Effects of pruning intensity on nonstructural carbohydrates of Populus alba × P. talassica in the arid desert region of Northwest China
Jun Zhang · Liqing Liu
Abstract Although pruning is important to obtain highquality, large-diameter timber, the effects of pruning on nonstructural carbohydrates (NSC) in aboveground organs of many timber species are not well understood. Three intensities of pruning (none, moderate and severe) were tested on poplars ( Populus alba × P. talassica) in the arid desert region of northwest China to compare the concentrations of soluble sugar (SS), starch (ST) and total nonstructural carbohydrate (TNC) in leaves, branches and trunks during the growing season. The concentration of NSC components after different pruning intensities varied similarly in seasonal patterns, increasing slowly at the beginning of the growing season, continuously declining in the middle, then gradually recovering by the end of the growing season. The monthly mean NSC concentration in poplar differed significantly among the three pruning intensities ( p < 0.05). The SS concentration in pruned trees was higher than in unpruned trees ( p < 0.05). For moderately pruned trees, the concentrations of ST and TNC in trunks and branches were higher than in unpruned and in severely pruned trees ( p < 0.05). Compared with no pruning, pruning changed the seasonal variation in NSC concentration. The orders of SS and TNC concentrations in aboveground organs were leaf > branch > trunk, while the order of ST concentration was trunk > leaf > branch, which was related to functional differences of plant organs. The annual average growth in height of unpruned, moderately pruned, and severely pruned poplars was 0.21 ± 0.06, 0.45 ± 0.09 and 0.24 ± 0.05 m, respectively, and the annual average growth in DBH were 0.92 ± 0.04, 1.27 ± 0.06 and 1.02 ± 0.05 cm, respectively. Our results demonstrate that moderate pruning may effectively increase the annual growth in tree height and DBH while avoiding damage caused by excessive pruning to the tree body. Therefore, moderate pruning may increase the NSC storage and improve the growth of timber species.
Keywords Pruning intensity · Nonstructural carbohydrate · Populus alba × P. talassica · Seasonal pattern
Introduction
Carbohydrates can be divided according to their functions into structural carbohydrates (SC) and nonstructural carbohydrates (NSC) (Song et al. 2016). NSC in plant tissues mainly consist of soluble sugar (SS) and starch (ST), which are the result of the balanced relationship between carbon supply and carbon demand (i.e., carbon assimilation and consumption) (Quentin et al. 2015). Thus, NSC plays a major role in the growth and metabolism of plant (Zhang et al. 2014), and further exploration of NSC concentration will deepen our understanding of the mechanisms underlying plant physiology, carbon budgeting, responses to environmental changes, and ultimately, ecosystem processes.
NSC concentrations in trees vary by season and organ (Schaberg et al. 2000; Hoch et al. 2003; Landh?usser and Lieffers 2003; Spann et al. 2008). As the main sites of photosynthesis, Leaves are the primary carbon sources for trees, and branches, trunks and roots are the main carbon sinks for the photosynthetic products (Mei et al. 2015). Because branches both store and transport photosynthates, branch growth is conducive for increases in leaf area and photosynthetic yield (Ishii et al. 2000). Branches first receive carbohydrates needed for growth and storage before excess carbohydrates are transported to other organs, while stored NSC provide energy for the growth of leaves and new shoots in the early growing season (Sch?del et al. 2009). Trunks not only function to support the canopy, but also to transport soluble carbohydrates. Although the concentration of NSC in the trunk is relatively low, the trunk accounts for a large proportion of the total biomass of the tree and stores a large amount of NSC (Mei et al. 2015). NSC concentrations in the trunk and branch vary asynchronously and are lower in the trunk than in branches during the growing season (Hoch et al. 2003).
Plants are vulnerable to external disturbances during growth, including herbivores, trampling (Song and Wang 2013), pests (Vanderklein and Reich 1999; Anderegg and Callaway 2012; Atkinson et al. 2014), and fires (Wiley et al. 2013). When wounded, plants quickly mobilize stored NSC for protection (Song and Wang 2013) and activate defense responses (León et al. 2001). After Reichenbacker et al. ( 1996) defoliated Populus saplings to simulate damage by leaf-feeding insects, tree vitality, as measured by total amount of nonstructural carbohydrates (TNC), was only slightly affected when the amount of defoliation increased. After herbivory, trees sprout new leaves and adopt other coping strategies, such as selective restriction of growth (Honkanen et al. 1994) and rapid mobilization of carbon reserves (Li et al. 2002), which lead to different response strategies in various organs. Other types of destruction to leaves are also likely to affect the NSC.
