The potential of arbuscular mycorrhizal fungi to conserve Kalappia celebica, an endangered endemic legume on gold mine tailings in Sulawesi, Indonesia
Husna · Faisal Danu Tuheteru · Asrianti Arif
Abstract Kalapi ( Kalappia celebica) is an endemic legume of Sulawesi and has been included in the endangered category since the early 1980s. Conservation of the species is possible through ex situ culture techniques. Arbuscular mycorrhizal fungi (AMF) can accelerate plant growth which in turn supports the conservation ofendangered species. This study aimed to assess the effi cacy of local AMF to accelerate the growth of kalapi and increase nutrient uptake in kalapi grown in gold mine tailing media. There were three AMF treatments, Glomus claroideum, Glomus coronatum, and a mixture of both, plus the control. Each treatment was replicated three times, each consisting of five plants. The results show that the highest AMF colony was obtained by kalapi seedlings inoculated with Glomus coronatum and the mixture of AMF. The range of mycorrhizae inoculation effect values was 59.7–71.3%. AMF inoculation increased growth and dry weight of 4-month-old seedlings compared to controls. Dry and total weights of kalapi inoculated with G. coronatum were significantly different from those inoculated with the AMF mixture. However, they are not significantly different from kalapi inoculated with G. claroideum. The results also show that AMF increased nitrogen and phosphorous uptake by the roots, as well as nitrogen, phosphorous, potassium, manganese and iron by the shoots. All AMF treatments decreased potassium uptake in the roots, except in kalapi inoculated with G. coronatum. The AMF mixture decreased iron contents the roots by 15%. AMF can be developed into biofertilizer to support the conservation of kalapi in tropical Indonesia.
Keywords Kalappia celebica · Glomeromycota · Glomus coronatum · Gold mine tailings · Southeast Sulawesi
Introduction
Land degradation and excessive exploitation of forest resources have often resulted in the endangered conservation status of tropical forest species high economic value (Edwards et al. 2019). Among tropical species with endangered conservation status is kalapi, Kalappia celebica (Kosterm.) (IUCN 1994; UNEP-WCMC 2007; Peraturan Menteri Kehutanan (the Minister of Forestry Regulation) No. P.57/Menhut-II/ 2008). Since the 1980s, kalapi has been heavily exploited in the Malili area of Sulawesi (Whitten et al. 1987). It is a tropical monotypic species of the Fabaceae family, and is endemic in Sulawesi (Whitten et al. 1987; Whitmore et al. 1989; Arifet al. 2016; Liam et al. 2019). The wood is used in furniture making because of its beautiful grain (Sosefet al. 1998). The species is categorized as endangered with a vulnerable status (VU D1 + 2c) based on the IUCN Red List Categories 1994.
Conservation of kalapi in tropical Indonesia needs to be addressed immediately. The mandate of the Minister of Forestry Regulation No. P.57/Menhut-II/2008 concerning the Direction of the National Species Conservation Strategy 2008–2018 states that research that must be priorities are ecological studies, silviculture, genetic research and wood properties. The diffi culties in the kalapi conservation program is that silviculture techniques for conserving kalapi have not yet been identified and it is believed that kalapi is a slow-growing species. To support the conservation program and to stimulate plant growth, it is necessary to use seed materials with beneficial microbes, including arbuscular mycorrhizal fungi (AMF).
Arbuscular mycorrhizal fungi (AMF) are mycorrhizal fungi species within the Glomeromycota division of the fungi kingdom, and develop a symbiotic relationship with root systems of vascular plants (Smith and Read 2008). AMF facilitate the uptake of mineral nutrients and water by host plants (Augé 2004; Smith and Read 2008). AMF are also beneficial to plant growth under various environmental stresses such as drought (Augé 2004; Zhu et al. 2012), salinity (Evelin et al. 2009; He et al. 2019), waterlogging (Tuheteru et al. 2015), and heavy metal toxicity (Barua et al. 2010; Miransari 2010; Husna et al. 2016, 2019; Wang 2017). With the conditions of gold mine tailings and soils contaminated with mercury, AMF assisted in the growth and uptake of nutrients (Or?owska et al. 2011 ; Madejon et al. 2012; Fiqri et al. 2016).
Previous studies indicate that AMF can be effective in improving the growth and culture ofendangered species (Sharma et al. 2008; Zubek et al. 2009; Husna et al. 2016, 2017a, 2019). AMF can also significantly accelerate the succession and survival of species in conservation and rehabilitation programs (Panwar and Tarafdar 2006; Fuchs and Haselwandter 2008; Zubek et al. 2009; Bothe et al. 2010; Husna et al. 2017a). In Indonesia, studies of AMF treatments for the conservation ofendangered species have been reported for Aquilaria malacensis Lam. and A. crasna Pierre (Turjaman et al. 2006a), A. filaria (Oken.) Merr. (Turjaman et al. 2006b), A. microcarpa Baill. (Santoso et al. 2007), Gonystylus bancanus (Miq.) Kurz (Muin 2003), and Pericopsis mooniana (Thw.) Thw. (Husna et al. 2018). Research on AMF applications for endangered legumes in Sulawesi such as P. mooniana has been carried out for several years (Husna et al. 2016, b, 2019). The present study was undertaken to assess the growth of kalapi inoculated with AMF and raised in gold mine tailing medium.
