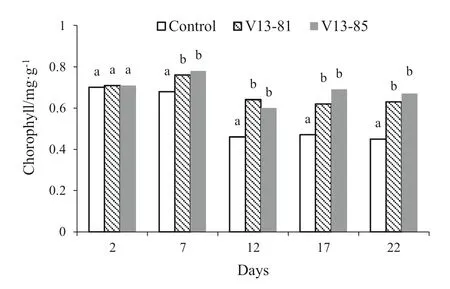
Fig.4 Mean (± SE) total chlorophyll content over time in control and transgenic poplar lines under waterlogging stress. N = 9 experiments. Different letters indicate significant differences from control plants at each time ( P < 0.05)
Discussion and conclusions
Expression of Vhb in transgenic tobacco significant affects the phenotype and metabolism, e.g., faster germination is faster, growth is promoted, and altered distribution of secondary metabolites such as the anabasine ontent is decreased (Holmberg et al. 1997). In D. innoxia, Vhb-expression lines had shorter germination times, increased growth rates and accelerated flowering compared with the controls when under low oxygen stress (Bülow et al. 1999). Petunia overexpressing Vhb had greater tolerance to low oxygen stress than controls did (Mao et al. 2003). In waterlogging tests, transgenic potato overexpressing Cry3A- Vhb also had increased tolerance to low oxygen stress (Zhou et al. 2004). In a study of Vhb-transgenic white poplar, only one of six transgenic lines had increased growth and biomass (Zelasco et al. 2006). In the present waterlogging tests, Vgb-expressing hybrid poplars had significantly increased survival rate, height, basal diameter and biomass compared with those of the control (Tables 1, 2, 3). Functional investigations of Vitreoscilla hemoglobin (VHb) suggest that, under limited oxygen, that it promotes the delivery oxygen, thus increasing productivity of certain oxygen-requiring metabolic pathways (Dikshit et al. 1992; Tsai et al. 1996, 2002; Guo et al. 2018). Plant hemoglobins are known to be involved in nitrogen and carbohydrate metabolism (Sturms et al. 2011; Leiva-Eriksson et al. 2014). Overexpression of Sugar beetnsHbs (BvHb) can improve root and shoot growth in seedlings treated with a NO donor (NO sodium nitroprussiate, SNP), BvHb can bind NO in the absence ofoxygen, to later form nitrate and increase gas exchange and oxygen levels (Hermann et al. 2007; Chen et al. 2017; Leiva et al. 2019). Thus, overexpression Vgb may improve gas exchange and oxygen level of transgenic poplar under waterlogging and enhance the growth and biomass of transgenic poplar.
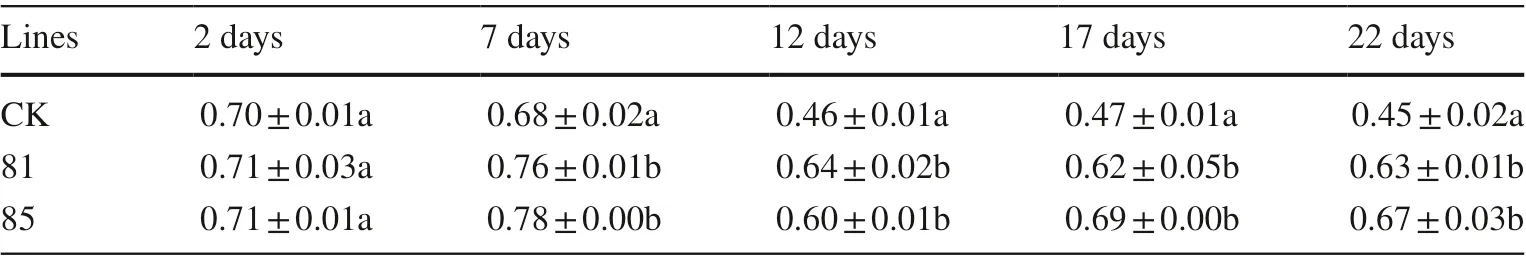
Table 4 Data of for chlorophyll content in individual plants of transgenic lines and control
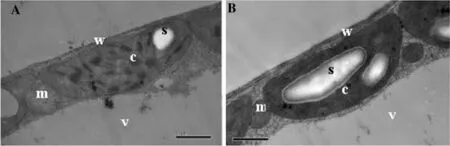
Fig.5 Transmission electron micrographs of mesophyll cells in leaves of wild-type (control) and transgenic poplars under waterlogging stress for 22 days. a The control line has several small starch grains and osmiophilic plastoglobuli in its chloroplasts. b The transgenic lines have relatively large starch grains in its chloroplasts. c, chloroplast; m, mitochondrion; s, starch; v, vacuole; and w, cell wall. Bar = 1.0 μm
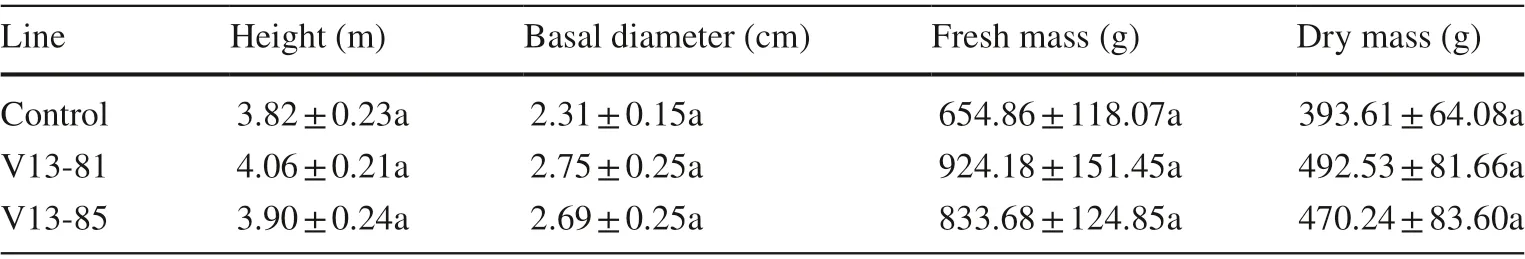
