Elevated CO 2 off sets the alteration of foliar chemicals ( n-icosane, geranyl acetate, and elixene) induced by elevated O 3 in three taxa of O 3 -tolerant eucalypts
Eka Novriyanti · Qiaozhi Mao · Evgenios Agathokleous · Makoto Watanabe · Yasuyuki Hashidoko · Takayoshi Koike
Abstract Eucalypts are important forest resources in southwestern China, and may be tolerant to elevated ground-level ozone (O 3 ) concentrations that can negatively affect plant growth. High CO 2 may off set O 3 -induced effects by providing excess carbon to produce secondary metabolites or by inducing stomatal closure. Here, the effects ofelevated CO 2 and O 3 on leaf secondary metabolites and other defense chemicals were studied by exposing seedlings of Eucalyptus globulus, E. grandis, and E. camaldulensis × E. deglupta to a factorial combination of two levels of O 3 (< 10 nmol mol ?1 and 60 nmol mol ?1 ) and CO 2 (ambient: 370 μmol mol ?1 and 600 μmol mol ?1 ) in open-top field chambers. GC-profiles of leafextracts illustrated the effect ofelevated O 3 and the countering effect of high CO 2 on compounds in leafepicuticular wax and essential oils, i.e., n-icosane, geranyl acetate and elixene, compounds known as a first-line defense against insect herbivores. n-Icosane may be involved in tolerance mechanisms of E. grandis and the hybrid, while geranyl acetate and elixene in the tolerance of E. globulus. Elevated O 3 and CO 2 , singly or in combination, affected only leaf physiology but not biomass of various organs. Elevated CO 2 impacted several leaf traits, including stomatal conductance, leaf mass per area, carbon, lignin, n-icosane, geranyl acetate and elixene. Limited effects ofelevated O 3 on leaf physiology (nitrogen, n-icosane, geranyl acetate, elixene) were commonly off set by elevated CO 2 . We conclude that E. globulus, E. grandis and the hybrid were tolerant to these O 3 and CO 2 treatments, and n-icosane, geranyl acetate and elixene may be major players in tolerance mechanisms of the tested species.
Keyword Carbon dioxide · Epicuticular wax · Eucalypts · Secondary metabolism · Tropospheric ozone
Introduction
High concentrations of tropospheric ozone (O3) can induce damage in plants and reduce forest productivity (Broadmeadow et al. 1999; Percy et al. 2003; Manning 2005; Proietti et al. 2016; Yuan et al. 2015, 2016). As a result of increasing O3concentrations, 50% (17 million km 2 ) of the world’s forest may be exposed to O3levels > 60 nmol mol?1and, thus, decrease photosynthetic productivities by the year 2100 (Fowler et al. 1999; Sitch et al. 2007). The considerable genetic variability among plants results in substantially different levels of damage in response to particular O3levels (Booker et al. 2009). At the same time, atmospheric carbon dioxide (CO2) concentrations have also increased and are predicted to reach 600 μmol mol –1 near the year 2060 (IPCC 2007). Elevated CO2may enhance photosynthetic rates and increase plant growth (e.g., Ainsworth and Long 2005). Hence, it is imperative to determine the interactive effects of CO2and O3on such representative species for future afforestation practices (e.g., Karnosky et al. 2003; Kitao et al. 2015; Shi et al. 2017).
Exposure to O3generates a response comparable to that of hypersensitive response of plants to pathogen attack; thus, O3is considered an abiotic “elicitor” of plant defense reaction, with the potential to alter the chemistry and metabolism of plant tissue (Sandermann et al. 1998; Matyssek et al. 2012; Agathokleous et al. 2019a). At elevated concentrations, O3is a strong oxidant, which reacts with leaf apoplast components and induces high production of reactive chemical species, leading to death of cells, inhibition of carbon assimilation and acceleration of senescence (Singh and Agrawal 2017). Conversely, elevated CO2can reduce water loss through transpiration, enhance carbon assimilation and the apparent quantum yield of CO2uptake and plant growth and yields, and improve water- and light-use effi ciency (Ainsworth and Long 2005; Eguchi et al. 2008). Elevated CO2and O3can also alter leaf chemistry, including secondary metabolites, in multiple ways (Booker and Maier 2001; Kopper and Lindroth 2003; Oksanen et al. 2005; Lindroth 2010; Novriyanti et al. 2012a; Shi et al. 2016, 2017; Araminien? et al. 2018).
Because CO2is a substrate of photosynthesis, elevated CO2may increase C availability for secondary metabolite production (Bryant et al. 1983; Herms and Mattson 1992; Mattson et al. 2005); thus, the altered secondary metabolites are more likely due to resource availability rather than induced defense. Hence, it is important to understand whether enhancement of growth and secondary metabolite concentration caused by elevated CO2can counteract damage caused by O3. Several studies have provided evidences ofelevated CO2alleviating the adverse effects ofelevated O3(Booker and Maier 2001; Karnosky et al. 2003; Kopper and Lindroth 2003; Paoletti and Grulke 2005). Moreover, as stomatal conductance can be suppressed by elevated CO2(e.g., Larcher 2003; Schulze et al. 2005), O3absorption is expected to be somewhat lessened. CO2amelioration of O3adverse effects is assumed to be related with the so-called fertilizer effect ofelevated CO2by which net photosynthesis increases and a C surplus is available for secondary metabolite production.
Eucalypts have been widely used in commercial plantation due to their fast growing traits and broad adaptability (e.g., Orwa et al. 2009) and are one of the most important forest resources in southwestern China (Wang and Koike 2019). Numerous studies have therefore investigated the effects of CO 2 on different eucalypt species, singly or in combination with other factors such as ambient air temperature, light availability, nitrogen deposition, soil nutrients, and water deficit (Lawler et al. 1996; Roden and Ball 1996; McKiernan et al. 2012; Novriyanti et al. 2012a; Murray et al. 2013; Plett et al. 2015; Ghini et al. 2015; Quentin et al. 2015; Xu et al. 2019). Despite the many studies on CO2interactive effects with other factors, the interactive effects of CO2and O3on eucalypts remain unknown, and studies on single effects of O3on eucalypts are very limited (Monk and Murray 1995). Furthermore, elevated CO2and O3alter the concentrations of secondary metabolites in trees in a gasspecific manner and with significant interactive effects of the two gases (Gleadow et al. 1998; Oksanen et al. 2005; McKiernan et al. 2012; Xu et al. 2019). The effects on leaf chemical defense, including secondary metabolites, are of high ecological relevance because they can drive plant–herbivore interactions (Gleadow et al. 1998; Bidart-Bouzat and Imeh-Nathaniel 2008). It is therefore important to investigate the effects of O3singly or in combination with CO2on leaf chemical defense, and especially secondary metabolites, in eucalypt species. Ifelevated CO 2 and O 3 affect the leaf chemicals of these promising afforestation species, their defense traits and fitness to grow and succeed in the changing environments should be of concern.
The present study aimed to examine the single and combined effects ofelevated CO2and O3on leaf chemical traits, including both primary and secondary chemistry, in representative afforestation eucalypt species. We hypothesized that, under elevated O3, a trade-off may occur between growth and secondary metabolites. Both growth and secondary metabolites concentrations would be enhanced by elevated CO2due to the fertilizer effect of CO2. We predicted that the stimulatory effect ofelevated CO2will moderate the inhibitory effect ofelevated O3on leaf chemicals. To test this prediction, three eucalypt species were grown in the field in open-top chambers (OTCs) supplied with ambient air or elevated CO2and/or O3. The selected eucalypts are fast-growing and widely planted in Asian forest plantations (Novriyanti et al. 2012a, b; Wang and Koike 2019) toward informing forestry applications in an O3-polluted, CO2-enriched world.
Materials and methods
Study sites and plant materials
The experiment was conducted at the experimental nursery of the Field Science Center of Hokkaido University, Sapporo, Japan (43° 0′ N, 141° 2′ E, 15 m a.s.l.). The seedlings were grown in 10-L pots filled with a commonly used nursery mixture of pumice soil and clay soil (1:1, v/v), with 200 mL of 500-fold diluted liquid fertilizer (balanced nutrients; 6:10:5, Hyponex Corp. JAPAN, Osaka, Japan) per plant at 2-week intervals to provide 192 mg N per pot. The pots were watered periodically to sustain the soil moisture.
Seedlings of Eucalyptus globulus (Glo) and Eucalyptus grandis (Gra) and cuttings of hybrid E. deglupta × E. camaldulensis (Hyb) were used because they are popular for plantations. The seeds were obtained from the Australian Tree Seed Centre of CSIRO, Australia. At the initiation of the experiment, all plants were 5 months old; average height and basal diameter was 25.0 cm and 2.4 mm for Glo, 24.3 cm and 3.0 mm for Gra, and 26.7 cm and 2.4 mm for Hyb. The experiment lasted from June to October 2010.
Gas treatment system
The seedlings were placed in 16 OTCs (1.2 × 1.2 × 1.2 m high; Dalton Co. Ltd. Sapporo, Japan) supplied with one of two levels of O3for 7 h during the daytime (ambient [AO]: < 10 nmol mol?1; elevated [EO]: 60 nmol mol?1) in combination with one of two levels of CO2(ambient [AC]: about 370 μmol mol?1; elevated [EC]: 600 μmol mol?1) during the daytime. Four chamber replications were deployed for each treatment; therefore, there were 16 OTCs.
Except for the EO chambers, the OTCs were equipped with charcoal filters to first clean the inlet air. The level of EO was selected because similar concentrations have been often observed in many regions in Japan. The CO2level of EC is the atmospheric level predicted near the year 2060 (IPCC 2007). The OTCs had an average temperature of 22.3 °C (max 29.8 °C and min 17.8 °C) and relative humidity of 76.4%.
Gas exchange measurements
Gas exchange rates were measured on third and fourth leaves from the shoot top using an open-type gas-exchange system (LI-6400, Li-Cor, Lincoln, NE, USA) with a photosynthetic photon flux of 1500 μmol m–2s –1 , the light level corresponding to the light-saturated photosynthetic rate for these plants in the growing environment. Leaf temperature was controlled at 25 °C ± 1 °C and leaf vapor pressure deficit (VPD) maintained at 1.2 ± 0.2 kPa to regulate stomatal conductance during the measurements. The leaves acclimated to the chamber conditions at growth CO2concentrations (i.e., 370 μmol mol?1for AC and 600 μmol mol?1for EC) for 15–20 min after clipping the leaf to the chamber. After the acclimation, light saturated net photosynthetic rate at growth CO2concentration ( Agrowth), stomatal conductance ( gs) and leaf transpiration rate ( E) were determined.
