Overexpression of Arabidopsis thaliana malonyl-CoA synthetase gene enhances cold stress tolerance by activating mitogen-activated protein kinases in plant cells
Wei Tang · Anna Y. Tang
Abstract Malonyl-CoA synthetases may modulate cell responses to abiotic stress by regulating stress-related signaling transduction pathways or activating expression of transcription factors. However, the molecular mechanism of cold stress tolerance enhanced by malonyl-CoA synthetase is not fully understood. Here, we report that overexpression of the Arabidopsis thaliana malonyl-CoA synthetase gene AAE13.1 resulted in increased cell viability and growth rate and decreased thiobarbituric acid reactive substances under cold stress in rice ( Oryza sativa L.), tobacco ( Nicotiana tabacum), and slash pine ( Pinus elliottii Engelm.). AAE13.1 was associated with cold stress tolerance by increasing the activity of ascorbate peroxidase, catalase, polyphenol oxidase, and peroxidase and the accumulation of acid phosphatase and alkaline phosphatase. Among six rice mitogenactivated protein kinase ( MAPK) genes examined, AAE13.1 overexpression increased the expression of OsMAPK genes during cold stress. AAE13.1 activated expression of stressresponse genes OsMAPK1, OsMAPK2, and OsMAPK3, indicating that AAE13.1 enhances cold stress tolerance by regulating expression of MAPK genes in plant cells. These results increase our understanding of cold stress tolerance in species of monocotyledons, dicotyledons, and gymnosperms.
Keywords Cold stress · Malonyl-CoA synthetase gene · MAPK transcription factor · Phosphatase · Pinus
Introduction
In response to abiotic stresses such as low temperature, high NaCl, drought, and heavy metals, cells regulate their biological activities at the transcriptional and translational levels. For example, in pea, transcription of DNA helicase 47 was induced in both shoots and roots under cold (4 °C) stress, and helicase activities also changed during distinct cellular processes in cold stressed conditions (Vashisht et al. 2005). Cell and plant growth rate were reduced and APOX capacity increased under cold stress in AtFad transgenic plants (Matos et al. 2007). In rice, the Osmyb4 gene is induced by cold stress and can activate stress responsive pathways in both Arabidopsis thaliana and apple ( Malus pumila Mill.) (Pasquali et al. 2008). A cDNA microarray of Alstroemeria flowers demonstrated that cold stress accelerates many changes in gene expression and activates the expression of 21 transcription factors (Wagstaff et al. 2010). In potato, cold stress also increases expression of transcription factorrelated genes and decreases expression of photosynthesisrelated genes (Evers et al. 2012). In A. thaliana, the cold signaling attenuator HIGH EXPRESSION OF OSMOTICALLY RESPONSIVE GENE1 negatively regulates cold responses via acting as a chromatin-remodeling factor under cold stress (Jung et al. 2013). In camelina ( Camelina sativa) and rapeseed ( Brassica napus), cold stress can increase H + -ATPase activity and H + -transport (Kim et al. 2013). In A. thaliana, a microarray analysis showed that 1780 genes were differentially regulated in CcCDR-transgenic plants, and CcCDR conferred tolerance to multiple abiotic stresses (Tamirisa et al. 2014). In durum wheat, cold stress increases the level of arabinose, fructose, glucose, raffi nose, sucrose, and hexose phosphates (Shahryar and Maali-Amiri 2016). In apple, different metabolic pathways such as methanol biosynthesis and ethylene biosynthesis pathways are affected by low temperature (Gapper et al. 2017).
Malonyl-CoA is important for the synthesis of fatty acids, phytoalexins, flavonoids, polyketides, anthocyanin pigments, and many malonylated compounds. Malonyl-CoA is derived from acetyl-CoA by acetyl-CoA carboxylase. In A. thaliana, recombinant AAE13, a malonyl-CoA synthetase encoded by the gene Acyl Activating Enzyme13 ( AAE13, AT3G16170), had high activity against malonic acid and is essential for growth and development, likely because it is involved in the detoxification of malonate (Chen et al. 2011). Metabolic engineering analysis demonstrated that malonyl-CoA synthetase increases expression of flavanone and flavone biosynthetic genes (Park et al. 2011). In gerbera ( Gerbera hybrida), expression of both GCHS1 and GCHS4 regulates expression of malonyl-CoA synthetase gene in the epidermal cells (Deng et al. 2014). Overexpression of AtAAE13 in Saccharomyces cerevisiae results in increased level of lipids, suggesting that malonyl-CoA is a critical target for fatty acid (Wang et al. 2014). Expression of a chalcone synthase gene ( PaCHS) in Marchantia paleacea increases the content of flavonoids, suggesting that PaCHS plays a key role in flavonoid biosynthesis under abiotic stress (Yu et al. 2015). Malonyl-CoA synthetase is encoded by AAE13 (AT3G16170) in A. thaliana is localized in the cytosol and the mitochondria, but only mitochondrial AAE13 is essential for plant growth (Guan and Nikolau 2016). In Petunia hybrida, expression of PhAAE13 is highest in corollas, and silencing of PhAAE13 reduces the levels of anthocyanins, fatty acids, and cuticular wax, demonstrating the involvement of PhAAE13 in anthocyanin biosynthesis (Chen et al. 2017).
Acid phosphatase and alkaline phosphatase are also important in the abiotic stress responses. Acid phosphatase activity significantly increases in tissues under abiotic stress (Malik and Sethi 1975; Erukainure et al. 2017; Zou et al. 2017). Plant-derived alkaline phosphatase also improves abiotic stress tolerance (Leyva et al. 2004). Alkaline phosphatase activity is higher in tomato than in cotton and cabbage under typical condition without stress, indicating that function of alkaline phosphatase is species dependent (Leyva et al. 2004; Sessions 2008; Widyowati et al. 2010; Yan et al. 2011; Sineshchekov et al. 2013). How the AAE13 gene coordinates with acid phosphatase and alkaline phosphatase during a stress response, however, has remained elusive.
Antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APOX) and guaiacol peroxidase (POX) also increase in cells of many plant species under abiotic stress, suggesting that increased activity of antioxidant enzymes is involved in increased tolerance stress (Gzyl et al. 2009; Arora et al. 2010; Sang and Kim 2011; Sirhindi et al. 2015). In A. thaliana, analysis of cat1, cat2, cat3, cat1 cat2, and cat2 cat3 T-DNA mutants demonstrated that stress has an indirect effect at the leaf level and that cell response to catalase deficiency is independent of the duration ofoxidative stress (Ray et al. 2012; Singh et al. 2012; Spanou et al. 2012; Murota et al. 2017; Yang et al. 2018). In transgenic tobacco and hybrid aspen, overexpression of the horseradish peroxidase gene results in increased stress tolerance (Yoshida et al. 2003). Activation of StPPO (polyphenol oxidase [PPO] derived from Solanum tuberosum) was caused by activation of a latent StPPO by chlorogenic acid quinones in a stress response (Nautiyal et al. 2008; Poiatti et al. 2009; Dirks-Hofmeister et al. 2013; Kuijpers et al. 2014; Molitor et al. 2016; Kampatsikas et al. 2017). How the AAE13 gene co-ordinates with antioxidant enzymes for stress response is not fully understood.
Here, we report that, during cold stress, overexpression of the A. thaliana malonyl-CoA synthetase gene AAE13.1, which encodes a protein of 608 amino acids (Guan and Nikolau 2016), in rice ( Oryza sativa L.), tobacco ( Nicotiana tabacum), and slash pine ( Pinus elliottii Engelm.) increased cell viability and growth rate and decreased the level of thiobarbituric acid reactive substances (TBARS), generated by lipid peroxidation. Measurement of TBARS is a well-established method for monitoring lipid peroxidation caused by cold stress. Lipid peroxidation is a well-defined mechanism of cellular damage in plant cells. Thiobarbituric acid reactive substances (TBARS) assay detects the level of malondialdehyde, the major product of lipid peroxidation and the degradation of unstable lipid peroxides (Tang et al. 2006). Our results showed that overexpression of AAE13.1 enhances cold stress tolerance by increasing the activity of APOX, CAT, PPO, and POD and accumulation of acid phosphatase and alkaline phosphatase and by activating expression of stress-response OsMAPK genes in rice cells.