Table 5 Growth variables for control and transgenic poplar trees in field study
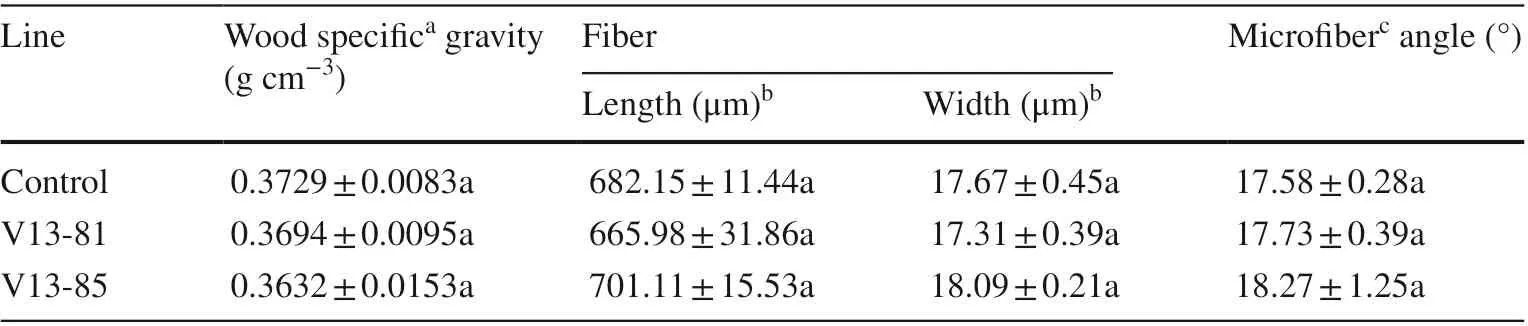
Table 6 Physical wood properties of transgenic and control poplar trees
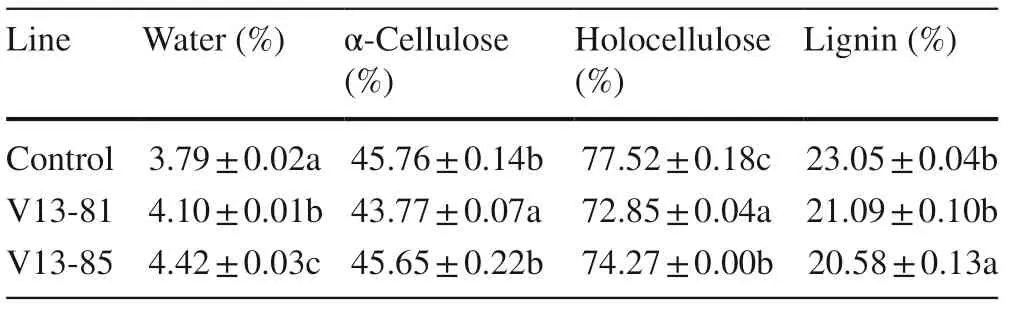
Table 7 Chemical wood properties of transgenic and control poplar trees
The expression of VHb favors host growth and improved production of some metabolic products under hypoxic conditions (Farrés and Kallio 2002; H?ggman et al. 2003; Wang et al. 2009; Rodrigo et al. 2015). Hbs may also help to maintain ATP levels, energy changes, and prevent the onset of cell death, and thus lead to higher levels of chlorophyll (Brewin and Flavell 1997; Leiva et al. 2019). Improved starch production in Vhb-expressing tobacco and hybrid aspen lines has been suggested to improve energy reservoirs and thus promote the growth of the transgenic lines (Wakabayashi et al. 1986; Farrés and Kallio 2002; H?ggman et al. 2003). In the present study, chlorophyll content was significantly higher in the leaves of the transgenic plants compared with those of the control plants under waterlogging stress. Starch and the osmiophilic plastoglobuli in the chloroplasts of the transgenic lines were more abundant than in the control. The control plants sustained heavier leaf damage compared with the transgenic lines under waterlogging stress. These results implied that under waterlogging stress, expression of the Vgb gene enhances the accumulation of chlorophyll and starch in the transgenic poplar, which would contribute to its growth and improve its ability to endure flooding.
In the field trials, the height, the slight increase in basal diameter and biomass of transgenic plants compared with the control plants was not significant; however, the analysis of wood properties showed that water content in transgenic line V13-85 was significantly higher and holocellulose and lignin contents significantly lower than the controls. The expression of VHb as a transgene in plants, animals, fungi and other bacteria leads to significant metabolic changes during aerobic growth (Stark et al. 2015; Guo et al. 2018). Wang et al. ( 2019) revealed that the expression of VHb in Lentinula edodes enhances the production of β-glucan and plant biomass degrading enzymes such as laccase and amylase. Thus, the expression of Vgb in transgenic poplar might increase the production of key enzymes in the metabolism of cellulose and lignin, and lead to a lower content of cellulose and lignin and a higher relative water content.
Authors’ contributionYL Li and WX Zhang projected and implemented all the experiments and drafted the manuscript. YL Li and WX Zhang are contributed equally to this work. XH Su, WX Zhu, BY Zhang, QJ Huang were involved in devising and directing the experiments and proofreading the manuscript. XH Su contributed to the concept of the research, gave constructive advice on the experiments, and finally completed the manuscript.
