Effects of phosphorus fertilizer application on phosphorus fractions in different organs of Cordia trichotoma
Matheus S. S. Kulmann · Lincon O. S. Stefanello · Raí A. Schwalbert · álvaro L. P. Berghetti · Maristela M. Araujo · Rogerio Piccin · Luciano C. Gatiboni · Tales Tiecher · Paulo A. A. Ferreira · Gustavo Brunetto
Abstract The application of phosphorus (P) to soil can increase its availability to plants and alter P fractions in annual and perennial organs of Cordia trichotoma. If a portion of P accumulates in perennial organs in organic fractions it can be used in the next growth season, possibly decreasing plant dependence on P derived from soil fertilization. However, if P is preferentially accumulated in inorganic fractions in annual organs, plants will be more dependent on phosphate fertilization. This study aimed to evaluate the distribution of P fractions in organs of C. trichotoma grown on sandy soil treated with 120 and 360 kg P 2 O 5 ha ?1 . The control was a zero application. After 24 months following fertilization, C. trichotoma seedlings were cut and separated into leaves, branches, stems and roots, dried, ground and subjected to chemical fractionation of P, which estimates fractions of total soluble P, soluble inorganic and organic P, lipid P, P associated ribonucleic acid and deoxyribonucleic acid, and residual P. P in annual organs, as leaves, accumulated preferentially in the soluble inorganic fraction in both treatments. In perennial organs such as stems and branches, P accumulated preferentially in the soluble organic fraction. The application of 300% of the recommended dosage (360 kg P 2 O 5 ha ?1 ) promoted the accumulation of P in soluble organic fractions which may contribute to annual growth the following season and be a strategy to reduce the dependence of 2-year-old stands on soil-derived P and on fertilizers.
Keywords Chemical fractionation · Phosphorus ·
Introduction
Phosphorous (P) is a limiting nutrient for the productivity of forest species (Fink et al. 2014; Carnevali et al. 2016), due to its low mobility in soil. It is strongly adsorbed to iron (Fe), aluminum (Al) and manganese (Mn) oxides in weathered soils and reduces the P uptake by the plant (Bortoluzzi et al. 2015; Fink et al. 2016; Freitas et al. 2017). However, P within the plant is mobile and can be transported to different annual organs (leaves and fine branches), or perennial organs (stems and roots) (Casali et al. 2011; Piccin et al. 2017a). Therefore, it is necessary to know the P requirement of forest crops and which fractions are preferentially accumulated in organs of forest species such as Cordia trichotoma (Vell.) Arrab. ex Steud., as some fractions may be redistributed throughout plant growth and development, possibly decreasing plant dependence on P fertilization (Stahl et al. 2013; Valadares et al. 2015; Oliveira et al. 2017).
Phosphorous may accumulate within plants as organic P metabolically active in the cytoplasm (PSO ), in phospholipids (PLIP), P associated with ribonucleic and deoxyribonucleic acids, RNA and DNA (PRNAand PDNA), as phosphoproteins (PRES), and as inorganic phosphorous (PSI) (Bieleski 1973; Veneklaas et al. 2012; Piccin et al. 2017a, 2017b). It is assumed that tropical forest species such as C. trichotoma on soils with increasing amounts of phosphorus fertilizer will accumulate higher levels of P in organs in the PSIfraction, especially in annual organs (leaves and branches) (Martinez et al. 2005; Casali et al. 2011; Lambers et al. 2011; Piccin et al. 2017a). The PLIPfraction represents phosphorous contained mainly in cell membranes (Bieleski 1973; Piccin et al. 2017b), and its increase occurs because of the increase of cell membrane complexes, especially thylakoid membranes at flowering (Thomas and Sadras; 2001; Casali et al. 2011; Niu et al. 2015; Oliveira et al. 2017) and in increased PSIcontent (Reefet al. 2010; Veneklaas et al. 2012; Noack et al. 2014). Phosphorous levels in the nucleic acids (PRNAand PDNA) generally vary between organs, tissues and cells. The highest concentrations are commonly diagnosed in organs undergoing intense cell division, such as leaves (Suzuki et al. 2001; Niklas 2006; Noack et al. 2014; Piccin et al. 2017a). However, when cell division ceases, P may accumulate in perennial organs in the PSO fraction and be redistributed to perennial organs such as roots and stems, which are important reserve organs (Valadares et al. 2015; Piccin et al. 2017a).
Lourdo-pardo ( Cordia trichotoma), belonging to the Boraginaceae family, is found in tropical and subtropical areas of Brazil, Paraguay and Argentina. It is a fast-growing species, reaching up to 35 m in height and 100 cm in diameter, with a straight stem and dense wood (Carvalho 2003; Berghetti et al. 2015, 2016; Cadorin et al. 2015; Machado et al. 2016). It is considered a potential species for commercial plantations (Sartoretto and Rossi 2014; Da Ros et al. 2015). C. trichotoma is adaptable to fertile and non-acidic soils (Sartoretto and Rossi 2014), and therefore, when a lack of fertility is diagnosed by soil analysis, liming and fertilization are carried out to enhance nutrient supply, especially for phosphorus (Rossa et al. 2015).
In growing C. trichotoma, it is not as yet clear whether the application of P and hence increased availability of P in the soil alters the distribution of P fractions in annual (leaves and branches) and perennial (roots and stems) organs. If P fractions accumulate in annual organs occurs, P reserves in perennial organs will be consumed, especially if the soil does not contain suffi cient phosphorous to meet plant demand (Lambers et al. 2011; Lima et al. 2011; Valadares et al. 2015). However, if P fractions are preferentially accumulated in reserve organs, it is assumed that they can contribute to annual organ growth in the following season, possibly reducing a need for fertilization. This study evaluates the distribution of P fractions in organs of C. trichotoma grown on soil with phosphate fertilization.
