Arbuscular mycorrhizal fungi communities associated with wild plants in a coastal ecosystem
Jinping Wang · Shilin Ma · G. Geoff Wang · Linhao Xu · Zhiyuan Fu · Juan Song · Jinchi Zhang
Abstract Arbuscular mycorrhizal fungi (AMF) form a near-ubiquitous mutualistic association with roots to help plants withstand harsh environments, and play a key role in the establishment of coastal beach plant communities. Yet little is known about the structure and composition of AMF communities on coastal beaches ofeastern China. In this study, we investigated the occurrence, community composition and diversity of AMF associated with common wild plants on a coastal beach of North Jiangsu, China. Almost all of the local wild species were colonized by AMF except for Chenopodium album L. Thirty-seven AMF species were isolated from the rhizosphere belonging to 12 genera in seven families. Glomus was the dominant genus and Funneliformis mosseae the dominant species. The colonization, spore composition and diversity of AMF were strongly related to edaphic factors. Sodium (Na + ) ions in the soil significantly and negatively affected the colonization rate by AMF and both soil Na + levels and pH had a significant negative effect on AMF spore density and evenness. However, there was a significant positive correlation between species richness and total organic carbon. The results provide insights into soil factors affecting native AMF communities in coastal beach habitats which could benefit vegetation recovery and soil reclamation efforts.
Keywords AMF diversity · Coastal beach · Edaphic factors · Mycorrhizal composition · Wild hosts
Introduction
Arbuscular mycorrhizal fungi (AMF) are a type of plant growth-promoting microorganisms that form a mutualistic relationship with more than 70% of plant species in terrestrial ecosystems (Brundrett and Tedersoo 2018). The mass ofextra-radical hyphae forms numerous extensive networks in the soil, which provide multiple benefits to the host plant (Cardoso et al. 2013) such as promoting plant growth (Wicaksono et al. 2017; Zhang et al. 2018) and improving plant stress resistance or tolerance (Sarkar et al. 2017; Hashem et al. 2018; Li et al. 2018). In addition, mycorrhization can increase soil aggregation by the action of hyphae and the glomalin they secrete (Purin and Rillig 2008; Leifheit et al. 2014). In these ways, AMF can play a key role in the recovery and re-establishment of plant communities in degraded areas.
Coastal areas represent special ecosystems which are between the ocean and the land, and AMF could help plant species withstand stress factors such as salinity (Wilson and Sykes 1999) or low water and nutrient availability (Ievinsh 2006). AMF play an important role in the establishment of plant communities in coastal areas (Rodríguez-Echeverría et al. 2008). As a consequence, there has been increasing attention to the diversity of AMF in coastal areas, and several reports on the diversity of AMF associated wit h plants in coastal dunes in the United States (Koske 1987; Friese and Koske 1991; Koske and Gemma 1996), in Brazil (Stürmer and Bellei 1994; Blaszkowski and Czerniawska 2011; Goto et al. 2012), in several European countries (Camprubí et al. 2010; Blaszkowski and Czerniawska 2011), and in India (Kamble 2012; Kamble et al. 2012). However, there have been few studies on the diversity of AMF on a beach ecosystem and none on AMF on coastal beaches of northern Jiangsu Province.
Beaches, although valuable recreational areas for humans, present harsh environments for vegetation. An important ecological strategy which plant species adopt to survive these harsh environments is to form associations with local AMF (Rodríguez-Echeverría et al. 2008). Considering the importance of AMF for the maintenance of vegetation in beach habitats, it is essential to study those occurring naturally in these habitats, such as in the dunes of Jiangsu Province, eastern China. Although AMF are widely distributed in terrestrial ecosystems, their communities are modulated by many factors. Some studies found that the composition of an AMF community was affected more by land use than by soil characteristics (Pereira et al. 2014), although a comparative study of land uses in forests in North America demonstrated that total precipitation and pH were the main factors which affected AMF species-richness (álvarez-Sánchez et al. 2012). de Oliveira Freitas et al. ( 2014) found that soil characteristics and plant species were the main factors which impacted AMF communities in an Amazonian forest. Bencherifet al. ( 2016) reported that seasonal variations in environmental factors affected AMF abundance but not diversity. AMF species richness was significantly higher after agricultural management (Turrini et al. 2017). Researches on factors affecting AMF communities are inconsistent, often due to the scale of research. On a global scale, climatic factors mainly shape AMF communities such as average annual temperatures and precipitation (Tedersoo et al. 2014), whereas at a local scale, AMF communities are mainly influenced by soil properties (da Silva et al. 2014). The research above indicates the importance of analyzing the factors that impact AMF communities and species distribution when studying AMF diversity at a local scale.
In this study, rhizosphere soils are characterized according to the composition of AMF communities associated with common wild plants of a coastal ecosystem in eastern China and investigated to determine ifedaphic factors interfere in mycorrhizal diversity and interface AMF-hosts. Given that AMF inoculated onto crops or tree species could help survival under harsh environments, the results of this study should provide basic information to facilitate the establishment and maintenance of beach plant communities.
Materials and methods
Study area and soil sampling
The study area was located within Dafeng City in central Jiangsu Province which has a 112 km coastline that includes tidal flats of 1000 km 2 . Field sampling was carried out over a 50 km 2 area at 33°05′ N, 120°49′ E, at 1–2 m above sea level (Sun et al. 2004). The area is an accumulation of silt mud beaches with saline soil. The vegetation is herbs and shrubs with the dominant species herbs such as Phragmites australis, Artemisia halodendron, and Solidago canadensis. The study area is in the subtropical, warm wet transition zone with a mean annual temperature of 14 °C, an average temperature in January of 0.4 °C, 26.4 °C in July, and a mean annual precipitation of 1058 mm (Ding et al. 2011). The annual average relative humidity is 81% and annual average wind speed 3.3 m/s (Zhao et al. 2010).
Soil salinity generally decreases with increasing shoreline distance. It changes the plant communities aboveground and belowground AMF communities. Four sampling locations (A to D) along a coast-to-inland gradient were selected (Fig.1), and plants were selected from each location (Table 1). Fine roots and rhizosphere soil (approximately 1500 g in each sample) were collected at a 5–30 cm depth from the rhizosphere of different species in the summer 2017. In order to collect fine roots and rhizosphere soil, the plants were dug up, shaken gently, and soil adhering to the coarse and fine roots placed in plastic bags. Four duplicate root and rhizosphere soil samples were collected for each species at each location and mixed as a composite sample. The distance between one sample and another was15 m and the distance between subsamples was10 m. The samples were air-dried at room temperature for 2 weeks and stored in plastic bags at 4 °C prior to analysis.
Measurement of soil chemical properties
Soil samples were sieved through a 2-mm grid and pH was measured with a digital pH meter in a 1:5 (w/v) soil–water suspension. Electrical conductivity (EC) was measured in the same soil suspension using a conductivity meter (DDS-11C, Shanghai Hong Yi Instrument Company, Shanghai, China). Sodium (Na + ) ion concentration was measured by flame photometry (Model 425 Flame Photometer, Sherwood, Chicago, IL, USA). Chloride (Cl ? ) ion concentration was determined by titration with AgNO3. Total organic carbon (TOC) was measured using an organic carbon analyzer. Nitrate nitrogen (NO3?–N) levels were determined by the phenol disulfonic acid method, while available phosphorus (AP) and total phosphorus (TP) concentrations were determined by molybdenum blue colorimetry (Olsen et al. 1954). Available potassium (AK) and total potassium (TK) were determined by flame photometry (Model 425 Flame Photometer; Sherwood Co. Ltd., Chicago, Il, USA).