References
Brewin NJ, Flavell RB (1997) A cure for anemia in plants. Nat Biotechnol 15:222–223
Bülow L, Holmberg N, Lilius G, Bailey JE (1999) The metabolic effects of native and transgenic hemoglobins on plants. Trends Biotechnol 17:21–24
Chen FH, Hao YQ, Yin ZZ, Wu GB, Jiang XX (2017) Transcriptome of wax apple ( syzygium samarangense) provides insights into nitric oxide-induced delays of postharvest cottony softening. Acta Physiol Plant 39(12):273
Dikshit RP, Dikshit KL, Liu Y, Webster DA (1992) The bacterial hemoglobin from Vitreoscilla can support the aerobic growth of Escherichia coli lacking terminal oxidases. Arch Biochem Biophys 293:241
Farrés J, Kallio PT (2002) Improved cell growth in tobacco suspension cultures expressing Vitreoscilla hemoglobin. Biotechnol Prog 18:229–233
Guo ZY, Tan HX, Lü ZY, Ji Q, Huang YX, Liu JJ, Chen DH, Diao Y, Si JP, Zhang L (2018) Targeted expression of Vitreoscilla hemoglobin improves the production of tropane alkaloids in Hyoscyamus niger hairy roots. Sci Rep 8:17969
H?ggman H, Frey AD, Ryyn?nen L, Aronen T, Julkunen-Tiitto R, Tiimonen H, Pihakaski-Maunsbach K, Jokipii S, Chen X, Kallio PT (2003) Expression of Vitreoscilla haemoglobin in hybrid aspen ( Populus tremula × tremuloides). Plant Biotechnol J 1:287–300
Hebelstrup KH, Igamberdiev AU, Hill RD (2007) Metabolic effects of hemoglobin gene expression in plants. Gene 398:86–93
Hebelstrup KH, Shah JK, Simpson C, Schjoerring JK, Mandon J, Cristescu SM, Harren FJ, Christiansen MW, Mur LA, Igamberdiev AU (2014) An assessment of the biotechnological use of hemoglobin modulation in cereals. Physiol Plant 150(4):593–603
Hermann K, Meinhard J, Dobrev P, Linkies A, Pesek B, Hess B, Machá?ková I, Fischer U, Metzger GL (2007) 1-Aminocyclopropane-1-carboxylic acid and abscisic acid during the germination of sugar beet ( Beta vulgaris L.): a comparative study of fruits and seeds. J Exp Bot 58(11):3047–3060
Holmberg N, Lilius G, Bailey JE, Bülow L (1997) Transgenic tobacco expressing Vitreoscilla hemoglobin exhibits enhanced growth and altered metabolite production. Nat Biotechnol 15:244–247
Inskeep WP, Bloom PR (1985) Extinction coeffi cients of chlorophyll a and b in N, N-dimethylformamide and 80% acetone. Plant Physiol 77:483–485
Leiva EN, Reeder BJ, Wilson MT, Bülow L (2019) Sugar beet hemoglobins: reactions with nitric oxide and nitrite reveal differential roles for nitrogen metabolism. Biochem J 476(14):2111–2125
Leiva-Eriksson N, Pin PA, Kraft T, Dohm JC, Minoche AE, Himmelbauer H, Bülow L (2014) Differential expression patterns of nonsymbiotic hemoglobins in sugar beet ( Beta vulgaris ssp. vulgaris). Plant Cell Physiol 55(4):834–844