Materials and methods
Description of the experimental area
The experiment was carried out in the city of Santa Maria, located in the Depress?o Central region of the state of Rio Grande do Sul, Brazil (29o 47′37″ S, 53o 40′08″ W). The climate is humid subtropical (Cfa) according to the K?ppen classification. According to data from the Instituto Nacional de Meteorologia (INMET), the average temperature in the hottest month is over 22 °C and the coldest is between ? 3 and 18 °C. Average annual rainfall is 1769 mm. The accumulated monthly values are presented in Fig.1.
The soil is an Argissolo Vermelho Distrófico arênico according to the Brazilian Soil Classification System (Embrapa 2013), and Ultisol by the USDA Soil Taxonomy (Soil Survey Staff 2010 ). Chemical and physical characteristics of the soil at 0–0.2 m were collected prior to the experiment (Table 1).
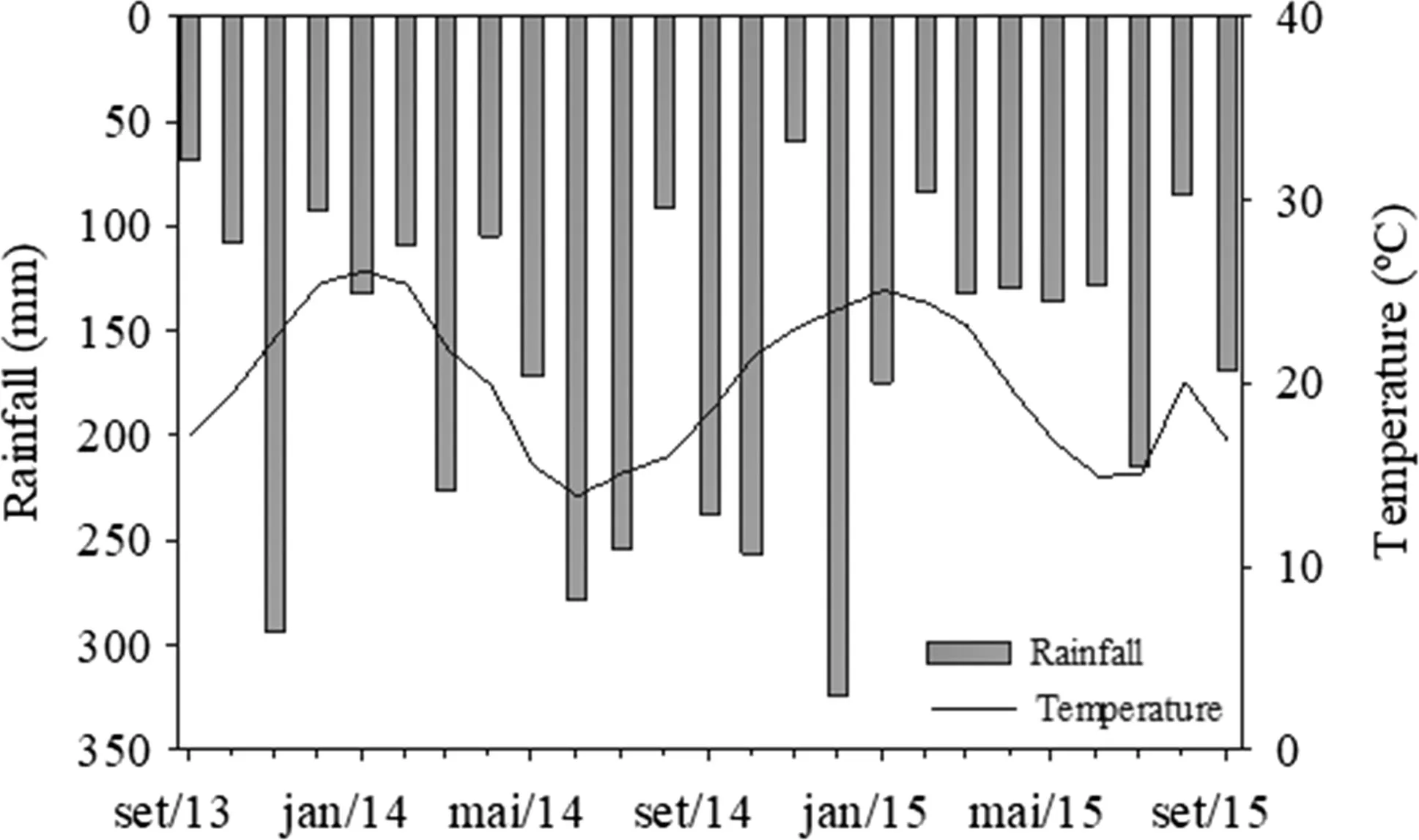
Fig.1 Average rainfall (mm) and air temperature (°C) during the experimental period (24 months)
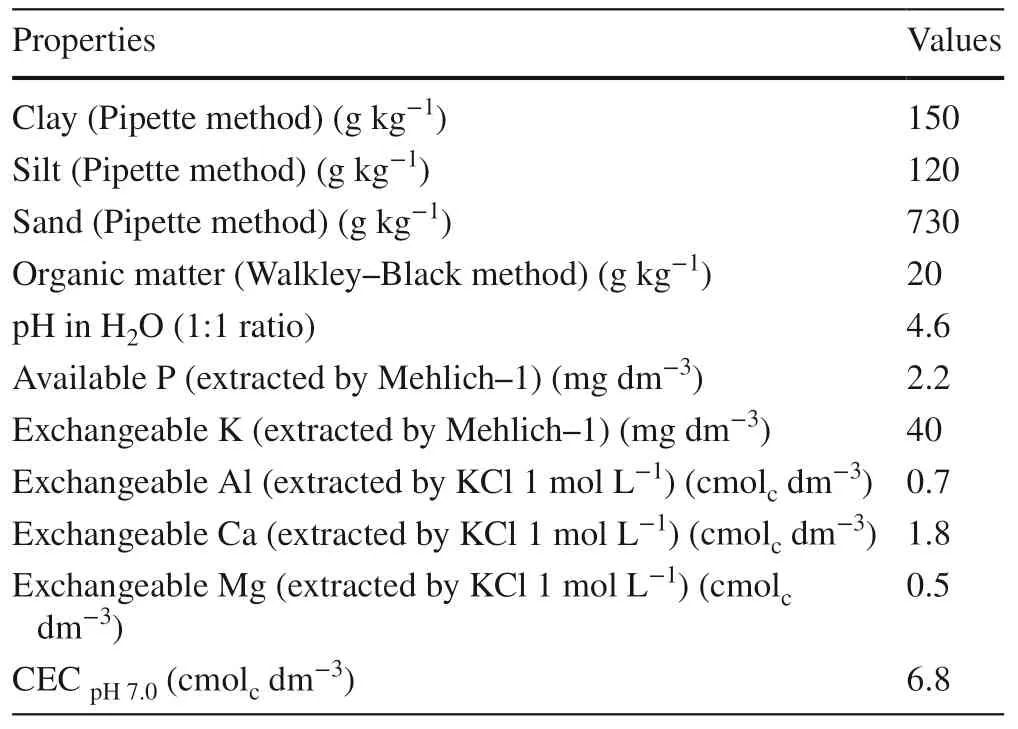
Table 1 Physical and chemical properties of the soil at 0.0–0.2 m
The vegetation consists predominantly of Vernonanthura tweedieana, Sida spp., Senna obtusifolia, and Bidens alba which were removed before the experiment began. Subsequently, 683 kg ha?1of limestone (76% PRNT) was applied to the soil to raise the pH to 5.5. Seedlings of C. trichotoma were produced in 280 cm 3 polypropylene tubes containing a mixture of subsurface soil, peat and carbonized rice husk (2:2:1 ratio). On September 3rd, 2013, uniform seedlings (40 cm height, 5.5 cm stem diameter) were selected and transplanted into 30 cm × 30 cm × 30 cm planting holes. Spacing was 2.0 m between plants and 3.0 m between rows. The experiment was arranged in four blocks, each consisting ofeight plots with 16 plants, of which four were evaluated. Soon after the planting, 1 L of water per pit was applied to eliminate air pockets around the soil and the root systems. On September 5th, 50 kg N ha?1and 45 kg K2O ha?1were applied into two lateral holes 0.15 m from the seedlings. The source of N and K were urea and potassium chloride (KCl), respectively. In addition, control, 120 and 360 kg P2O5ha?1were applied into the holes. The source of phosphorous was triple superphosphate (46% P2O5). As there was no recommendation for the levels of P for C. trichotoma, the amounts were equivalent to control, 100 and 300% of the recommended P2O5for eucalypt crops by the CQFS-RS/SC ( 2004), since there was no recommendation for P for C. trichotoma.
Throughout the cultivation period (up to 24 months), three applications of glyphosate ( N-(phosphonomethyl) glycine) were applied in the weeds. In the initial phase of growth the seedlings were protected. Each spray application consisted of 3.5 L ha?1of glyphosate.