To obtain the response curve for net photosynthetic rate ( A) to intercellular CO2concentration ( Ci), i.e., the Agrowth/ Cicurve, 12 levels ofexternal CO2concentration were supplied to the chamber (60–1500 μmol mol –1 ), and the corresponding values for maximum rate of carboxylation ( V cmax) and maximum rate ofelectron transport ( Jmax) were determined (Farquhar et al. 1980; Long and Bernacchi 2003). The Rubisco Michaelis constants for CO2( Kc) and O2( Ko) and the CO2compensation point in the absence of dark respiration (Γ*) for the analysis of the Agrowth/ Cicurve were obtained from Bernacchi et al. ( 2001). All gas-exchange variables were expressed on the basis of the projected (onesided) leaf area covered.
Total phenolic and condensed tannin measurements
Total phenolics were determined using the Folin–Ciocalteu method as modified by Julkunen-Tiitto ( 1985), and condensed tannins were measured using the proanthocyanidin method of Bate-Smith ( 1977) (Matsuki et al. 2004). A powdered, freeze-dried leaf sample (20 mg) was placed in 5 mL of 50% methanol in an ultrasonic machine (ST-02M, Sonic Tech, Tokyo, Japan) at 40 °C for 1 h.
For measuring condensed tannins, 1 mL of the methanolic extract solution was placed in a test tube with 4 mL HCl–1-butanol (1: 19). The solution was then boiled for 2 h, then cooled before the optical density at 55 nm was measured with a spectrophotometer (UV-2700, Shimadzu Kyoto, Japan).
For measuring total phenolics, 50 μL of the filtered methanolic extract solution was placed in a test tube with 2.25 mL of deionized water, 0.25 mL of 50% phenol reagent and 2.5 mL of 20% w/v Na2CO3, and the mixture was mixed thoroughly. After the solution rested for approximately 15 min, the optical density at 760 nm was measured with the spectrophotometer.
The total condensed tannins (mg g –1 ) content was calculated as (4.5 A/4 + 0.011)/(20.255 × 5000)/B, and total phenolics (mg g –1 ) as ( A/2 ? 0.03)/12.281 × 100,000/B, where A is the absorbance reading and B is the mass of the sample (± 20 mg).
Gas chromatography
A powdered sample of fresh leaves was soaked 3 times in 15 mL methanol (MeOH), then 1 mL of the MeOH extract was rotoevaporated (< 35 °C and 80 Pa), dissolved in 1 mL CHCl3, then added to 3 mL Sep-Pak silica cartridge (Waters Corp., Milford, MA, USA) eluted with CHCl3. The eluate was then collected and evaporated. This fraction contained the less-polar phenolics such as waxes, fats, and other hydrocarbons. A MeOH standard solution was made by dissolving 100 mg m- tert-butylphenol in 100 mL MeOH, then diluted 10 times with MeOH. The extract was then dissolved in the MeOH-standard solutions to make 1 mL (volumetric) of sample solution.
One microliter of sample solution was injected into a gas chromatograph (GC-2025, Shimadzu, Kyoto, Japan) with a DB-1 column (20 m × 0.25 mm i.d., 0.25 μm, silica coated; J&W Scientific, Folsom, CA, USA) and helium as the carrier gas. The injector and detector temperature were maintained at 250 °C. The column temperature was initially 70 °C, with a hold-time of 2 min, then increased at 5 °C/min to 275 °C, and finally at 2.5 °C/min to 280 °C, with a final hold-time of 5 min. Peaks detected during the 58 min procedure were compared and analyzed to check the effects of the treatments. There were four replications ofeach sample solution, and an analysis of variance (ANOVA) was carried out to determine the significant difference among peak patterns in the GC profiles ( α = 0.05).
Gas chromatography–mass Spectrometry
After the GC-profiles revealed peak patterns affected by the treatments, he representative sample solutions from each species were subjected to gas chromatography–mass spectrometry (GC/MS) using Agilent Technologies 6890 series GC (Hewlett-Packard, Palo Alto, CA, USA) coupled to an electron ionization-mass spectrometer (EI-MS) (JEOL JMS-700TZ) with He as the carrier gas. Data acquisition parameters were flow rate 1.5 mL/min; injection volume 1 μL (splitless); inlet temperature 250 °C; ZB-1MS (phenomenex) 30 m × 0.25 mm i.d. × 0.25 μm column; temperature program: 70 °C for 2 min, increasing 5 °C/min to 275 °C, then 2.5 °C/min to 280 °C with a final hold time of 5 min; electron ionization (EI) mode, electron energy 70 eV, 300 μA, source temperature 230 °C, scan mode, mass range at m/z 40–500 Da. The acquired mass spectra were used to search the mass spectral library NIST/EPA/NIH/EINECS/IRDB to identify compounds.
Data analysis
The statistical significance was set a priori to an alpha level of 0.05. The data for each trait and species were averaged per OTC to provide four replicates per gas treatment. Data were tested against the requirements of parametric statistical tests, and, when needed, subjected to a Box–Cox transformation (Box and Cox 1964) according to the methodology explained by Agathokleous et al. ( 2016a). Statistical hypothesis testing was done with Spiegel’s Method I sum of squares-adjusted (Howell and McConaughy 1982) general linear models (GLM) where species and gas treatment were fixed factors and OTC was a random factor. For significant species, treatment or species × treatment interactions, Bonferroni post-hoc test was applied for multiple comparisons among the experimental groups. The results are shown as means ± SD. Data processing and statistics were performed with EXCEL 2010 (Microsoft, Redmond, CA, USA) and STATISTICA v.10 (StatSoft, Tulsa, OK, USA).
Results
Biomass
Biomasses varied significantly among species but were not significantly affected by gas treatment; the species × treatment interaction was also not significant (Fig.1).
Gas exchange, leaf mass per area (LMA) and photosynthetic pigments
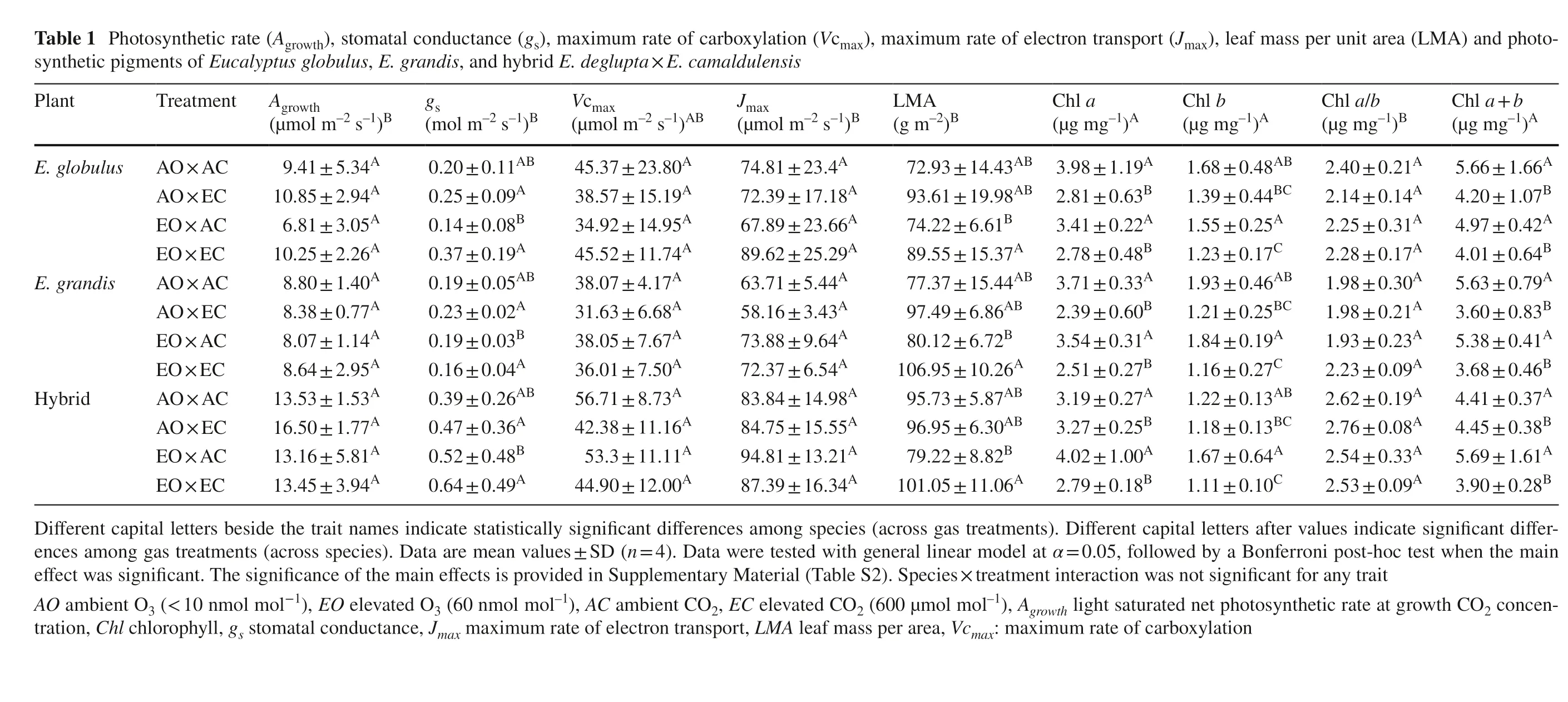
Agrowth, gs, V cmaxand Jmaxvaried significantly among species; and values were greater for the hybrid than for the other two species, except that V cmaxfor E. globulus did not differ significantly from E. grandis and the hybrid (Table 1). Gas treatment was a significant factor only for gs. In particular, EC did not significantly affect gsin AO but did increase gsin EO, suggesting a significant interaction between CO2and O3(Table 1).
LMA varied among species and among gas treatments (Table 1). E. grandis and the hybrid had a higher LMA than E. globulus did. EC tended to increase LMA, compared to AC, in AO; however, variation was large, and the difference was not significant. The EC-induced increase in LMA was significant in EO (Table 1).
Chlorophyll a, b and a + b contents did not vary significantly among species (Table 1). However, the chlorophyll a to b ratio ( a/ b) was in the order hybrid > E globulus > E. grandis. Gas treatment was a significant factor for chlorophyll a, b and a + b contents but not for chlorophyll a/ b. EC significantly decreased chlorophyll a and a + b levels, but not chlorophyll b in AO (Table 1). However, EC significantly decreased chlorophyll a, b and a + b content in EO. EO per se did not affect the photosynthetic pigments.