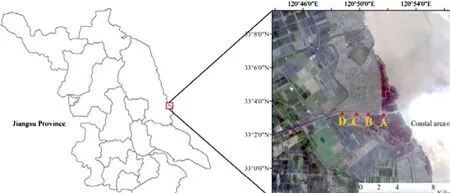
Fig.1 Sampling sites on a coastal beach of Jiangsu province, eastern China

Table 1 Host plants in each site
Estimation of mycorrhizal colonization
Fine roots were washed and fixed in standard FAA solution (10% formaldehyde, 50% ethanol, 5% acetic acid, 35% water) for more than 24 h. They were then cut into 1-cm segments, soaked in 10% (w/v) KOH and incubated in a 90 °C water bath for 50 min. The roots were cooled, washed with water, and stained with basic H2O2(solution of 30 mL 10% (v/v) H2O2, 3 mL concentrated NH4OH and 60 mL water) for 25 min. The roots were then soaked in 1% (w/v)hydrochloric acid for 3 min and washed with water. After decanting the hydrochloric acid, the roots were stained with 0.05% (w/v) trypan blue solution and placed in a 90 °C water bath for 30 min (Phillips and Hayman 1970). The roots were then soaked in lactic acid–glycerol (1:1) to eliminate excess Trypan blue solution. Fifty roots per sample were examined for the presence of AMF structures at 100–400 × magnification with a semi-automatic digital microscope (Giovannetti and Mosse 1980). The percentage of root colonization was calculated using the following equation:
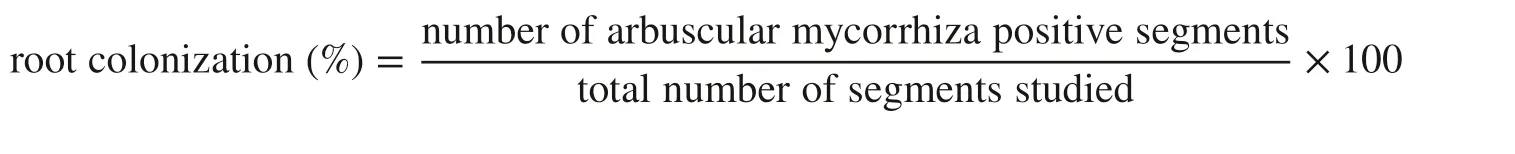
Quantification and identification of AMF spores
AMF spores were extracted from 100 g air-dried samples by wet sieving (Gerdemann and Nicolson 1963) and sucrose centrifugation (Jenkins 1964). Intact healthy spores were collected on filter paper and counted under a dissecting microscope at 45× magnification. The isolated spores were mounted in polyvinyl lactoglycerol (PVLG) and PVLG + Melzer’s reagent (1:1, v/v) (Morton 1988) and structural features were recorded, including spore size, color, ornamentations, wall layers, and hyphal attachments. The spores were identified according to identification manuals provided by Schenck and Perez ( 1990), by recent advances in Glomeromycota taxonomy (Schü?ler and Walker 2010; Oehl et al. 2011; Krüger et al. 2012; Wang and Liu 2017), and by the reference culture descriptions ( http://invam .wvu.edu/the-fungi/class ifica tion and http://www.zor.zut.edu.p1/Glome romyc ota).
Diversity studies
The isolation frequency (IF), relative abundance (RA), importance value (IV), spore density (SD), species richness (SR), Shannon–Wiener index (H) and diversity index or evenness (E) were used to estimate the structure of the AMF community. The parameters were calculated with the following equations:
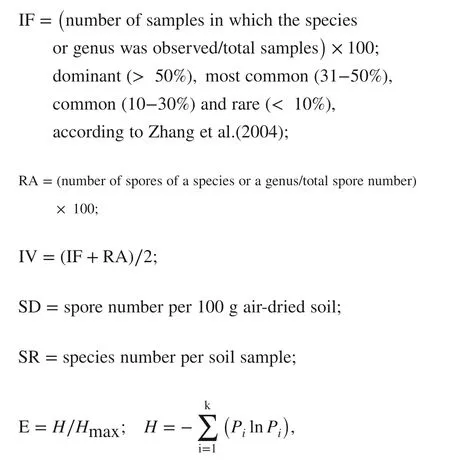
where Pi= ni/ N with nithe spore numbers of a species and N the total number of identified spore samples (Shannon et al. 1949); Hmax= ln S, where S is the total number of identified species.
Statistical analysis
A species accumulation curve was developed in Origin 8.5 to evaluate whether the number of soil samples satisfactorily reflected the AMF community structure. A measure of the Pearson correlation coeffi cient was performed in Excel 2007 to determine the relationship between the diversity of AMF and soil properties. A redundancy analysis (RDA) was carried out using CANOCO for Windows (version 5.0) to reveal the influence ofedaphic properties on the composition of AMF communities. The AMF parameters RA, SD, H were defined as “species variables,” while pH, EC, Na + , Cl ? , TOC, NO3?–N, AP, TP, AK and TK were defined as “environmental variables.” For this analysis, rare species (IF < 10%) were removed, the AMF spore number data were transformed using log (x + 1), and the Shannon index was transformed using EXP (x). The Monte Carlo permutation test (4999 permutations) was used to calculate the significance ofenvironmental variables and species variables.
Results
AMF species and the species-accumulation curve