Material collection and sample preparation
On September 3rd 2015, 2 years after transplanting of seedlings, the shoots of four plants per treatment were cut close to the soil surface. The shoots were separated into leaves, branches and stem. Afterwards, the roots were collected manually and soil removed by washing in running water and then with distilled water. The material was oven-dried at 65 °C until a constant weight was reached. The samples were ground in a Wiley-type mill and passed through a 2-mm mesh sieve and the reserved for the analysis of P fractions.
Fractionation of P in tissues
The chemical fractionation of phosphorous in organ tissues was performed according to Miyachi and Tamiya ( 1961), and adapted by Casali et al. ( 2011). The P fractions were: total soluble P (PST); soluble inorganic P (PSI); soluble organic P (PSO ), the difference between PSTand PSI; lipid P (PLIP); P associated with RNA (PRNA) with DNA (PDNA); and, residual P (PRES). To accomplish this, 0.2 g of dry tissue matter was weighed in triplicate, placed into Falcon 15 mL round-bottom tubes, and 10 mL of 0.2 mol L?1HClO4added. The mixture was immediately shaken for 5 min and centrifuged at 5000× g for 10 min in a temperature-controlled centrifuge. The extract was filtered through quantitative filter with 8- micron pores and an additional 5 mL of 0.2 mol L?1HClO4was added to the remaining material in the tubes. Centrifugation and filtration was repeated and P analysis was carried out to obtain the PSTfraction according to Murphy and Riley ( 1962). Two mL were removed from the extract to determine PSIby digestion with 2- mL of concentrated H2SO4and 1- mL of 30% H2O2in a digester block (Tedesco et al. 1995). The PSO fraction was obtained by the difference between PSTand PSI.
A 6- mL solution ofether–ethanol–chloroform (E + E + C) was added (2:2:1 ratio, respectively), to the remaining sample of the first extraction, kept in the 15 mL tubes and remained in the water-bath at 50 °C for 1 h. Subsequently, the samples were centrifuged at 5000× g for 10 min, and the extract reserved in a 25- mL flask. Four mL of cold ether (4 °C) was added to the remaining samples which were centrifuged again at 5000× g for 10 min. The extract from this second centrifugation was combined to the first in a 25 mL flask. The flasks remained in an air extraction hood for 24 h to facilitate ether evaporation. Subsequently, the samples were placed in an oven with forced air circulation at 27 °C for 3 h. Six mL of distilled water were added and 2 mL of this extract was removed for digestion with 2 mL of concentrated H2SO4and 2 mL of 30% H2O2in a digester to determine the PLIPfraction.