Materials and methods
Growth medium
Gold tailings were obtained from the disposal site of PT. Panca Logam Makmur, Bombana District, Southeast Sulawesi. Chemical and physical characteristics were analyzed at the Soil and Plant Laboratory of SEAMEO BIOTROP, Bogor. The analysis showed a pH of 6.1, organic carbon content 0.10%, total N 0.05%, C/N ratio 2.1, and available P2O511.5 mg kg?1. Ca, Mg, K, Na levels were 1.45, 0.73, 0.13, and 0.09 cmol kg?1, respectively. The cation exchange capacity was 4.77, base saturation 50.4%; Al 3+ and H + of 0.00 meq 100 g?1and 0.10 meq 100 g?1, respectively. The tailings consisted of 79.2% sand, 16.2% silt, and 4.6% clay. Total metal contents were Mn (487 mg kg?1), Fe (0.73%), Cd (0.6 mg kg?1), and Pb [9.8 mg kg?1(HNO3–HClO4)–AAS]. Total Hg was < 0.00004 mg kg?1(method of Cool Vapor-AAS). The gold tailings medium was mixed with soil and vermicompost (4:2:1).
Preparation and germination of kalapi seeds
Kalapi fruits were collected in 2016 from parent plants in the natural forest of Abuki Subdistrict, Konawe District of Southeast Sulawesi. Prior to planting, the seeds were filed, soaked in water for 6 h germinated in plastic containers containing sterile sand. Germination was carried out in the greenhouse of the Indonesian Mycorrhizas Association, Southeast Sulawesi Branch, Kendari.
Inoculation preparation and AMF inoculation
The AMF inoculums were Glomus claroideum Schenk & Smith (B), Glomus coronatum Giovann. (C), and an AMF mixture of both which were isolated from the rhizosphere of P. mooniana (Husna et al. 2015). AMF inoculums were propagated in zeolite media and Pueraria javanica (Benth.) Benth. host for three months in the greenhouse. Ten grams of AMF inoculums were inoculated in the root of kalapi seedlings grown in 15 × 20 cm polybags containing sterile gold tailing soil media. The inoculated seedlings were maintained, watered, and observed for 4 months. Daily temperatures varied between 27 and 47 °C and relative humidity was 45–79%.
Experimental design
A completely randomized design was used, consisting of a control (A) and three treatments, Glomus claroideum (B), Glomus coronatum (C), and a mixture of the two (D). Each treatment had three replications, each consisting of five seedlings for a total of 60 seedlings.
Measured parameters
Heights were recorded and diameters measured 1 cm above the medium. The number of leaves were counted and leaf area calculated based on leaf length and width. Shoots and roots were separately harvested and ovendried at 70 °C for 48 h and weighed.
Phosphorus (P) contents were determined using the SLMU-TT-05 (Bray I/II) method, while nitrogen (N) contents were analyzed by the SN 13-4721-1998 (Kjeldahl) method. Manganese (Mn) and iron (Fe) contents were analyzed using HNO3–HClO4method (Carter 1993). N, P, and pottasium (K) uptakes were calculated by multiplying nutrient contents by plant dry weights. The metal transport factor (TF) was calculated using C aerial/C root, where C aerial is metal concentration in the shoot (stems and leaves) and C root is metal concentration in the root.
The increase/decrease of nutrient uptake of AMF-treated seedlings relative to the controls was calculated basing on the formula: [metal absorption of AMF plant—metal absorption of non-mycorrhizal plant/metal absorption of nonmycorrhizal plant] × 100% (Wang et al. 2005). Seed quality index (SQI) was calculated according to Duryea and Brown ( 1984): Seed quality index: (SQI) = [shoot dry weight + dry root weight]/[(height/diameter) + (dry weight of shoot/root dry weight)]. Seedlings are high quality if SQI ≥ 0.09.
The shoot and root ratio compare shoot dry weight and root dry weight colonized by AMF. The kalapi roots were rinsed under running water, soaked in 10% KOH for 24 h, then acidified in 2% HCl for 30 min, and finally stained with trypan blue. AMF colonies were calculated using the formula of Brundrett et al. ( 1996): [Σ number of fields of view colonized/Σ total observed field of view] × 100%. Mycorrhizae inoculation effect (MIE) was calculated using the formula of Habte and Manjunath ( 1991): [dry weight of mycorrhizal plant—dry weight of non-mycorrhizal plant/dry weight of mycorrhizal plant] × 100%.
Data analyses
The data were analyzed using F test, followed by the least significant difference (LSD) test at 95% confidence level.
Results
AMF colonization and MIE
Roots of 4-month old kalapi had higher numbers of colonies of Glomus coronatum and mixed AMF compared to the other treatments (Table 1). The G. claroideum treatment was significantly different from controls. MIE values ranged from 59.7 to 71.3% (Table 1).
Seedling growth
Inoculation with local AMF increased height and diameter of 4-month old seedlings (Table 2). The treatments resulted in significant differences in height and diameter compared to the untreated controls. There was no significant effecton the total number of leaves between non-inoculated and inoculated seedlings (Fig.1).