Pruning is an important management practice to produce high-quality, no-section timber with large diameters and regulate the distribution of photosynthates among organs (Wang and Zeng 2016). In Camellia sinensis, Bore et al. ( 2003) found that the NSC concentration in the leaves, stems, and roots decreases soon after pruning, then gradually increases over the next 30 days. Chesney and Vasquez ( 2007) adopted different pruning intensities for Gliricidia sepium and Erythrina poeppigiana and found that pruning could lead to a large consumption of starch in stem tissues. In the branches and trunks of Aer saccharinum and Acer platanodies during the growing season, Ramirez et al. ( 2018) found that pruning has no effect on the concentrations of SS and ST in the trunks of the two species; However, the concentrations of SS and ST in the branches of A. platanoides is higher in April, decreases in June, and peaks in October. In contrast, in A. saccharinum, SS and ST concentrations increase slowly throughout the growing season. At present, relatively little is known about the effect of pruning in poplar plantations on the dynamics of NSC reserves.
Poplar ( Populus alba × P. talassica; Salicaceae), a deciduous tree species that is resistant to cold, drought, wind, and salt, is widely planted in arid desert regions in Northwest China and is an important afforestation tree species for soil improvement, desertification control, and water and soil conservation. Since the establishment of a shelter forest in 1999, the lack of forest management practices in poplar plantations has led to poor ventilation and light transmittance, so it is necessary to adopt pruning measures of reasonable intensity to improve the quality of tree. Here, we sample leaves, branches, and trunks of a poplar subject to different pruning intensities and explore the following hypotheses: (1) Severe pruning causes greater depletion in carbohydrate reserves than does moderate pruning; (2) NSC concentrations in different organs respond differentially to pruning intensities.
Materials and methods
Study area
The research site is located in the Linzhi base of the agricultural comprehensive development zone in Karamay City (45°22′–45°40′ N, 84°50′–85°20′ E) to the southeast of Mount Gayle and Genghis Khan in the western mountainous area of Junggar. The terrain elevation varies little, with a high in the southwest of 275 m a.s.l. and a low in the northeast of 259 m a.s.l. The average annual precipitation is about 157.4 mm (Fig.1) and is mostly concentrated from June to August, which accounts for 12.5–25.2% of the annual total. The annual potential evaporation is 1492 mm, about 9.5 times the annual precipitation, and the annual average temperature is 8.9 °C. The annual sunshine duration is about 2704 h, the annual average wind speed is about 3.2 m/s, and the frost-free period is 180–220 days. The region is a typical continental arid desert, and the soil in the forest belt of the study area is sandy loam with a depth of about 35 cm, and the groundwater depth is about 8.85 m (Zhang et al. 2013).
Sample area
The poplar plantation was established in 1999 with 2-year-old saplings and row spacing of 2 × 4 m. The
Fig.1 Air temperature, humidity, and precipitation at the research site in Northwest Chinastand age was 12 when sampled. The branches of poplar are dense with poor ventilation and light transmission in the forest belt. The height of natural pruning is less than 3.5 m. Low groundcover plants such as Ceratocarpus, Halogeton arachnoideus, Halogeton glomeratus and Malcolmia africana are grown in the poplar plantation.
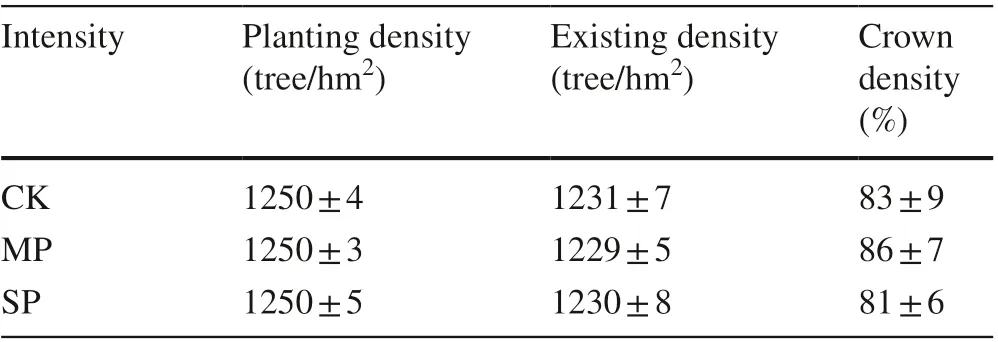
Table 1 Survey of poplar plantation with different pruning intensities
In early March 2012, pruning was adopted in a 20 m × 40 m space in the plantation with three intensities: no pruning (control, CK), moderate pruning (pruning branches to 1/3 of tree height from the ground, MP), and severe pruning (pruning branches to 2/3 of tree height from the ground, SP). Each pruning intensity was replicated three times. Pruning was done by cutting branches even with the trunk to ensure smooth incisions and minimize damage to the bark around the incision. After pruning, the wound was painted to prevent wound infection. A wireless weather station (Vantage Pro2; American Davis) was set up approximately 10 m away from the study area, approximately 2.5 m off the ground, to continuously observe and record meteorological factors such as air temperature, air humidity and rainfall. The information of the poplar plantation is listed in Table 1.