Lodhi MA, Ye GN, Weeden NF, Reisch B (1994) A simple and effi cient method for DNA extraction from grapevine cultivars and Vitis species. Plant Mol Biol Report 12:6–13
Mao ZC, Hu YL, Zhong J, Wang LX, Guo JY, Lin ZP (2003) Improvement of the hydroponic growth and waterlogging tolerance of Petunias by the introduction of Vhb gene. Acta Bot Sin 45:205–210
Martinelli L, Vaccari I, Dallacosta L, Poletti V, Masuero D, Vrhovsek U (2013) Combining molecular and metabolomic analysis to evaluate transgenic Vitis vinifera plants expressing the Vitreoscilla haemoglobin (VHb). Vitis 52:77–84
Misra AN, Vladkova R, Singh R, Misra M, Dobrikova AG, Apostolova EL (2014) Action and target sites of nitric oxide in chloroplasts. Nitric Oxide 39:35–45
Rodrigo ML, Marvin M, Vikas Y, Marcelo FL (2015) Improved biomass and protein production in solid-state cultures of an Aspergillus sojae strain harboring the Vitreoscilla hemoglobin. Appl Microbiol Biotechnol 99:9699–9708
Shi XZ, Wang XL, Peng FT, Zhao Y (2012) Molecular cloning and characterization of a nonsymbiotic hemoglobin gene (GLB1) from Malus hupehensis Rehd. with heterologous expression in tomato. Mol Biol Rep 39:8075–8082
Stark BC, Pagilla KR, Dikshit KL (2015) Recent applications of Vitreoscilla hemoglobin technology in bioproduct synthesis and bioremediation. Appl Microbiol Biotechnol 99:1627–1636
Sturms R, DiSpirito AA, Fulton DB, Hargrove MS (2011) Hydroxylamine reduction to ammonium by plant and cyanobacterial hemoglobins. Biochemistry 50:10829–10835
Su XH, Chu YG, Li H, Hou YJ, Zhang BY, Huang QJ, Hu ZM, Huang RF, Tian YC (2011) Expression of multiple resistance genes enhances tolerance to environmental stressors in transgenic poplar ( Populus × euramericana ‘Guariento’). PLoS ONE 6:e24614. https://doi.org/10.1371/journ al.pone.00246 14
Tang QY, Feng MG (2002) DPS data processing system for practical statistics. Science Press, Beijing
Thiel J, Rolletschek H, Friedel S, Lunn JE, Nguyen TH, Feil R, Tschiersch H, Muller M, Borisjuk L (2011) Seed-specific elevation of non-symbiotic hemoglobin AtHb1: beneficial effects and underlying molecular networks in Arabidopsis thaliana. BMC Plant Biol 11:48
Trevisan S, Manoli A, Begheldo M, Nonis A, Enna M, Vaccaro S, Caporale G, Ruperti B, Quaggiotti S (2011) Transcriptome analysis reveals coordinated spatiotemporal regulation of hemoglobin and nitrate reductase in response to nitrate in maize roots. New Phytol 192:338–352
Tsai PS, Rao G, Bailey JE (1995) Improvement of Escherichia coli microaerobic oxygen metabolism by Vitreoscilla hemoglobin: new insights from NAD(P)H fluorescence and culture redox potential. Biotechnol Bioeng 47:347–354
Tsai P, Hatzimanikatis V, Bailey JE (1996) Effect of Vitreoscilla hemoglobin dosage on microaerobic Escherichia coli carbon and energy metabolism. Biotechnol Bioeng 49:139–150