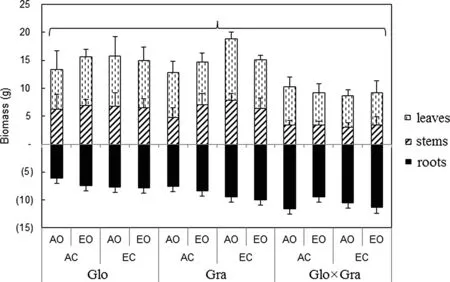
Fig.1 Biomass of leaves, stems and roots of Eucalyptus globulus (Glo), E. grandis (Gra), and E. deglupta × E. camaldulensis (Glo × Gra). Error bar is standard deviation with n = 4. AO ambient O 3 (< 10 nmol mol ?1 ), EO elevated O 3 (60 nmol mol –1 ), AC ambient CO 2 , EC elevated CO 2 (600 μmol mol –1 ). Only species was a significant factor according to general linear model tests (statistical results are provided in Supplementary Material, Table S1)
The interaction between species and gas treatment was not significant for any trait for gas exchange and photosynthetic pigments as for LMA (Table 1).
Phenolics, tannins, C and N
Total phenolics varied only among species; the hybrid had lower phenolic content than the other two species (Table 2). Condensed tannins varied among species and among gas treatments; however, the species × treatment interaction was not significant. The hybrid and E. grandis had similar content, significantly more than that of E. globulus. While EC did not significantly affect tannin content in AO, it increased tannin content in EO. EO did not significantly affect the total phenolics or tannins in AC or EC.
Regarding C and N traits, there were significant differences among species and among gas treatments, but the interaction between species and treatments was not significant (Table 2). In both AO and EO, EC significantly increased the area-based C, but there was a trend toward lower mass-based C content, which was significant only in EO. EO did not affect Careaand Cmassin either AC or EC. EC decreased Nareaand Nmassin both AO and EO, although for Nareathe difference was not significant in EO. EO decreased significantly both Nareaand Nmassin AC, but this effect was off set by EC (no significant differences between AO × EC and EO × EC). Interestingly, EC significantly increased the C/N ratio in both AO and EO, but this effect was mainly due to a decrease in N content than an increase in C content by EC. EO increased the C/N ratio in AC but did not affect C/N ratio in EC where C/N ratio was driven by EC.
Lignin content was significantly decreased by EC in EO but was not significantly affected by EC in AO (Table 2). However, this difference seems to be due to higher lignin content in EO × AC (compared to AO × AC), although the difference between the two groups was not significant. EO did not significantly affect lignin. While species and treatments had significant effects on lignin, the interaction of the two factors was not significant. Interestingly, the hybrid that tended to have high biomass was also the species with the highest lignin content and lowest phenolic content; it also had high tannin content (across gas treatments).
GC profiles of leafextracts
Peaks in the GC profile for E. globulus were the most abundant, compared with those of hybrid and E. grandis (Fig.2). Although the GC profiles differed among the species and hybrid, all showed the same peak pattern at retention time (RT) 34.8 min (hereafter compound 1).
Regarding E. grandis, EO increased the peak height of compound 1 in AC but not in EC (Fig.3 a), indicating that EC off sets the EO-induced effect. Regarding the hybrid, EO increased the peak height of compound 1 in both AC and EC, while EC decreased it in both AO and EO; nonetheless, EC did not fully off set the EO-induced effect because the value was still higher in EO × EC than in AO × EC (Fig.3 b). As for E. globulus, there were no significant differences in the relative peak height of compound 1 among treatments (Fig.4 a). However, E. globulus displayed an EO-induced increase in the relative concentration of compounds at RT 17.2 min (hereafter compound 2; Fig.4 b) and 24.1 min (hereafter compound 3; Fig.4 c) in AC. This EO-induced effect did not appear in EC, where there were no significant differences between AO × EC and EO × EC, due to CO2mediation. Similarly with EO, EC significantly increased the relative concentration of compound 2 in AO; the value decreased when EO and EC were combined in such an extent that it was not significantly different from AO × AC (Fig.4 b). On the other hand, EC significantly decreased the relative concentration of compound 3 in AO, and this CO2-induced effect also prevailed in EO, ruling out the EOinduced effect (Fig.4 c).
?
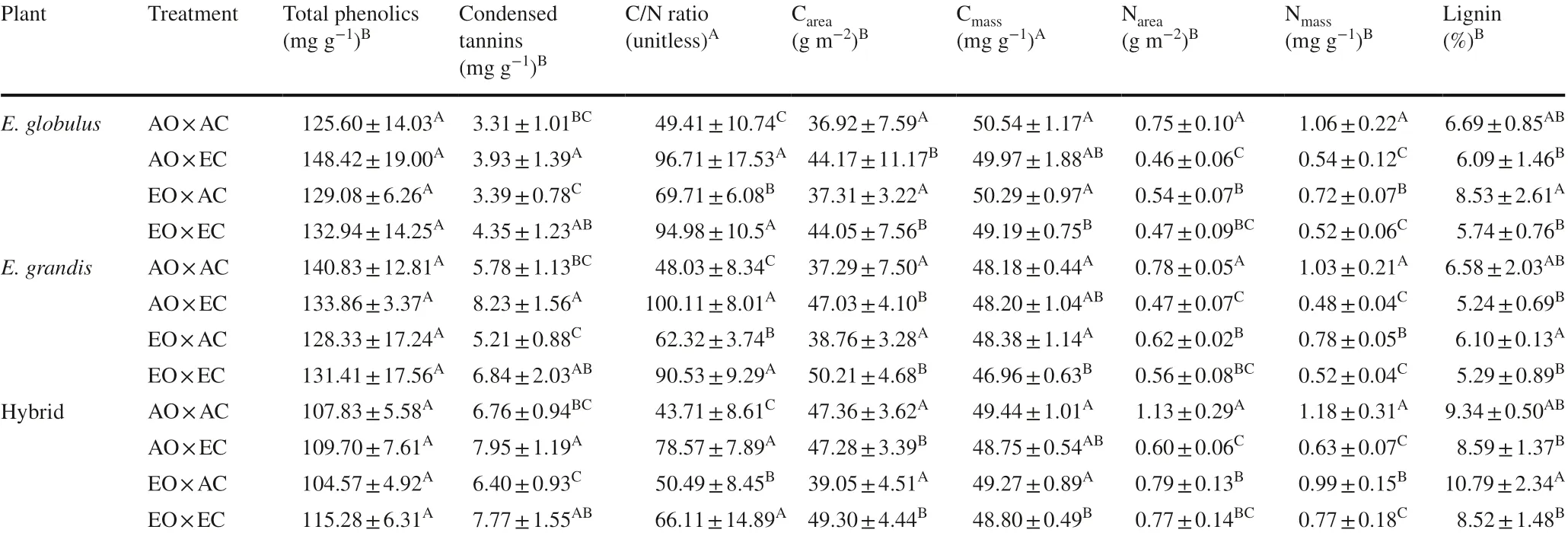
Table 2 Leaf chemistry of Eucalyptus globulus, E. grandis, and hybrid E. deglupta × E. camaldulensis
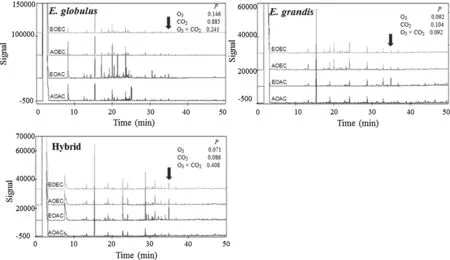
Fig.2 Gas chromatography profile of Eucalyptus globulus, E. grandis, and hybrid E. deglupta × E. camaldulensis. n = 4. AOAC ambient O 3 + CO 2 , EOAC elevated O 3 + ambient CO 2 , AOEC ambient O 3 + elevated CO 2 , EOEC elevated O 3 + elevated CO 2
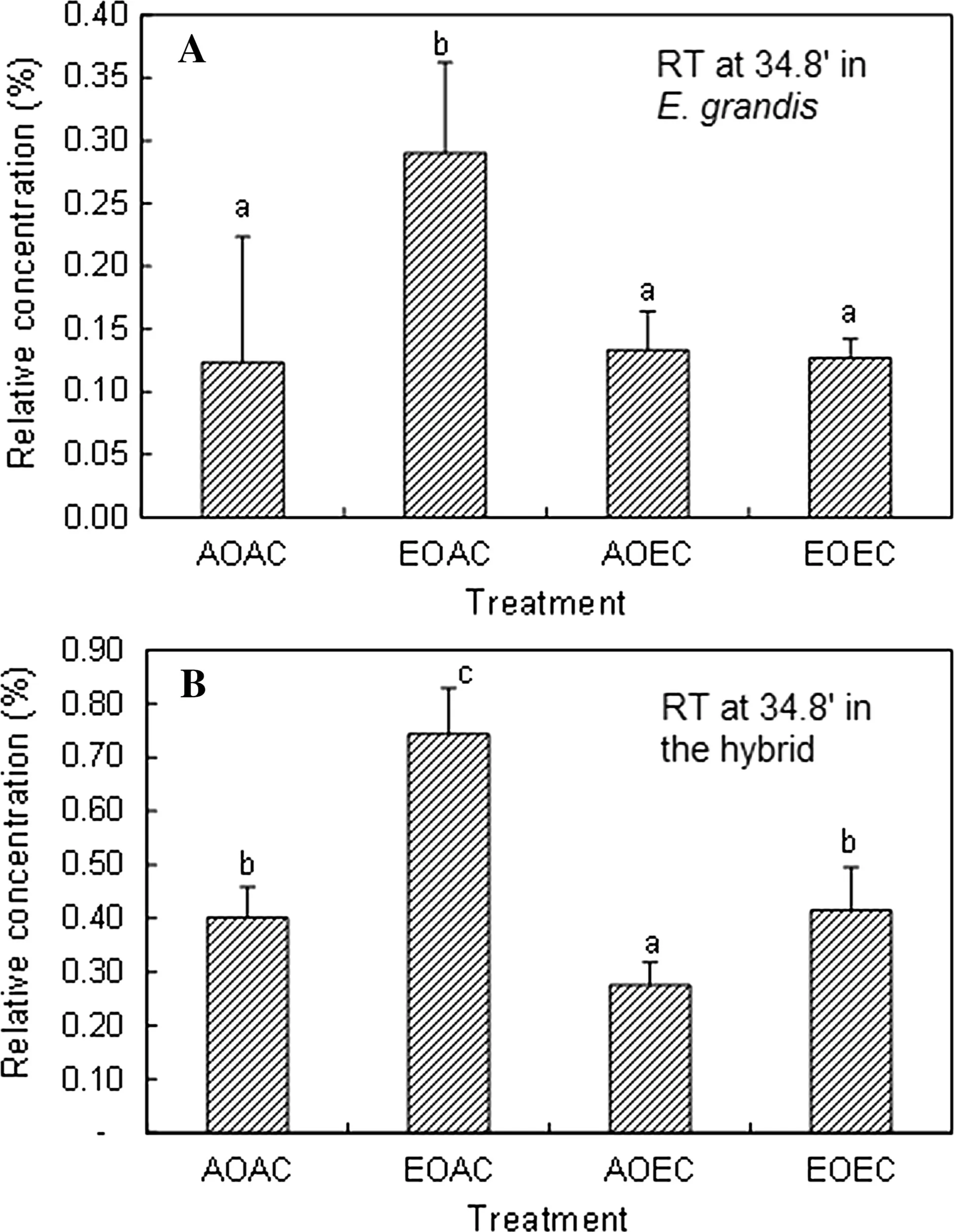
Fig.3 Mean (± SD) relative concentration of compound 1 calculated from GC profiles for peak at RT 34.8 min. a Eucalyptus grandis. b E. deglupta × E. camaldulensis. Different letters above bars indicate means differed significantly ( n = 4). AOAC ambient O 3 and CO 2 , EOAC elevated O 3 + ambient CO 2 , AOEC ambient O 3 + elevated CO 2 , EOEC elevated O 3 + elevated CO 2 . The data for E. globulus are presented in Fig.4 because of its distinct peaks
GC/MS analysis revealed that compound 1 is an alkane, n-icosane (Fig.5). Based on the mass fragmentation resulted from GC/MS analysis, the library provided a match for compounds 2 and 3: 2,6-octadien-1-ol, 3,7-dimethyl-, acetate, (E)-geranyl acetate or geranyl acetate (Fig.6) and cyclohexane, 1-ethenyl-1-methyl-2-(1-methylethenyl)-4-(1-methylethylidene) or elixene (Fig.7). These terpenes are commonly found in plant essential oils.
