Estimation of carbon pools in secondary tropical deciduous forests of Odisha, India
Subhashree Pattnayak · M. Kumar · N. K. Dhal · Sudam C. Sahu
Abstract Secondary tropical forests sequester atmospheric CO 2 at relatively faster rates in vegetation and in soil than old-growth primary forests. Spatial understanding of biomass and carbon stocks in different plant functional types of these forests is important. Structure, diversity, composition, soil features and carbon stocks in six distinct plant functional types, namely: Moist Mixed-Deciduous Forest, Peninsular Sal Forest (PSF), Semi-Evergreen Forest (SEF), Planted Teak Forest, Bamboo Brakes (BB), and Degraded Thorny Shrubby Forest were quantified as secondary tropical deciduous forests of the Chandaka Wildlife Sanctuary, Eastern Ghats of Odisha, India. Seventy-one species ≥ 10 cm Girth at breast height (GBH) were recorded, belonging to 38 families and 65 genera. Above- ground biomass carbon and soil organic carbon ranged from 2.1–72.7 Mg C ha ?1 and 20.6–67.1 Mg C ha ?1 , respectively, among all plant functional types. Soil organic carbon and important value index were positively correlated with above- ground biomass carbon. Maximum carbon allocation was in SOC pool (51–91%), followed by the above- ground biomass pool (9–52%), indicating SOC is one of the major carbon sinks in secondary dry forests. The results highlight the importance of secondary tropical deciduous forests in biodiversity conservation and ecological importance in reducing greenhouse gases.
Keywords Chandaka wildlife Sanctuary · Diversity indices · Soil organic carbon · Above- ground biomass carbon · Correlation study
Introduction
Tropical forests are the primary carbon sinks of terrestrial ecosystems and possess higher biomass in comparison to temperate and boreal forests. These forests cover about 7% of the land surface and are habitat to more than half of the world’s biotic species (Galley 2014). Being the richest in biodiversity, tropical forests contribute above one-third of global primary productivity (Laurance et al. 2012). These forests are, however, disappearing at the rate of 0.8–2% per year (Sagar et al. 2003), and are being converted into open forest with remnant patches of undisturbed primary forest (Gove et al. 2005). These areas subsequently become secondary forest through natural regeneration. Forest types and areas, rates of carbon dioxide (CO2) sequestration, and amounts of carbon stored by plant and in soil are some of the factors affecting the global carbon cycle (Brown and Lugo 1982). The basic parameter for forest ecosystems (structural and functional aspects) and carbon stock measurements are above- ground biomass (Ketterings et al. 2001). Stand structure, species diversity and composition, plant functional types (PFTs) and their living biomass regulate forest dynamics (Condit et al. 1996; Laurance et al. 2004; Tchouto et al. 2006; Mohandass and Davidar 2009). PFTs reflect the different eco-physiological input variables for net and gross primary productivity linked to a vegetation model (Woodward et al. 2004). Physiological, morphological, phenological and resource competition differentiate the PFTs of a forest and significantly affect soil organic carbon (SOC) (Deyn et al. 2008). Fast-growing species are larger sources of soil carbon compared to slow grow-growing species (Berhongaray et al. 2018). SOC pools vary widely across the forest biome namely, desert and savannas < warm temperate and dry tropical forests < temperate grasslands and forests < wet tropical forests < boreal forests (Deyn et al. 2008). SOC stock (20-cm depth) contribution for shrub lands, grasslands and forest ecosystems were 33%, 42%, and 50%, respectively, (Jobbagy and Jackson 2000). Estimation of carbon stock is primarily by indirect methods and is concerned with the control of terrestrial carbon exchange factors (Kasischke et al. 2013).
In India, forests cover over 708,000 km 2 (21.5%) of the total land area (ISFR 2017) and 72% are tropical moist deciduous, dry deciduous and tropical wet evergreen forest. The latter has the maximum carbon stock/ha (157.2 t C ha?1), followed by [tropical moist deciduous forest (91.4 t C ha?1) and dry deciduous forest (95.5 t C ha?1)], respectively, (FAO 2010). The Odisha forest covers 5.0 million hectares and it represents 28.6% of the total forested area of the country. The total carbon stock was estimated as 444.3 million tonnes (Mt) with plant biomass carbon at 159.8 Mt and SOC at 284.3 Mt (Sahu et al. 2015). Chandaka Wildlife Sanctuary is located in the north-eastern edge of the Eastern Ghats, India. It was earlier classified as a semi-evergreen forest by Champion and Seth ( 1968). Due to anthropogenic activities, several patches within this forest have been transformed into shrub jungles and thorn brush. It is now considered a secondary regenerated deciduous forest.