To obtain the PRNAfraction, 6 mL of 0.5 mol L?1KOH were added to the samples remaining in the Falcon tubes of the second extraction (E + E + C). The tubes were then closed, shaken for 1 min, and placed in a forced air circulation oven at 37 °C for 17 h. A 1-mL solution of 3.0 mol L?1HCl and 1-mL of 70% HClO4were added and the tubes centrifuged at 5000× g for 10 min. The extract was transferred to 20 mL acrylic tubes, 5 mL of 0.5 mol L?1HClO4were added to the remaining sample of the Falcon tubes, centrifuged at 5000× g for 10 min, joining the two extracts in the acrylic tubes. A 2-mL amount of this extract was removed and placed into 50 mL digestion tubes together with 2 mL of concentrated H2SO4and 1 mL of 30% H2O2in a digester block.
For the PDNAfraction, 5 mL of 0.5 mol L?1HClO4was added to the samples remaining in the Falcon tubes and then immediately shaken for 1 min. The samples were suspended in a water bath at 100 °C for 15 min. Afterwards, the Falcon tubes were centrifuged at 5000× g for 10 min, and the extract was stored. Two mL of this extract was removed and placed into 50 mL tubes. For digestion, 2 mL of H2SO4and 1 mL of 30% H2O2were used in a digester block.
To obtain the PRESfraction, the residual tissue was transferred to 50 mL tubes for digestion with 2 mL of concentrated H2SO4and 2 mL of 30% H2O2in a digester block.
Phosphorous determination from the extracts was done at 882 nm in a UV–visible spectrophotometer according to Murphy and Riley ( 1962).
Analysis of soil phosphorous
After 24 months of cultivation, four 0.0–0.2 m soil samples were collected in each treatment, air-dried and passed through a 2-mm mesh sieve. The phosphorous was determined by Mehlich-1 (0.05 mol L?1HCl + 0.0125 mol L?1H2SO4) (Tedesco et al. 1995). The concentration of available P was determined at 882 nm in a UV–visible spectrophotometer (Model SF325NM—Bel Engineering, Italy), according to Murphy and Riley ( 1962).
Statistical analysis
The results of the P fractions (PSI, PSO , PLIP, PRNA, PDNA, PRES)at each P dosage were compared by analysis of variance using R software (R Core Team 2017). By considering possible correlations among samples from the same organ and the same fraction, the means were fit using the “nlme” package (Pinheiro et al. 2014) and compared by the Tukey test at 5% using the multcomp package (Hothorn et al. 2008). Lastly, principal component analysis (PCA) was performed combining all phosphorus fractions, Cordia trichoma organs and phosphorus dosages using CANOCO software, version 4.5 (Ter Braak and Smilauer 2002).
Results
Distribution of P fractions in organs at the same P dosage
In C. trichotoma grown in soil without phosphorous application, the highest concentrations of PSI(soluble inorganic P) were found in leaves and roots (Table 2), while the highest PSO (soluble organic P) concentrations were in branches and stems of the same plants. The highest PLIP(lipid P) and PRNA(P associated with ribonucleic acid) concentrations were in the stems and branches, respectively. Levels of PDNA(P associated with deoxyribonucleic acid) did not differ statistically among the organs. The highest concentrations of PRES(residual P) were found in the leaves and roots.
In plants grown in soil treated with 120 kg P2O5ha?1, the highest amounts of PSIwere in the leaves and roots, while the highest PSO concentrations were in the branches and stems. The highest PLIPconcentrations were in the stems and roots, while the highest PRNAlevels were in the branches and stems. PDNAconcentrations were not statistically different ( p ≥ 0.05) among the organs. The highest amounts of PRESwere in the leaves, followed by the branches and roots.
In seedlings grown in soil treated with 360 kg P2O5ha?1, the highest levels of PSIwere in the leaves, while the highest PSO concentrations were in the shoots and stems. The highest levels of PSI, PSO and PLIPwere in the roots. The highest PRNAconcentration was in the stems. PDNAamounts did not differ statistically among the organs. The highest levels of PRESwere in the branches, followed by the roots and leaves.
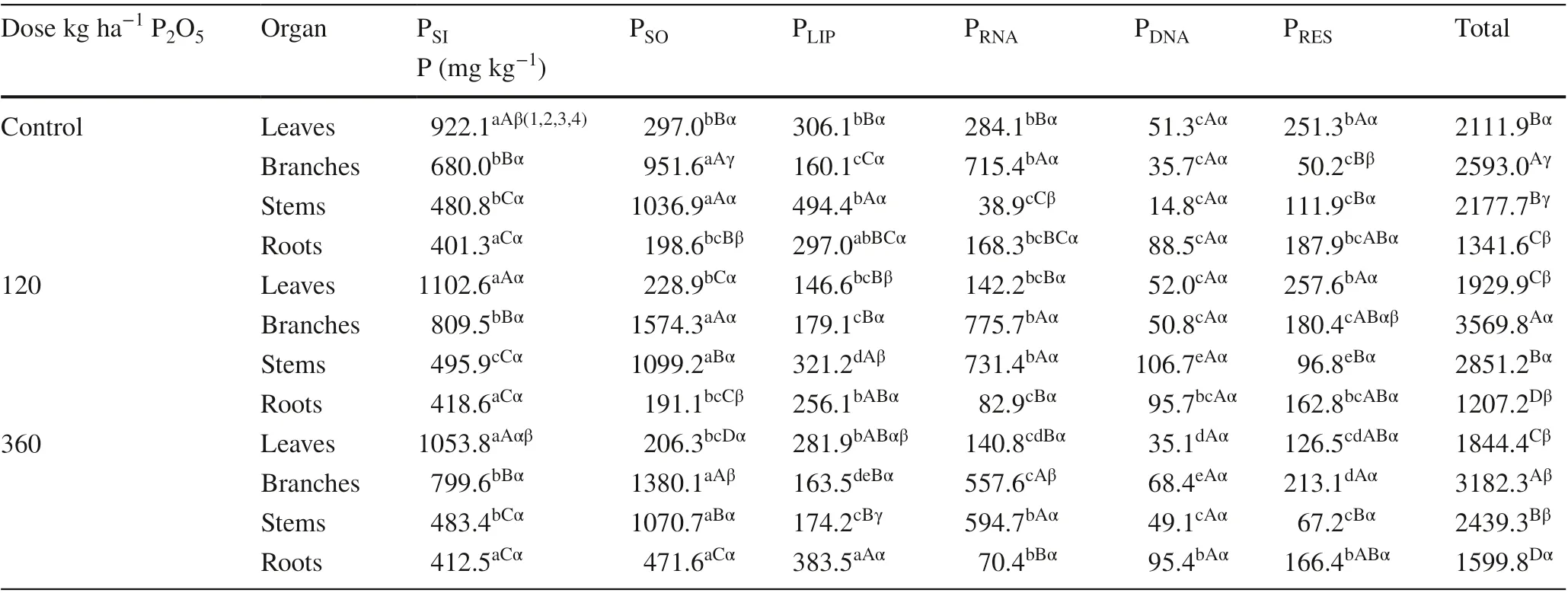
Table 2 Phosphorus fractions in leaves, branches, stems and roots of Cordia trichotoma on sandy soil treated with phosphorus
Distribution of P fractions in the same organ of C. trichotoma with different P doses