Discussion
Despite the species-specific biomass Agrowth, V cmax, and Jmax, the fact that the eucalypts biomasses Agrowth, V cmax, and Jmaxwere not significantly affected by gas treatments suggests that the three species are not susceptible to EO and EC (Agathokleous and Saitanis 2020). This hypothesis is further supported by lack of a significant effect on the R/S ratio, indicating no adverse single or combined effect of EO and EC (Agathokleous et al. 2016b, 2019b). E. globulus has been found to be tolerant to acute O3exposures, as indicated by unaffected physiological measures (including photosynthetic traits and lipoxygenase pathway emission rates) when exposed to 0.3–2.0 μmol mol?1O3for a few hours (O’Connor et al. 1975; Kanagendran et al. 2018). In a different study, E. globulus had neither visible injury nor biomass reduction after exposure to diurnally varied concentrations of 26 or 172 nil?1for 7 h day?1, 5 days every 14 days, for 18 weeks (Monk and Murray 1995). Although that study with a chronic exposure (Monk and Murray 1995) and other studies with acute exposures (O’Connor et al. 1975; Kanagendran et al. 2018) indicate that E. globulus is tolerant to EO, a different study suggested an “extreme sensitivity”, with reduced biomass, Agrowthand gs, after seedlings were exposed to 50 nmol mol?1(7 h day?1) for 37 days under low light and controlled temperature (20 °C) (Pearson 1995); however, the susceptibility of the plants might have been affected by the low light condition and/or the controlled temperature. Chronic and acute exposure to O3can differ in their effects on plant physiology due to secondary acclimation responses (Liu et al. 2019). However, our study and the study of Monk and Murray ( 1995) indicate that E. globulus is tolerant to O3, and the tolerance of E. grandis and the hybrid seem to be similar that of E. globulus to EO. It is therefore important to pinpoint biological mechanisms underpinning the tolerance of these eucalypts to the gas treatments.
The absence of a significant effect of the gas treatments on Agrowth, V cmaxand Jmaxmay indicate acclimation, as reported for many species (Koike et al. 1996; Tissue et al. 1999; Watanabe et al. 2011). This may explain why no significant biomass enhancement or inhibition was induced by EC and EO, respectively. Not only elevated O3(Kitao et al. 2009; Koike et al. 2012), but also elevated CO2is known to induce stomatal closure (Chater et al. 2015; Dusenge et al. 2019). However, this was not the case in our experiment with the three eucalypts, where EC did not affect gsin AO but increased gsin EO. This EC-induced increase in gsin EO, however, may indicate a permanent stomatal impairment when EO is combined with EC (Hoshika et al. 2019).
Interestingly, EC tended to increase LMA, especially in EO where the effect was significant. This increase has been observed often in plants grown in elevated CO2concentrations and may result from accumulation of carbohydrates that are not used for plant growth (Hikosaka et al. 2005). Increased LMA may favor plant growth in atmospheres with elevated CO2concentrations by compensating for otherwise reduced leaf N concentration per unit mass (Hikosaka et al. 2005), which may account for the observed EC-induced decrease in Nmassin both AO and EO. Increased LMA may also indicate enhanced stored carbohydrates (Booker 2001).
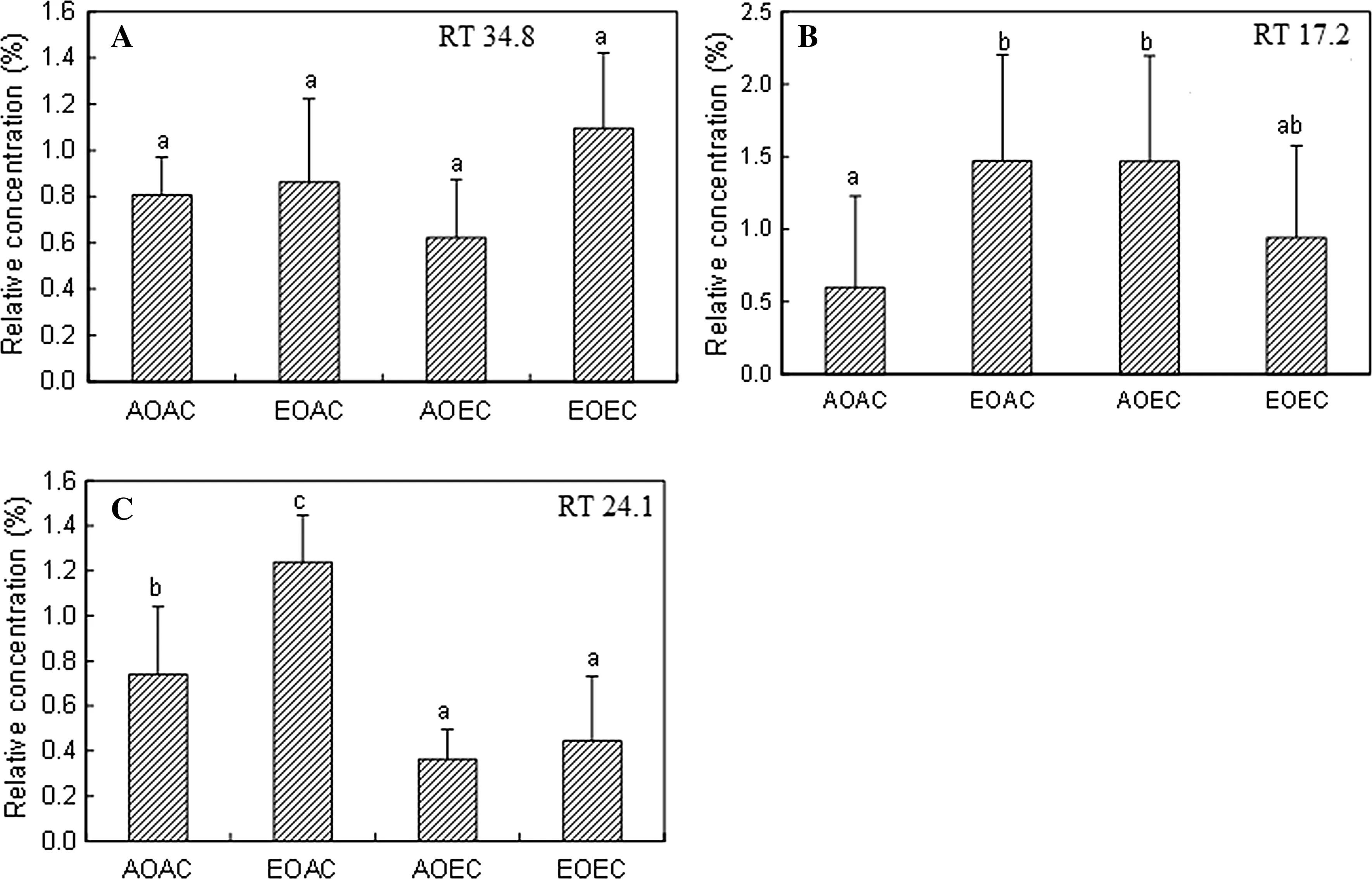
Fig.4 Mean (± SD) relative concentration of compounds calculated from GC profiles for distinctive peaks on Eucalyptus globulus. a Peak at RT 34.8 min. The RT is the same as the peaks in Fig.3. b Peak at RT 17.2 min. c Peak at RT 24.1 min. Different letters above bars indicate means differed significantly ( n = 4). AOAC ambient O 3 and CO 2 , EOAC elevated O 3 + ambient CO 2 , AOEC ambient O 3 + elevated CO 2 , EOEC elevated O 3 + elevated CO 2
Elevated O3exposures have been extensively shown to decrease the level of chlorophylls, an effect associated with inhibition of quantum yield (Saitanis et al. 2001; Li et al. 2017). However, we found no significant effect of the tested EO exposure on the three eucalypt species. We postulate that the three eucalypts might have maintained the chlorophyll content as a tolerance mechanism to maintain the photosynthetic activity at homeostatic levels. Conversely, EC decreased chlorophyll levels in AO and EO. The CO2effect on chlorophyll pigments depends on CO2concentration (concentration-specific), and our findings agree with those ofother studies that show an EC-induced negative effect on chlorophyll levels that can result from reallocation of limiting resources away from the photosynthetic apparatus (Ong et al. 1998; Grams et al. 1999; Ormrod et al. 1999; Pritchard et al. 2000). Since we analyzed chlorophyll levels only at the end of the experiment, the EC-induced decrease in chlorophyll contents in AO and EO may also be due to accelerated development induced by EC (Centritto and Jarvis 1999), although there are no empirical data to support this hypothesis.