Tree sampling
Canopy density, DBH, and total height were measured at the end of the 2011 and 2012 growing seasons (Table 3). Leaves, branches, and trunks were sampled from five standard trees every month from May to September; sampling was completed within 2 days. Canopy branches and leaves were obtained by climbing to the crown of the tree and using high branch scissors to remove branches randomly from the upper and lower canopy of the south-facing side of the crown. Branches and leaves were evenly mixed before making measurements. Growth cones with an inner diameter of 5 mm were used to drill tree cores at chest height and the middle of the crown on south-facing side of the trunk, and were likewise evenly mixed. After drilling the tree core, the resulting hole was smeared with butter and filled in quickly to avoid the invasion of diseases and pests. Samples were placed into self-sealing bags and temporarily kept in a lowtemperature refrigerator. On the same day, all samples were taken to the laboratory, heated in an 800-W microwave oven for 90 s (Hoch et al. 2003), dried to constant mass in an oven at 65 °C, pulverized with a high speed pulverizer, and passed through a 100-mesh screen for NSC analysis.
Data and statistical analyses
Samples were analyzed for SS and ST. The sum of SS and ST was referred here as TNC. SS and ST were measured using a modified phenol–sulfuric acid method (Buysse and Merckx 1993; Zhang et al. 2014). The NSC concentration is reported as a percentage of dry mass. Analysis of variance (ANOVA) and Tukey–Kramer honestly significant difference (HSD) test were used to compare the significance of differences in NSC concentrations among different treatments, sampling periods and organs. SPSS version 18.0 (SPSS Inc., Chicago) was used for all test, and SigmaPlot 10.0 software (SYSTAT, San Jose, CA, USA) to plot all graphs.
Results
Seasonal dynamics of NSC concentrations
The SS, ST and TNC concentrations in aboveground organs fluctuated during the growing season regardless of the pruning intensities of poplars (Fig.2). Regardless of pruning intensity, the concentrations of NSC components in leaves of poplars had similar seasonal patterns; ST and TNC in leaves rose early in the growing season and began to decline in June. As the growth rate of poplars increased, ST and TNC of leaves declined, reaching the lowest value in July. When the growth rate of poplars slowed, ST and TNC in leaves recovered, with the greatest rebound observed in severely pruned trees. SS in leaves decreased in the early growth stage to a minimum in July. Similarly, SS in leaves also began to recover as growth slowed, and the severely pruned trees showed the largest rebound. The