The AMF species-accumulation curve demonstrates that the number of soil samples was suffi cient to detect the majority of AMF species (Fig.2). Altogether, 37 species were detected, belonging to 12 genera in seven families, of which 14 species belonged to the genus Glomus, four each to Archaeospora and Rhizophagus, three to Acaulospora, and two each to Septoglomus, Claroideoglomus, Funneliformis and Ambispora, and one each to Entrophospora, Gigaspora, Paraglomus and Sclerocystis. Spore number, IF, RA and IV of the AMF species are presented in Table 2. The spore numbers of F. mosseae, R. intraradices and S. deserticola were much larger than those for other AMF species. The IF value of F. mosseae was 53.1%, meaning that F. mosseae was dominant species in this study. F. mosseae was also the most abundant species, followed by R. intraradices and S. deserticola (Table 2). Funneliformis, Glomus and Rhizophagus were the dominant genera, with IF values greater than 50%, with all three genera belonging to the Glomeraceae family. Among them, Funneliformis was the most abundant genus, followed by Rhizophagus (Table 2).
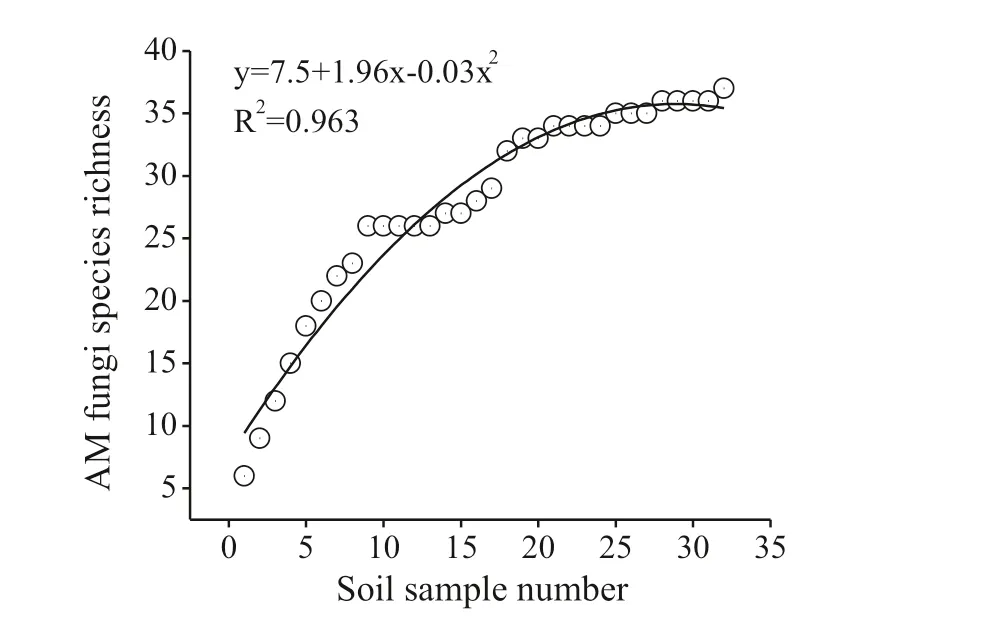
Fig.2 AMF species accumulation curve for the sampling area
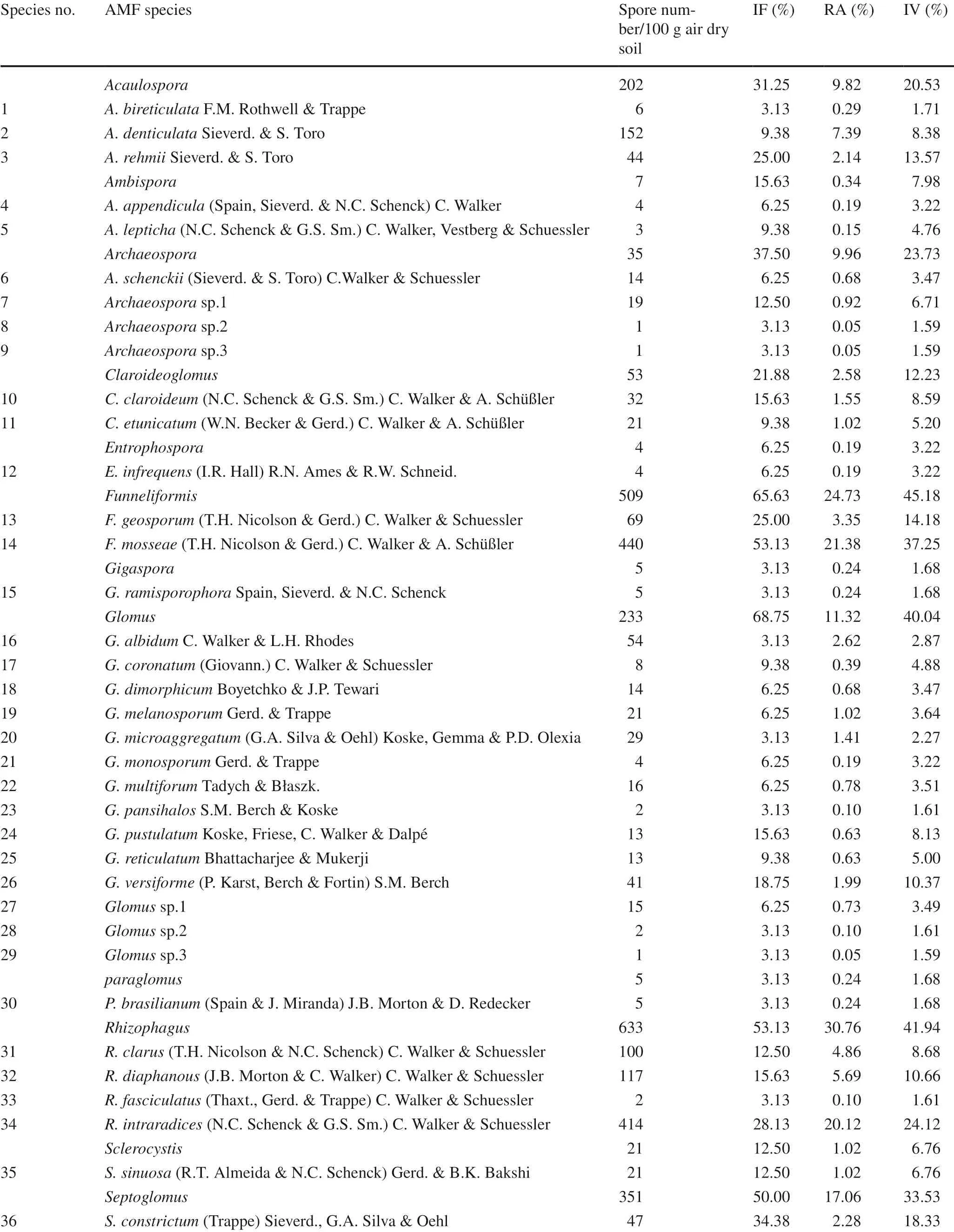
Table 2 Isolation frequency, relative abundance and important value of AMF species for the sampling area

Table 2 (continued)
Root colonization, spore density and diversity of AMF
Root colonization, spore density (SD), SR, H and E of AMF in the 32 rhizosphere samples from the roots of 19 wild plant species are shown in Table 3. The highest root colonization of 100% was by P. hieracioides, M. offi cinalis and D. carota. Only one species, C. album, was not colonized by AMF.Root colonization of P. australis and T. chinensis was the most variable. Spore density (SD) ranged from 10.0–644.7, with an average of 126.6 per 100 g air-dried soil. SD exhibited a significant positive correlation with root colonization ( r = 0.47, P < 0.01), and a significant negative correlation with evenness E ( r = ? 0.50, P < 0.01). The species richness (SR) in rhizosphere samples from different plant species was variable, with a median of 4 (from a range between 2 and 6), and showed a positive correlation ( r = 0.71, P < 0.01) with the Shannon–Wiener index (H), a measure of species diversity, which reflects both species abundance and evenness. The SR of P. australis differed considerably, (ranging from 2 to 6), among the four locations. The dominant AMF differed among the various plant species as well as among locations for some species. P. australis plants on site B were dominated by the fungus R. intraradices and plants on site C were dominated by G. versiforme.
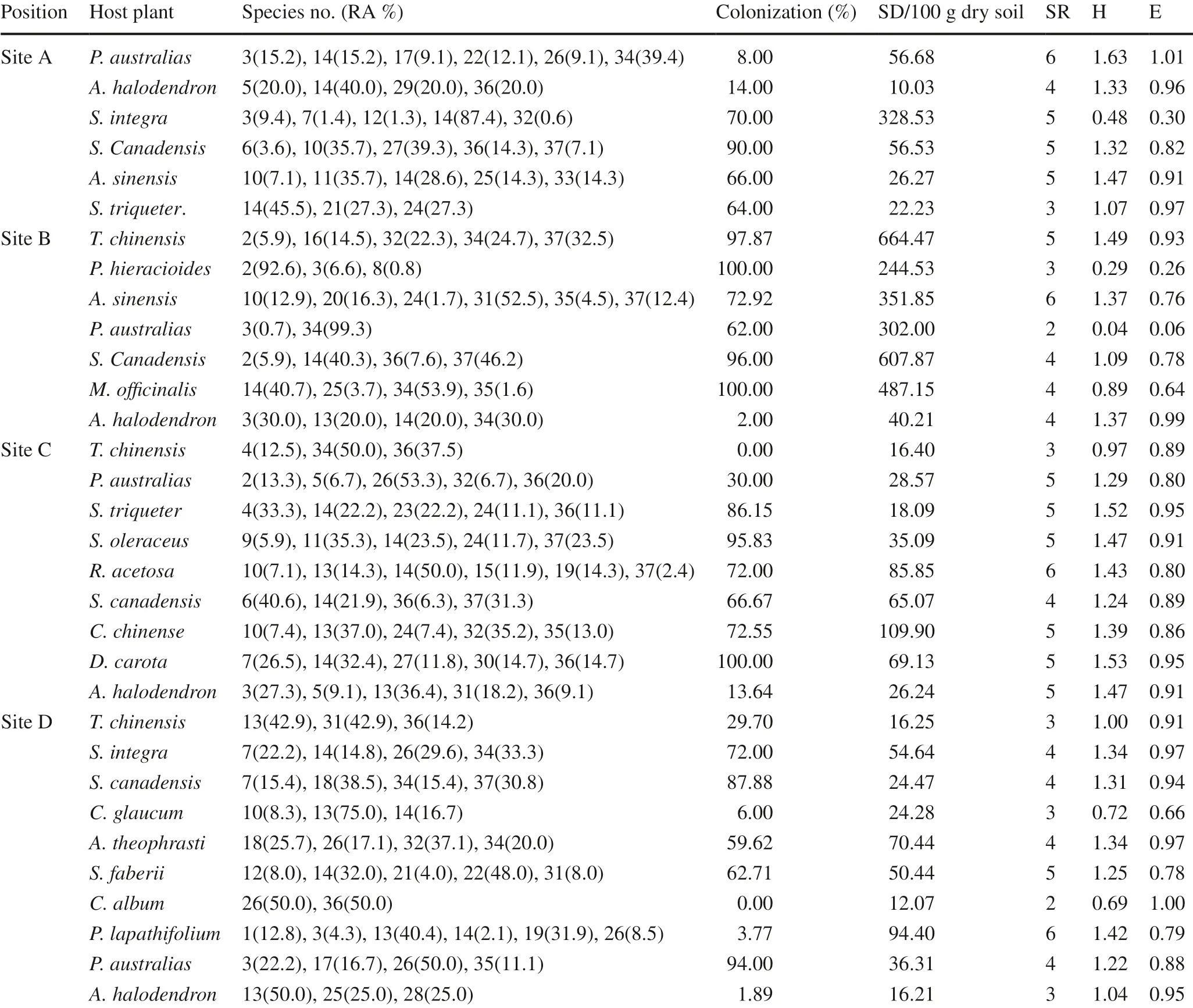
Table 3 Spore density, relative abundance, diversity of AMF and root colonization in different host plants
Effect of edaphic factors on diversity and community of arbuscular mycorrhizal fungi
Correlation analysis revealed that Na + ion concentration was negatively correlated with rate of root colonization (RLC) ( r = ? 0.42, P < 0.05), and SD ( r = ? 0.37, P < 0.05), while pH was negatively correlated with spore density (SD) ( r = ? 0.50, P < 0.05) and evenness (E) ( r = ? 0.41, P < 0.05) (Table 4). Total organic carbon (TOC) was positively correlated with species richness (SR) ( r = 0.30, P < 0.05). There were no significant correlations between any edaphic factor and the Shannon–Wiener diversity index (H). Redundancy analysis revealed that edaphic factors could explain only 29.5% of the variation in AMF species composition and diversity (Fig.3). According to the redundancy analysis, TOC was positively correlated with the occurrence of Archaeospora sp.1, A. rehmii, F. mosseae, F. geosporum and G. pustulatum, while it was negatively correlated with the abundance of S. constrictum and G. versiforme. The edaphic parameters pH, EC, NO3?–N, Na + and Cl ? exhibited negative correlations with the relative abundance of C. claroideum, S. sinuosa, S. deserticola and R. clarus. The concentration of available phosphorus (AP) was positively correlated with the occurrence of R. diaphanus and R. intraradices.