The finding that phenolics and tannins were not significantly affected by EO, but tannins were increased by EC when O3was elevated, suggests that total phenolics were insensitive but tannins were sensitive to the gas treatment. This finding also suggests that when EO and EC are combined, plant–herbivore interactions might be affected because increased tannins would decrease leaf palatability.
Elevated O3and CO2can alter C and N metabolism, stock, and allocation to different plant organs (Uddling et al. 2006; Ainsworth et al. 2007; Dusenge et al. 2019; Shang et al. 2019a, b). However, Careaand Cmassin the three eucalypts were not significantly affected by EO in our study. The results suggest that EC drove the C response in EO as indicated by increased Careaand decreased Cmass. The fact that the EO-induced decrease in Nareaand Nmassand the increased in C/N ratio were off set by EC, indicates that N response is regulated by EC but not by EO in elevated O3. The increased C/N ratio (commonly in both AO and EO) by EC was due to decreased Nareaand Nmass. Bloom et al. ( 2010) reported that elevated CO2inhibited the assimilation of nitrate into organic N compound in leaves of wheat and Arabidopsis. This inhibition of nitrate assimilation may lead to the decreased organic N content and may play an important role in the photosynthetic acclimation under EO (Bloom et al. 2010). The EC-induced decrease in leaf N might also be due to inhibited N resorption. For example, elevated O3substantially impairs N resorption in birch leaves before leaf abscission (Uddling et al. 2006; Shi et al. 2017), causing a significant loss in foliar N. The impairment in N resorption may be due to impaired phloem-loading in leaves due to accumulated starch along leaf veins (Uddling et al. 2006). That the species × treatment interaction was not significant while the single effects of species and treatment were significant suggests that C and N metabolism was an important common mechanism among the three eucalypts under the tested gas treatments.
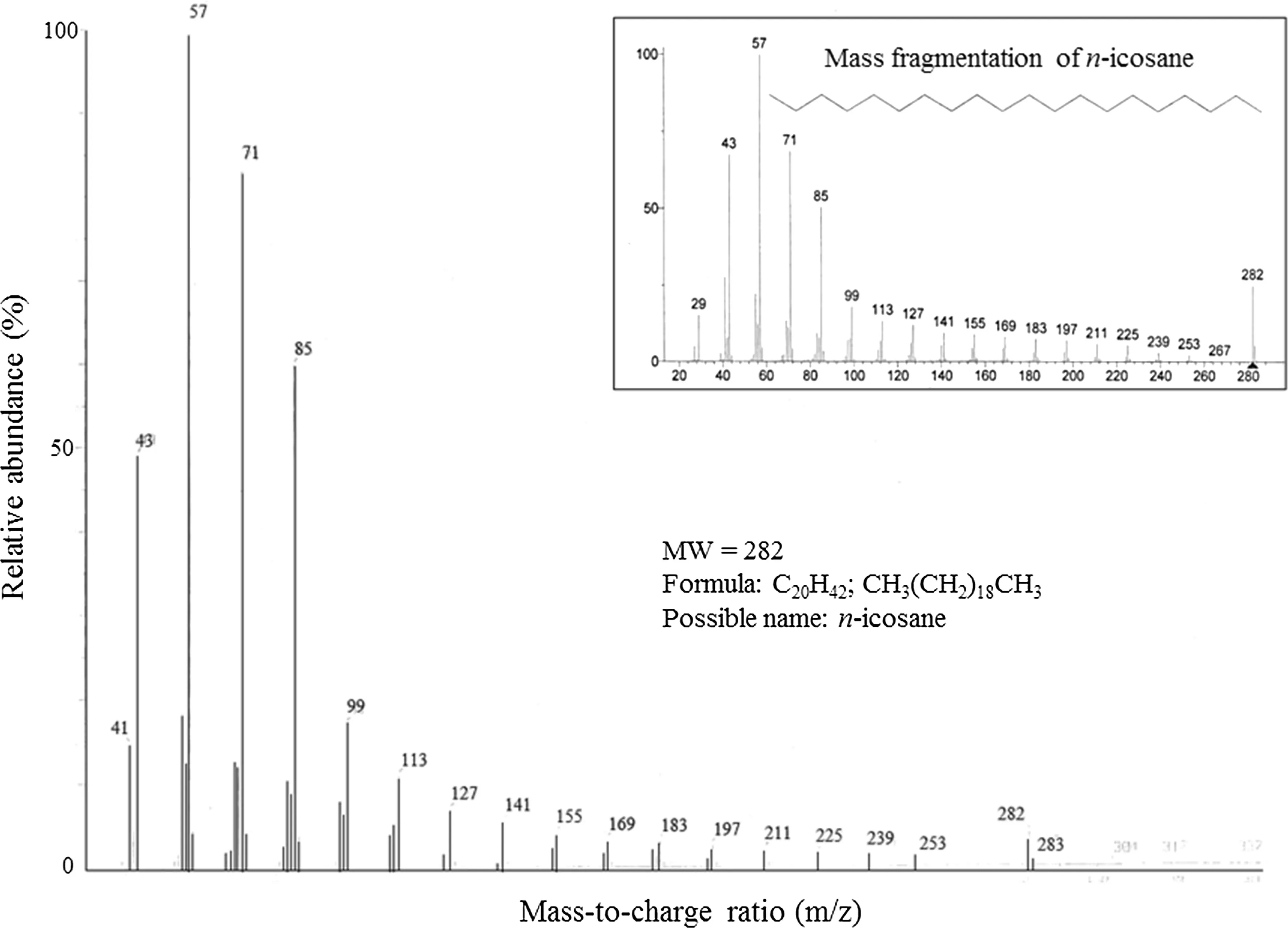
Fig.5 Mass fragmentation of compound 1 at RT 34.8 min in GC/MS ofextracts from Eucalyptus globulus. Possible chemical name is n-icosane based on comparison with GC/MS library. Insert is from the NIST/EPA/NIH/EINECS/IRDB library
Area-based traits are important for studying photosynthesis-related processes that are measured as a flux per unit of leaf area, whereas mass-based traits are important for studying leafeconomy in terms of biomass investment for carbon fixation (Hikosaka 2004; Shang et al. 2019b). Thylakoid N is proportional to the chlorophyll content (e.g., 50 mol thylakoid N per mol chlorophyll); hence, there is a positive correlation between chlorophyll content and total leaf N under typical growth conditions (Evans 1989). A recent study on O3effects on two poplar clones revealed that chlorophyll per unit mass was negatively correlated with Nmass, whereas chlorophyll per unit area was positively correlated with Narea(Shang et al. 2019b). Conversely to the negative correlation that Shang et al. ( 2019b) found in poplars, a regression analysis with the data of all eucalypt species and gas treatments from our study revealed that chlorophyll per unit mass was positively correlated with Nmass( y = 0.1349 x + 0.1456, r = 0.571, F = 22.2, P < 0.001); interestingly, the R 2 value was nearly identical with that found by Shang et al. ( 2019b). The finding that EC increased the Careabut tended to decrease Cmasscontent (although significant only in EO) and the findings of Shang et al. ( 2019b) suggest that care should be exercised when selecting the appropriate unit for assessing C and N responses to gaseous treatments.
Lignin content was significantly decreased by EC in EO but was not significantly affected by EC in AO. However, this difference seems to be due to higher lignin content in EO × AC (compared to AO × AC),
Elevated CO2can modify lignin concentration in leaves, but the effect is species specific, and no general conclusion can be drawn (Co?teaux et al. 1999; Norby et al. 2001; Zheng et al. 2019). In the present study, EC decreased lignin in EO but not in AO; however, this difference seems to be due to more lignin in EO × AC (compared to AO × AC). Blaschke et al. ( 2002) reported that lignin in seedlings grown with suffi cient nutrients was unaffected or even decreased by elevated CO2. Because nutrients were normally supplied in the present study and lignification is a physiologically important process during growth, development and tissue maturation in woody plants (e.g., Blaschke et al. 2002), impairment of lignification may be related to the unaffected biomass in high CO2. Interestingly, a regression analysis of the data of all eucalypt species and gas treatments from our study revealed that lignin was negatively correlated with leaf biomass ( y = ? 0.535 x + 11.602, r = 0.537, F = 18.6, P < 0.001) and with stem biomass ( y = ? 0.626 x + 10.093, r = 0.606, F = 26.7, P < 0.001), but was not correlated with root biomass ( y = 0.296 x + 6.742, r = 0.222, F = 2.4, P = 0.130).