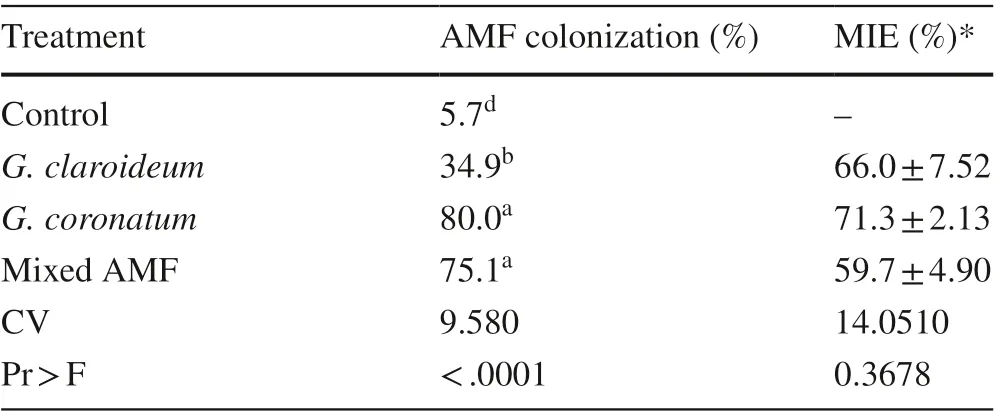
Table 1 AMF colonization and MIE of 4-month old K. celebica seedlings
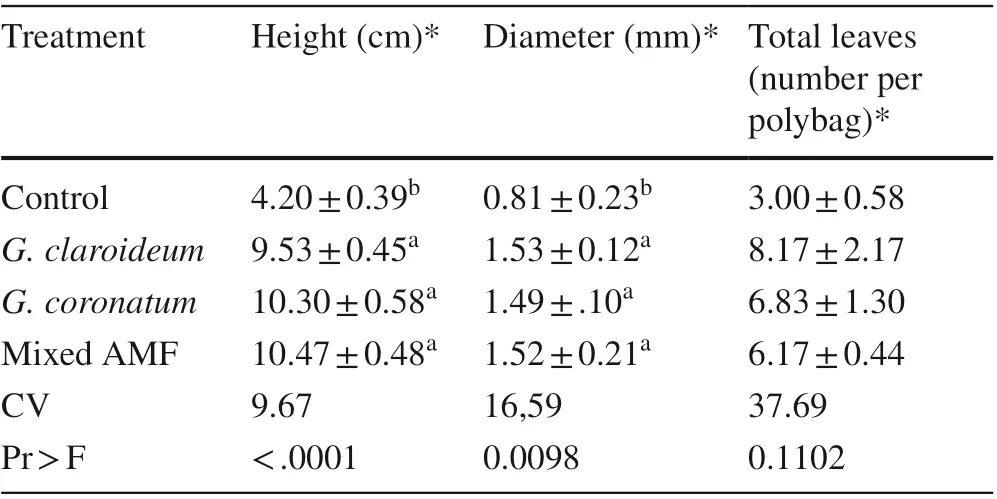
Table 2 Height and diameter growth and total number of leaves of non-inoculated and inoculated K. celebica seedlings
Dry weight
Inoculation with AMF increased root, shoot and total dry weight as well as seed quality index (SQI) of 4-month old kalapi seedlings compared to controls (Table 3). The G. coronatum treatment was significantly different from mixed AMF treatment for root and total dry weight but not significantly different from the G. claroideum treatment. There was no significant effect on shoot–root ratios between noninoculated and inoculated seedlings (Table 3).
Nutrient contents and uptake
There were no significant effects on N and P contents between non-inoculated and inoculated seedlings (Table 4). Mn and Fe levels were significantly higher in the control roots (Table 4). The controls showed no significant difference in K content compared with the G. coronatum and mixed AMF treatments.

Fig.1 Performance of 4-month old kalapi seedlings (left) and AMF of G. claroideum colonization (right). Notes: A—control, B— G. claroideum, C— G. coronatum, D—mixed AMF ( G. claroideum, G. coronatum), Hi—internal hyphae
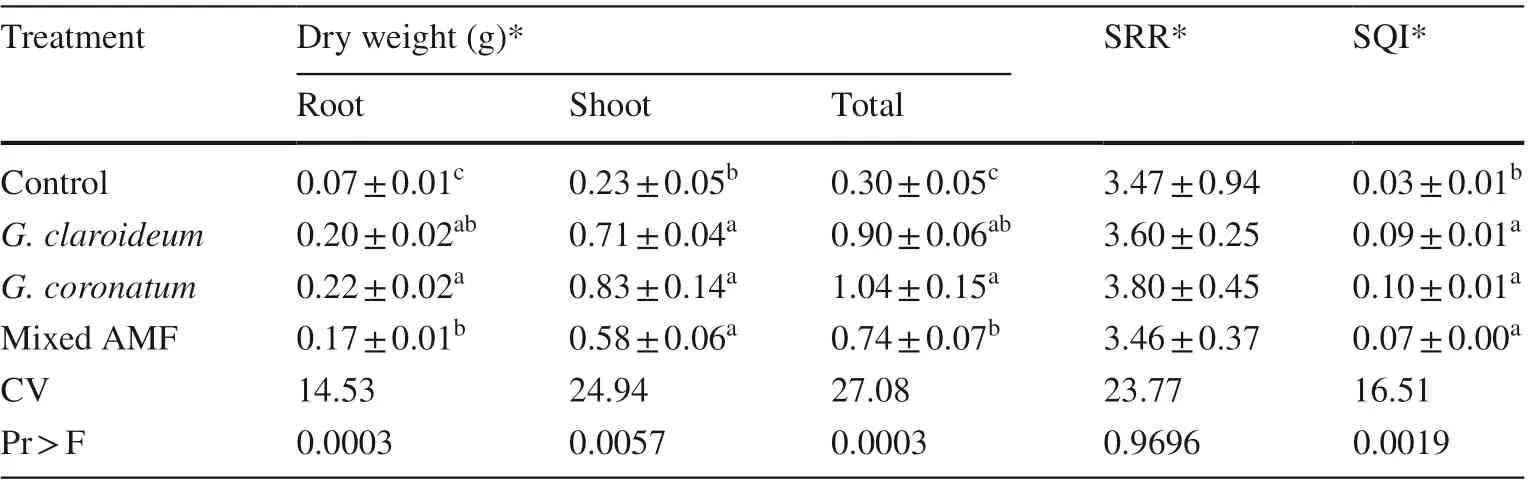
Table 3 Dry weight, shoot–root ratio (SRR), and seed quality index (SQI) of 4-month old K. celebica seedlings

Table 4 Nutrient contents in roots of 4-month old kalapi seedlings
G. coronatum inoculation significantly increased N, P, K uptakes by the roots (Table 5). The G. claroideum and mixed AMF treatments were significantly different from the controls for N and P uptakes (Table 5). There were no significant effects between the non-inoculated and inoculated seedlings on Mn and Fe uptake.