Discussion
Arbuscular mycorrhizal fungi(AMF) play key roles in the recovery and restoration of plant communities in degraded areas, including coastal areas. In coastal saline environments, these fungi promote plant growth and salinity tolerance by employing various mechanisms such as enhancing water and nutrient absorption, adjusting physiological and biochemical properties of the host plant, and maintaining ion balance (Evelin et al. 2009). The occurrence of mycorrhizal colonization of wild plants on saline soils has been reported by Hildebrandt et al. ( 2001), Wang et al. ( 2004), and Estrada et al. ( 2013b). Plant families such as Chenopodiaceae, Brassicaceae, Cyperaceae, Juncaceae and Urticaceae were considered to be non-host plants (Brundrett 1991; Vierheilig et al. 1996). However, there are several controversial mechanisms to explain the absence of AMF colonization of non-host plants, such as the presence of toxic compounds of root exudates (Allen et al. 1989), an intrinsic barrier of the root cortex or epidermis or lack of root exudates (Nagahashi and Douds 1999). In this study, all the plant species were colonized by AMF with the exception of C. album (Chenopodiaceae), which suggests that most wild plants of coastal beaches associate with AMFto survive. The absence of AMF colonization of C. album may be due to root exudates inhibiting on spore germination and hyphal spreading, as previous studies have reported that plants belonging to the Chenopodiaceae family, such as Salsola kali Pall and Spinacea oleracea L. cv. Butterflay, accumulated phenolic or glucosinolates compounds with an inhibitory effect on the mycorrhizal fungus (Allen et al. 1989; Vierheilig and Ocampo 1990). Although C. glaucum (Chenopodiaceae) has often been presumed to be non-mycorrhizal or rarely mycorrhizal (Wang et al. 2001), it was colonized by AMF in the current study, which supports the finding that members of the Chenopodiaceae could be colonized by AMF in saline soils (Hildebrandt et al. 2001) and is consistent with the conclusion that these members are variable in terms of their mycorrhizal association (Tian et al. 2009). Polygonaceae was previously found to be not colonized by AMF (Tian et al. 2009), but in our study, hyphae were present in the roots of P. lapathifolium. The phenomenon of some nonmycorrhizal Chenopodiaceae and Polygonaceae to be slightly infected (cortical mycelium and vesicle, but no arbuscules), might be attribute to the presence of nearby mycorrhizal host plants causing clumps ofendophyte mycelium on their root surfaces, usually attached to entry points which had often aborted (Ocampo et al. 1980).
Our study indicates that mycorrhizal colonization of roots by AMF varied greatly among host species, ranging from zero to 100%, which indirectly supports the observation that plants have different strategies for survival under stressful environments. Some plants can alter their morphology and physiology while others form mutualistic relationships with soil microbes such as AMF (Miransari 2011; Qin et al. 2011). Differences in colonization rates of the same plant species by AMF at different sampling sites were also observed. This may be caused by variations in Na ion concentrations of the soil, as salinity would reduce hyphal growth and decrease plant growth (less carbohydrates) (Juniper and Abbott 2006), an observation supported by the negative correlation between Na ion concentration and root colonization (RLC). Similar results have been reported by other researchers (Talaat and Shawky 2014; Liu et al. 2016).
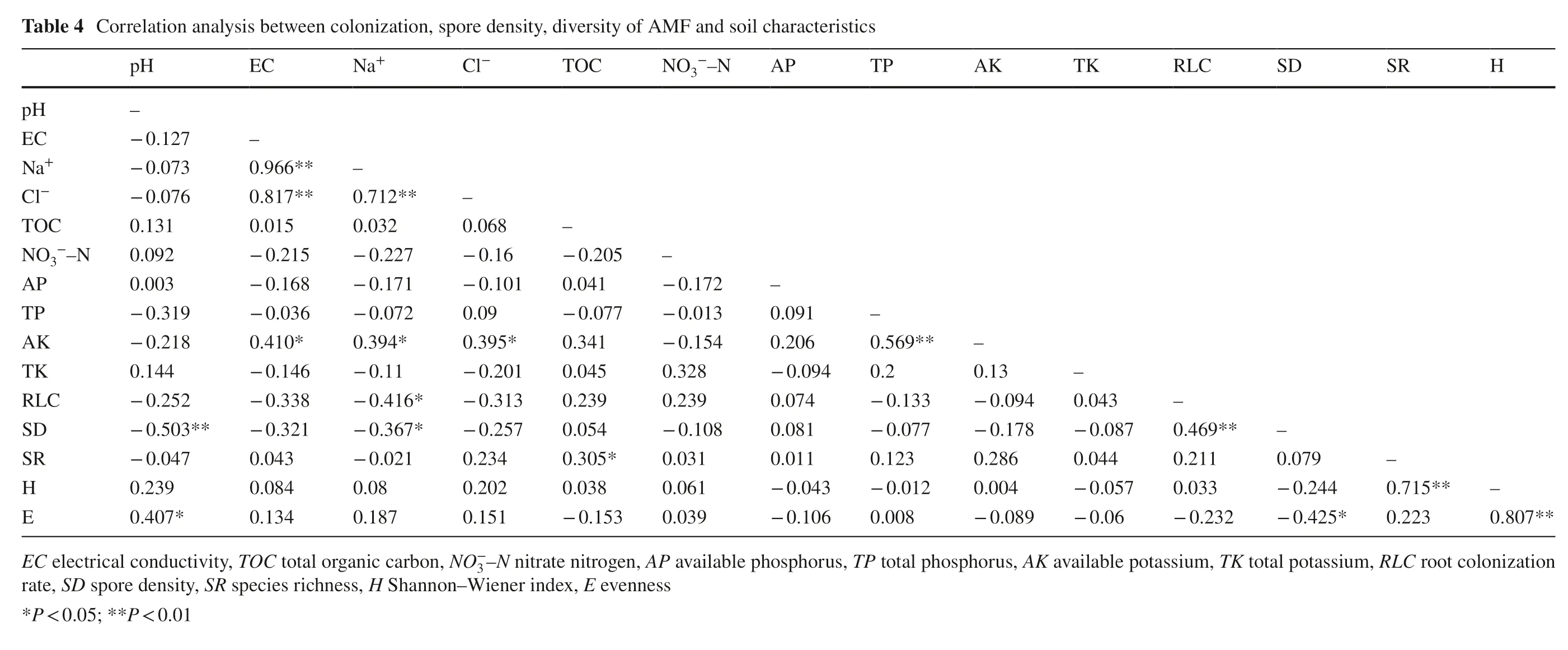
?