Materials and methods
Plasmid constructs
The full-length A. thaliana AAE13.1 coding sequence (1827 bp) was cloned into pBI121 as previously described (Chen et al. 2011; Guan and Nikolau 2016). Vector pBI121 and AAE13.1 were digested by restriction enzymes KpnI and XbaI (Promega, Madison, WI, USA) at 37 °C, then purified using a QIAquick Gel Extraction Kit (QIAGEN, Valencia, CA, USA) and ligated to generate the expression vector (Tang et al. 2005a, b, 2006). The resulting expression vector, designated pBI- AtAAE13.1, was introduced into Agrobacterium tumefaciens strain LBA4404 using electroporation.
Transformation of rice, tobacco, and pine cells
AtAAE13.1-transgenic cell lines of three plant species rice ( O. sativa), tobacco ( N. tabacum ), and pine ( P. elliottii) were generated as described before (Tang and Page 2013), using A. tumefaciens strain LBA4404 carrying pBI- AtAAE13.1 (Tang et al. 2006, 2007a, b). Cell cultures of rice, tobacco, and pine were then grown for 7 weeks before further analysis.
Polymerase chain reaction analyses of transgenic cells
Genomic DNA was extracted from 7 g of transgenic rice, tobacco, and pine cells using a Genomic DNA Isolation Kit (Sigma). For the PCR using the method of Tang and Page ( 2013), neomycin phosphotransferase II gene ( NPTII) was amplified using forward primer nfp and reverse primer nrp; AtAAE13.1 was amplified using forward primer zaf and reverse primer zar. The PCR mixture, the PCR conditions, and gel electrophoresis have been described previously (Tang et al. 2006, 2007a, b). The PCR was run in a PTC-100TM machine (MJ Research, San Francisco, CA, USA) using 300 ng of genomic DNA as a template.
Southern blot analysis of transgenic cells
Southern blots were done as previously described (Tang et al. 2006, 2007a, b). Genomic DNA was extracted from 9 g of control cells or transgenic cells of rice, tobacco, and pine using a Genomic DNA Isolation Kit (Sigma). Twenty-eight micrograms of DNA for each was digested with the restriction enzyme XbaI (Boehringer Mannheim) for 16 h at 37 °C. The molecular probe (1827 bp fragment of AtAAE13.1) were labeled with digoxigenin (DIG) (Roche Diagnostics, Indianapolis, IN, USA).
RNA isolation and northern blot analysis
Total RNA was extracted from transgenic and control cells using an RNeasy Mini Plant Kit (Qiagen, Germantown, MD, USA) and the instructions, and 9 μg of total RNA was used for northern blotting as described (Tang et al. 2007a, b). The DIG-labelled AtAAE13.1 DNA fragment (1827 pb) (Roche Diagnostics) was used as the hybridization probe. Tobacco rRNA was used as the loading control for RNA samples.
Cold treatment
The AtAAE13.1-transgenic cell lines of O. sativa, N. tabacum, and P. elliottii and wild-type control cells were incubated in growth incubators at ? 10, ? 4, 10, or 24 °C in the dark for 24 h. Cells were then moved to typical growth conditions incubated at 24 °C with 16 h light/8 h dark.
Determination of cell viability and growth rate
After the 24-h temperature treatment and 7 days in typical growth conditions incubated at 24 °C with 16 h light/8 h dark, the influence of cold stress on cell growth and viability was analyzed as previously described (Tang et al. 2006, 2007a, b). The average growth rate was expressed as mg/g FW/day. Cell samples from both chilled and control cell cultures were collected with 3 biological replicates. Cells were imaged using a confocal microscope. In a preliminary experiment, cell viability of nontransgenic cells of O. sativa, N. tabacum, and P. elliottii was evaluated at 0, 1, 3, 5, 7, and 9 days after the cold treatment to select an appropriate assay time (7 days). The effect of cold stress at a shorter time (2 days after cold treatment) was also verified.
Measurement of thiobarbituric acid reactive substances
Thiobarbituric acid reactive substances (TBARS) were quantified using the thiobarbituric acid (TBA) reaction as described previously (Tang et al. 2005a, b; Tang and Page 2013) using 3 g of transgenic or control cells from each species. The sample was heated at 95 °C for 30 min and centrifuged at 10,000 × g for 15 min before absorbance was measured at 532 nm. A preliminary time-course study was done as described for cell viability to determine the assay time (7 days).
Activity of APOX, CAT, PPO, and POD
Activity of ascorbate peroxidase (APOX), catalase (CAT), polyphenol oxidase (PPO), and peroxidase (POD) was measured as previously described (Tang et al. 2005a, b, 2006; Tang and Page 2013) 7 days after the temperature treatment using 5 g of control or transgenic cells. Three biological replicates were assayed for each treatment.
Activity of acid phosphatase and alkaline phosphatase
Activity of acid phosphatase and alkaline phosphatase in a methanolic extract of cells (Ma et al. 2013; Selin-Rani et al. 2016) was measured as previously described (Kaida et al. 2009; Johnson et al. 2015) 7 days after the temperature treatment of control and transgenic cells of the three species and quantified by the method of Sakthivel and Guruvayoorappan ( 2013) was subjected to the measurement of acid phosphatase and alkaline phosphatase enzymatic activities (Ma et al. 2013; Selin-Rani et al. 2016). Three biological replicates were used for each treatment.
Expression of mitogen-activated protein kinase genes
Expression of mitogen-activated protein kinase genes in control and transgenic cells of the three species 7 days after treatment was quantified using qPCR as previously described (Wan et al. 2007). Total RNA was extracted from frozen cells using TRIzol reagent according to the manufacturer’s protocol (Invitrogen). First-strand cDNA was synthesized in a 50-μL reaction containing 2.5 mM oligo (dT) primers, 2.5 mM random hexamer, and 2.5 μg of total RNA. The PrimeScript RT reagent kit (TaKaRa, Ohtsu, Japan) was used according to the instructions. Samples were analyzed in triplicate on the Applied Biosystems 7900HT System as described in the manufacturer’s manual. Primers for qPCR are listed in Table 1. Small noncoding RNA U6 was used as an internal control for normalizing data. The delta–delta Ct method was used to obtain expression value. Three biological replicates were used for each treatment.
Statistical analyses
The General Linear Model procedure and ANOVA in SAS 9.4m4 (SAS Institute, Cary, NC, USA) were used to analyze significant differences among means between the control and transgenic cell lines for each species at 5% level of probability.
Results
Molecular analysis of transgenic cells
Among the 27 AAE13.1-transgenic cell lines of O. sativa, 33 of N. tabacum, and 38 transgenic cell lines of P. elliottii obtained, transgenic cell lines Os1, Os2, and Os3 of O. sativa, Nt1, Nt2, and Nt3 of N. tabacum, and Pe1, Pe2, and Pe3 of P. elliottii were selected for PCR analysis of NPTII (Fig.1 b) and AAE13.1 (Fig.1 c) and found to be positive for the genes. The lines were then examined by Southern blot analysis using the DNA fragment of AAE13.1 as probe (Fig.1 d) and by Northern blot analysis for AAE13.1 (Fig.1 e). These cell lines were then functionally analyzed after a cold stress.