The results of this study show that there were no significant effects on K and Fe contents in the shoots between non-inoculated and inoculated seedlings (Table 6). The G. coronatum and mixed AMF treatments significantly increased P contents in the shoots and both these treatments were significantly different from the G. claroideum and control treatments (Table 6). The controls had significantly higher K and Mn levels in the shoots and was not significantly different in K contents from the G. coronatum treatment (Table 6).
Inoculation of local AMF significantly increased N and Fe uptakes in the shoots (Table 7). The controls showed significant differences in P uptake from the mycorrhized plants. Kalapi inoculated with G. coronatum showed high P uptake (Table 7). With regards to K and Mn uptakes, there were no significant differences between the noninoculated and inoculated seedlings (Table 7).
Transport factors
Nitrogen (N) and potassium (K) elements had transport factors (TF) > 1, while phosphorus (P), manganese (Mn) and iron (Fe) had transport factors < 1 (Table 8).
Nutrient increase and decrease
AMF inoculation increased N and P uptakes in the roots and N, P, K, Mn and Fe uptake in the shoots. All inoculation treatments decreased K uptake in the roots except for the G.coronatum treatment (Table 9). The mixed AMF treatment decreased Fe contents in the roots by 15% (Table 9).
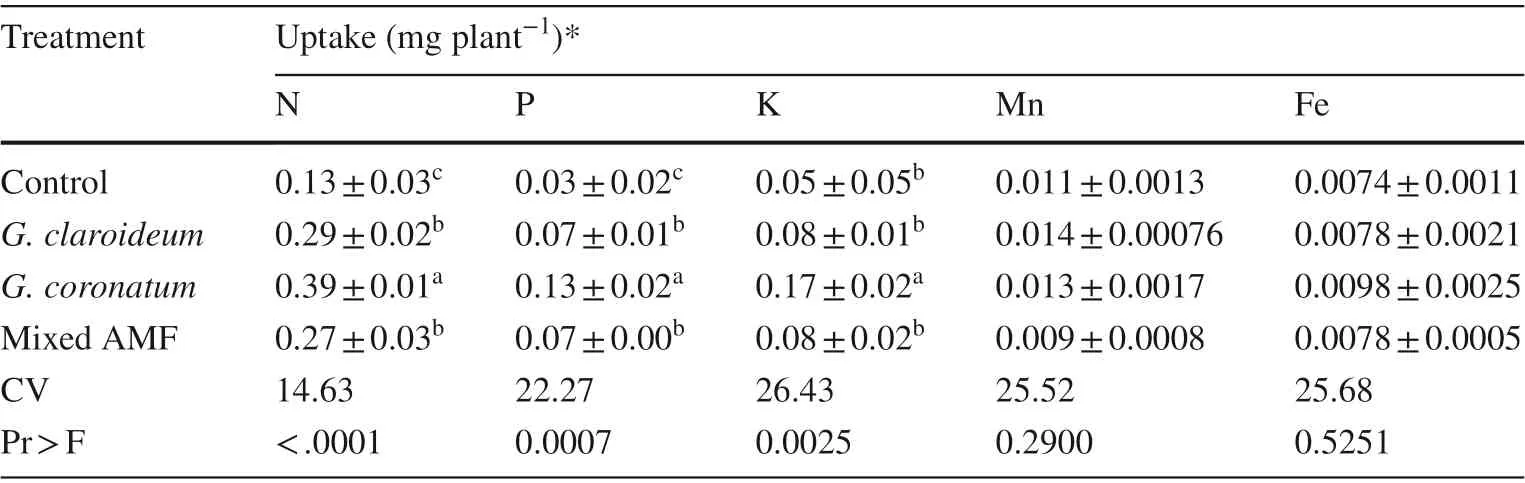
Table 5 Nutrient uptake by roots of 4-month old kalapi seedlings
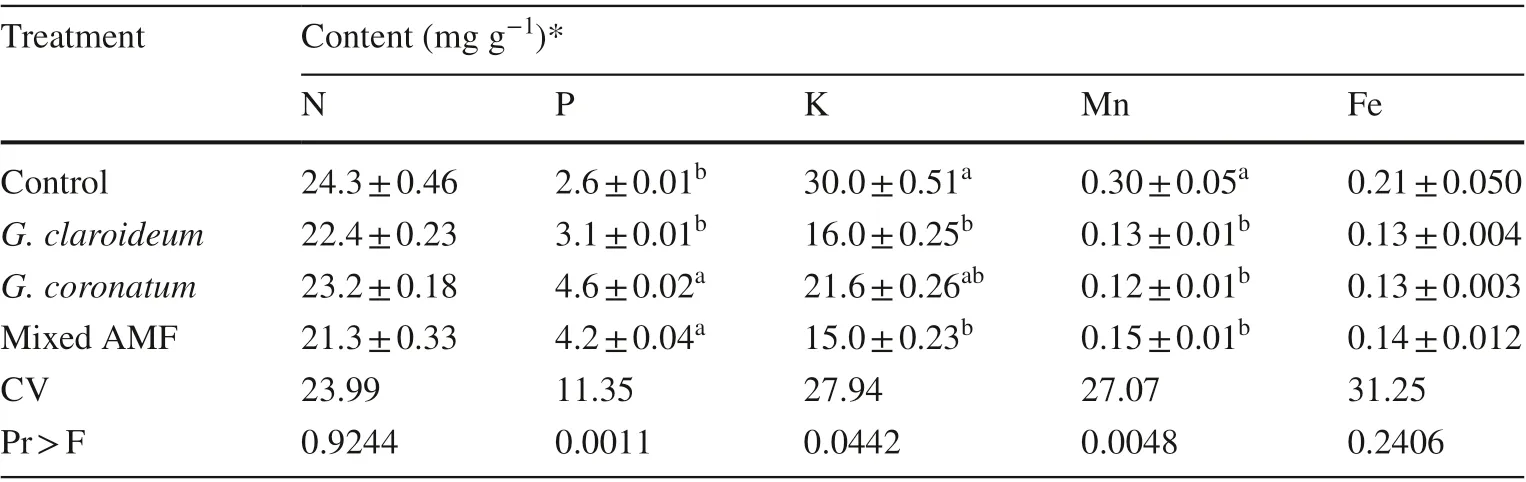
Table 6 Nutrient contents in shoots of 4-month old kalapi seedlings