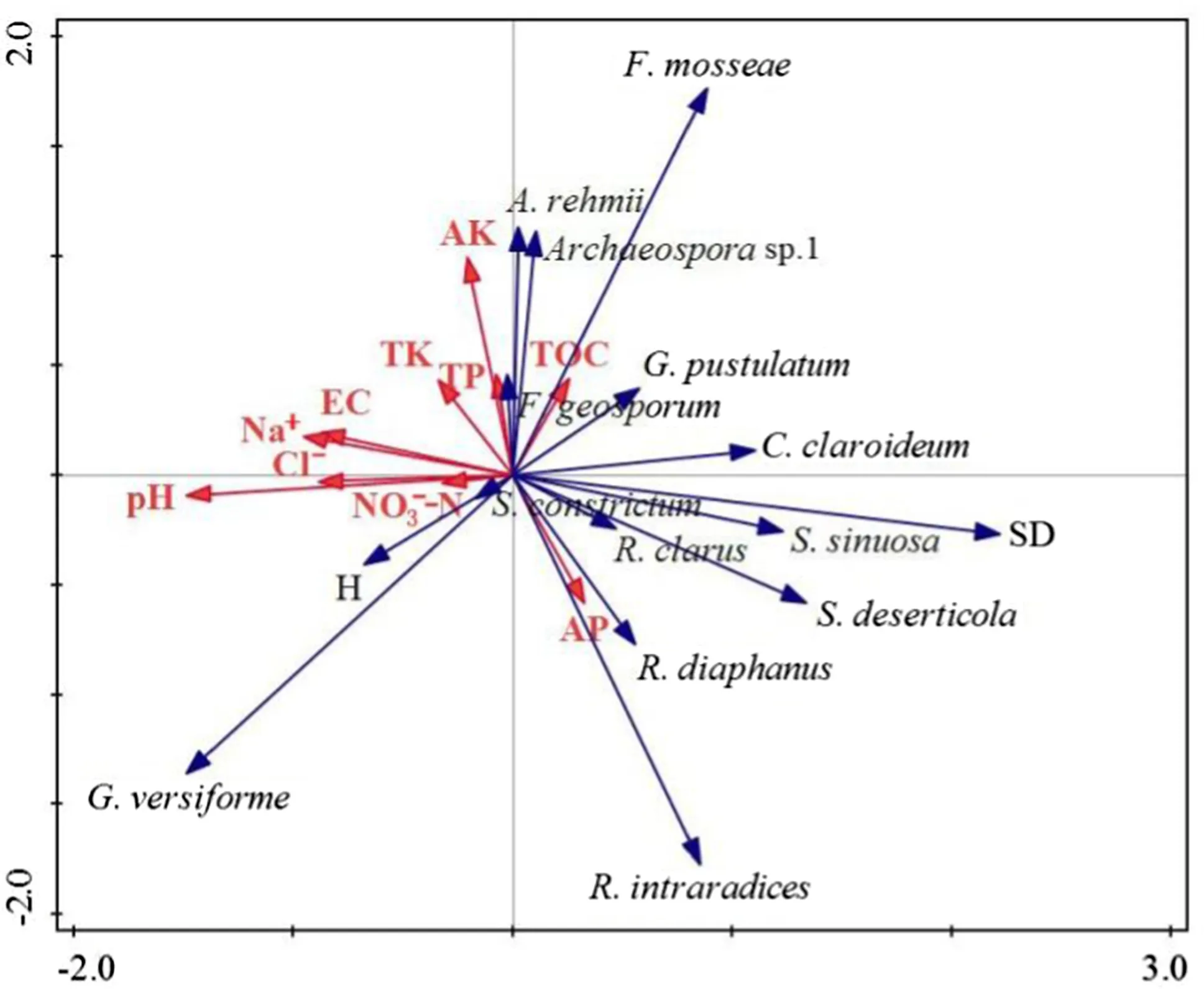
Fig.3 Redundancy analysis (RDA) of the composition, diversity (Shannon–Weaver index, species richness, and evenness) and spore density of AMF communities in relation to soil characteristics (n = 32). EC, soil electric conductivity; Na + , sodium ion content; Cl ? , chloride ion content; TOC, dissolved organic carbon; NO 3 ? –N, nitrate nitrogen; AP, available phosphorus; TP, total phosphorus; AK, available potassium; TK, total potassium
The variation in spore numbers among different arbuscular mycorrhizal fungi was considerable, and may best be explained by spore production capacity is different among AMF species (Clapp et al. 2010) because spore density was not consistently correlated with the percentage of root colonization (Tian et al. 2009). We found that spore density was significantly and positively correlated with root colonization, a finding supporting that of Cardoso et al. ( 2003) but contradicting Tian et al. ( 2009). These inconsistent results should not be surprising because numerous biological and environmental factors could affect the relationship between spore density and root colonization (Liu and Wang 2003). Previous studies have shown that extractable soil P was negatively correlated to AMF spore density (Kahiluoto et al. 2001; Soka and Ritchie 2018), although Chen et al. ( 2012) reported that available phosphorous was positively correlated with spore density. There was no significant correlation in this study between spore density and soil P, probably because the phosphorous levels in all our samples were not high enough to influence mycorrhizal development (Muleta et al. 2007). Both pH and water-soluble sodium ion concentrations were negatively correlated with spore density, findings which support previous studies by Chen et al. ( 2012) and Estrada et al. ( 2013b). Soil salinity may delay the germination of spores and limit growth of hyphae (Juniper and Abbott 2006).
AMF richness, diversity and community structure were investigated, with individual AMF species identified on morphological grounds, as Glomeraceae spores have key characters permitting identification at the species level. Several studies of AMF have been carried out using this approach (Pontes et al. 2017; da Silva et al. 2017). A total of 37 taxa representing 12 AMF genera were identified. Similar results have been reported from other coastal areas. For example, 46 AMF species distributed across five genera were reported from Brazil (Trufem 1990); in China, 33 species representing three AMF genera were isolated from saline–alkaline soils of the Yellow River Delta (Wang et al. 2004). In other saline–alkaline soils in China, 28 species representing four AMF genera were isolated from the Gansu, Ningxia and Inner Mongolia provinces (Sheng et al. 2011), while 26 species were isolated from the rhizosphere of 13 plant species in different types of saline soil in Inner Mongolia (Tang 2007). A total of 11 AMF species were identified from 15 natural saline–alkaline Leymus chinensis grasslands in 12 regions of the western Songnen Plain (Zhang et al. 2015).
Among AMF species, Glomus and Acaulospora are widely distributed across a variety of natural environments, including arid deserts (Wang et al. 2017 ), semiarid areas (del Mar Alguaci et al. 2016), alpine grasslands (Cai et al. 2010) and maritime dunes (Stürmer et al. 2013). They are distributed worldwide owing to their great adaptability (Daniell et al. 2001) and to the high infectivity of their propagules (Hart and Reader 2002). Glomus has been commonly reported from the coastal areas of South America, including Brazil (Trufem 1990; Stürmer et al. 2013), as well as from China’s Yellow River Delta (Wang et al. 2004). However, the dominant AMF species differed among coastal areas: in the west coastal dunes of India, the dominant AMF species were
G. ramisporophora, G. albidum, G. clarum and S. gregaria (Kulkarni et al. 1997), whereas in the Yellow River Delta of China, G. caledonium was often found (Wang et al. 2004). In our study, Glomus was the most common genus and it contributes to the clay soil in this area (Lekberg et al. 2007). Generally, AMF species with isolation frequencies greater than 50% are considered dominant; the dominant species in the study area was F. mosseae with high isolation frequency and relative abundance values, indicating that it was more competitive in this area (Zhao et al. 2017).