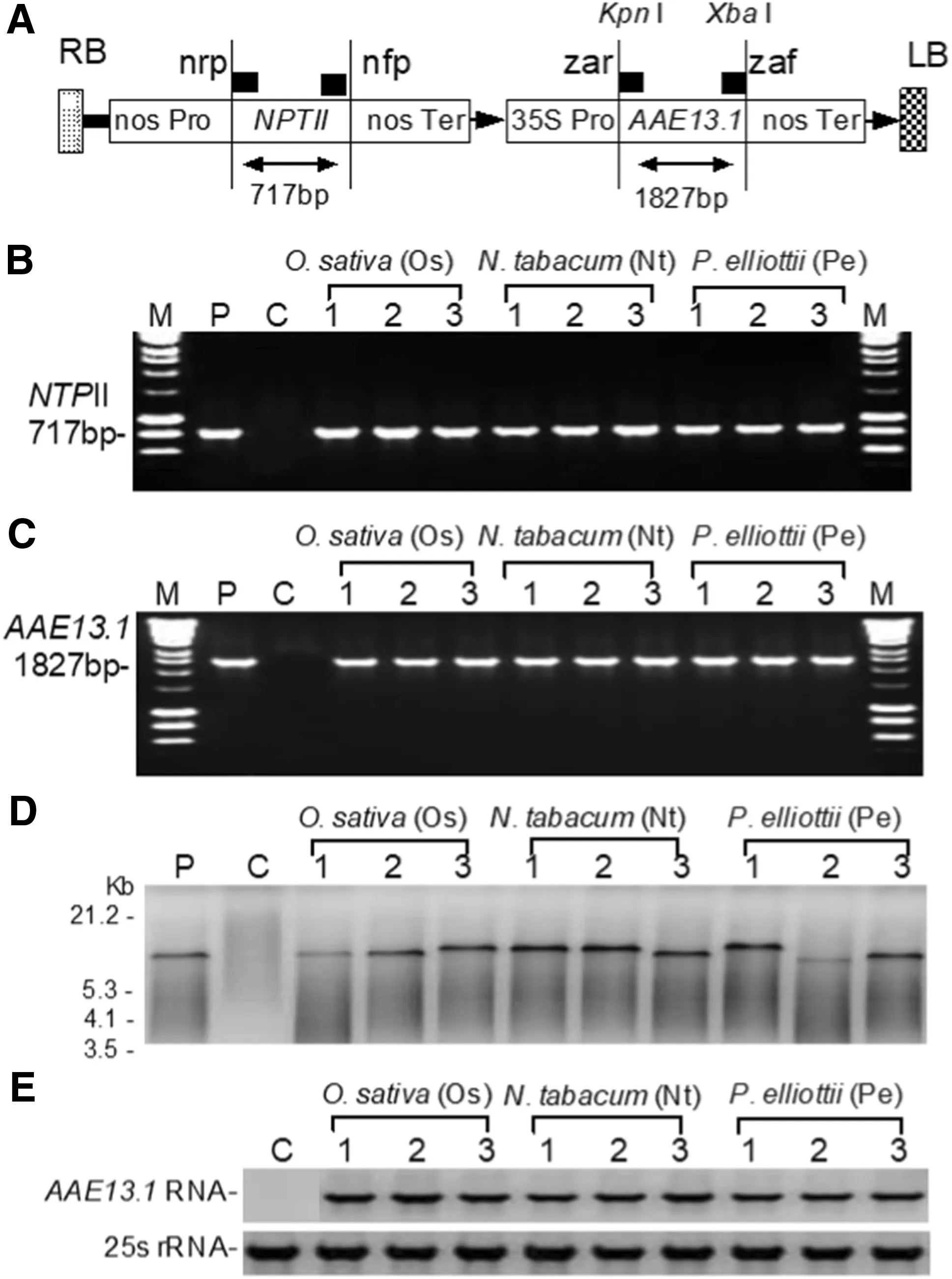
Fig.1 Expression vector and molecular analyses of transgenic cells. a Expression vector showing the T-DNA region that includes right border (RB), left border (LB), the nos promoter (nos Pro), the NPTII gene ( NPTII), the nos terminator (nos Ter), the 35 S promoter (35S Pro), and the AAE13.1 gene ( AAE13.1). PCR of b NPTII gene (717 bp) and c AAE13.1 (1827 bp) in O. sativa, N. tabacum, and P. elliottii. d Southern blots and northern blots of AAE13.1 gene in O. sativa, N. tabacum, and P. elliottii. Tobacco 25S rRNA was used as the loading control. Lane P: plasmid positive control; C negative control
Overexpression of AAE13.1 increases growth rate and viability and decreases TBARS during cold stress
Cell viability and TBARS were evaluated through a time course (0, 1, 3, 5, 7, 9 days after cold treatment) using nontransgenic cells of O. sativa, N. Tabacum, and P. elliottii to determine the assay time point (Fig.2 a-f). Compared tothe growth rate of the control cells, cell growth rate was 76–91% higher in the three transgenic lines of O. sativa (Fig.2 g), 28–39% higher in three transgenic lines of N. tabacum (Fig.2 h), and 35–43% higher in the three transgenic lines of P. elliottii (Fig.2 i) after the treatment with ? 10 °C and ? 4 °C. Compared to viability of the control cells, cell viability of O. sativa transgenic cell lines was 31–43% higher (Fig.2 j), 32–41% higher for the N. tabacum transgenic cell lines (Fig.2 k), and 21–46% higher for the three P. elliottii transgenic cell lines (Fig.2 l) after treatment with ? 10 °C and ? 4 °C. Overexpression of AAE13.1 also decreased TBARS in transgenic cells of O. sativa by 12–19% (Fig.2 m), N. tabacum by 13–18% (Fig.2 n), P. elliottii by 13–17% (Fig.2 o) after treatment with ? 10 °C and 4 °C.