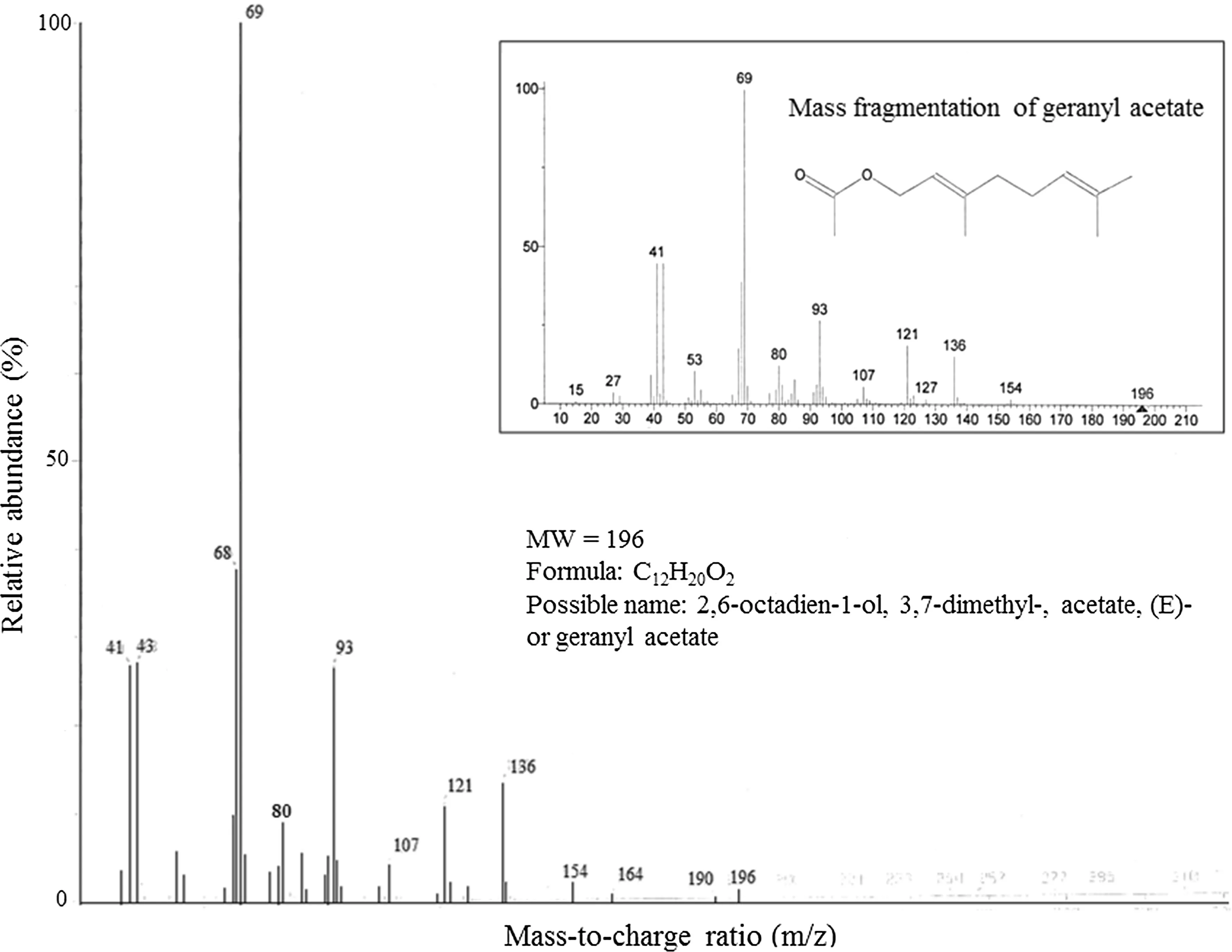
Fig.6 Mass fragmentation of compound 2 at RT 17.2 min in GC/MS ofextracts from Eucalyptus globulus. Possible identity of compound is based on comparison with GC/MS library. Insert is from the NIST/EPA/NIH/EINECS/IRDB library
GC profiles of the leafextracts suggest that the compounds n-icosane, geranyl acetate and elixene seem to be involved in the eucalypts tolerance to O3and CO2. In particular, n-icosane appears to be involved in the response mechanisms of E. grandis and the hybrid, and its O3-induced increase was partly or fully off set by EC. n -Icosane is a saturated aliphatic hydrocarbon (a component of wax that is commonly found in leafepicuticle; Dubis et al. 2001). Epicuticular waxes act as physical barrier against pathogen penetration. Air pollutants, such as O3, could alter the structure of leaf waxes from crystallite to amorphous with longer carbon chains (e.g., Karnosky et al. 2002). The increased relative concentration of n-icosane induced by EO may indicate a defensive mode for preventing O3penetration into the leaves. Epicuticular waxes can also be found surrounding the stomata (photo data not shown). EO may enhance the synthesis of n-icosane for accumulation in higher concentrations around the stomata (and therefore O3uptake through stomata to be reduced). Geranyl acetate and elixene appear to be involved in the O3response mechanisms of E. globulus. The relative concentration of geranyl acetate and elixene were increased by EO in AC. However, EC increased the relative concentration of geranyl acetate and decreased the relative concentration ofelixene in AO and ruled out an O3effect and acted as the primary control of geranyl acetate and elixene in EO. A recent study also revealed that six formylated phloroglucinol compounds (metabolites of Myrtaceae), including five macrocarpals and one sideroxylonal, showed distinct patterns to single and combined effects of acute O3exposure (5 μmol mol?1× 3 h) and wounding in E. globulus; total macrocarpals and total formylated phloroglucinol compounds increased by single elevated O3(Liu et al. 2019). Geranyl acetate is a component of plant essential oils that are released from fresh plants as a possible defense mechanism against damage from insects (e.g., Carpino et al. 2004; Pe?aflor et al. 2011). Elixene is also a terpene essential oil and insecticidal (e.g., Wang et al. 2011). O3can act as an abiotic elicitor of plant defense reaction and generate adaptive responses, which precondition plants for more severe environmental challenges, by activating defense signaling networks and enhancing “stress coping skills”, such as antioxidative systems (Sandermann et al. 1998; Agathokleous et al. 2019a). Such organismic responses can involve fitness trade-off s between defense and growth/reproduction (Karabourniotis et al. 2014; Agathokleous and Calabrese 2020); however, based on the studied traits, none of the tested eucalypts displayed a negative fitness response to the gas treatments.
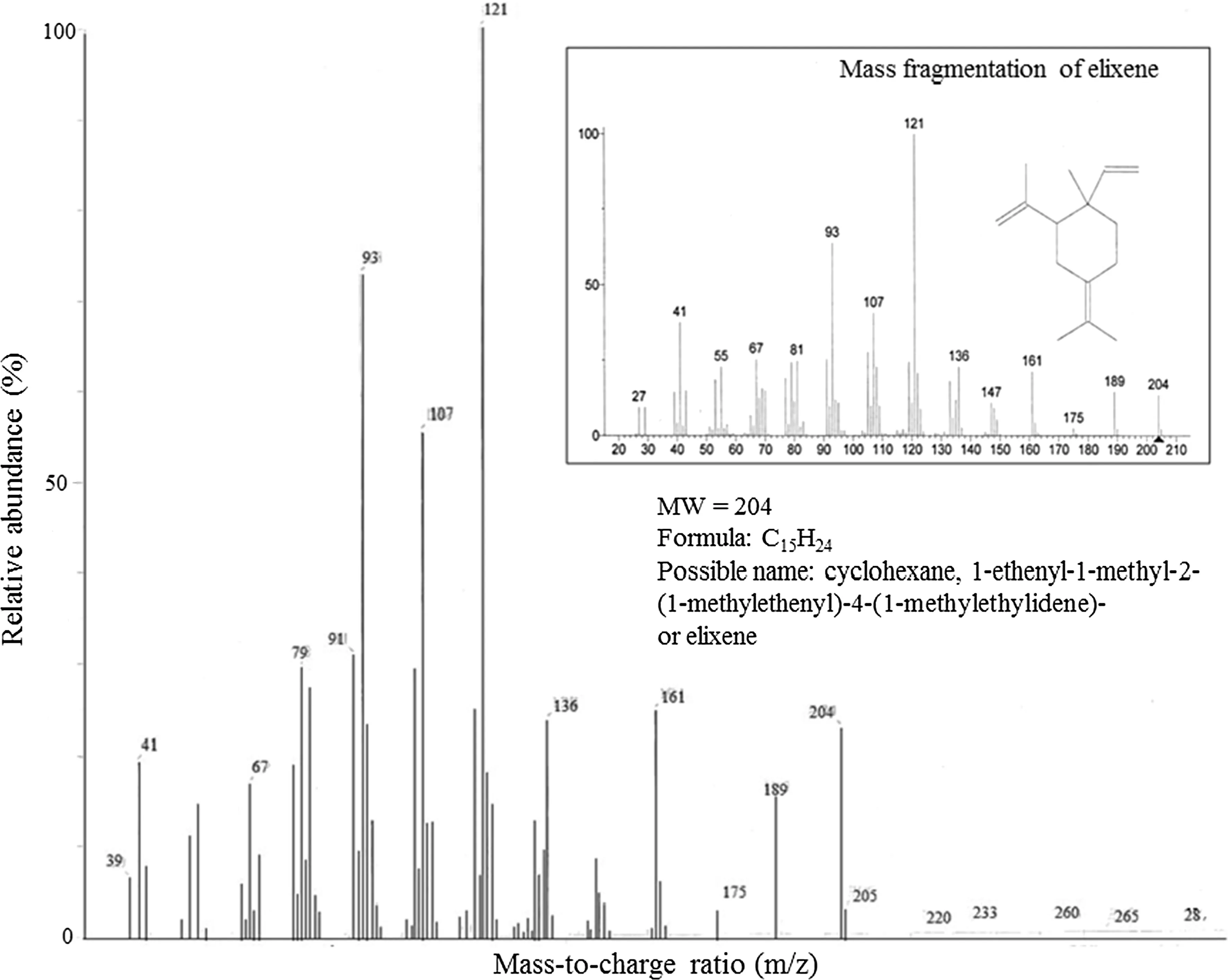
Fig.7 Mass fragmentation of compound 3 at RT 24.1 min in GC/MS ofextracts from Eucalyptus globulus. Possible chemical name is based on comparison with the GC/MS library. Insert is from the NIST/EPA/NIH/EINECS/IRDB library
Conclusion
Overall, EO and EC, singly and in combination, had no effect on the biomass of the three eucalypts. EO had limited effects on leaf physiology (Narea, Nmass, n-icosane, geranyl acetate, elixene) in AC. EC affected more traits of leaf physiology ( gs, LMA, tannins, Carea, Cmass, lignin, n-icosane, geranyl acetate and elixene) and off set most of the limited EO effects on leaf physiology. E. globulus, E. grandis and the hybrid eucalypt appear to be tolerant to a chronic realistic exposure of O3and CO2. n-Icosane seems to be involved in the tolerance mechanisms of E. grandis and the hybrid, and geranyl acetate and elixene seem to be involved in the tolerance of E. globulus. Although the mass fragmentation of the compounds mentioned herein is similar to those in the GC/MS library, further studies are needed to verify whether these specific compounds in plants are involved in the mechanisms of tolerance to O3and CO2.
AcknowledgementsE.A acknowledges multi-year support from the National Natural Science Foundation of China (NSFC) (Grant No. 31950410547) and The Startup Foundation for Introducing Talent of Nanjing University of Information Science and Technology (NUIST), Nanjing, China (Grant No. 003080). Q.M. was supported by China Scholarship Council (CSC), China. T.K. and M.W. acknowledge support from the Strategic International Collaborative Research Program (SICORP) of the Japan Science and Technology Agency (JST), Japan (Grant No. JPMJSC18HB).
Open AccessThis article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creat iveco mmons.org/licen ses/by/4.0/.