Edaphic factors play an important role in AMFcommunities (Yamato et al. 2009; da Silva et al. 2014). A field experiment of tropical agricultural systems suggested that N fertilizer significantly increased AMF species diversity (Jefwa et al. 2006). Zhao et al. ( 2017) studied the species diversity of AMF communities in a semi-arid mountain region in China and found that concentrations of soil water, available phosphorus, and available potassium were the most crucial factors that affected community composition. In this study, species richness was positively correlated with soil organic carbon ( P < 0.05). A similar result was also reported by Chen et al. ( 2012) on the diversity of AMF in the rhizosphere of three host plant species in a farming-pastoral zone of northern China. A possible mechanism may be that different host plants delivered different kinds or levels of carbon to the soil which indirectly affected sporulation, growth, and survival of different AMF species (de Oliveira Freitas et al. 2014; Wang et al. 2019). Redundancy analysis in the current study showed that only 24.8% of the variation in AMF community species composition and diversity could be explained by the edaphic factors measured, which suggests that other soil factors such as texture (Carvalho et al. 2012), water content (Zhao et al. 2017), seasonality (Sylvia and Will 1988), enzymes (Atul-Nayyar et al. 2009), and biotic factors (Alguacil et al. 2008) might affect AMF community composition. Indicator species analysis is an index that combines the relative abundance and the frequency ofoccurrence of species (Dufrêne and Legendre 1997). Based on this analysis, F. mosseae would be considered as an indicator species of this coastal region. According to redundancy analysis, S. constrictum and G. versiforme also had a close relationship with soil electric conductivity and with concentrations of Na + and Cl ? . Does this mean that these two arbuscular mycorrhizal fungi species in the coastal dunes of Jiangsu province have higher salt tolerance than other species? This question will need to be addressed in future research.
Numerous studies have shown that AMF alleviated salt stress to plants have considerable potential in vegetation restoration on saline–alkaline soils (Rodríguez-Echeverría et al. 2008; Liu et al. 2016; Zhang et al. 2018), and the symbiotic effectiveness of indigenous AMF was better than exogenous AMF (Estrada et al. 2013a; Liu et al. 2016). In this study, the occurrence, community composition and diversity of AMF associated with common wild plants on coastal beaches of eastern China were first investigated. Glomus was the dominant genus and F. mosseae the dominant species. Hence, our study is meaningful for the selection of indigenous, dominant arbuscular mycorrhizal fungi species to reestablish vegetation in coastal areas, especially in eastern China. Simultaneously, the high mycorrhizal colonization of P. hieracioides, M. offi cinalis, D. carota and S. canadensis indicates that these species could form good symbiotic relationships with native AMF, thus becoming pioneer plants in vegetation restoration at a local area. Total soil organic carbon was significantly and positively correlated with species richness, suggesting that we could improve AMF species richness by increasing soil organic carbon. Higher arbuscular mycorrhizal fungi species richness was beneficial to functional complementation in vegetation restoration because these AMF species had different positive effects on host plants (van der Heijden et al. 1998).
In conclusion, almost all local wild plants are colonized by AMF except for C. album. This suggests that AMF play important roles in maintaining plant diversity of coastal beaches. Thirty-seven AMF species, belonging to 12 genera in seven families isolated from the rhizosphere, indicates high AMF diversity in this ecosystem. In addition, colonization, spore community composition and diversity are significantly related to edaphic factors and host plants. Na ion concentrations and pH significantly and negatively affect mycorrhizal colonization rate, spore density and evenness, total soil organic carbon is significantly and positively correlated with species richness. Hence, soil pH, Na ions and total organic carbon levels should be considered when introducing arbuscular mycorrhizal fungi for vegetation restoration of coastal ecosystems.
AcknowledgementsThe authors thank Dr Nan Yang of Nanjing Forestry University for his suggestions in writing this manuscript, and Dafeng Forest Farm for assistance in field work. We thank International Science Editing ( http://www.inter natio nalsc ience editi ng.com) for editing this manuscript.
References
Alguacil MM, Lumini E, Roldán A, Sakinas-Garcia JR, Bonfante P, Bianciotto V (2008) The impact of tillage practices on arbuscular mycorrhizal fungal diversity in subtropical crops. Ecol Appl 18(2):527–536
Allen MF, Allen EB, Friese CF (1989) Responses of the non-mycotrophic plant Salsola kali to invasion by vesicular-arbuscular mycorrhizal fungi. New Phytol 111(1):45–49
álvarez-Sánchez J, Johnson NC, Antoninka A, Chaudhary VB, Lau MK, Owen SM, Guadarrama P, Castillo S (2012) Large-scale diversity patterns in spore communities of arbuscular mycorrhizal fungi. In: Pagano M (ed) Mycorrhiza: occurrence in natural and restored environments. Nova Science Publishers Inc., New York, pp 29–47
Atul-Nayyar A, Hamel C, Hanson K, Germida J (2009) The arbuscular mycorrhizal symbiosis links N mineralization to plant demand. Mycorrhiza 19(4):239–246
Bencherif K, Boutekrabt A, Dalpé Y, Sahraoui AH (2016) Soil and seasons affect arbuscular mycorrhizal fungi associated with Tamarix rhizosphere in arid and semi-arid steppes. Appl Soil Ecol 107:182–190
Blaszkowski J, Czerniawska B (2011) Arbuscular mycorrhizal fungi (Glomeromycota) associated with roots of Ammophila arenaria growing in maritime dunes of Bornholm (Denmark). Acta Soc Bot Pol 80(1):63–76
Brundrett MC (1991) Mycorrhizas in natural ecosystems. Adv Ecol Res 21:171–313
Brundrett MC, Tedersoo L (2018) Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol 220(4):1108–1115
Cai XB, Peng YL, Gai JP (2010) Ecological distribution of arbuscular mycorrhizal fungi in alpine grasslands of Tibet Plateau. Chin J Appl Ecol 21(10):2635–2644 (in Chinese)
Camprubí A, Calvet C, Cabot P, Pitet M, Estaún V (2010) Arbuscular mycorrhizal fungi associated with psammophilic vegetation in Mediterranean coastal sand dunes. Span J Agric Res 8(1):96–102
Cardoso IM, Boddington C, Janssen BH, Oenema O, Kuyper TW (2003) Distribution of mycorrhizal fungal spores in soils under agroforestry and monocultural coffee systems in Brazil. Agrofor Syst 58(1):33–43
Cardoso EJBN, Bini D, Vasconcellos RLF, Bini D, Miyauchi MYH, dos Santos CA, Alves PRL, de Paula AM, Nakatani AS, Pereira JM, Nogueira MA (2013) Soil health: looking for suitable indicators. What should be considered to assess the effects of use and management on soil health? Sci Agric 70(4):274–289
Carvalho F, de Souza FA, Carrenho R, de Souza Moreira FM, da Con?ei??o Jesus E, Fernandes GW (2012) The mosaic of habitats in the high-altitude Brazilian rupestrian fields is a hotspot for arbuscular mycorrhizal fungi. Appl Soil Ecol 52:9–19
Chen Z, He XL, Guo HJ, Yao XQ, Chen C (2012) Diversity of arbuscular mycorrhizal fungi in the rhizosphere of three host plants in the farming-pastoral zone, north China. Symbiosis 57(3):149–160
Clapp JP, Young JPW, Merryweather JW, Fitter AH (2010) Diversity of fungal symbionts in arbuscular mycorrhizas from a natural community. New Phytol 130(2):259–265