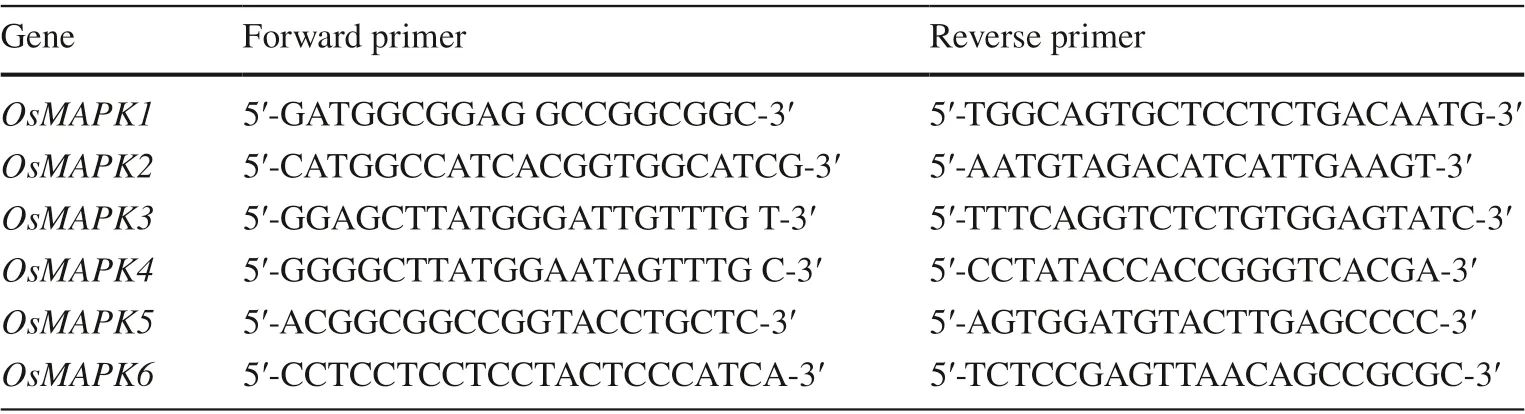
Table 1 Primers used in qRTPCR in this study
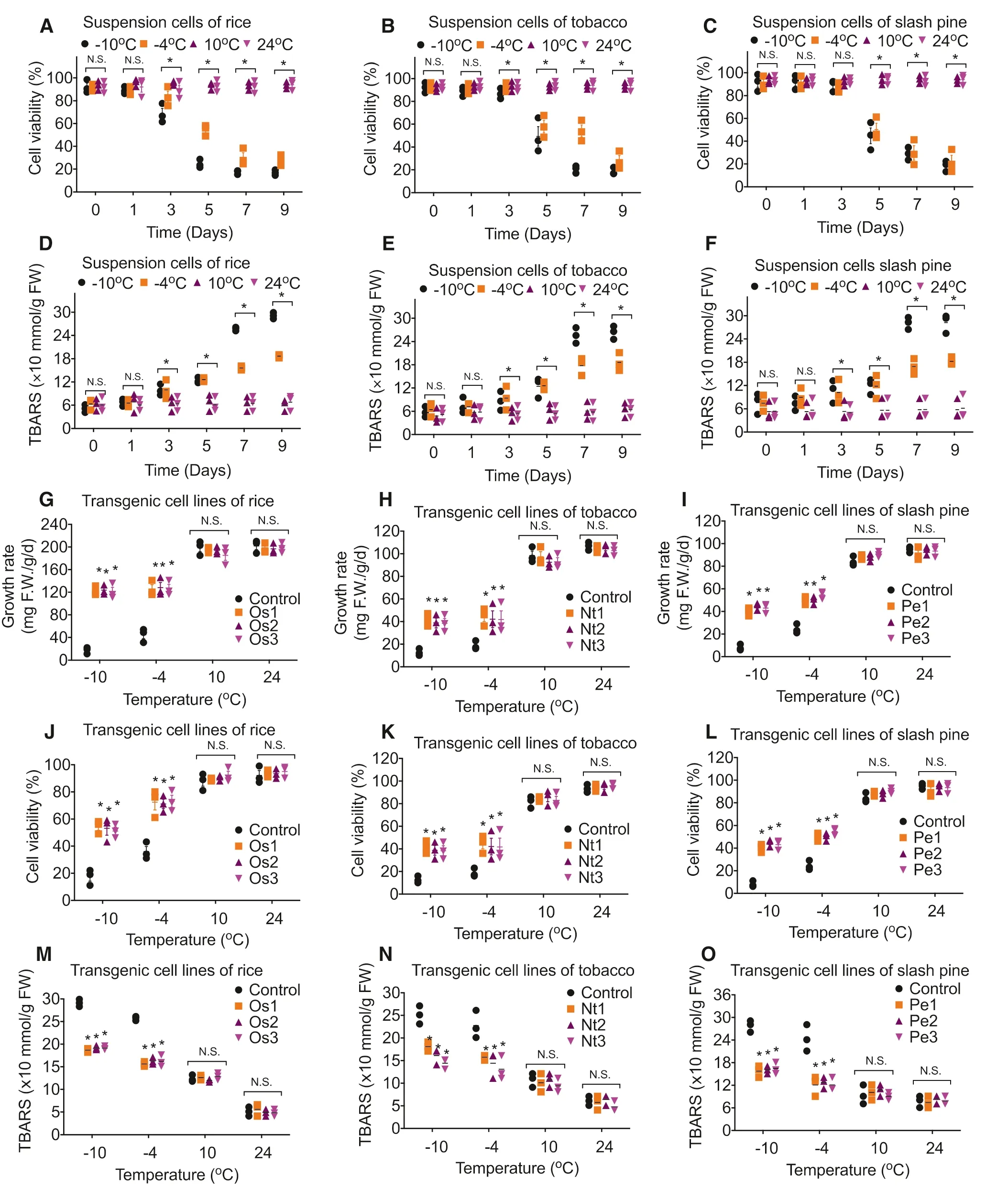
Fig.2 Time course of cell viability ( a– c) and TBARS ( d– f) of O. sativa, N. tabacum, and P. elliottii cells under cold treatment. Overexpression of AAE 13.1 increases cell growth rate and viability, and decreases TBARS levels after cold treatments in transgenic rice ( g, j, m), tobacco ( h, k, n), and pine ( i, l, o)
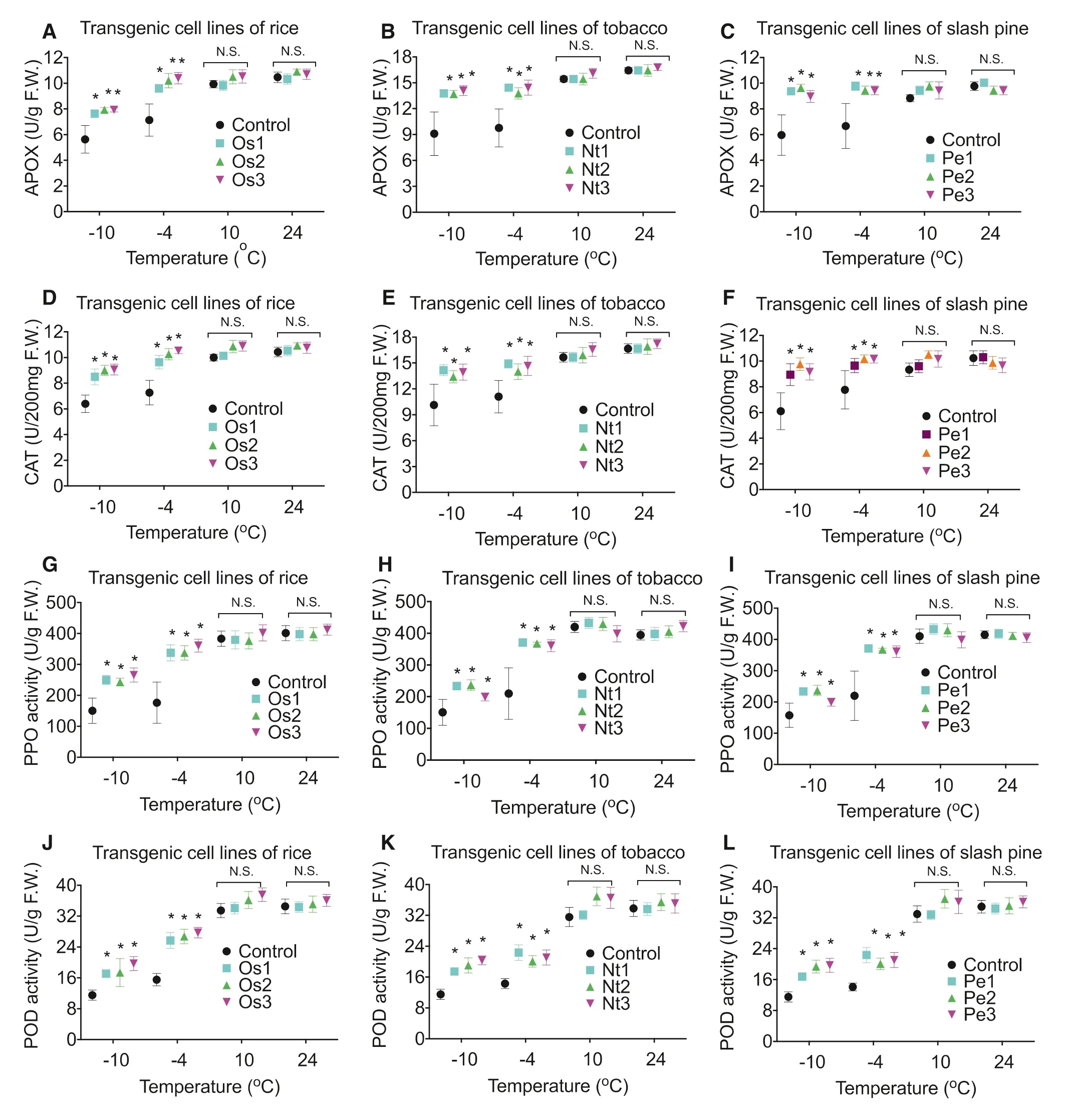
Fig.3 Effect of AAE13.1 on activity of APOX, CAT, PPO, and POD in transgenic cells of rice ( a, d, g, j), tobacco ( b, e, h, k), and pine ( c, f, i, l). Data are means of three independent experiments; error bars represent standard deviations. One-way ANOVA was used to test for significant differences among treatments. * P < 0.05, significant relative to control as assessed by a t test
Activity of ascorbate peroxidase (APOX), catalase (CAT), polyphenol oxidase (PPO), and peroxidase (POD)
Overexpression of AAE13.1 increased the activity of APOX, CAT, PPO, and POD in transgenic cell lines of O. sativa (Fig.3 a, d, g, j), N. tabacum (Fig.3 b, e, h, k), and P. elliottii (Fig.3 c, f, i, d) after treatment at ? 10 °C and ? 4 °C but activity did not change at 10 °C or 24 °C compared to controls.
Acid phosphatase and alkaline phosphatase of transgenic cell lines
Compared to the activity in the controls, activity of acid phosphatase and alkaline phosphatase increased significantly in the transgenic cell lines of O. sativa (Fig.4 a, d), N. tabacum (Fig.4 b, e), and P. elliottii (Fig.4 c, f) after treatment with ? 10 °C and ? 4 °C but not after treatment with 10 °C and 24 °C.