Tsai PS, Nageli M, Bailey JE (2002) Intracellular expression of Vitreoscilla hemoglobin modifies microaerobic Escherichia coli metabolism through elevated concentration and specific activity of cytochrome o. Biotechnol Bioeng 79:558–567
Vigeolas H, Huhn D, Geigenberger P (2011) Nonsymbiotic hemoglobin-2 leads to an elevated energy state and to a combined increase in polyunsaturated fatty acids and total oil content when overexpressed in developing seeds of transgenic Arabidopsis plants. Plant Physiol 155:1435–1444
Wakabayashi S, Matsubara H, Webster DA (1986) Primary sequence of a diametric bacterial hemoglobin from Vitreoscilla. Nature 322:481–483
Wang ZN, Xiao Y, Chen WS, Tang KX, Zhang L (2009) Functional expression of Vitreoscilla hemoglobin (VHb) In Arabidopsis relieves submergence, nitrosative, photo-oxidative stress and enhances antioxidants metabolism. Plant Sci 176:66–77
Wang XT, Ding YT, Gao XY, Liu HH, Zhao K, Gao YQ, Qiu LY (2019) Promotion of the growth and plant biomass degrading enzymes production in solid-state cultures of Lentinula edodes expressing Vitreoscilla hemoglobin gene. J Biotechnol 302:42–47
Webster DA (1987) Structure and function of bacterial hemoglobin and related proteins. In: Eichorn GC, Marzilli LG (eds) Advances in inorganic biochemistry, vol 7. Elsevier, New York, pp 245–265
Zelasco S, Reggi S, Callihari P, Balestrazzi A, Bongiorni C, Quattrini E, Delia G, Bisoffi S, Fogher C, Confalonieri M (2006) Expression of the Vitreoscilla hemoglobin (VHb)-encoding gene in transgenic white poplar: plant growth and biomass production, biochemical characterization and cell survival under submergence, oxidative and nitrosative stress conditions. Mol Breed 17:201–216
Zhang BY, Su XH, Li YL, Huang QJ, Zhang XH, Zhang L (2005) Regeneration of vgb-transgenic poplar ( populus alba × p. glandulosa) and the primary observation of its growth. J Agric Biotechnol 13:288–293
Zhou ZZ, Zhou YG, He CZ, Mang KQ, Tian YC (2004) Expression of cry3A and vhb genes in transgenic potato plants. Prog Biochem Biophys 31:741–745
 Journal of Forestry Research2021年2期
Journal of Forestry Research2021年2期
- Journal of Forestry Research的其它文章
- Stem taper functions for Betula platyphylla in the Daxing’an Mountains, northeast China
- Leaf phenotypic variation of endangered plant Tetracentron sinense Oliv. and influence of geographical and climatic factors
- Ecological variations of woody species along an altitudinal gradient in the Qinling Mountains of Central China: area-based versus mass-based expression of leaf traits
- Variations in stem radii of Larix principis-rupprechtii to environmental factors at two slope locations in the Liupan Mountains, northwest China
- A review of ecological mechanisms for management practices of protective forests
- Variation of basic density, calorific value and volumetric shrinkage within tree height and tree age of Ugandan grown Eucalyptus grandis wood