da Silva IR, de Mello CMA, Alves Ferreira Neto R, Alves da Silva DK, de Melo AL, Oehl F, Maia LC (2014) Diversity of arbuscular mycorrhizal fungi along an environmental gradient in the Brazilian semiarid. Appl Soil Ecol 84:166–175
da Silva IR, de Souza FA, Alves da Silva DK, Oehl F, Maia LC (2017) Patterns of arbuscular mycorrhizal fungal distribution on mainland and island sandy coastal plain ecosystems in Brazil. Microb Ecol 74(3):654–669
Daniell TJ, Husband R, Fitter AH, Yong JPW (2001) Molecular diversity of arbuscular mycorrhizal fungi colonising arable crops. FEMS Microbiol Ecol 36(2–3):203–209
de Oliveira Freitas R, Buscardo E, Nagy L, dos Santos Maciel AB, Carrenbo R, Luiz?o RC (2014) Arbuscular mycorrhizal fungal communities along a pedo-hydrological gradient in a Central Amazonian terra firme forest. Mycorrhiza 24(1):21–32
del Mar Alguacil M, Torres MP, Montesinos-Navarro A, Roldán A (2016) Soil characteristics driving arbuscular mycorrhizal fungal communities in semiarid Mediterranean soils. Appl Environ Microb 82(11):3348–3356
Ding NN, Wang BS, Liang ZH, Liu DH (2011) Effects of different amelioration measures on coastal saline soil in the David’s deer reserve of Dafeng county of Jiangsu province. Soils 43(3):487–492 (in Chinese)
Dufrêne M, Legendre P (1997) Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol Monogr 67(3):345–366
Estrada B, Aroca R, Azón-Aguilar C, Barea JM, Ruiz-Lozano JM (2013a) Importance of native arbuscular mycorrhizal inoculation in the halophyte Asteriscus maritimua for successful establishment and growth under saline condition. Plant Soil 370:175–185
Estrada B, Beltrán-Hermoso M, Palenzuela J, Iwase K, Ruiz-Lozano JM, Barea JM, Oehl F (2013b) Diversity of arbuscular mycorrhizal fungi in the rhizosphere of Asteriscus maritimus (L.) Less., a representative plant species in arid and saline Mediterranean ecosystems. J Arid Environ 97:170–175
Evelin H, Kapoor R, Giri B (2009) Arbuscular mycorrhizal fungi in alleviation of salt stress: a review. Ann Bot 104(7):1263–1280
Friese CF, Koske RE (1991) The spatial dispersion of spores of vesicular-arbuscular mycorrhizal fungi in a sand dune: microscale patterns associated with the root architecture of American beach grass. Mycol Res 95(8):952–957
Gerdemann JW, Nicolson TH (1963) Spores of mycorrhizal endogone species extracted from soil by wet sieving and decanting. Trans Br Mycol Soc 46(2):235–244
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol 84(3):489–500
Goto BT, Jardim JG, da Silva GA, Furrazola E, Torres-Arias Y, Oehl F (2012) Glomus trufemii (Glomeromycetes), a new sporocarpic species from Brazilian sand dunes. Mycotaxon 120(1):1–9
Hart MM, Reader RJ (2002) Taxonomic basis for variation in the colonization strategy of arbuscular mycorrhizal fungi. New Phytol 153(2):335–344
Hashem A, Alqarawi AA, Radhakrishnan R, Al-Arjani AF, Aldehaish HA, Egamberdieva D, Abd-Allah EF (2018) Arbuscular mycorrhizal fungi regulate the oxidative system, hormones and ionic equilibrium to trigger salt stress tolerance in Cucumis sativus L. Saudi J Biol Sci 25(6):1102–1114
Hildebrandt U, Janetta K, Ouziad F, Renne B, Nawrath K, Bothe H (2001) Arbuscular mycorrhizal colonization of halophytes in Central European salt marshes. Mycorrhiza 10(4):175–183
Ievinsh G (2006) Biological basis of biological diversity: physiological adaptations of plants to heterogeneous habitats along a sea coast. Acta Univ Latv 710:53–79
Jefwa JM, Sinclair R, Maghembe JA (2006) Diversity of glomale mycorrhizal fungi in maize/sesbania intercrops and maize monocrop systems in Southern Malawi. Agrofor Syst 67(2):107–114
Jenkins WR (1964) A rapid centrifugal-flotation technique for separating nematodes from soil. Plant Dis Rep 48(9):692
Juniper S, Abbott LK (2006) Soil salinity delays germination and limits growth of hyphae from propagules of arbuscular mycorrhizal fungi. Mycorrhiza 16(5):371–379
Kahiluoto H, Ketoja E, Vestberg M, Saarela I (2001) Promotion of AM utilization through reduced P fertilization 2. Field studies. Plant Soil 231(1):65–79
Kamble VR (2012) Status of AM fungi in some medicinal plants from panambur beach mangalore India. J Pharm Biol Sci 3(4):1–4
Kamble VR, Agre DG, Dixit GB (2012) Incidence of arbuscular mycorrhizal fungi in indian squill: drimia indica from coastal sand dunes of Konkan, India. J Pharm Biol Sci 4(3):31–36
Koske RE (1987) Distribution of VA mycorrhizal fungi along a latitudinal temperature gradient. Mycologia 79(1):55–68
Koske RE, Gemma JN (1996) Arbuscular mycorrhizal fungi in Hawaiian sand dunes: Island of Kauai. Pac Sci 50(1):36–45
Krüger M, Krüger C, Walker C, Stockinger H, Schüssler A (2012) Phylogenetic reference data for systematic and phylotaxonomy of arbuscular mycorrhizal fungi from phylum to species level. New Phytol 193(4):970–984
Kulkarni SS, Raviraja NS, Sridhar KR (1997) Arbuscular mycorrhizal fungi of tropical sand dunes of west coast of India. J Coast Res 13(3):931–936
Leifheit EF, Veresoglou SD, Lehmann A, Morris EK, Rillig MC (2014) Multiple factors influence the role of arbuscular mycorrhizal fungi in soil aggregation—a meta-analysis. Plant Soil 374:523–537
Lekberg Y, Koide RT, Rohr JR, Aldrich-Wolfe L, Morton JB (2007) Role of niche restrictions and dispersal in the composition of arbuscular mycorrhizal fungal communities. J Ecol 95(1):95–105
Li JL, Sun YQ, Jiang XL, Chen BD, Zhang X (2018) Arbuscular mycorrhizal fungi alleviate arsenic toxicity to Medicago sativa by influencing arsenic speciation and partitioning. Ecotoxicol Environ Saf 157:235–243
Liu RJ, Wang FY (2003) Selection of appropriate host plants used in trap culture of arbuscular mycorrhizal fungi. Mycorrhiza 13(3):123–127
Liu S, Guo XL, Feng G, Maimaitiaili B, Fan JL, He XH (2016) Indigenous arbuscular mycorrhizal fungi can alleviate salt stress and promote growth of cotton and maize in saline fields. Plant Soil 398(1–2):195–206
Miransari M (2011) Interactions between arbuscular mycorrhizal fungi and soil bacteria. Appl Microbiol Biot 89(4):917–930
Morton JB (1988) Taxonomy of VA mycorrhizal fungi: classification, nomenclature, and identification. Mycotaxon 32:267–324
Muleta D, Assefa F, Nemomissa S, Granhall U (2007) Composition of coffee shade tree species and density of indigenous arbuscular mycorrhizal fungi (AMF) spores in Bonga natural coffee forest, southwestern Ethiopia. For Ecol Manag 241(1–3):145–154
Nagahashi G, Douds DD (1999) Rapid and sensitive bioassay to study signals between root exudates and arbuscular mycorrhizal fungi. Biotechnol Tech 13(12):893–897
Ocampo JA, Martin J, Hayman DS (1980) Influence of plant interactions on vesicular-arbuscular mycorrhizal infections. I. Host and non-host plants grown together. New Phytol 84(1):27–35
Oehl F, Sieverding E, Palenzuela J, Ineichen K, da Silva GA (2011) Advances in Glomeromycota taxonomy and classification. IMA Fungus 2(2):191–199
Olsen SR, Cole CV, Watanabe FS, Dean LA (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. USDA Circular No 939, US Gov Print Offi ce, Washington
Pereira CMR, Alves da Silva DK, de Almeida Ferreira AC, Goto BT, Maia LC (2014) Diversity of arbuscular mycorrhizal fungi in Atlantic forest areas under different land uses. Agric Ecosyst Environ 185(3):245–252
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55(1):158–161
Pontes JS, Oehl F, Pereira CD, Machado CT, Coyne D, da Silva DK, Maia LC (2017) Diversity of arbuscular mycorrhizal fungi in the Brazilian’s Cerrado and in soybean under conservation and conventional tillage. Appl Soil Ecol 117–118:178–189
Purin S, Rillig MC (2008) The arbuscular mycorrhizal fungal protein glomalin: limitations, progress, and a new hypothesis for its function. Pedobiologia 51(2):123–130