Effect of AAE13.1 gene on the morphology of transgenic cells
Overexpression of the AAE13.1 gene decreased the size of transgenic cells in rice (Fig.5 a–d), tobacco (Fig.5 e–h), and pine (Fig.5 i–l), after treatment with ? 10 °C and ? 4 °C compared to the transgenic cells of O. sativa, N. tabacum, and P. elliottii treated at 10 °C, and 24 °C.
Expression of MAPK genes in transgenic cells
Compared to expression of the respective MAPK genes in the controls, the expression of OsMAPK1 (Fig.6 a), OsMAPK2 (Fig.6 b), OsMAPK3 (Fig.6 c), OsMAPK4 (Fig.6 d), OsMAPK5 (Fig.6 e), and OsMAPK6 (Fig.6 f) was significantly higher in transgenic rice cells after treatment with ? 10 °C and ? 4 °C, but there was no difference after treatment with 10 °C and 24 °C. At 2 days after the cold treatment, results were similar (Fig.7).
Discussion
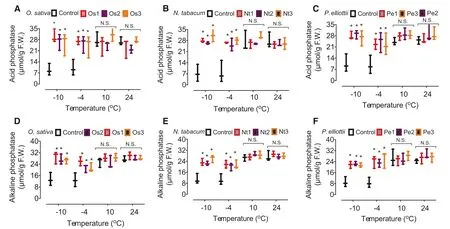
Fig.4 Effect ofoverexpression of AAE13.1 on activity of acid phosphatase and alkaline phosphatase in transgenic cells of rice ( a, d), tobacco ( b, e), and pine ( c, f). Data are means of three independent experiments; error bars represent standard deviations. One-way ANOVA was used to test for significant differences among treatments. * P < 0.05, significant relative to control as assessed by a t test
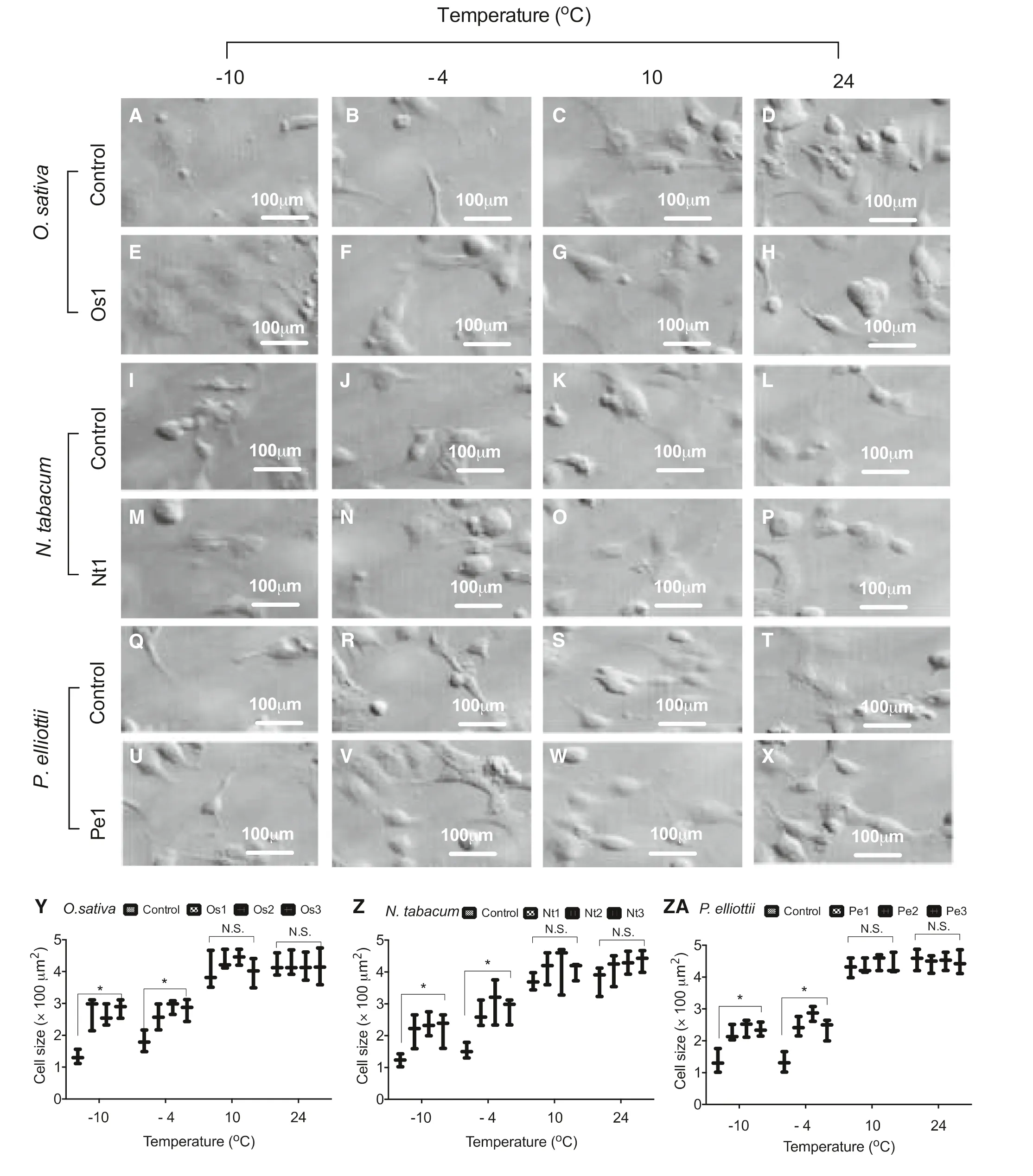
Fig.5 Effect ofoverexpression of AAE13.1 on morphology of transgenic cells of rice ( a– h), tobacco ( i– p), and pine ( q- x) and cell size ( y– za) after treatment with different temperature (? 10 °C, ? 4 °C, 10 °C, and 24 °C)
In response to signal transduction during abiotic stress, cells regulate their biological activities at the transcriptomic and proteomic levels. Increased activity of antioxidant enzymes is involved in tolerance to stress. In our genetic and biochemical assays to explore the effect ofoverexpression of AAE13.1 after cold stress (? 10, ? 4, and 10 °C) in different plant species, overexpression increased cell viability and growth rate, and decreased TBARS in transgenic cells of rice, tobacco, and pine. Thus, overexpression of the AAE13.1 gene has the potential to enhance cold stress tolerance by decreasing TBARS and might be achieved in different plant species.
The activity of APOX, CAT, PPO, and POD is related to cold stress tolerance in many plant species (Yang et al. 2018; Faltin et al. 2010; Correa-Aragunde et al. 2015). In a study of PPO activity in 60 plant or fungal species including S. tuberosum and Agaricus bisporus, RP-UHPLC-MS analysis showed that the extracts of O. sativa, N. tabacum, and P. elliottii cells contained a mixture of phenolic compounds, and the phenolic fraction was mainly responsible for inhibiting PPO (Nautiyal et al. 2008; Poiatti et al. 2009; Dirks-Hofmeister et al. 2013; Kuijpers et al. 2014; Molitor et al. 2016; Kampatsikas et al. 2017). In our present study, overexpression of AAE13.1 during cold stress was associated with increased activity of APOX, CAT, PPO, and POD. Among the six MAPK genes analyzed in O. sativa, only OsMAPK1 (Fig.6 a), OsMAPK2 (Fig.6 b), OsMAPK3 (Fig.6 c) increased significantly; the other did not change after the treatment with ? 10 °C and ? 4 °C; thus, OsMAPK1 (Fig.6 a), OsMAPK2 (Fig.6 b), OsMAPK3 (Fig.6 c) apparently are involved in AAE13.1-enhanced cold stress tolerance.