The highest concentrations of PSIwere found in leaves of seedlings on soil subject to a dose of 120 kg P2O5ha?1. They were not statistically different ( p ≥ 0.05) for the branches, leaves and roots. PSO levels increased in branches of seedlings grown in soil treated with 120 kg P2O5ha?1. PSO concentrations were not statistically different ( p ≥ 0.05) in the leaves and stems. However, the highest concentrations of PSO in the roots were found in plants grown with the 360 kg P2O5ha?1treatment. PLIPlevels were highest in the leaves in plants grown without P application, i.e., the controls. PLIPconcentrations in branches and roots did not differ statistically among dosages. The highest PLIPconcentrations in the stems were in the controls. There was no statistically significant differences ( p ≥ 0.05) in concentrations of PRNAin the leaves and branches among doses. The branches of the controls and those treated with 120 kg P2O5ha?1had higher levels of PRNAcompared to plants treated with 360 kg P2O5h?1. The highest concentrations of PRNAin the stems were found in plants grown in soil treated with 120 and 360 kg P2O5ha?1. PDNAlevels in all organs were not statistically different ( p ≥ 0.05) between the two doses. PRESwas highest in branches in plants grown in soil treated with 360 kg P2O5ha?1. However, for the other organs, PRESconcentrations were not statistically different ( p ≥ 0.05) among the doses.
Distribution of P SI and P SO in organs of C. trichotoma
Leaves of all plants had higher concentrations of PSIcompared to PSO (Fig.2). However, the branches and stems of all plants had higher concentrations of PSO compared to PSI. The roots of all plants showed higher concentrations of the PSIfraction than PSO .
Principal component analysis (PCA) of phosphorus fractions in organs of Cordia trichotoma
Principal component analysis was carried out by extracting only the first two components (PC1 and PC2), which explained 69.4% of the original variability of the results (PC1 43.6%; PC2 25.8%) (Fig.3). The separation of the points occurred according to the different organs of C. trichotoma. Points referring to the leaves and roots were distributed to the right of PC1, while those related to the stems and branches were distributed to the left. The organic phosphorus and PRNAfractions were grouped, showing a positive correlation with each other and with the total phosphorus
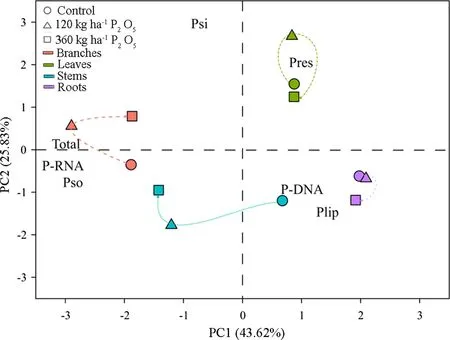
Fig.3 Relationship between principal component 1 (PC1) and component 2 (PC2) for phosphorus fractions in leaves, branches, stems and roots of Cordia trichotoma subject to phosphorus doses in sandy soil
Fig.2 Distribution of soluble inorganic P (PSI) and soluble organic P (PSO) in organs of Cordia trichotoma subjected to applications of phosphorus in sandy soil. Letters indicate significant difference among the doses in each organ and fraction by the Tukey test ( p < 0.05) content, which also had a strong influence on P distribution in branches and stems. PLIPand PDNAfractions were positively correlated and showed greater influence on P distribution in roots. It was also possible to identify similar patterns between the dosages of P applied to the soil for branches and stems, which also occurred for leaves and roots.
Discussion
The highest concentrations of total soluble phosphorous (PSI) in the leaves of C. trichotoma grown in soil with control treatment, 120 and 360 kg P2O5ha?1represented 44; 58 and 57% of total P, respectively. This may be attributed to intense cell division and expansion of the leaves in the initial development stage of 2-year-old plants. This stimulated the increase in dry matter (Table 3), becoming a P drain (Martinez et al. 2005; Tagliavini et al. 2005; Lambers et al. 2011).
In shoots and stems of plants grown in the control and in the two treatments, soluble organic phosphorous (PSO ) predominated among the P fractions (Fig.1). This possibly occurred because young plants accumulate some phosphorous in a reserve and some in a maintenance fraction (organic fraction), especially in perennial organs such as stem and branches (Martinez et al. 2005; Santos et al. 2008; Veneklaas et al. 2012). After leaf senescence, part of the organic P was mobilized and redistributed to growing perennial organs (branches), decreasing the dependence of soil P (Veneklaas et al. 2012; Piccin et al. 2017a). However, PSO reserves were higher in branches than in stems because the stem is an organ where nutrients such as phosphorus are mobile (Casali et al. 2011; Piccin et al. 2017a).