Qin F, Shinozaki K, Yamaguchi-Shinozaki K (2011) Achievements and challenges in understanding plant abiotic stress responses and tolerance. Plant Cell Physiol 52(9):1569–1582
Rodríguez-Echeverría S, Hol WHG, Freitas H, Eason WR, Cool R (2008) Arbuscular mycorrhizal fungi of Ammophila arenaria (L.) link: spore abundance and root colonisation in six locations of the European coast. Eur J Soil Biol 44(1):30–36
Sarkar A, Asaeda T, Wang QY, Kaneko Y, Rashid MH (2017) Response of Miscanthus sacchariflorus to zinc stress mediated by arbuscular mycorrhizal fungi. Flora 234:60–68
Schenck NC, Perez Y (1990) Manual for the identification of VA mycorrhizal fungi, 3rd edn. Synergistic Publications, Corvallis
Schü?ler A, Walker C (2010) The Glomeromycota : a species list with new families and genera. Libraries at the royal botanical garden Edinburgh, the royal botanic garden Kew, botanische staatssammlung Munich, and Oregon State University
Shannon CE, Weaver W, Wiener N (1949) The mathematical theory of communication. The University of Illinois Press, Urbana
Sheng M, Tang M, Zhang FF, Huang YH (2011) Effect of soil factors on arbuscular mycorrhizal fungi in saline alkaline soils of Gansu, Inner Mongolia and Ningxia. Biodivers Sci 19(1):85–92
Soka GE, Ritchie ME (2018) Arbuscular mycorrhizal spore composition and diversity associated with different land uses in a tropical savanna landscape, Tanzania. Appl Soil Ecol 125:222–232
Stürmer SL, Bellei MM (1994) Composition and seasonal variation of spore populations of arbuscular mycorrhizal fungi in dune soils on the island of Santa Catarina, Brazil. Can J Bot 72(3):359–363
Stürmer SL, Stürmer R, Pasqualini D (2013) Taxonomic diversity and community structure of arbuscular mycorrhizal fungi ( Phylum Glomeromycota) in three maritime sand dunes in Santa Catarina state, south Brazil. Fungal Ecol 6(1):27–36
Sun L, Zhu ZS, Liu Y, Zhang JL (2004) Evaluation of service values of the intertidal land eco-system of Dafeng city. Rural Ecoenviron 20(3):10–14 (in Chinese)
Sylvia DM, Will ME (1988) Establishment of vesicular-arbuscular mycorrhizal fungi and other microorganisms on a beach replenishment site in Florida. Appl Environ Microbiol 54(2):348–352
Talaat NB, Shawky BT (2014) Protective effects of arbuscular mycorrhizal fungi on wheat ( Triticum aestivum L.) plants exposed to salinity. Environ Exp Bot 98:20–31
Tang M (2007) Diversity and distribution of arbuscular mycorrhizal fungi in saline alkaline soil, Inner Mongolia. Acta Pedol Sin 44(6):1105–1110 (in Chinese)
Tedersoo L, Bahram M, P?lme S, K?ijalg U, Yorou NS, Wijesundera R, Ruiz LV, Vasco-Palacios AM, Thu PQ, Suija A, Smith ME, Sharp C, Saluveer E, Saitta A, Rosas M, Riit T, Ratkowsky D, Pritsch K, P?ldmaa K, Piepenbring M, Phosri C, Peterson M, Parts K, P?rtel K, Otsing E, Nouhra E, Njouonkou AL, Nilsson NRH, Morgado LN, Mayor J, May TW, Majuakim L, Lodge DJ, Lee SS, Larsson K, Kohout P, Hosaka K, Hiiesalu I, Henkel TW, Harend H, Guo LD, Greslebin A, Grelet G, Geml J, Gates G, Dunstan W, Dunk C, Drenkhan R, Dearnaley J, Kesel AD, Dang T, Chen X, Buegger F, Brearley FQ, Bonito G, Anslan S, Abell S, Abarenkov K (2014) Global diversity and geography of soil fungi. Science 346(6213):1–10
Tian H, Gaia JP, Christie P, Li XL (2009) Arbuscular mycorrhizal fungi associated with wild forage plants in typical steppe ofeastern Inner Mongolia. Eur J Soil Biol 45(4):321–327
Trufem SFB (1990) Aspectos ecológicos de fungos micorrízicos vesículo-arbusculares da mata tropical úmida da Ilha do Cardoso, SP, Brasil. Acta Bot Bras 4(2):31–45
Turrini A, Agnolucci M, Palla M, Tomé E, Tagliavini M, Scandellari F, Giovannetti M (2017) Species diversity and community composition of native arbuscular mycorrhizal fungi in apple roots are affected by site and orchard management. Appl Soil Ecol 116:42–54
van der Heijden MGA, Klironomos JN, Ursic M, Moutoglis P, Strietwolf-Engel R, Boller T, Wiemken A, Sanders IR (1998) Mycorrhizal fungi diversity determines the plant diversity, ecosystem variability and productivity. Nature 396:69–72
Vierheilig H, Ocampo JA (1990) Role of root extract and volatile substances of non-host plants on vesicular-arbuscular mycorrhizal spore germination. Symbiosis 9:199–202
Vierheilig H, Iseli B, Alt M, Raikhel N, Wiemken A, Boller T (1996) Resistance of Urtica dioica to mycorrhizal colonization: a possible involment of Urtica dioica agglutinin. Plant Soil 183:131–136
Wang YS, Liu RJ (2017) A checklist of arbuscular mycorrhizal fungi in the recent taxonomic system of Glomeromycota. Mycosystema 36(7):820–850 (in Chinese)
Wang S, Lin X, Shi YQ (2001) Effects of arbuscular mycorrhiza on resistance of plants to environmental stress. Chin J Ecol 20(3):27–30 (in Chinese)
Wang FY, Liu RJ, Lin XG, Zhou JM (2004) Arbuscular mycorrhizal status of wild plants in saline-alkaline soils of the Yellow River Delta. Mycorrhiza 14(2):133–137
Wang JJ, Qiang W, Liu HY, Ge JL, Zuo YL, He XL (2017) Effects of plants pecies on diversity of arbuscular mycorrhizal fungi in extremely arid desert environment. Mycosystema 36(7):861–869 (in Chinese)
Wang JP, Wang GG, Zhang B, Yuan ZM, Fu ZY, Yuan YD, Zhu LJ, Shilin Ma, Zhang JC (2019) Arbuscular mycorrhizal fungi associated with tree species in a planted forest ofeastern China. Forests 10(5):424
Wicaksono WA, Sansom CE, Jones EE, Perry NB, Monk J, Ridgway HJ (2017) Arbuscular mycorrhizal fungi associated with Leptospermum scoparium (mānuka): effects on plant growth and essential oil content. Symbiosis 75:39–50
Wilson B, Sykes MT (1999) Is zonation on coastal sand dunes determined primarily by sand burial or by salt spray? A test in New Zealand dunes. Ecol Lett 2(4):233–236
Yamato M, Ikeda S, Iwase K (2009) Community of arbuscular mycorrhizal fungi in drought-resistant plants, Moringa spp., in semiarid
regions in Madagascar and Uganda. Mycoscience 50(2):100–105
Zhang Y, Guo LD, Liu RJ (2004) Survey of arbuscular mycorrhizal fungi in deforested and natural forest land in the subtropical region of Dujiangyan, southwest China. Plant Soil 261:257–263
Zhang YF, Qi BI, Yang YF, Zhang ZH, Hu CJ, Zhao SS, Wang XG (2015) Arbuscular mycorrhizal fungi diversity in saline-alkaline Leymus chinensis grasslands on the Songnen Plain. Acta Prataculturae Sin 24(9):80–88 (in Chinese)
Zhang T, Hu YJ, Zhang K, Tian CY, Guo JX (2018) Arbuscular mycorrhizal fungi improve plant growth of Ricinus communis by altering photosynthetic properties and increasing pigments under drought and salt stress. Ind Crop Prod 117:13–19
Zhao XF, Yang JS, Yao RJ (2010) Relationship between soil salt dynamics and factors of water balance in the typical coastal area of Northern Jiangsu Province. Trans CSAE 26(3):52–57 (in Chinese)
Zhao H, Li XZ, Zhang ZM, Zhao Y, Yang JT, Zhu YW (2017) Species diversity and drivers of arbuscular mycorrhizal fungal communities in a semi-arid mountain in China. Peer J 5(12):e4155
 Journal of Forestry Research2021年2期
Journal of Forestry Research2021年2期
- Journal of Forestry Research的其它文章
- Stem taper functions for Betula platyphylla in the Daxing’an Mountains, northeast China
- Leaf phenotypic variation of endangered plant Tetracentron sinense Oliv. and influence of geographical and climatic factors
- Ecological variations of woody species along an altitudinal gradient in the Qinling Mountains of Central China: area-based versus mass-based expression of leaf traits
- Variations in stem radii of Larix principis-rupprechtii to environmental factors at two slope locations in the Liupan Mountains, northwest China
- A review of ecological mechanisms for management practices of protective forests
- Variation of basic density, calorific value and volumetric shrinkage within tree height and tree age of Ugandan grown Eucalyptus grandis wood