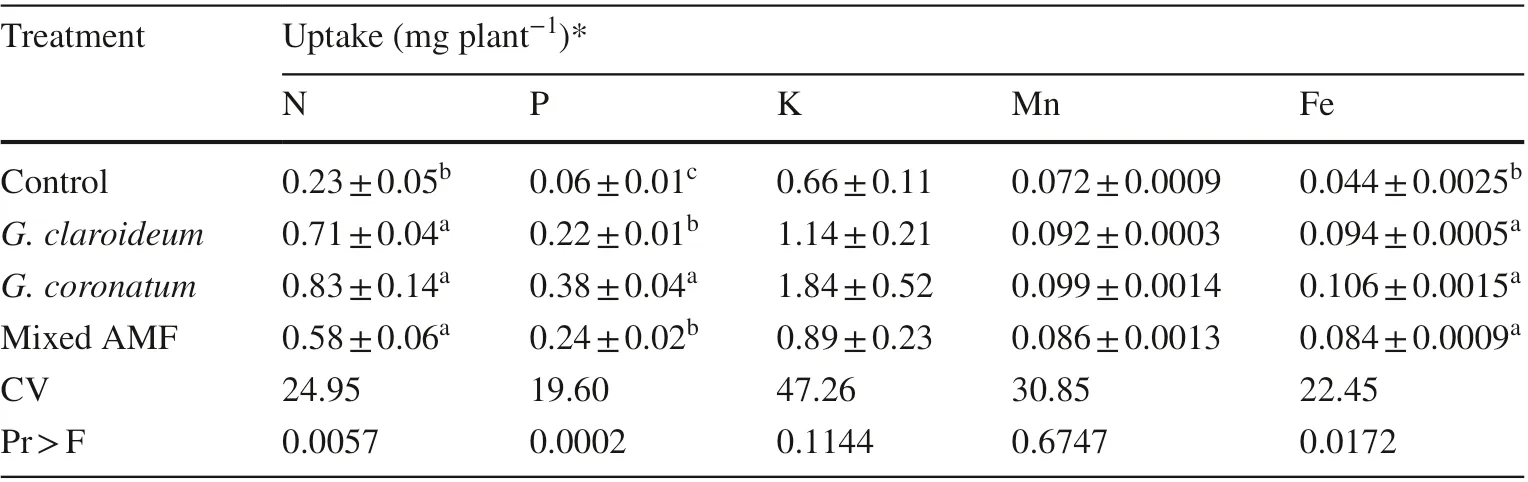
Table 7 Nutrient uptake in shoots of 4-month old kalapi seedlings
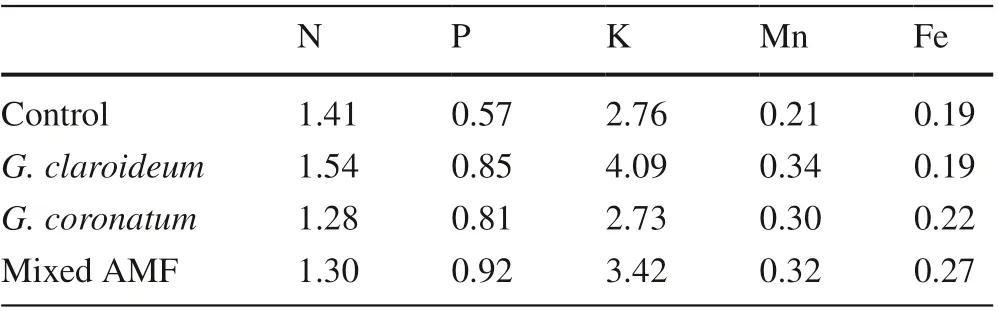
Table 8 Transport factors ofelements in 4-month old kalapi seedlings
Discussion
The results show that local arbuscular mycorrhizal fungi colonized roots of 4-month old kalapi seedlings. The AMF structures in the roots were internal and external hyphae, coil, and vesicles. These structures important in accelerating the growth and survival of the inoculated seedlings. AMF colonization increased the growth and dry weight of kalapi. Previous studies have reported that increasing growth of mychorrized kalapi was due to improvements in water status (Augé 2004), nutrient contents (Wulandari et al. 2016; Husna et al. 2019), and soil characteristics (Abbott and Johnson 2017), as well as the decrease in heavy metal toxicity (Husna et al. 2016; Miransari 2017). The inoculated seedlings had higher N and P uptakes in the roots and higher N, P, K contents compared to the controls (Table 9).
The results of this study are consistent with reports that AMF inoculation increased growth and nutrient uptake of species grown in mercury-contaminated gold mine tailings. AMF inoculation increased the growth of sugargum ( Eucalyptus cladacalyx F. Muell.) seedlings on sulfidic arseniccontaminated gold mine tailings (Madejon et al. 2012). Inoculation also increased the dry weight and survival of Dodonaea viscosa (L.) Jacq., Andropogon eucomus Nees, and Imperata cylindrica (L.) Raeusch grown on alkaline gold mine tailings (Or?owska et al. 2011) and Paspalum (Fiqri et al. 2016).