Carbon stock in relation to plant distribution, composition and PFTs has been less focused in secondary forests compared to primary forests (Ketterings et al. 2001; Lasco et al. 2004; Ekoungoulou et al. 2014). As data are lacking on carbon stocks of tropical secondary forests, the information that can be collected by a carbon pool study of different plant functional types is essential both in regional and in global climate modelling. This knowledge can be implemented for enhancing carbon stocks in natural forests and for reducing CO2in the atmosphere. This study aimed: (1) to assess above-ground biomass carbon (AGBC) and soil organic carbon for different PFTs of the Chandaka Wildlife Sanctuary; and, (2) to study the correlation of AGBC with importance value index, SOC and other soil physicochemical parameters.
Materials and methods
Study area
The Chandaka Wildlife Sanctuary (CWS) is situated in the north-western fringe of Bhubaneswar City and is part of the Khurdha uplands of the Eastern Ghats biotic region in the State of Odisha. The forest (175.8 km 2 ) within the CWS is spread over small sprawling hillocks of Khurdha and Cuttack districts between 20°12′ 30″ N to 20°26′ 03″ N and 85°49′35″ E to 85°34′ 42″ E. Elevation varies from 35 to 219 m. The prominent soil types are sandy loam, red clay loam, and red clay, but primarily lateritic. The climate is tropical with distinct three seasons (summer, rainy and winter) with temperatures 11° to 42° C, annual average precipitation 1500 mm and humidity 77% (Report of forest range offi ce, CWS).
Plant functional types of the CWS
The flora of the CWS is a mixture of semi-ever green and deciduous species. Phytodiversity is dominated by angiosperms (Biswal et al. 2005). The area is divided into six plant functional types (PFTs) based on species composition and dominance:
Moist- mixed deciduous forest ( MMDF)
This PFT is associated with intermingled species such as Xylia xylocarpa (Roxb.) W.Theob., Strychnos nux- vomica L., Syzygium cumini (L.) Skeels, Tectona Grandis L. f., Careya arborea Roxb., Holarrhena pubescens Wall. Ex G.Don, Polyalthia cerasoides (Roxb.) Benth. & Hook. f. ex Bedd., Woodfordia fruticosa (L.) Kurz, Glycosmis pentaphylla (Retz.) DC., Aegle marmelos (L.) Corrêa, Casearia graveolens Dalzell, Tarenna asiatica (L.) Kuntze ex K.Schum. and Ziziphus xylopyrus (Retz.) Willd., Combretum roxburghii Spreng. It is primarily found on the Chandaka and Dampara ranges.
Semi-evergreen forest (SEF)
The tropical semi- evergreen forest is dominated by Xylia xylocarpa but not in pure stands. A major portion of the sanctuary is covered with this type of vegetation. Notable tree species are Strychnos nux- vomica L., Lagerstroemia parviflora Roxb., Diospyros sylvatica Roxb., Alangium salviifolium (L.f.) Wangerin,, Terminalia elliptica Willd., Cassia fistula L., Bridelia retusa (L.) A.Juss., Careya arborea Roxb., Syzygium cumini (L.) Skeels, Terminalia bellirica (Gaertn.) Roxb., Lepisanthes tetraphylla (Vahl) Radlk., Shorea robusta Gaertn., Morinda coreia Buch.-Ham., and Antidesma acidum Retz. This forest type is found primarily in the Mahuriabari, Ambakhali, Pithakhia, Godibari areas.
Planted teak forest ( PTF)
Large plantations of teak ( Tectona grandis), 20–25 yearsold, are found in Jhalara, Ambilla, Bualigada areas of the sanctuary.
Peninsular sal forest (PSF)
This type of forest is encountered in the drier habitats of Godibari, Salabari and Morihabanka areas. The dominant species is Shorea robusta. Other notable species are Diospyros melanoxylon Roxb., Chloroxylon swietenia DC., Senegalia pennata (L.) Maslin., Albizia odoratissima (L.f.) Benth.,, Benkara malabarica (Lam.) Tirveng., Semecarpus anacardium L.f., Piliostigma racemosum (Lam.) Benth., Buchanania cochinchinensis (Lour.) M.R. Almeida., Vitex pinnata L., Madhuca longifolia var. latifolia (Roxb.) A. Chev., Carissa spinarum L. and Cipadessa baccifera (Roth) Miq.
Bamboo brakes ( BB)
Bamboo brakes are characterized by species found in the Bharatpur range, Kochilabarana, Dhalabandha, Kochilabaran Watch Tower, Balimada Chowk. Bambusa bambos (L.) Voss is found in thickets near villages in the boundary of the sanctuary. In the bamboo brakes, saplings of numerous species of trees and shrubs are found but their numbers are few. Dendrocalamus strictus (Roxb.) Nees species are also found in some plantation areas.
Degraded thorn shrub forest ( DTSF)
This type of forest is found on degraded sites of Bhimakhala, Jamuchawk, Malima tower, t of the Bharatpur range. There are few numbers of species and represent a degraded stage of dry deciduous forest. The major species are Ziziphus xylopyrus, Casearia graveolens Dalzell, Cipadessa baccifera and Phoenix acaulis Roxb.