The concentrations of soluble inorganic phosphorous (PSI) in roots of the control seedlings and those growing in soil with 120 kg P2O5ha?1represented 33 and 40% of the total P, respectively. PSIintensified in the roots in response to cell division and expansion, thus promoting root growth in 2-year-old seedlings, consequently becoming a P sink (Williams 1987; Borém and Ramos 2002; Tagliavini et al. 2005; Lambers et al. 2011). However, in seedlings grown in soil fertilized with 360 kg P2O5ha?1, concentrations of PSIand PSO in the roots were similar (26% and 27%, respectively). This reinforces the premise that part of phosphorous absorbed from the soil is stored in the organic fraction in perennial organs (Marschner 2012; Piccin et al. 2017a). This suggests that phosphorous may be absorbed in amounts greater than the metabolic needs of the plant, characterizedby luxury consumption (Chapin and Bieleski 1982; Silveira and Gava 2004; Pereira et al. 2008; Lambers et al. 2011; Veneklaas et al. 2012).
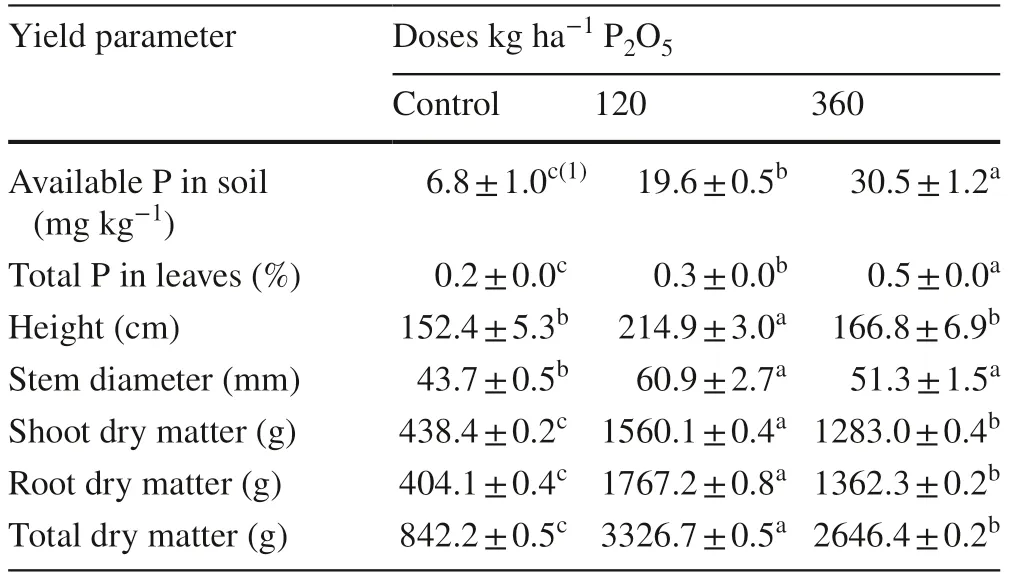
Table 3 Available phosphorus in soil, morphological parameters and total phosphorus content in Cordia trichotoma subjected to phosphorus applications in sandy soil
In control seedlings, the highest concentrations of lipid phosphorous (PLIP) were found in the stems, possibly due to lower P contents in the soil (Table 2) where the uptake and accumulation of P is aimed solely to meet the demands of cellulose synthesis (Silveira and Gava 2004). However, in seedlings growing in soil fertilized with 360 kg P2O5ha?1, the highest concentrations of PLIPwere in the roots, representing a phosphorous reserve. This is possibly because shoots had sufficient phosphorous to meet metabolic demands, and thus the transport of P to leaves and stems was not critical (Bieleski 1973; Thomas and Sadras 2001; Marschner 2012; Veneklaas et al. 2012).
In control seedlings, the highest concentrations of phosphorous associated with ribonucleic acid (PRNA) were in the branches, while seedlings fertilized with 120 and 360 kg P2O5ha?1, the highest concentrations were in the branches and in the stems. The accumulation of PRNAwas due to the increase in cell division in these organs provided by ribonucleic acid, fulfilling the function of protein synthesis (Suzuki et al. 2001; Niklas 2006; Reefet al. 2010). The lack of deoxyribonucleic acid (PDNA) increase in plants under both controls and fertilization treatments may be attributed to the recalcitrance of this fraction.
Residual phosphorous (PRES) is associated with compounds with protein structures and represents the most stable fraction of P in plant tissues (Casali et al. 2011). In the leaves and roots, the highest concentrations of PRESwere in unfertilized seedlings. These are valid results because annual organs such as leaves and fine roots are phosphorous drain (Casali et al. 2011; Piccin et al. 2017a). In contrast, the highest PRESconcentration was in seedlings branches fertilized with 360 kg P2O5ha?1. This may be because PRESis associated with an increase in dry matter of annual organs, among which are the branches, as it contributes to the synthesis of sugar phosphates and phospholipids (Marschner 2012; Taiz and Zeiger 2013; Carnevali et al. 2016).
Principal component analysis showed a positive relationship between distributions of inorganic phosphorus (PSI) with organs of intense cell division such as leaves. The development of these organs has a high demand for adenosine triphosphate (ATP), an energy currency dependent on the availability of soil P, and its absorption and concentration in vegetative growth (Piccin et al. 2017a). However, the concentration of phosphorous in the PSO, PRNAand PDNAfractions is positively correlated with perennial organs such as stems and branches, which have the possibility of secondary growth (Valadares et al. 2015; Piccin et al. 2017b). The presence oforganic fractions normally linked to the reserve lipids were found in organelles. These fractions remain available in periods of greater physiological demand (e.g., the budding stage), but can also be linked to PRNAwhere they are associated with the transport or coding of genes from the nucleus or even the Golgi complex (Taiz et al. 2017).