Environmental stresses can alter cell cycle regulation by shortening the G2 period and thus altering growth (Matia et al. 2010; Herranz and Medina 2014; de Buanafina et al. 2017; Lee et al. 2017; Majda et al. 2017). In embryogenic cell suspensions of wheat ( Triticum aestivum L.), cold stress led to an abnormal chromosome number and low cell viability (Ahmed and Sagi 1993). Cell viability and cell size are important for increased cold stress tolerance (Tang et al. 2006). In the present study, growth rate was significantly increased in cultures with high viability. Increased sensitivity of the cells to stresses was associated with a 35% increase in cell size of Medicago sativa plants (Steward et al. 1999). In our study, overexpression of the AAE13.1 gene increased the size of transgenic cells in rice (Fig.5 a–d), tobacco (Fig.5 e–h), and pine (Fig.5 i–l) after low temperature (? 10 °C and ? 4 °C) treatment, but not increased cell size under growth at 24 °C.
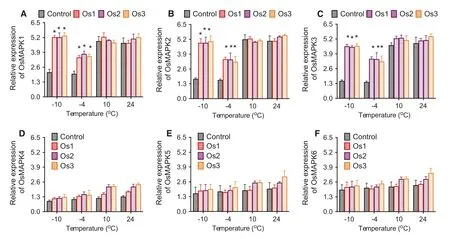
Fig.6 MAPK expression is regulated by overexpression of AAE13.1 gene in transgenic rice cells. OsMAPK1 ( a), OsMAPK2 ( b), OsMAPK3 ( c), OsMAPK4 ( d), OsMAPK5 ( e), and OsMAPK6 ( f). Data are means of three independent experiments; error bars represent standard deviations. One-way ANOVA was used to test for significant differences among treatments. * P < 0.05, significant relative to control as assessed by a t test
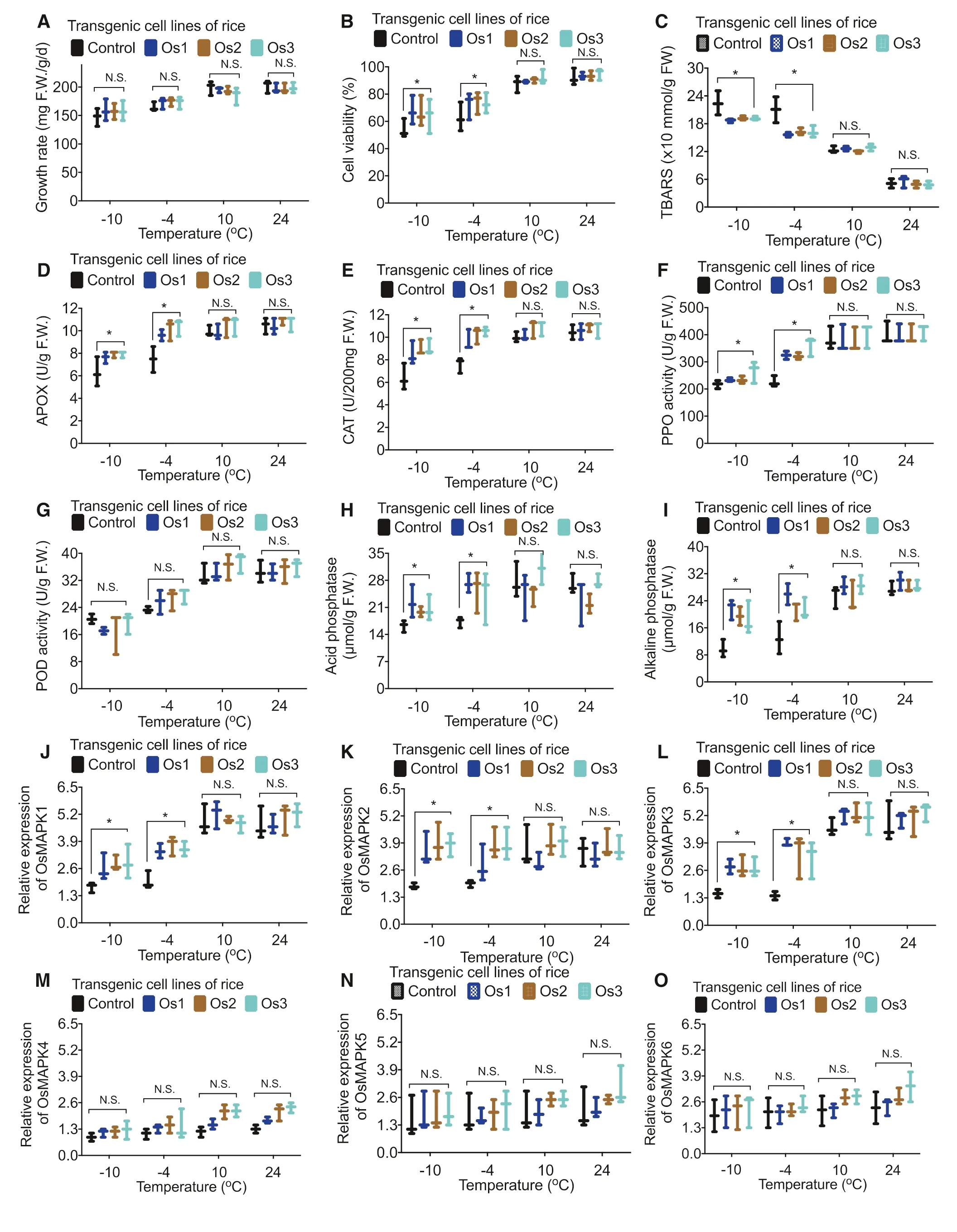
Fig.7 Verification ofeffect of cold stress in transgenic rice cells. Effect of a shorter cold stress (2 days) on growth rate ( a), cell viability ( b), TBARS ( c), APOX ( d), CAT ( e), PPO ( f), POD ( g), acid phosphatase ( h), alkaline phosphatase ( i), OsMAPK1 ( j), OsMAPK2 ( k), OsMAPK3 ( l), OsMAPK4 ( m), OsMAPK5 ( n), and OsMAPK6 ( o) in control and transgenic rice cell lines. Data are means of three independent experiments; error bars represent standard deviations. One-way ANOVA was used to test for significant differences among treatments. * P < 0.05, significant relative to control as assessed by a t test
The effects of chilling can also affect newly formed cells that were not stressed. On the basis of previous reports and our own experimental results here, we propose a mechanism by which previous chilling can affect new cells: (1) Cold stress affects cell wall formation and microtubules organization when the new cell wall is formed during the division of cold-stress-treated cells (Lee et al. 2017; Majda et al. 2017). The effect on this molecular machinery may last a few generations. (2) Cold stress affects cell metabolism and the biosynthesis of stress-related proteins that are associated with the growth of cells and their internal structures, such as the formation of the mitochondria envelope (Deng et al. 2014 ). (3) Cold stress may lead to alterations in the ultrastructure of nuclei, plasmalemma, and chromatin (Johnson et al. 2015). (4) Cold stress causes DNA damage and affects the formation of secondary structures of RNA that are related to the process of cell death (Johnson et al. 2015; Majda et al. 2017).
Conclusion
Overexpression of the AAE13.1 gene increased cold stress tolerance in association with increased cell viability and growth rate and lower levels of TBARS in rice, tobacco, and slash pine. Activity of APOX, CAT, PPO, and POD and accumulation of acid phosphatase and alkaline phosphatase also increased. Among six MAPK genes in rice, AAE13.1 overexpression changed expression of all examined MAPK genes after cold stress. AAE13.1 counteracts cold stress by activating expression of stress-response genes OsMAPKs, indicating that AAE13.1 enhances cold stress tolerance by regulating expression of transcription factor genes in plant cells. These findings may provide new information for our understanding of the AAE13.1 gene-related cold stress tolerance in different plant species including monocotyledonous, dicotyledonous, and gymnosperm plants.
AcknowledgementsThe authors are grateful to Dr. T. Bradshaw, Dr. R. Lischewski, and Dr. D. Thompson for their critical reading and suggestions during the preparation of this manuscript.