concentrations of NSC components in branches of poplars among different pruning intensities showed similar seasonal pattern changes. SS in branches decreased early to a minimum in June, recovered to a peak value in August, and then decreased slowly. ST also peaked in August before declining. TNC in branches began to increase early in the year and peaked 1 month earlier (July) in severely pruned trees than in trees with the other treatments (August). NSC patterns in trunk were qualitatively similar across treatments: NSC increaseduntil July and then declined relatively abruptly in August and maintained or slightly increased in September. Compared with unpruned poplars, moderately and severely pruned trees showed a great magnitude of seasonal variation in NSC concentrations in leaves, branches, and trunks to some extent.
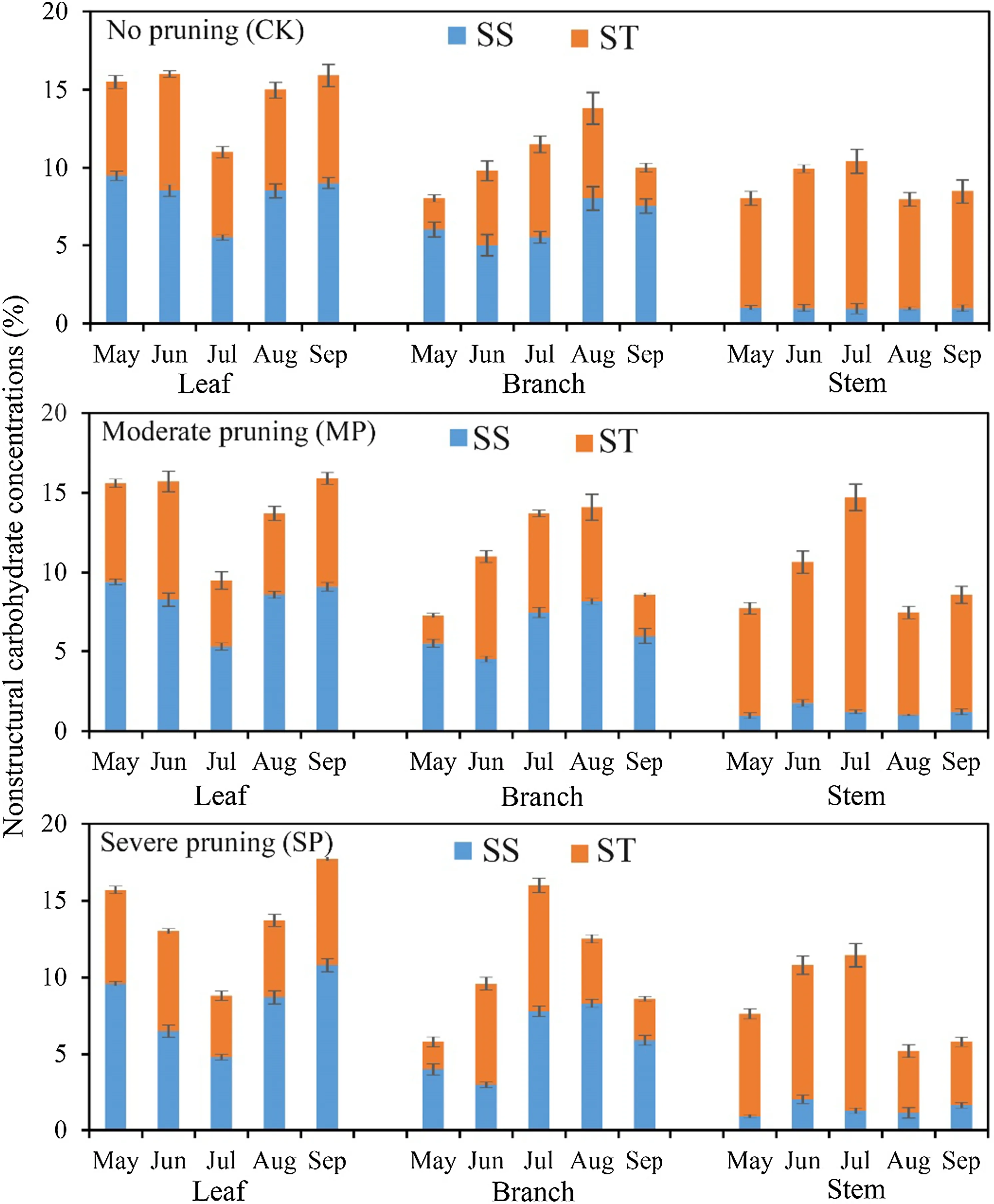
Fig.2 Nonstructural carbohydrates in leaves, branches, and trunks of poplars during the growing season after different pruning intensities. ( SS soluble sugars, ST starch, TNC total nonstructural carbohydrates)
NSC concentrations in different organs
In addition to its seasonal variation (Fig.2), NSC also varied across treatments and between organs (Table 2, Fig.3). Except for SS concentrations in trunks, the mean concentration of NSC components in leaves, branches, and trunks differed significantly ( p < 0.05, Table 2), and NSC concentrations of poplars with moderate and severe pruning were significantly higher than that of unpruned trees ( p < 0.05, Fig.3). The mean concentrations of ST and TNC in trunks differed significantly between treatments, and generally in the order MP > CK > SP, as did SS, ST, and TNC in branches. The patterns were different in the leaves: mean SS concentrations, and the mean concentrations of ST and TNC were in the order CK > MP > SP ( p < 0.05, Fig.3). Within pruning treatments, SS and TNC concentrations in aboveground organs were in the order leaf > branch > trunk, while ST concentrations were generally in the order trunk > leaf > branch.
Effects of different pruning intensities on tree growth
We measured canopy density, tree height, and DBH of trees within each treatment at the end of the growing season (Table 3). There were significant differences in the average growth of tree height and DBH between moderate and severepruning at the end of the growing season ( p < 0.05), but the average growth in tree height and DBH did not differ significantly between the severe pruning and no pruning treatments ( p > 0.05). Due to the greater canopy opening after pruning, nutrient area, ventilation, and light transmittance were improved. Thus, moderate pruning apparently promoted poplar growth.