Conclusions
The application of 120 kg P2O5ha?1to a 2-year-old stand promoted preferentially the accumulation of the soluble inorganic (PSI) fraction in annual organs such as leaves, stimulating the initial growth of Cordia trichotoma.
The application of 300% of the recommended dose 360 kg P2O5ha?1resulted in the accumulation preferentially of soluble organic (PSO ) and lipid (PLIP) fractions in reserve organs such as roots. This contributed to organ growth in the following growth season and may be a strategy to reduce dependence of young stands on phosphate fertilizers during the initial years.
References
Berghetti áLP, Araujo MM, Bovolini MP, Tonetto TDS, Muniz MFB (2015) Seedling morphology and control of pathogens in seeds of Cordia trichotoma. Florest Amb 22:99–106
Berghetti áLP, Araujo MM, da Silva Tonetto TI, Aimi SC, Navroski MC, Turchetto F, Zavistanovicz TC (2016) Growth of Cordia trichotoma seedlings in different sizes of recipients and doses of fertilizer. Afr J Agric Res 11:2450–2455
Bieleski RL (1973) Phosphate pools, phosphate transport, and phosphate availability. Annu RevPlant Physiol 24:225–252
Borém RAT, Ramos DP (2002) Varia??o estacional e topográfica de nutrientes na serapilheira de um fragmento de Mata Atlantica. Cerne 8:42–59
Bortoluzzi EC, Pérez CA, Ardisson JD, Tiecher T, Caner L (2015) Occurrence of iron and aluminum sesquioxides and their implications for the P sorption in subtropical soils. Appl Clay Sci 104:196–204
Cadorin DA, Malavasi UC, Coutinho PWR, Dranski JAL, Malavasi MDM (2015) Methyl jasmonate and stem bending, hardening and initial growth of Cordia trichotoma seedlings. Cerne 21:657–664
Carnevali NHDS, Marchetti ME, Vieira MDC, Carnevali TDO, Ramos DD (2016) Eficiência nutricional de mudas de Stryphnodendron polyphyllum em fun??o de nitrogênio e fósforo. Cienc Florest 26:449–461
Carvalho PER (2003) Espécies arbóreas brasileiras, 1st edn. Academic Press, Brasília
Casali CA, Kaminski J, Piccin R, Arbugeri FE, Doneda A (2011) A mineraliza??o das formas de fósforo do tecido de plantas de cobertura. Inform Agron 135:21–24
Chapin FS, Bieleski RL (1982) Mild phosphorus stress in barley and a related low-phosphorus-adapted barley grass: phosphorus fractions and phosphate absorption in relation to growth. Physiol Plant 54:309–317
CQFS RS/SC (2004) Comiss?o, de química e fertilidade do solo. Manual de aduba??o e calagem para os Estados do Rio Grande do Sul e de Santa Catarina. 10 Ed. Porto Alegre, RS, Brazil: Sociedade Brasileira de Ciência do Solo, p 400
Da Ros CO, Rex FE, Ribeiro IR, Kafer PS, Rodrigues AC, da Silva RF, Somavilla L (2015) Uso de substrato compostado na produ??o de mudas de Eucalyptus dunnii e Cordia trichotoma. Florest Amb 22:549–558
Embrapa (2013) Sistema Brasileiro de Classifica??o de Solos, 3rd edn. Embrapa, Brasília, p 353
Fink JR, Inda AV, Bayer C, Torrent J, Barrón V (2014) Mineralogy and phosphorus adsorption in soils of south and central-west Brazil under conventional and no-tillage systems. Acta Sci-Agron 36:379–387
Fink JR, Inda AV, Bavaresco J, Barrón V, Torrent J, Bayer C (2016) Adsorption and desorption of phosphorus in subtropical soils as affected by management system and mineralogy. Soil Tillage Res 155:62–68
Freitas ECSD, Paiva HND, Leite HG, Oliveira Neto SND (2017) Effect of phosphate fertilization and base saturation of substrate on the seedlings growth and quality of Plathymenia foliolosa Benth. Rev árvore 41:1–9
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50:346–363
Lambers H, Finnegan PM, Laliberté E, Pearse SJ, Ryan MH, Shane MW, Veneklaas EJ (2011) Phosphorus nutrition of Proteaceae in severely phosphorus-impoverished soils: Are there lessons to be learned for future crops? Plant Physiol 156:1058–1066
Lima R, Severino LS, Cazetta JO, de Azevedo CA, Sofiatti V, Arriel NH (2011) Redistribui??o de nutrientes em folhas de pinh?omanso entre estádios fenológicos. Rev Bras Eng Agr Amb 11:1175–1179
Machado GG, Pastorini LH, Souza LA, Barbeiro C, Santos LS (2016) Germina??o de diásporos e crescimento inicial de Cordia trichotoma (Vell.) Arrab. Ex Steud. (Boraginaceae). Iheringia Ser Bot 70:279–286
Marschner H (2012) Mineral nutrition of higher plants, 3rd edn. Elsevier, London, p 889
Martinez HEP, Novais RF, Rodrigues LA, Sacramento LVSD (2005) Phosphate forms in plant and their internal buffering in five soybean cultivars. Rev Bras Cienc Solo 29:249–257
Miyachi S, Tamiya H (1961) Distribution and turnover of phosphate compounds in growing chlorella cells. Plant Cell Physiol 2:405–414
Murphy J, Riley JP (1962) A modified single solution methods for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Niklas JK (2006) Plant allometry, leaf nitrogen and phosphorus stoichiometry, and interspecific trends in annual growth rates. Ann Bot 9:155–163
Niu F, Zhang D, Li Z, Van Iersel MW, Alem P (2015) Morphological response of Eucalyptus seedlings to phosphorus supply through hydroponic system. Sci Hortic 194:295–303