References
Agathokleous E, Calabrese EJ (2020) A global environmental health perspective and optimisation of stress. Sci Total Environ 704:135263
Agathokleous E, Saitanis CJ (2020) Plant susceptibility to ozone: a tower of Babel? Sci Total Environ 703:134962
Agathokleous E, Saitanis CJ, Stamatelopoulos D, Mouzaki-Paxinou A-C, Paoletti E, Manning WJ (2016a) Olive oil for dressing plant leaves so as to avoid O3injury. Water Air Soil Pollut 227:282
Agathokleous E, Saitanis CJ, Wang X, Watanabe M, Koike T (2016b) A review study on past 40 years of research on effects of tropospheric O3on belowground structure, functioning, and processes of trees: a linkage with potential ecological implications. Water Air Soil Pollut 227:33
Agathokleous E, Belz RG, Calatayud V, De Marco A, Hoshika Y, Kitao M, Saitanis CJ, Sicard P, Paoletti E, Calabrese EJ (2019a) Predicting the effect ofozone on vegetation via the linear non-threshold (LNT), threshold and hormetic dose-response models. Sci Total Environ 649:61–74
Agathokleous E, Belz RG, Kitao M, Koike T, Calabrese EJ (2019b) Does the root to shoot ratio show a hormetic response to stress? An ecological and environmental perspective. J For Res 30:1569–1580
Ainsworth EA, Long SP (2005) What have we learned from 15 years of free-air CO2enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol 165:351–372
Ainsworth EA, Rogers A, Leakey AD, Heady LE, Gibon Y, Stitt M, Schurr U (2007) Does elevated atmospheric [CO2] alter diurnal C uptake and the balance of C and N metabolites in growing and fully expanded soybean leaves? J Exp Bot 58:579–591
Araminien? V, Varnagiryt?-Kaba?inskien? I, Stak?nas V (2018) Pilot study on the effects ofelevated air temperature and CO2on artificially defoliated silver birch saplings. J For Res 29:1507–1518
Bate-Smith EC (1977) Astringent tannins of Acer species. Phytochemistry 16:1421–1426
Bernacchi CJ, Singsaas EL, Pimentel C, Portis AR, Long SP (2001) Improved temperature response functions for models of Rubiscolimited photosynthesis. Plant Cell Environ 24:253–259
Bidart-Bouzat MG, Imeh-Nathaniel A (2008) Global change effects on plant chemical defences against insect herbivores. J Integr Plant Biol 50:1339–1354
Blaschke L, Forstreuter M, Sheppard LJ, Leith IK, Murray MB, Polle A (2002) Lignification in beech ( Fagus sylvatica) grown at elevated CO2concentrations: interaction with nutrient availability and leaf maturation. Tree Physiol 22:469–477
Bloom AJ, Burger M, Asensio JSR, Cousins AB (2010) Carbon dioxide enrichment inhibits nitrate assimilation in wheat and Arabidopsis. Science 328:899–903
Booker FL (2001) Influence of carbon dioxide enrichment, ozone and nitrogen fertilization on cotton ( Gossypium hirsutum L.) leaf and root composition. Plant Cell Environ 23:573–583
Booker FL, Maier CA (2001) Atmospheric carbon dioxide, irrigation, and fertilization effects on phenolic and nitrogen concentrations in loblolly pine ( Pinus taeda) needles. Tree Physiol 21:609–616
Booker F, Muntifering R, McGrath M, Burkey K, Decoteau D, Fiscus E, Manning W, Krupa S, Chappelka A, Grantz D (2009) The ozone component of global change: potential effects on agricultural and horticultural plant yield, product quality and interactions with invasive species. J Integr Plant Biol 51:337–351
Box GEP, Cox DR (1964) An analysis of transformations. J R Stat Soc B 26:211–252
Broadmeadow MSJ, Heath J, Randle TJ (1999) Environmental limitations to O3uptake—some key results from young trees growing at elevated CO2concentrations. Water Air Soil Pollut 116:299–310
Bryant JP, Chapin FSIII, Klein DR (1983) Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos 40:357–368
Carpino S, Mallia S, La Terra S, Melilli C, Licitra G, Acree TE, Barbano DM, Van Soest PJ (2004) Composition and aroma compounds of ragusano cheese: native pasture and total mixed rations. J Dairy Sci 87:816–830
Centritto M, Jarvis PG (1999) Long-term effects ofelevated carbon dioxide concentration and provenance on four clones of Sitka spruce ( Picea sitchensis). II. Photosynthetic capacity and nitrogen use effi ciency. Tree Physiol 19:807–814
Chater C, Peng K, Movahedi M, Dunn JA, Walker HJ, Liang YK, McLachlan DH, Casson S, Isner JC, Wilson I, Neill SJ, Hedrich R, Gray JE, Hetherington AM (2015) Elevated CO2-induced responses in stomata require ABA and ABA signaling. Curr Biol 25:2709–2716
Co?teaux MM, Kurz C, Bottner P, Raschi A (1999) Influence of increased atmospheric CO2concentration on quality of plant material and litter decomposition. Tree Physiol 19:301–311
Dubis EN, Dubis AT, Pop?awski J (2001) Determination of the aromatic compounds in plant cuticular waxes using FT-IR spectroscopy. J Mol Struct 596:83–88
Dusenge ME, Duarte AG, Way DA (2019) Plant carbon metabolism and climate change: elevated CO2and temperature impacts on photosynthesis, photorespiration and respiration. New Phytol 221:32–49
Eguchi N, Karatsu K, Ueda T, Funada R, Takagi K, Hiura T, Sasa K, Koike T (2008) Photosynthetic responses of birch and alder saplings grown in a free air CO2enrichment system in northern Japan. Trees 22:437–447
Evans JR (1989) Photosynthesis and nitrogen relationships in leaves of C3plants. Oecologia 78:9–19
Farquhar GD, von Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2assimilation in leaves of C3species. Planta 149:78–90
Fowler D, Cape JN, Coyle M, Flechard C, Kuylenstierna J, Hicks K, Derwent D, Johnson C, Stevenson D (1999) The global exposure of forests to air pollutants. Water Air Soil Pollut 116:5–32
Ghini R, MacLeod REO, Santos MS, Silva CEO (2015) Elevated atmospheric carbon dioxide concentration increases eucalyptus plantlets growth and reduces diseases severity. Proc Environ Sci 29:206–207
Gleadow RM, Foley WJ, Woodrow IE (1998) Enhanced CO2alters the relationship between photosynthesis and defence in cyanogenic Eucalyptus cladocalyx F. Muell. Plant Cell Environ 21:12–22
Grams TEE, Anegg S, Haberle K-H, Langebartels C, Matyssek R (1999) Interactions of chronic exposure to elevated CO2and O3levels in the photosynthetic light and dark reactions of European beech ( Fagus sylvatica). New Phytol 144:95–107
Herms DA, Mattson WJ (1992) The dilemma of plants: to grow or defend. Q Rev Biol 67:283–335
Hikosaka K (2004) Interspecific difference in the photosynthesis–nitrogen relationship: patterns, physiological causes, and ecological importance. J Plant Res 117:481–494
Hikosaka K, Onoda Y, Kinugasa T, Nagashima H, Anten NPR, Hirose T (2005) Plant responses to elevated CO2concentration at different scales: leaf, whole plant, canopy, and population. Ecol Res 20:243–253
Hoshika Y, De Carlo A, Baraldi R, Neri L, Carrari E, Agathokleous E, Zhang L, Fares S, Paoletti E (2019) Ozone-induced impairment of night -time stomatal closure in O3-sensitive poplar clone is affected by nitrogen but not by phosphorus enrichment. Sci Total Environ 692:713–722
Howell DC, McConaughy SH (1982) Nonorthogonal analysis of variance: putting the question before the answer. Educ Psychol Meas 42:9–24
IPCC (2007) Summary for policy makers. In: Metz B, Davidson OR, Bosch PR, Dave R, Meyer LA (eds) Climate change 2007: mitigation. Contribution of Working Group III to the Fourth Assessment Report of the Intergovernmental panel on Climate Change. Cambridge University Press, New York
Julkunen-Tiitto R (1985) Phenolic constituents in the leaves of northern willows: methods for the analysis of certain phenolics. J Agric Food Chem 33:213–217
Kanagendran A, Pazouki L, Niinemets ü (2018) Differential regulation of volatile emission from Eucalyptus globulus leaves upon single and combined ozone and wounding treatments through recovery and relationships with ozone uptake. Environ Exp Bot 145:21–38
Karabourniotis G, Liakopoulos G, Nikolopoulos D, Bresta P, Stavroulaki V, Sumbele S (2014) “Carbon gain vs. water saving, growth vs. defence”: two dilemmas with soluble phenolics as a joker. Plant Sci 227:21–27
Karnosky DF, Percy KE, Xiang B, Callan B, Noormets A, Mankovska B, Hopkin A, Sober J, Jones W, Dickson RE, Isebrands JG (2002) Interacting elevated CO2and tropospheric O3predisposes aspen ( Populus tremuloides Michx.) to infection by rust ( Melampsora medusae f. sp. tremuloidae). Glob Change Biol 8:329–338
Karnosky DF, Zak DR, Pregitzer KS, Awmack CS, Bockheim JG, Dickson RE, Hendrey GR, Host GE, King JS, Kopper BJ, Kruger EL, Kubiske ME, Lindroth RL, Mattson WJ, McDonald EP, Noormets A, Oksanen E, Parsons WFJ, Percy KE, Podila GK, Riemenschneider DE, Sharma P, Thakur R, S?ber A, S?ber J, Jones WS, Anttonen S, Vapaavuori E, Mankovska B, Heilman W, Isebrands JG (2003) Tropospheric O3moderates responses of temperate hardwood forests to elevated CO2: a synthesis of molecular to ecosystem results from the Aspen FACE project. Funct Ecol 17:289–304
Kitao M, L?w M, Heerdt C, Grams TEE, H?berle K-H, Matyssek R (2009) Effects of chronic elevated ozone exposure on gas exchange responses of adult beech trees ( Fagus sylvatica) as related to the within-canopy light gradient. Environ Pollut 157:537–544
Kitao M, Komatsu M, Yazaki K, Kitaoka S, Tobita H (2015) Growth overcompensation against O3exposure in two Japanese oak species, Quercus mongolica var. crispula and Quercus serrata, grown under elevated CO2. Environ Pollut 206:133–141
Koike T, Lei TT, Maximov TC, Tabuchi R, Takahashi K, Ivanov BI (1996) Comparison of the photosynthetic capacity of Siberian and Japanese birch seedlings grown in elevated CO2and temperature. Tree Physiol 16:381–385
Koike T, Mao Q, Inada N, Kawaguchi K, Hoshika Y, Kita K, Watanabe M (2012) Growth and photosynthetic responses of cuttings of a hybrid Larch ( Larix gmelinii var. japonica x L. kaempferi ) to elevated ozone and/or carbon dioxide. Asian J Atmos Environ 6:104–110
Kopper B, Lindroth R (2003) Effects ofelevated carbon dioxide and ozone on the phytochemistry of aspen and performance of an herbivore. Oecologia 134:95–103