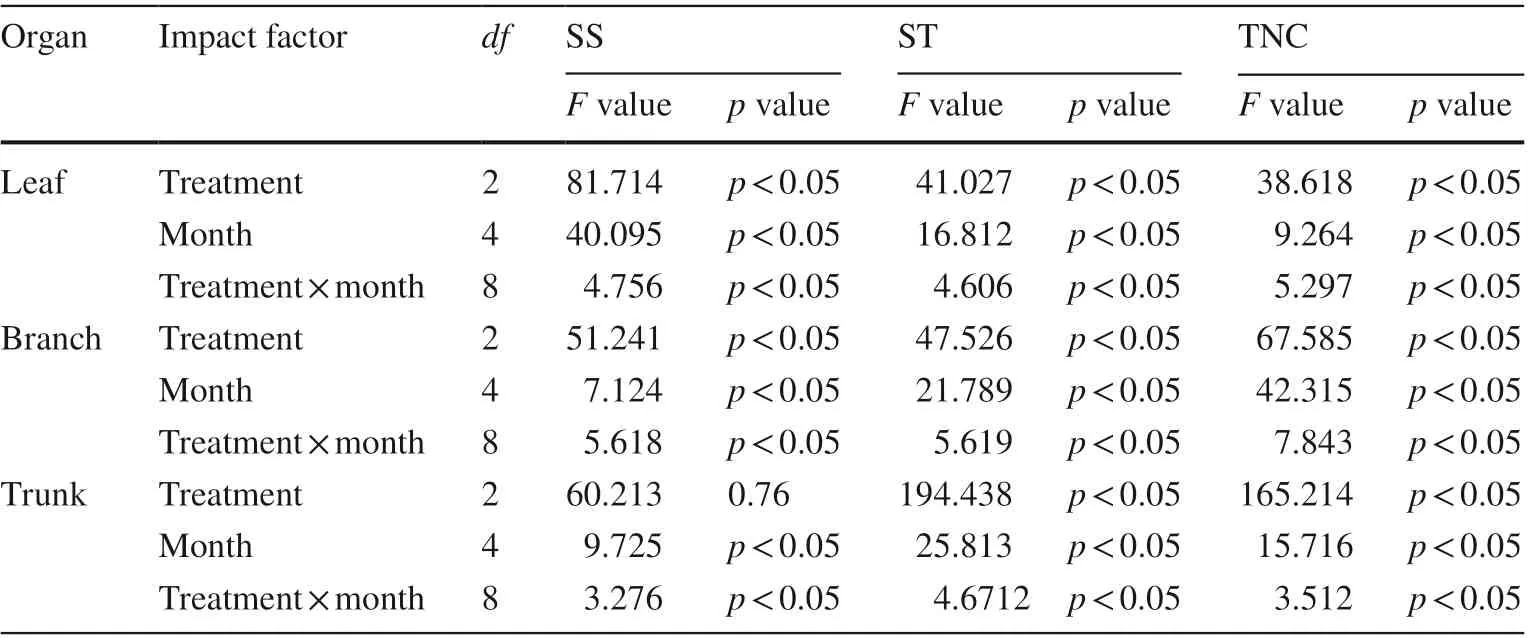
Table 2 ANOVA of concentrations of nonstructural carbohydrate components
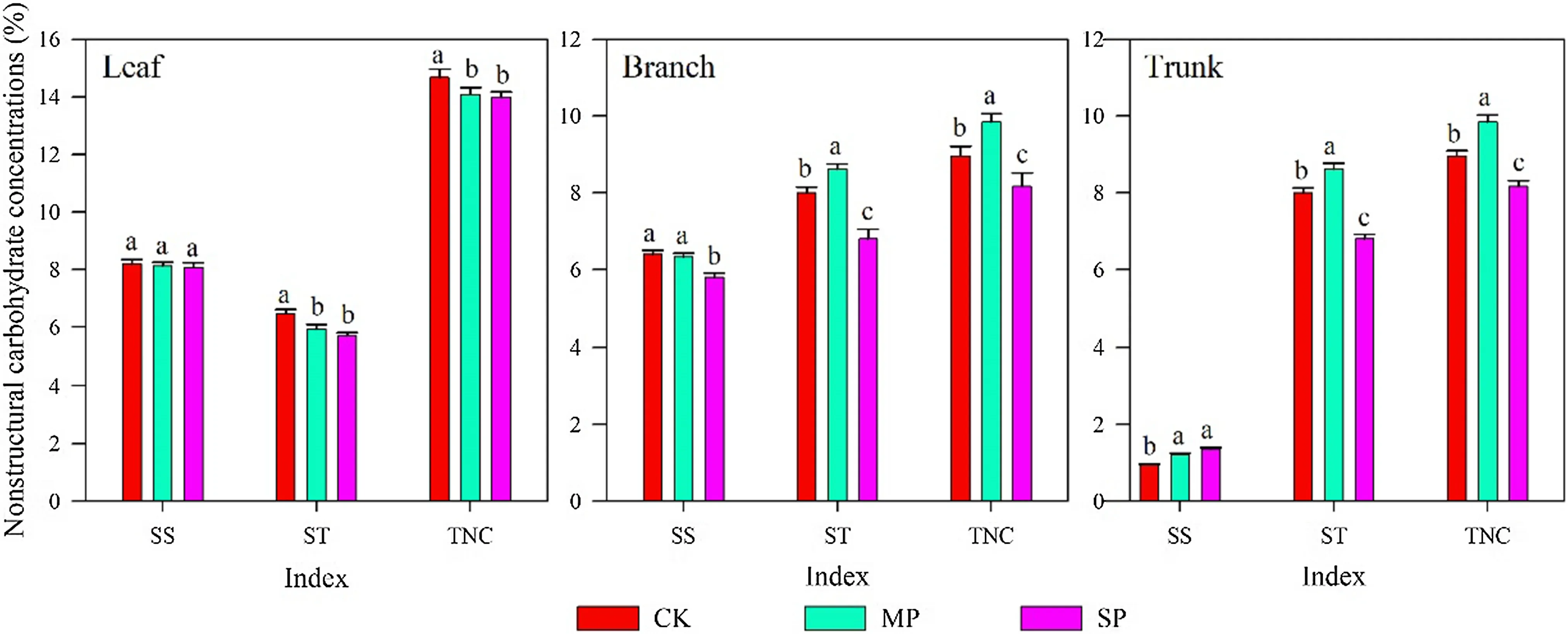
Fig.3 Comparison of seasonal mean values of nonstructural carbohydrates in leaves, branches, and trunks of poplar after different pruning intensities. Errors bars are standard errors of the mean. Bars labeled with the same letter within each index are not significantly different (Tukey’s test at p < 0.05). SS soluble sugars, ST starch, TNC total nonstructural carbohydrates
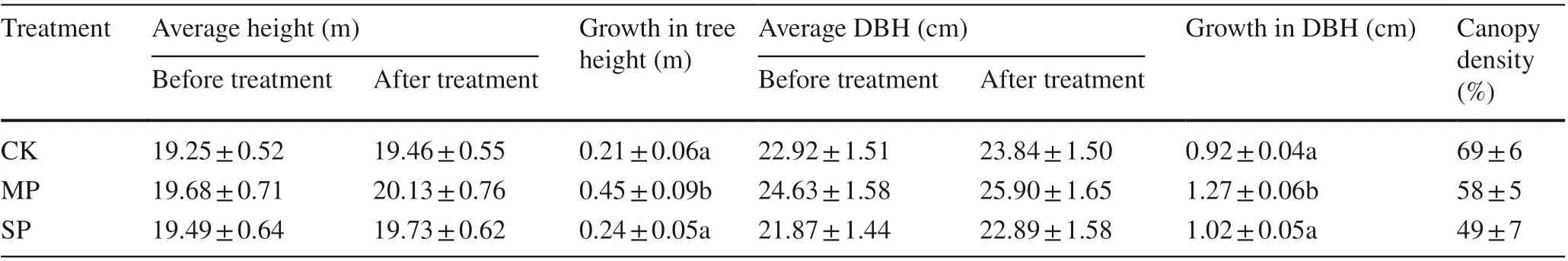
Table 3 Effect of pruning intensities on tree growth
Discussion
Effects of pruning on carbohydrate concentrations
As we expected, carbohydrate reserves in branches of poplars that were severely pruned were more depleted than in those of trees that were moderately pruned (Fig.2). The stasis of carbohydrate reserves after pruning may be explained by the intensity of the perturbation, which modulates the response of carbohydrates (Atkinson et al. 2014; Eyles et al. 2009). In contrast to severe pruning, moderate pruning did not reduce the percentage of biomass enough to mobilize the carbohydrate reserves to maintain metabolic demand and compensatory growth. Poplars with severe pruning transferred more NSC from the branches early in the growing season—for growth (i.e., new shoots and foliage)—than did the other pruning intensities (Fig.2). This result may indicate a high carbon loading of trees, and supports the idea that trees tend not to be limited by carbon supply (Hoch et al. 2003). Additionally, new foliage produced after pruning could have contributed to the recovery of the pruned trees (carbon autonomy of branches, Landh?usser and Lieffers 2012). Indeed, 1 year after pruning, trees had recovered their crown entirely without any sign of a reduction in growth (Lecigne 2013). These results were surprising because the moderately pruned treatment was close to the pruning limits proposed by the American National Standards Institute to maintain the health of planted forests (Johnson 2007). Current guidelines suggest that poplar plantations should not be pruned more than 30%, because disease, insect attacks, or even death may occur due to inappropriate conditions for growth in pure poplar stands (ANSI 2001). It remains unknown whether and how repeated pruning, such as done on target-trees near our research site, may affect trees over a long period, which would have implications for the growth of large diameter timber.
This first study of NSC dynamics in trees in the arid desert region of Northwest China showed that, despite the high external disturbances to which they are exposed (Gillner er al. 2014; Ramirez et al. 2018), seasonal dynamics of trees with different pruning intensities reflected what had been documented previously in forest trees (Martinez-Vilalta et al. 2016; Maurin and Desrochers 2013). Whatever the pruning intensity, SS concentrations decreased in the middle of the growing season, then reached high levels by the end of the growing season, where they remained until the end of dormancy. In contrast, ST after all pruning intensities increased continuously through the middle of the growing season, then declined to low levels by the end of the growing season, where they remained until the end of dormancy. The initial decrease in sugar concentrations may result from photosynthates being mobilized to meet metabolic and growth demands, rather than being stored early in the growing season (Carbone et al. 2013). In the middle of summer, the photosynthes are allocated to reserves, and NSC concentrations increase because of the reduction in sink strength (Gaucher et al. 2005).