References
Ahmed KZ, Sagi F (1993) Culture of and fertile plant regeneration from regenerable embryogenic suspension cell-derived protoplasts of wheat ( Triticum aestivum L.). Plant Cell Rep 12:175–179
Arora P, Bhardwaj R, Kumar Kanwar M (2010) 24-Epibrassinolide induced antioxidative defense system of Brassica juncea L. under Zn metal stress. Physiol Mol Biol Plants 16:285–293
Chen H, Kim HU, Weng H, Browse J (2011) Malonyl-CoA synthetase, encoded by ACYL ACTIVATING ENZYME13, is essential for growth and development of Arabidopsis. Plant Cell 23:2247–2262
Chen GJ, Liu HP, Wei Q, Zhao HN, Liu JX, Yu YX (2017) The acylactivating enzyme PhAAE13 is an alternative enzymatic source of precursors for anthocyanin biosynthesis in petunia flowers. J Exp Bot 68:457–467
Correa-Aragunde N, Foresi N, Lamattina L (2015) Nitric oxide is a ubiquitous signal for maintaining redox balance in plant cells: regulation of ascorbate peroxidase as a case study. J Exp Bot 66:2913–2921
de Buanafina MMO, Iyer PR, Buanafina MF, Shearer EA (2017) Reducing cell wall feruloylation by expression of a fungal ferulic acid esterase in Festuca arundinacea modifies plant growth, leaf morphology and the turnover of cell wall arabinoxylans. PLoS ONE 12:e0185312
Deng X, Bashandy H, Ainasoja M, Kontturi J, Pietiainen M, Laitinen RA, Albert VA, Valkonen JP, Elomaa P, Teeri TH (2014) Functional diversification of duplicated chalcone synthase genes in anthocyanin biosynthesis of Gerbera hybrida. New Phytol 201:1469–1483
Dirks-Hofmeister ME, Kolkenbrock S, Moerschbacher BM (2013) Parameters that enhance the bacterial expression of active plant polyphenol oxidases. PLoS ONE 8:e77291
Erukainure OL, Mopuri R, Oyebode OA, Koorbanally NA, Islam MS (2017) Dacryodes edulis enhances antioxidant activities, suppresses DNA fragmentation in oxidative pancreatic and hepatic injuries; and inhibits carbohydrate digestive enzymes linked to type 2 diabetes. Biomed Pharmacother 96:37–47
Evers D, Legay S, Lamoureux D, Hausman JF, Hoff mann L, Renaut J (2012) Towards a synthetic view of potato cold and salt stress response by transcriptomic and proteomic analyses. Plant Mol Biol 78:503–514
Faltin Z, Holland D, Velcheva M, Tsapovetsky M, Roeckel-Drevet P, Handa AK, Abu-Abied M, Friedman-Einat M, Eshdat Y, Perl A (2010) Glutathione peroxidase regulation of reactive oxygen species level is crucial for in vitro plant differentiation. Plant Cell Physiol 51:1151–1162
Gapper NE, Hertog M, Lee J, Buchanan DA, Leisso RS, Fei ZJ, Qu GQ, Giovannoni JJ, Johnston JW, Schaffer RJ, Nicolai BM, Mattheis JP, Watkins CB, Rudell DR (2017) Delayed response to cold stress is characterized by successive metabolic shifts culminating in apple fruit peel necrosis. BMC Plant Biol 17:77
Guan X, Nikolau BJ (2016) AAE13 encodes a dual-localized malonyl-CoA synthetase that is crucial for mitochondrial fatty acid biosynthesis. Plant J 85:581–593
Gzyl J, Rymer K, Gwozdz EA (2009) Differential response of antioxidant enzymes to cadmium stress in tolerant and sensitive cell line of cucumber ( Cucumis sativus L.). Acta Biochim Polonica 56:723–727
Herranz R, Medina FJ (2014) Cell proliferation and plant development under novel altered gravity environments. Plant Biol 16(Suppl 1):23–30
Johnson KL, Ramm S, Kappel C, Ward S, Leyser O, Sakamoto T, Kurata T, Bevan MW, Lenhard M (2015) The Tinkerbell (Tink) mutation identifies the dual-specificity MAPK phosphatase INDOLE-3-BUTYRIC ACID-RESPONSE5 (IBR5) as a novel regulator oforgan size in Arabidopsis. PLoS ONE 10:e0131103
Jung JH, Park JH, Lee S, To TK, Kim JM, Seki M, Park CM (2013) The cold signaling attenuator HIGH EXPRESSION OF OSMOTICALLY RESPONSIVE GENE1 activates FLOWERING LOCUS C transcription via chromatin remodeling under short-term cold stress in Arabidopsis. Plant Cell 25:4378–4390
Kaida R, Satoh Y, Bulone V, Yamada Y, Kaku T, Hayashi T, Kaneko TS (2009) Activation of beta-glucan synthases by wall-bound purple acid phosphatase in tobacco cells. Plant Physiol 150:1822–1830
Kampatsikas I, Bijelic A, Pretzler M, Rompel A (2017) Three recombinantly expressed apple tyrosinases suggest the amino acids responsible for mono- versus diphenolase activity in plant polyphenol oxidases. Sci Rep 7:8860
Kim HS, Oh JM, Luan S, Carlson JE, Ahn SJ (2013) Cold stress causes rapid but differential changes in properties of plasma membrane H + -ATPase of camelina and rapeseed. J Plant Physiol 170:828–837
Kuijpers TF, van Herk T, Vincken JP, Janssen RH, Narh DL, van Berkel WJ, Gruppen H (2014) Potato and mushroom polyphenol oxidase activities are differently modulated by natural plant extracts. J Agric Food Chem 62:214–221
Lee DJ, Choi HJ, Moon ME, Chi YT, Ji KY, Choi D (2017) Superoxide serves as a putative signal molecule for plant cell division: overexpression of CaRLK1 promotes the plant cell cycle via accumulation of O2( ? ) and decrease in H2O2. Physiol Plant 159:228–243
Leyva A, Hernandez N, Gonzalez T, Sanchez JC, Franco A, Delgado I, Montanez M, Valdes R (2004) Standardization and validation of an alkaline phosphatase-linked immunoassay to quantify a plantderived antibody directed against the hepatitis B virus surface antigen. Biochem Biophys Res Commun 325:1438–1442
Ma HR, Wei HB, Chen Z et al (2013) The estrogenic activity of isoflavones extracted from chickpea Cicer arietinum L sprouts in vitro. Phytother Res 27:1237–1242
Majda M, Grones P, Sintorn IM, Vain T, Milani P, Krupinski P, Zagorska-Marek B, Viotti C, Jonsson H, Mellerowicz EJ, Hamant O, Robert S (2017) Mechanochemical polarization of contiguous cell walls shapes plant pavement cells. Develop Cell 43(290–304):e294
Malik CP, Sethi RS (1975) Histochemical studies in stomatal apparatus of Phaseolus mungo linn. IV. Mechanism of stomatal action. Acta Histochem 53:1–11
Matia I, Gonzalez-Camacho F, Herranz R, Kiss JZ, Gasset G, van Loon JJ, Marco R, Javier Medina F (2010) Plant cell proliferation and growth are altered by microgravity conditions in spaceflight. J Plant Physiol 167:184–193
Matos AR, Hourton-Cabassa C, Cicek D, Reze N, Arrabaca JD, Zachowski A, Moreau F (2007) Alternative oxidase involvement in cold stress response of Arabidopsis thaliana fad2 and FAD3 + cell suspensions altered in membrane lipid composition. Plant Cell Physiol 48:856–865
Molitor C, Mauracher SG, Rompel A (2016) Aurone synthase is a catechol oxidase with hydroxylase activity and provides insights into the mechanism of plant polyphenol oxidases. Proc Natl Acad Sci USA 113:E1806–E1815
Murota K, Shimura H, Takeshita M, Masuta C (2017) Interaction between Cucumber mosaic virus 2b protein and plant catalase induces a specific necrosis in association with proteasome activity. Plant Cell Rep 36:37–47
Nautiyal CS, Govindarajan R, Lavania M, Pushpangadan P (2008) Novel mechanism of modulating natural antioxidants in functional foods: involvement of plant growth promoting Rhizobacteria NRRL B-30488. J Agric Food Chem 56:4474–4481
Park SR, Ahn MS, Han AR, Park JW, Yoon YJ (2011) Enhanced flavonoid production in Streptomyces venezuelae via metabolic engineering. J Microbiol Biotechnol 21:1143–1146