This study showed that there were no significant effects of AMF inoculation on growth variables apart from dry weight. Single AMF inoculation had a more significant effect in increasing root and total plant dry weight compared to the mixed AMF inoculation. Under heavy metal stress conditions, seedling response to AMF colonization would depend on the type of plants, the AMF inoculums, biotic and abiotic conditions, as well as the heavy metal concentrations. Effectivness of AMF inoculums would be affected by the type of arbuscular mycorrhizal fungi (Kafkas and Ortas 2009). Or?owska et al. ( 2005) reported that the fungal strain, depending on ecotypes, had various effects on plant development when grown on mining soil media.
Kalapi is a slow-growing species without root nodules, which leads to the assumption that natural kalapi regeneration would be slow, hindering a kalapi conservation program. Research by Choosa-Nga et al. ( 2019) revealed that leguminous species in natural ecosystems showed poor regeneration and slow growth.
Previous studies reported that mycorrhizal fungi improved the growth of kalapi trees, shown by high mycorrhiza inoculation effects (MIE) ranging from 59.7 to 71.3% (Habte and Manjunath 1991). High MIE values indicate kalapi growth and survival depend on its symbiosis with AMF. The degree of dependency degree by plants towards AMF varies among species (especially root morphology), soil conditions and climate. Kalapi appears to have a high dependency on mycorrhiza due to limited lateral roots and root hairs as well as the low fertility of gold mine tailing soil. High dependency on AMF has also been reported for several endangered tree species such as A. malaccensis and A. crasna (Turjaman et al. 2006a) and P. mooniana (Husna et al. 2015, 2016, 2019).
This study showed that phosphorous, iron and manganese levels were higher in roots compared to shoots (TF < 1), while nitrogen and potassium contents were higher in the shoots (TF > 1). The AMF mixture decreased iron content in the roots by 15%. A rhizofiltration technique, the filtering of contaminated water, was assumed to be the mechanism used by the 4-month old kalapi seedlings to adapt under iron and manganese stress conditions (TF < 1). The survival ability and exclusion characteristic of kalapi was assumed to detoxify heavy metals from the trees’ surroundings, resulting in root exudates which functioned as metal chelation to store metal elements in the roots. The root exudates were amino acid (histidine) and carboxylic acid (citrate) (Brown 2006; Lambers et al. 2008).
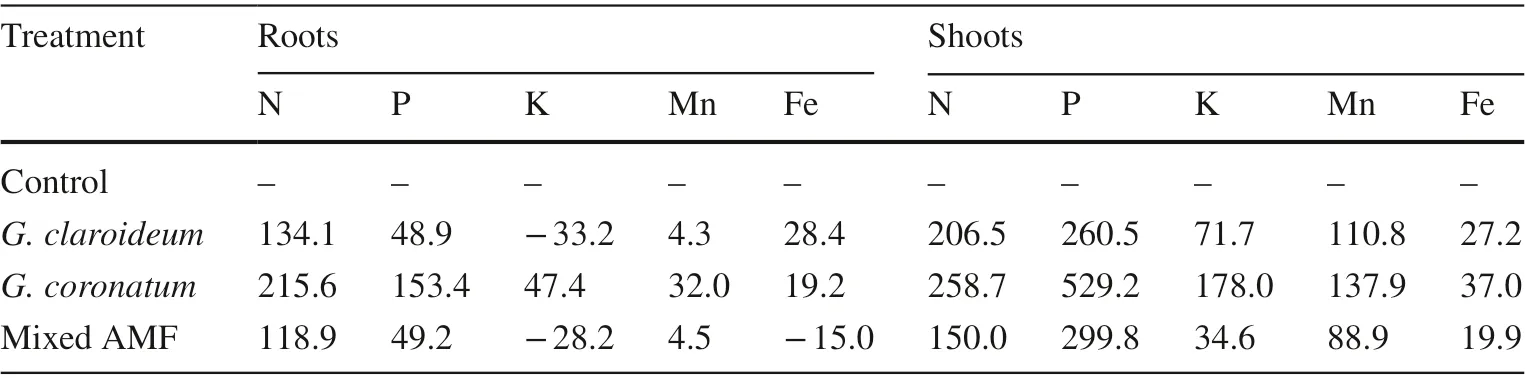
Table 9 Increase/decrease of nutrient uptake in roots and shoots of 4-month old kalapi seedlings
AMF-inoculated kalapi seedlings showed higher manganese and iron uptake by roots and shoots compared to noninoculated seedlings. The mixed AMF decreased iron levels in the roots by 15%. The arbuscular mycorrhizal fungi used in this study showed phytostabilization and phytoextraction mechanisms. Heavy metal phytostabilization by AMF can be done by a metal chelating process to stop heavy metal flow to the plant so that concentrations will not become excessive. The heavy metal elements are assumed to be stored in the AMF structure located in the roots (vacuoles, hyphae and vesicles) (Joner and Leyval 1997) or stored in the form of a chemical compound secreted by the fungi, such as glomalin that can reduce the content of metals (González-Chávez et al. 2004) and be absorbed in the cell walls of the fungi.
Conclusions
Local arbuscular mycorrhizal fungi are effective in improving the quality and growth of kalapi as well as nutrient uptake. Arbuscular mycorrhizal fungi have the potential to be developed as a biofertilizer for kalapi cultivation and for the conservation in general ofendangered tropical species and could be adopted by large scale nursery jointly with reforestation programs. The effectiviness of AMF at a nursery scale should be tested at the field level such as previous mining land.