Noack SR, McLaughlin MJ, Smernik RJ, McBeath TM, Armstrong RD (2014) Phosphorus speciation in mature wheat and canola plants as affected by phosphorus supply. Plant Soil 378:125–137
Oliveira RAD, Comin JJ, Tiecher T, Piccin R, Somavilla LM, Loss A, Lourenzi CR, Kurtz C, Brunetto G (2017) Release of phosphorus forms from cover crop residues in agroecological no-till onion production. Rev Bras Cienc Solo 41:1–16
Pereira JM, Cambraia J, Júnior EMF, Ribeiro C (2008) Efeito do alumínio sobre a absor??o, o acúmulo e o fracionamento do fósforo em sorgo. Bragantia 67:961–967
Piccin R, Couto RDR, Bellinaso RJS, Gatiboni LC, Conti LD, Rodrigues LAT, Michelon LS, Kulmann MSS, Brunetto G (2017a) Phosphorus forms in leaves and their relationships with must composition and yield in grapevines. Pesqui Agropecu Bras 52:319–327
Piccin R, Kaminski J, Ceretta CA, Tiecher T, Gatiboni LC, Bellinaso RJS, Marchezan C, Souza ROS, Brunetto G (2017b) Distribution and redistribution of phosphorus forms in grapevines. Sci Hortic 218:125–131
Pinheiro J, Bates D, DebRoy S, Sarka D, R Core Team (2014) Nlme: linear and nonlinear mixed effects models. R Found Stat Comput 3:1–118
R Core Team (2017) R: a language and environment for statistical computing. https://www.r-proje ct.org/
Reef R, Ball MC, Feller IC, Lovelock CE (2010) Relationship between RNA: DNA ratio, growth and elemental stoichiometry in mangrove trees. Funct Ecol 24:1064–1072
Rossa üB, Angelo AC, Bognola IA, Westphalen DJ, Milani JE (2015) Fertilizante de libera??o lenta no desenvolvimento de mudas de Eucalyptus grandis. Rev Floresta 45:85–96
Santos JZL, Resende áV, Neto AEF, Corte EF (2008) Crescimento, acúmulo de fósforo e fra??es fosfatadas em mudas de sete espécies arbóreas nativas. Rev árvore 32:799–807
Sartoretto LM, Rossi E (2014) Caracteriza??o de três espécies florestais de importancia econ?mica. Unoesc Cienc-Acet 5:145–152
Silveira RLVA, Gava JL (2004). Nutri??o e aduba??o fosfatada em eucalipto. In: Yamada T, Abdalla SRS. Fósforo na agricultura brasileira. 2. Ed. Piracicaba, SP, Brazil: Associa??o brasileira para pesquisa da potassa e do fosfato, pp 495–536
Staff , Soil Survey (2010) Keys to soil taxonomy, 12th edn. Department of Agriculture, Soil Conservation Service, Washington, DC, p 360
Stahl J, Ernani PR, Gatiboni LC, Chaves DM, Neves CU (2013) Produ??o de massa seca e eficiência nutricional de clones de Eucalyptus dunnii e Eucalyptus benthamii em fun??o da adi??o de doses de fósforo ao solo. Cienc Florest 23:287–295
Suzuki Y, Makino A, Mae T (2001) An effi cient method for extraction of RNA from rice leaves at different ages using benzyl chloride. J Exp Bot 52:1575–1579
Tagliavini M, Baldi E, Lucchi P, Antonelli M, Sorrenti G, Baruzzi G, Faedi W (2005) Dynamics of nutrients uptake by strawberry plants (Fragaria × ananassa Dutch.) grown in soil and soilless culture. Eur J Agron 23:15–25
Taiz L, Zeiger E (2013) Fisiologia Vegetal, 5th edn. Artmed, Porto Alegre, p 954
Taiz L, Zeiger E, M?ller IM, Murphy A (2017) Fisiologia e desenvolvimento vegetal, 6th edn. Artmed, Porto Alegre, p 858
Tedesco MJ, Gianello C, Bissani CA, Bohnen H, Volkweiss SJ (1995) Análise de solo, plantas e outros materiais. Departamento de solos, Universidade Federal do Rio Grande do Sul, Porto Alegre
Ter Braak CJ, Smilauer P (2002). CANOCO Reference manual and CanoDraw for windows user’s guide: software for Canonical Community Ordination (version 4.5). www.canoc o.com
Thomas H, Sadras V (2001) The capture and gratuitous disposal of resources by plants. Funct Ecol 15:3–12
Valadares SV, da Silva LF, Valadares RV, Fernandes LA, Neves JCL, Sampaio RA (2015) Plasticidade fenotípica e fra??es fosfatadas em espécies florestais como resposta à aplica??o de fósforo. Rev árvore 39:225–232
Veneklaas EJ, Lambers H, Bragg J, Finnegan PM, Lovelock CE, Plaxton WC, Price CA, Scheible WR, Shane MW, White PJ, Raven JA (2012) Opportunities for improving phosphorus-use effi ciency in crop plants. New Phytol 95:306–320
Williams LE (1987) Growth of ‘Thompson seedless’ grapevines: II. Nitrogen distribution. J Am Soc Hortic Sci 112:330–333
 Journal of Forestry Research2021年2期
Journal of Forestry Research2021年2期
- Journal of Forestry Research的其它文章
- Stem taper functions for Betula platyphylla in the Daxing’an Mountains, northeast China
- Leaf phenotypic variation of endangered plant Tetracentron sinense Oliv. and influence of geographical and climatic factors
- Ecological variations of woody species along an altitudinal gradient in the Qinling Mountains of Central China: area-based versus mass-based expression of leaf traits
- Variations in stem radii of Larix principis-rupprechtii to environmental factors at two slope locations in the Liupan Mountains, northwest China
- A review of ecological mechanisms for management practices of protective forests
- Variation of basic density, calorific value and volumetric shrinkage within tree height and tree age of Ugandan grown Eucalyptus grandis wood