Pasquali G, Biricolti S, Locatelli F, Baldoni E, Mattana M (2008) Osmyb4 expression improves adaptive responses to drought and cold stress in transgenic apples. Plant Cell Rep 27:1677–1686
Poiatti VA, Dalmas FR, Astarita LV (2009) Defense mechanisms of Solanum tuberosum L. in response to attack by plant-pathogenic bacteria. Biol Res 42:205–215
Ray M, Mishra P, Das P, Sabat SC (2012) Expression and purification of soluble bio-active rice plant catalase-A from recombinant Escherichia coli. J Biotechnol 157:12–19
Sakthivel KM, Guruvayoorappan C (2013) Acacia ferruginea inhibits tumor progression by regulating inflammatory mediators-(TNF-a, iNOS, COX-2, IL-1beta, IL-6, IFN-gamma, IL-2, GM-CSF) and pro-angiogenic growth factor- VEGF. Asian Pac J Cancer Prevent 14:3909–3919
Sang MK, Kim KD (2011) Biocontrol activity and primed systemic resistance by compost water extracts against anthracnoses of pepper and cucumber. Phytopathol 101:732–740
Selin-Rani S, Senthil-Nathan S, Revathi K, Chandrasekaran R, Thanigaivel A, Vasantha-Srinivasan P, Ponsankar A, Edwin ES, Pradeepa V (2016) Toxicity of Alangium salvifolium Wang chemical constituents against the tobacco cutworm Spodoptera litura Fab. Pesticide Biochem Physiol 126:92–101
Sessions A (2008) Immunohistochemistry on sections of plant tissues using alkaline-phosphatase-coupled secondary antibody. CSH Protoc 2008:pdb prot4946
Shahryar N, Maali-Amiri R (2016) Metabolic acclimation of tetraploid and hexaploid wheats by cold stress-induced carbohydrate accumulation. J Plant Physiol 204:44–53
Sineshchekov V, Koppel L, Shor E, Kochetova G, Galland P, Zeidler M (2013) Protein phosphatase activity and acidic/alkaline balance as factors regulating the state of phytochrome A and its two native pools in the plant cell. Photochem Photobiol 89:83–96
Singh S, Braus-Stromeyer SA, Timpner C, Valerius O, von Tiedemann A, Karlovsky P, Druebert C, Polle A, Braus GH (2012) The plant host Brassica napus induces in the pathogen Verticillium longisporum the expression of functional catalase peroxidase which is required for the late phase of disease. Mol Plant Microbe Interact 25:569–581
Sirhindi G, Mir MA, Sharma P, Gill SS, Kaur H, Mushtaq R (2015) Modulatory role of jasmonic acid on photosynthetic pigments, antioxidants and stress markers of Glycine max L. under nickel stress. Physiol Mol Biol Plants 21:559–565
Spanou CI, Veskoukis AS, Stagos D, Liadaki K, Aligiannis N, Angelis A, Skaltsounis AL, Anastasiadi M, Haroutounian SA, Kouretas D (2012) Effects of Greek legume plant extracts on xanthine oxidase, catalase and superoxide dismutase activities. J Physiol Biochem 68:37–45
Steward N, Martin R, Engasser JM, Goergen JL (1999) Determination of growth and lysis kinetics in plant cell suspension cultures from the measurement ofesterase release. Biotechnol Bioengineer 66:114–121
Tamirisa S, Vudem DR, Khareedu VR (2014) Overexpression of pigeonpea stress-induced cold and drought regulatory gene (CcCDR) confers drought, salt, and cold tolerance in Arabidopsis. J Exp Bot 65:4769–4781
Tang W, Page M (2013) Transcription factor AtbZIP60 regulates expression of Ca 2+ -dependent protein kinase genes in transgenic cells. Mol Biol Rep 40:2723–2732
Tang W, Charles TM, Newton RJ (2005a) Overexpression of the pepper transcription factor CaPF1 in transgenic Virginia pine ( Pinus Virginiana Mill.) confers multiple stress tolerance and enhances organ growth. Plant Mol Biol 59:603–617
Tang W, Peng XX, Newton RJ (2005b) Enhanced tolerance to salt stress in transgenic loblolly pine simultaneously expressing two genes encoding mannitol- 1-phosphate dehydrogenase and glucitol-6-phosphate dehydrogenase. Plant Physiol Biochem 43:139–146
Tang W, Newton RJ, Lin J, Charles TM (2006) Expression of a transcription factor from Capsicum annuum in pine calli counteracts the inhibitory effects of salt stress on adventitious shoot formation. Mol Genet Genom 276:242–253
Tang W, Newton RJ, Li C, Charles TM (2007a) Enhanced stress tolerance in transgenic pine expressing the pepper CaPF1 gene is associated with the polyamine biosynthesis. Plant Cell Rep 26:115–124
Tang W, Newton RJ, Weidner DA (2007b) Genetic transformation and gene silencing mediated by multiple copies of a transgene in eastern white pine. J Exp Bot 58:545–554
Vashisht AA, Pradhan A, Tuteja R, Tuteja N (2005) Cold- and salinity stress-induced bipolar pea DNA helicase 47 is involved in protein synthesis and stimulated by phosphorylation with protein kinase C. Plant J 44:76–87
Wagstaff C, Bramke I, Breeze E, Thornber S, Harrison E, Thomas B, Buchanan-Wollaston V, Stead T, Rogers H (2010) A specific group of genes respond to cold dehydration stress in cut Alstroemeria flowers whereas ambient dehydration stress accelerates developmental senescence expression patterns. J Exp Bot 61:2905–2921
Wan BL, Lin YJ, Mou TM (2007) Expression of rice Ca2+-dependent protein kinases (CDPKs) genes under different environmental stresses. FEBS Lett 581:1179–1189
Wang Y, Chen H, Yu O (2014) A plant malonyl-CoA synthetase enhances lipid content and polyketide yield in yeast cells. Appl Microbiol Biotechnol 98:5435–5447
Widyowati R, Tezuka Y, Miyahara T, Awale S, Kadota S (2010) Alkaline phosphatase (ALP) enhancing iridoid glucosides from the Indonesian medicinal plant Barleria lupulina. Nat Product Commun 5:1711–1716
Yan Y, Peng L, Liu WX, Wan FH, Harris MK (2011) Host plant effects on alkaline phosphatase activity in the whiteflies, Bemisia tabaci Biotype B and Trialeurodes vaporariorum. J Insect Sci 11:9
Yang Z, Mhamdi A, Noctor G (2018) Analysis of catalase mutants underscores the essential role of CATALASE2 for plant growth and day length-dependent oxidative signalling. Plant Cell Environ 42:688–700
Yoshida K, Kaothien P, Matsui T, Kawaoka A, Shinmyo A (2003) Molecular biology and application of plant peroxidase genes. Appl Microbiol Biotechnol 60:665–670
Yu HN, Wang L, Sun B, Gao S, Cheng AX, Lou HX (2015) Functional characterization of a chalcone synthase from the liverwort Plagiochasma appendiculatum. Plant Cell Rep 34:233–245
Zou Y, Aboshora W, Li J, Xiao TC, Zhang LF (2017) Protective effects of Lepidium meyenii (Maca) Aqueous extract and lycopene on Testosterone Propionate-induced prostatic hyperplasia in mice. Phytother Res 31:1192–1198
 Journal of Forestry Research2021年2期
Journal of Forestry Research2021年2期
- Journal of Forestry Research的其它文章
- Stem taper functions for Betula platyphylla in the Daxing’an Mountains, northeast China
- Leaf phenotypic variation of endangered plant Tetracentron sinense Oliv. and influence of geographical and climatic factors
- Ecological variations of woody species along an altitudinal gradient in the Qinling Mountains of Central China: area-based versus mass-based expression of leaf traits
- Variations in stem radii of Larix principis-rupprechtii to environmental factors at two slope locations in the Liupan Mountains, northwest China
- A review of ecological mechanisms for management practices of protective forests
- Variation of basic density, calorific value and volumetric shrinkage within tree height and tree age of Ugandan grown Eucalyptus grandis wood