References
Abbott LK, Johnson NC (2017) Introduction: perspectives on mycorrhizas and soil fertility. In: Johnson NC, Gehring C, Jansa J (eds) Mycorrhizal mediation of soil fertility, structure, and carbon storage. Academic Press, New York, pp 93–105
Arif A, Tuheteru FD, Kandari AM, Husna Mekuo IS, Masnun (2016) Status and culture of arbuscular mycorrhizal fungi isolated from rhizosphere ofendemic and endangered species of Kalapi ( Kalappia celebica Kosterm). Eur J Sus Dev 5(4):395–402
Augé RM (2004) Arbuscular mycorrhizae and soil/plant water relations. Can J Soil Sci 84:373–381
Barua A, Gupta SD, Mridha MAU, Bhuiyan MK (2010) Effect of arbuscular mycorrhizal fungi on growth of Gmelina arborea in arsenic-contaminated soil. J For Res 21(4):423–432
Bothe H, Turnau K, Regvar M (2010) The potential role of arbuscular mycorrhizal fungi in protecting endangered plants and habitats [review]. Mycorrhiza 20:445–457
Brown PH (2006) Nickel. In: Barker AV, Pilbeam DJ (eds) Handbook of plant nutrition. CRC Taylor & Francis, New York, pp 395–410
Brundrett M, Bougher N, Deu B, Grove T, Majalaczuk (1996) Working
with mycorrhizas in forestry and agriculture. Australian Centre for International Agriculture Research, Canberra
Carter MR (1993) Soil sampling and methods of analysis Boca Raton. Lewis Publishers, USA
Choosa-Nga P, Sangwanit U, Kaewgrajang T (2019) The arbuscular mycorrhizal fungi’s diversity in fabaceous trees species of Northeastern Thailand. Biodiversitas 20(2):405–412
Duryea ML, Brown GN (1984) Seedling physiology and reforestation success. In: Proceeding of the physiology working group Technical Session. Dr. W. Juck Publishers, Boston
Edwards DP, Socolar JB, Mills SC, Burivaloka Z, Koh LP, Wilcove DS (2019) Conservation of tropical forests in the anthropocene. Curr Biol Rev 29:R1008–R1020
Evelin H, Kapoor R, Giri B (2009) Arbuscular mycorrhizal fungi in alleviation of salt stress: a review. Ann Bot 104:1263–1280
Fiqri A, Utomo WH, Handayanto E (2016) Effect of arbuscular mycorrhizal fungi on the potential of three wild plant species for phytoextraction of mercury from small-scale gold mine tailings. J Degrade Min Land Manag 3(3):551–558
Fuchs B, Haselwandter K (2008) Arbuscular mycorrhiza ofendangered plant species: potential impacts on restoration strategies. In: Varma A (ed) Mycorrhiza. Springer, Berlin
González-Chávez MC, Carrillo-Gonzales R, Wright SF, Nichols KA (2004) The role glomalin, a protein produced by mycorrhizal fungi, in sequestering potentially toxic elements. Environ Pollut 130:317–323
Habte M, Manjunath A (1991) Categories of vesicular–arbuscular mycorrhizal dependency of host species. Mycorrhiza 1:3–12
He WY, Fan XX, Zhou ZX, Zhang HH, Gao X, Song FQ, Geng G (2019) The effect of Rhizophagus irregularis on salt stress tolerance of Elaeagnus angustifolia roots. J For Res 5:1–10. https://doi.org/10.1007/s1167 6-019-01053-1
Husna Budi RSW, Mansur I, Kusmana C (2015) Growth response of kayu kuku ( Pericopsis mooniana (Thw.) Thw) seedling to indigenous arbuscular mycorrhizal fungi inoculation. Jurnal Pemuliaan Tanaman Hutan 9(3):131–148
Husna, Budi RSW, Mansur I, Kusmana C (2016) Growth and nutrient status of kayu kuku ( Pericopsis mooniana Thw.) with micorrhiza in soil media of nickel post mining. Pak J Biol Sci 19:158–170
Husna, Tuheteru FD, Arif A (2017a) Arbuscular mycorrhizal fungi and plant growth on serpentine soils. In: Wu QS (ed) Arbsucular mycorrhizas and stress tolerance of plants. Springer, Singapore, pp 293–303
Husna, Tuheteru FD, Wigati E (2017b) Growth response and dependency ofendangered nedum tree species ( Pericopsis mooniana) affected by indigenous arbuscular mycorrhizal fungi inoculation. Nusant Biosci 9(1):57–61
Husna, Tuheteru FD, Arif A (2018) Arbuscular mycorrhizal fungi symbiosis and conservation ofendangered tropical legume trees. In: Giri B et al (eds) Root biology, soil biology 52. Springer, Germany, pp 465–486
Husna, Mansur I, Budi RSW, Tuheteru FD, Arif A, Tuheteru EJ, Albasri (2019) Effects of arbuscular mycorrhizal fungi and organic material on growth and nutrient uptake by Pericopsis mooniana in coal mine. Asian J Plant Sci 18(3):101–109
IUCN (1994) IUCN Red List Categories. Prepared by the IUCN Species Survival Commission. IUCN, Gland Switzerland
Joner EJ, Leyval C (1997) Uptake of 109 Cd by roots and hiphae of a Glomus mosseae/Trifolium subterraneum Mycorrhiza from soil amended with high and low concentration of cadmium. New Phytol 135:353–360
Kafkas S, Ortas I (2009) Various mycorrhizal fungi enhance dry weights, P and Zn uptake of four Pistacia species. J Plant Nutr 32:146–159
Lambers H, Chapin FS III, Pons TL (2008) Plant physiology ecology, 2nd edn. Springer, New York
Liam AT, Arif A, Clark RP, Girmansyah D, Kintamani E, Prychid CJ, Pujirahayu N, Rosmarlinansiah, Brearly FQ, Utteridge TMA, Lewis GP (2019) An enigmatic genus on an enigmatic island: the re-discovery of Kalappia on Sulawesi. Ecology 100(11):e02793