Effects of pruning on NSC concentrations in organs
In contrast to our expectation, the NSC concentrations of different organs did not respond to pruning intensities. NSC concentrations in leaves, branches, and trunks clearly differed across pruning intensities. We found consistencies in the relative magnitudes of NSC components regardless of pruning intensity; for example, the order of concentrations of ST was trunk > leaf > branch. Here, the concentration of SS in leaves was higher than in branches and trunks, consistent with previous findings (Landh?usser and Lieffers 2003). The trunk is an important carbon storage organ and carbon sink, and its lignification degree is higher than other organs. In the trunk, NSC is mainly stored in the form of starch, which is maintained at higher concentrations than in the leaves and branches.
Conclusion
The dynamics of carbohydrate reserves have been evaluated widely in trees grown under natural conditions. To our knowledge, this is the first study that evaluates the dynamics of tree NSC in the arid desert region of Northwest China and, additionally, the first study to evaluate carbohydrate concentrations in three plant organs (leaves, branches, and stems) of severely pruned, moderately pruned and unpruned trees. The concentrations of NSC in aboveground organs had similar seasonal variation regardless of pruning intensity; indicating that the amplitude, rather than the patterns of seasonal variation in NSC are not affected by pruning. We further found that the monthly mean values of NSC concentrations in the aboveground organs of poplars varied according to pruning intensity. The concentration of SS in trunks of pruned poplars was higher than unpruned trees, and the concentrations of ST and TNC in trunks and branches of moderately pruned poplars were higher than those ofother treatments. Regardless of pruning intensity, the order of concentrations of SS and TNC in poplars was leaf > branch > trunk, and ST was trunk > leaf > branch, which may reflect the functional differences of plant organs. Therefore, moderate pruning can both increase NSC storage and improve the growth of poplars and may effectively increase growth in tree height and DBH, while avoiding the damage caused by excessive pruning to the tree body.
AcknowledgementsWe thank Ian Gilman at Yale University for his assistance with the English.
References
Anderegg WR, Callaway ES (2012) Infestation and hydraulic consequences of induced carbon starvation. Plant Physiol 159:1866–1874
ANSI (2001) American National Standard for Tree Care Operations—tree, shrub, and other woody plant maintenance: standards practices (pruning) (part 1). American National Standards Institute, New York
Atkinson RRL, Burrell MM, Rose KE, Osborne CP, Rees M (2014) The dynamics of recovery and growth: how defoliation affects stored resources? Proc R Soc B Biol Sci 281(1783):140–147
Bore JK, Isutsa DK, Itulya FM, Ngetich WK (2003) Effects of pruning time and resting period on total non-structural carbohydrates, regrowth and yield of tea ( Camellia sinensis L.). J Hortic Sci Biotechnol 78(2):272–277
Buysse J, Merckx R (1993) An improved colorimetric method to quantify sugar content of plant tissue. J Exp Bot 44(10):1627–1629
Carbone MS, Czimczik CL, Keenan TF, Murakami PF, Pederson N, Schaberg PG, Xu X, Richardson AD (2013) Age, allocation and availability of nonstructural carbon in mature red maple trees. New Phytol 200:1145–1155
Chesney P, Vasquez N (2007) Dynamics of non-structural carbohydrate reserves in pruned Erythrina poeppigiana and Gliricidia sepium trees. Agrofor Syst 69(2):89–105
Eyles A, Pinkard EA, Mohammed C (2009) Shifts in biomass and resource allocation patterns following defoliation in Eucalyptus globulus growing with varying water and nutrient supplies. Tree Physiol 29(6):753–764
Gaucher C, Gougeon S, Mauffette Y, Messier C (2005) Seasonal variation in biomass and carbohydrates partitioning of understory sugar maple ( Acer saccharum) and yellow birch ( Betula alleghaniensis) seedlings. Tree Physiol 25:93–100
Gillner S, Braüning A, Roloff A (2014) Dendrochronological analysis of urban trees: climatic response and impact of drought on frequently used tree species. Trees 28:1079–1093
Hoch G, Richter A, K?rner C (2003) Non-structural carbon compounds in temperate forest trees. Plant, Cell Environ 26(7):1067–1081
Honkanen T, Haukioja E, Suomela J (1994) Effects of simulated defoliation and debudding on needle and shoot growth in Scots pine ( Pinus sylvestris): implications of plant source/sink relationships for plant-herbivore studies. Funct Ecol 8:631–639
Ishii H, Clement JP, Shaw DC (2000) Branch growth and crown form in old coastal Douglas-fir. For Ecol Manag 131(1):81–91