Larcher W (2003) Physiological plant ecology: ecophysiology and stress physiology of functional groups, 4th edn. Springer, Berlin
Lawler IR, Foley WJ, Woodrow IE, Cork SJ (1996) The effects of elevated CO2atmospheres on the nutritional quality of Eucalyptus foliage and its interaction with soil nutrient and light availability. Oecologia 109:59–68
Li P, Feng Z, Calatayud V, Yuan X, Xu Y, Paoletti E (2017) A metaanalysis on growth, physiological and biochemical responses of woody species to ground-level ozone highlights the role of plant functional types. Plant Cell Environ 40:2369–2380
Lindroth R (2010) Impacts ofelevated atmospheric CO2and O3on forests: Phytochemistry, trophic interactions, and ecosystem dynamics. J Chem Ecol 36:2–21
Liu B, dos Santos BM, Kanagendran A, Jakobsen Neilson EH, Niinemets ü (2019) Ozone and wounding stresses differently alter the temporal variation in formylated phloroglucinols in Eucalyptus globulus leaves. Metabolites 9:46
Long SP, Bernacchi CJ (2003) Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources oferror. J Exp Bot 54:2393–2401
Manning WJ (2005) Establishing a cause and effect relationship for ambient ozone exposure and tree growth in the forest: progress and an experimental approach. Environ Pollut 137:443–454
Matsuki S, Sano Y, Koike T (2004) Chemical and physical defence in early and late leaves in three heterophyllous birch species native to Northern Japan. Ann Bot 93:141–147
Mattson JW, Julkunen-Tiitto R, Herms DA (2005) CO2enrichment and carbon partitioning to phenolics: do plant responses accord better with the protein competition or the growth differentiation balance models? Oikos 111:337–347
Matyssek R, Schnyder H, Obwald W, Ernst D, Munch JC, Pretzsch H (2012) Growth and defence in plants: resource allocation at multiple scales. Springer, Heidelberg, p 470. ISBN 978-3-642-30645-7
McKiernan AB, O’Reilly-Wapstra JM, Price C, Davies NW, Potts BM, Hovenden MJ (2012) Stability of plant defensive traits among populations in two Eucalyptus species under elevated carbon dioxide. J Chem Ecol 38:204–212
Monk RJ, Murray F (1995) The relative tolerance of some Eucalyptus species to ozone exposure. Water Air Soil Pollut 85:1405–1411
Murray TJ, Ellsworth DS, Tissue DT, Riegler M (2013) Interactive direct and plant-mediated effects ofelevated atmospheric [CO2] and temperature on a eucalypt-feeding insect herbivore. Glob Change Biol 19:1407–1416
Norby RJ, Cotrufo MF, Ineson P, Neill EGO, Canadell JG (2001) Elevated CO2, litter chemistry, and decomposition: a synthesis. Oecologia 127:153–165
Novriyanti E, Watanabe M, Kitao M, Utsugi H, Uemura A, Koike T (2012a) High nitrogen and elevated [CO2] effects on the growth, defense and photosynthetic performance of two eucalypt species. Environ Pollut 170:124–213
Novriyanti E, Watanabe M, Makoto K, Takeda T, Hashidoko Y, Koike T (2012b) Photosynthetic nitrogen and water use effi ciency of acacia and eucalypt seedlings as afforestation species. Photosynthetica 50:273–281
O’Connor JA, Parbery DG, Strauss W (1975) The effects of phytotoxic gases on native Australian plant species: part 2. Acute injury due to ozone. Environ Pollut 7:7–23
Oksanen E, Riikonen J, Kaakinen S, Holopainen T, Vapaavuori E (2005) Structural characteristics and chemical composition of birch ( Betula pendula) leaves are modified by increasing CO2and ozone. Glob Change Biol 11:732–748
Ong B-L, Koh CK-K, Wee Y-C (1998) Effects of CO2on growth and photosynthesis of Pyrrosia piloselloides (L.) Price gametophytes. Photosynthetica 35:21–27
Ormrod DP, Lesser VM, Olszyk DM, Tingey DT (1999) Elevated temperature and carbon dioxide affect chlorophylls and carotenoids in Douglas-fir seedlings. Int J Plant Sci 160:529–534
Orwa C, Mutua A, Jamnadass R, Anthony S (2009) Agroforestry database: a tree reference and selection guide version 4.0. World Agroforestry Centre, Nairobi. http://www.world agroforest ry.org/outpu t/agroforest ree-datab ase
Paoletti E, Grulke NE (2005) Does living in elevated CO2ameliorate tree response to ozone? A review on stomatal responses. Environ Pollut 137:483–493
Pearson M (1995) Effects ofozone on growth and gas exchange of Eucalyptus globulus seedlings. Tree Physiol 15:207–210
Pe?aflor M, Erb M, Robert C, Miranda L, Werneburg A, Dossi F, Turlings T, Bento J (2011) Oviposition by a moth suppresses constitutive and herbivore-induced plant volatiles in maize. Planta 234:207–215
Percy KE, Legge AH, Krupa SV (2003) Tropospheric ozone: a continuing threat to global forest? In: Karnosky DF, Percy KE, Chapelka AH, Simpson C, Pikkarainen J (eds) Air pollution, global change and forest in the new millennium. Elsevier, Oxford, pp 85–118
Plett JM, Kohler A, Khachane A, Keniry K, Plett KL, Martin F, Anderson IC (2015) The effect ofelevated carbon dioxide on the interaction between Eucalyptus grandis and diverse isolates of Pisolithus sp. is associated with a complex shift in the root transcriptome. New Phytol 206:1423–1436
Pritchard SG, Ju Z, van Santen E, Qiu J, Weaver DB, Prior SA, Rogers HH (2000) The influence ofelevated CO2on the activities of antioxidative enzymes in two soybean genotypes. Austral J Plant Physiol 27:1061–1068
Proietti C, Anav A, De Marco A, Sicard P, Vitale M (2016) A multisites analysis on the ozone effects on gross primary production of European forests. Sci Total Environ 556:1–11
Quentin AG, Crous KY, Barton CVM, Ellsworth DS (2015) Photosynthetic enhancement by elevated CO2depends on seasonal temperatures for warmed and non-warmed Eucalyptus globulus trees. Tree Physiol 35:1249–1263
Roden JS, Ball MC (1996) The effect ofelevated [CO2] on growth and photosynthesis of two Eucalyptus species exposed to high temperatures and water deficits. Plant Physiol 111:909–919
Saitanis CJ, Riga-Karandinos AN, Karandinos MG (2001) Effects of ozone on chlorophyll and quantum yield of tobacco ( Nicotiana tabacum L.) varieties. Chemosphere 42:945–953
Sandermann H Jr, Ernst D, Heller W, Langebartels C (1998) Ozone: An abiotic elicitor of plant defence reactions. Trends Plant Sci 3:47–50
Schulze E-D, Beck E, Müller-Hohenstein K (2005) Plant ecology. Springer, Berlin
Shang B, Yuan X, Li P, Xu Y, Feng Z (2019a) Effects ofelevated ozone and water deficit on poplar saplings: changes in carbon and nitrogen stocks and their allocation to different organs. For Ecol Manag 441:89–98
Shang B, Xu Y, Dai L, Yuan X, Feng Z (2019b) Elevated ozone reduced leaf nitrogen allocation to photosynthesis in poplar. Sci Total Environ 657:169–178
Shi C, Eguchi N, Meng F, Watanabe T, Satoh F, Koike T (2016) Retranslocation of foliar nutrients of deciduous tree seedlings in different soil condition under free-air O3fumigation. iForest 9:835–841
Shi C, Watanabe T, Koike T (2017) Leaf stoichiometry of deciduous tree species in different soils exposed to free-air O3enrichment over two growing seasons. Environ Exp Bot 138:148–163
Singh AA, Agrawal SB (2017) Tropospheric ozone pollution in India: effects on crop yield and product quality. Environ Sci Pollut Res 24:4367–4382
Sitch S, Cox PM, Collins WJ, Huntingford C (2007) Indirect radiative forcing of climate change through ozone effects on the landcarbon sink. Nature 448:791–794
Tissue DT, Griffi n KL, Ball JT (1999) Photosynthetic adjustment in field-grown ponderosa pine trees after six years ofexposure to elevated CO2. Tree Physiol 19:221–228
Uddling J, Karlsson PE, Glorvigen A, Selldén G (2006) Ozone impairs autumnal resorption of nitrogen from birch ( Betula pendula) leaves, causing an increase in whole-tree nitrogen loss through litter fall. Tree Physiol 26:113–120
Wang YN, Koike T (2019) Current status ofeucalypts and broadleaved trees in southeastern part of China and their future. For Tech 932:30–33 (in Japanese)
Wang CF, Yang K, Zhang HM, Cao J, Fang R, Liu ZL, Du SS, Wang YY, Deng ZW, Ligang Z (2011) Components and insecticidal activity against the Maize Weevils of Zanthoxylum schinifolium fruits and leaves. Molecules 16:3077–3088
Watanabe M, Watanabe Y, Kitaoka S, Utsugi H, Kita K, Koike T (2011) Growth and photosynthetic traits of hybrid larch F1( Larix gmelinii var. japonica × L. kaempferi) under elevated CO2concentration with low nutrient availability. Tree Physiol 31:965–975
Xu H, Xie H, Wu S, Wang Z, He K (2019) Effects ofelevated CO2and increased N fertilization on plant secondary metabolites and chewing insect fitness. Front Plant Sci 10:739
Yuan X, Calatayud V, Jiang L, Manning WJ, Hayes F, Tian Y, Feng Z (2015) Assessing the effects of ambient ozone in China on snap bean genotypes by using ethylenediurea (EDU). Environ Pollut 205:199–208
Yuan X, Calatayud V, Gao F, Fares S, Paoletti E, Tian Y, Feng Z (2016) Interaction of drought and ozone exposure on isoprene emission from extensively cultivated poplar. Plant Cell Environ 39:2276–2287
Zheng Y, Li F, Hao L, Yu J, Guo L, Zhou H, Ma C, Zhang X, Xu M (2019) Elevated CO2concentration induces photosynthetic downregulation with changes in leaf structure, non-structural carbohydrates and nitrogen content of soybean. BMC Plant Biol 19:255
 Journal of Forestry Research2021年2期
Journal of Forestry Research2021年2期
- Journal of Forestry Research的其它文章
- Stem taper functions for Betula platyphylla in the Daxing’an Mountains, northeast China
- Leaf phenotypic variation of endangered plant Tetracentron sinense Oliv. and influence of geographical and climatic factors
- Ecological variations of woody species along an altitudinal gradient in the Qinling Mountains of Central China: area-based versus mass-based expression of leaf traits
- Variations in stem radii of Larix principis-rupprechtii to environmental factors at two slope locations in the Liupan Mountains, northwest China
- A review of ecological mechanisms for management practices of protective forests
- Variation of basic density, calorific value and volumetric shrinkage within tree height and tree age of Ugandan grown Eucalyptus grandis wood