Madejon E, Doronila AI, Madejon P, Baker AJM, Woodrow IE (2012) Biosolids, mycorrhizal fungi and eucalypts for phytostabilization of arsenical sulphidic mine tailings. Agrofor Syst 84(3):389–399
Miransari M (2010) Contribution of arbuscular mycorrhizal symbiosis to plant growth under different types of soil stress. Plant Biol 12:563–569
Miransari M (2017) Arbuscular mycorrhizal fungi and heavy metal tolerance in plants. In: Wu QS (ed) Arbuscular mycorrhizas and stress tolerance of plants. Springer, Singapore, pp 147–162
Muin A (2003) Pertumbuhan anakan Ramin ( Gonystylus bancanus (Miq.) Kurz)) dengan inokulasi cendawan mikoriza arbuskula (CMA) pada berbagai intensitas cahaya dan dosis fosfat alam [dissertation]. Program Pascasarjana Institut Pertanian Bogor
Or?owska E, Jurkiewicz A, Anielska T, Godzik B, Turnau K (2005) Influence of different arbuscular mycorrhiza fungal (AMF) strains on heavy metal uptake by Plantago lanceolat a (Plantaginaceae). Pol Bot Stud 19:65–72
Or?owska E, Or?owski D, Mesjasz-Przyby?owicz J, Turnau K (2011) Role of ycorrhizal colonization in plant establishment on an alkaline gold mine tailing. Int J Phytoremediat 13:185–205
Panwar J, Tarafdar JC (2006) Distribution of three endangered medicinal plant species and their colonization with arbuscular mycorrhizal fungi. J Arid Environ 65:337–350
Peraturan Menteri Kehutanan (the Minister of Forestry Regulation) No. P.57/Menhut-II/2008 (2008) tentang Arahan Strategi Konservasi Spesies Nasional 2008–2018
Santoso E, Gunawan AW, Turjaman M (2007) Kolonisasi cendawan mikoriza arbuskula pada bibit tanaman penghasil gaharu Aquilaria microcarpa Baill. Jurnal Penelitian Hutan dan Konservasi ALam, IV 5:499–509
Sharma D, Rupan K, Bhatnagar AK (2008) Arbuscular mycorrhizal (AM) technology for the conservation of Curculigo orchioides Gaertn.: an endangered medicinal herb. World J Microbiol Biotechnol 24:395–400
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Academic Press, London
Sosef MSM, Hong LT, Prawirohatmodjo S (1998) Timber trees: lesserknown timbers No 5 (3). Prosea, Bogor
Tuheteru FD, Kusmana C, Mansur I, Iskandar (2015) Response of lonkida ( Nauclea orientalis L.) towards mycorrhizal inoculum in waterlogged condition. Biotropia 22(1):61–71
Turjaman M, Santosa E, Sumarna Y (2006a) Arbuscular mycorrhizal fungi increased early growth of gaharu wood species Aquilaria malaccensis and A. crasna under greenhouse conditions. J For Res 3(2):139–148
Turjaman M, Tamai Y, Santoso E, Osaki M, Tawaraya K (2006b) Arbuscular mycorrhizal fungi incresead early growth of two nontimber forest product species Dyera polyphylla and Aquilaria filaria under greenhouse conditions. Mycorrhiza 16:459–464
UNEP-WCMC (2007) Strategies for the sustainable use and management of timber tree species subject to international trade: South East Asia. Cambridge
Wang FY (2017) Occurrence of arbuscular mycorrhizal fungi in mining-impacted sites and their contribution to ecological restoration: Mechanisms and applications. Crit Rev Environ Sci Technol 47:1901–1957
Wang FY, Lin XG, Yin R (2005) Heavy metal uptake by arbuscular mycorrhizas of Elsholtzia splendens and the potential for phytoremediation of contaminated soil. Plant Soil 269:225–232
Whitmore TC, Tantra IGM, Sutisna U (1989) Tree flora of Indonesia check list for Sulawesi. Forest Research and Development Centre, Forestry of Departemen, Bogor
Whitten AJ, Mustafa M, Henderson GS (1987) Ekologi Sulawesi. Gajah Mada University Press, Yogyakarta
Wulandari D, Saridi ChengWG, Tawaraya K (2016) Arbuscular mycorrhizal fungal inoculation improves Albizia saman and Paraserianthes falcataria growth in post-opencast coal mine in East Kalimantan, Indonesia. For Ecol Manag 376:67–73
Zhu XC, Song FB, Liu SQ, Liu TD, Zhou X (2012) Arbsucular mycorrhizae improves photosynthesis and water status of Zea mays L. under drought stress. Plant Soil Environ 58(4):186–191
Zubek S, Turnau K, Tsimilli-Michael M, Strasser RJ (2009) Response ofendangered plant species to inoculation with arbuscular mycorrhizal fungi and soil bacteria. Mycorrhiza 19:113–123
 Journal of Forestry Research2021年2期
Journal of Forestry Research2021年2期
- Journal of Forestry Research的其它文章
- Stem taper functions for Betula platyphylla in the Daxing’an Mountains, northeast China
- Leaf phenotypic variation of endangered plant Tetracentron sinense Oliv. and influence of geographical and climatic factors
- Ecological variations of woody species along an altitudinal gradient in the Qinling Mountains of Central China: area-based versus mass-based expression of leaf traits
- Variations in stem radii of Larix principis-rupprechtii to environmental factors at two slope locations in the Liupan Mountains, northwest China
- A review of ecological mechanisms for management practices of protective forests
- Variation of basic density, calorific value and volumetric shrinkage within tree height and tree age of Ugandan grown Eucalyptus grandis wood