Johnson DL (2007) Pruning. In: Kuser JE (ed) Urban and community forestry in the Northeast. Springer Netherlands, Dordrecht, pp 46–57
Landh?usser SM, Lieffers VJ (2003) Seasonal changes in carbohydrate reserves in mature northern Populus tremuloides clones. Trees 17(6):471–476
Landh?usser SM, Lieffers VJ (2012) Defoliation increases risk of carbon starvation in root systems of mature aspen. Trees 26(2):653–661
Lecigne B (2013) Effets des tailles de dégagement des reseaux électriques sur la colonization de l’espace par les arbres, développement et mise en application d’analysis de données T-LidAR. Master thesis, Université du Québec a Montréal (UQAM), Montreal, pp 24–32
León J, Rojo E, Sánchez-Serrano JJ (2001) Wound signalling in plants. J Exp Bot 52(354):1–9
Li H, Hoch G, K?rner C (2002) Source/sink removal affects mobile carbohydrates in Pinus cembra at the Swiss treeline. Trees 16:331–337
Martinez-Vilalta J, Sala A, Asensio D, Galiano L, Hoch G, Palacio S, Piper FL, Lloret F (2016) Dynamics of non-structural carbohydrates in terrestrial plant: a global synthesis. Ecol Monogr 86(4):495–516
Maurin V, Desrochers A (2013) Physiological and growth responses to pruning season and intensity of hybrid poplar. For Ecol Manag 304:399–406
Mei L, Xiong YM, Gu JC, Wang ZQ (2015) Whole-tree dynamics of non-structural carbohydrate and nitrogen pools across different seasons and in response to girdling in two temperate trees. Oecologia 177(2):333–344
Quentin AG, Pinkard EA, Ryan MG, Tissue DT, Baggett S (2015) Non-structural carbohydrates in woody plants compared among laboratories. Tree Physiol 35(11):1–20
Ramirez JA, Handa IT, Posada JM, Delagrange S, Messier C (2018) Carbohydrate dynamics in roots, stems, and branchs after maintenance pruning in two common urban tree species of North America. Urban For Urban Green 30:24–31
Reichenbacker RR, Schultz RC, Hart ER (1996) Artificial defoliation effect on populus growth, biomass production, and total nonstructural carbohydrate concentration. Environ Entomol 25(3):632–642
Schaberg PG, Snyder MC, Shane JB, Donnelly JR (2000) Seasonal patterns of carbohydrate reserves in red spruce seedlings. Tree Physiol 20(8):549–555
Sch?del C, Bl?chl A, Richter A, Hoch G (2009) Short-term dynamics of nonstructural carbohydrates and hemicelluloses in young branches of temperate forest trees during bud break. Tree Physiol 29(7):901–911
Song H, Wang CQ (2013) Mechanism of wound-induced defense response and signal transduction in plants. Chin Bull Bot 48(4):461–469 (with abstract in English)
Song XZ, Peng CH, Zhou GM, Gu HH, Zhang C (2016) Dynamic allocation and transfer of non-structural carbohydrates, a possible mechanism for the explosive growth of Moso bamboo ( Phyllostachys heterocycla). Sci Rep 6:25908–25915
Spann TM, Beede RH, Dejong TM (2008) Seasonal carbohydrate storage and mobilization in bearing and non-bearing pistachio ( Pistacia vera) trees. Tree Physiol 28(2):207–213
Vanderklein DW, Reich PB (1999) The effect of defoliation intensity and history on photosynthesis, growth and carbon reserves of two conifers with contrasting leaf lifespans and growth habits. New Phytol 144:121–122
Wang CS, Zeng J (2016) Research advances in forest tree pruning. World For Res 29(3):65–70 (with abstract in English)
Wiley E, Huepenbecker S, Casper BB, Helliker BR (2013) The effects of defoliation on carbon allocation: can carbon limitation reduce growth in favour of storage? Tree Physiol 33(11):1216–1228
Zhang J, Li XF, Li JG, Wang H, Huang CT, Min SJ, Li G, Zhang FH, Tian X, Kong J (2013) Sap flow dynamics of Populus alba L. × P. talassica plantation in arid desert area. Acta Ecol Sin 33(18):5655–5660 (with abstract in English)
Zhang HY, Wang CK, Wang XC (2014) Spatial variations in nonstructural carbohydrates in stems of twelves temperate tree species. Trees 28(1):77–89
 Journal of Forestry Research2021年2期
Journal of Forestry Research2021年2期
- Journal of Forestry Research的其它文章
- Stem taper functions for Betula platyphylla in the Daxing’an Mountains, northeast China
- Leaf phenotypic variation of endangered plant Tetracentron sinense Oliv. and influence of geographical and climatic factors
- Ecological variations of woody species along an altitudinal gradient in the Qinling Mountains of Central China: area-based versus mass-based expression of leaf traits
- Variations in stem radii of Larix principis-rupprechtii to environmental factors at two slope locations in the Liupan Mountains, northwest China
- A review of ecological mechanisms for management practices of protective forests
- Variation of basic density, calorific value and volumetric shrinkage within tree height and tree age of Ugandan grown Eucalyptus grandis wood