Field survey and sampling
Periodic field surveys and sampling were undertaken during 2015 to 2017 in selected sites in the six plant functional types. In each PFT, eight 0.1 ha (20 × 50 m) plots were randomly set out according to Mueller-Dombois and Ellenberg ( 1974). Forty-eight plots (4.8 ha) were selected in an undisturbed area, leaving 100-m buffer zone from any anthropogenic structures like roads, settlements or dams. The plots were tagged for geographical positioning, i.e., latitude and longitude, using a GARMIN- Global Positioning System (GPS). Samples of twigs, leaves, flowers, and fruit were collected from the plots using various implements and brought to the laboratory and identified using the Flora of Presidency Madras (Gamble and Fischer 1935) and Flora of Orissa (Saxena and Brahmam 1996). The Herbarium (RRL-B) at CSIR-IMMT was referred for classification and identification of specimens. Woody plants ≥ 10 cm girth at breast height (GBH) taken at 1.3 m above ground were selected to calculate basal area and above- ground biomass (AGB). Plants < 5 cm GBH were categorized as saplings (Marimon et al. 2002; Mishra et al. 2005).
Soil samples (up to 30 cm depth), from 48 plots, five/plot, covering the six PFTs, were randomly collected using a soil auger and stored in polythene bags. A total of 240 soil samples were collected from the study area. The number of soil samples taken was based on topography, canopies, and types of soil in each plot.
Phytosociological analysis
Frequency, density, and dominance of plant species were estimated to reflect the relative contribution of a particular species to the total stand as well as their importance value index (IVI) (Mishra 1968). GBH classes were classified as 10–20, 21–30, 31–40, 41–50, 51–60, 61–70, 71–80, 81–90, 91–100, 101–110, and > 111 cm for determination of density, basal area and above-ground biomass. The structure and composition of the six PFTs were quantified through species diversity indices such as Species Richness Index, Shannon–Wiener Diversity Index, Simpson’s Diversity Index, and Species Evenness Index. The Species Richness Index ( d) denotes the average number of species (Margalef 1958). Shannon–Wiener Diversity Index ( H) and Simpson’s Diversity Index ( D) were calculated for community structure to account for abundance, evenness and dominance of species (Tripathi et al. 2010). Pielou’s ( 1966) formula for Species Evenness, i.e., the distribution of individual species, was used. The following formulae were used for calculating IVI and diversity indices:
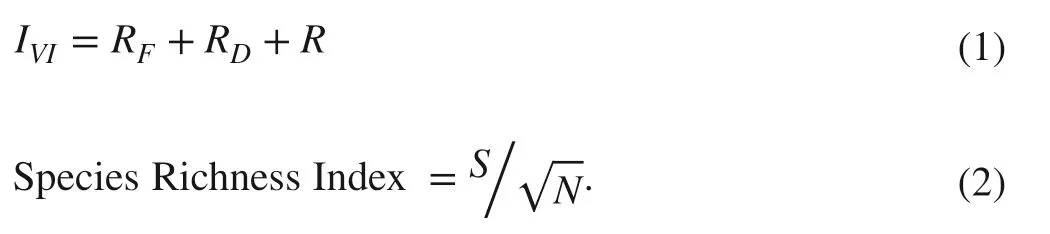
where, IVIis Importance Value Index; RFis Relative Frequency; RDis Relative Density; R is Relative Dominance; S is number of species; N is number of individuals of all speciesShannon–Wiener’s index:

Simpson’s index:


where, n is number of individuals of a particular species; H is Shannon–Wiener’s index; N is total number of individuals in all sample plots; S is number of species.
Biomass and carbon stock
Allometric equations were used to estimate above-ground biomass (AGB) using diameter at breast height (DBH) measurements (Chave et al. 2005). DBHs were derived from GBH. In the case of bamboo, the DBH of the clump (aggregates of culms) were used for AGB estimation with allometric equations (Kumar et al. 2006). The carbon stock of the AGB can be calculated assuming 50% of its weight is carbon (Dixon Dixon et al 1994; Ravindranath et al. 1997). The allometric equation for AGB estimation is:
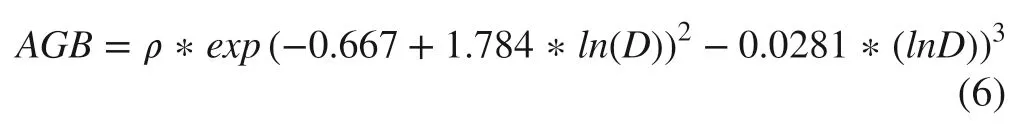
where, AGB is above-ground biomass, ρ = wood specific gravity (grams/cm 3 ), ln is natural logarithm, D is DBH (cm).For bamboo species:

Soil physicochemical characterization
Soil samples were air-dried and plant debris removed before sieving with 500, 250 and 200 μm (< 0.2 mm) mesh. The pH and electrical conductivity (EC) were determined with pH and EC meters (Systronics 371) in 1: 2.5 w/v, soil: water. Moisture content l (MCS %) was estimated by gravimetric oven (104.5 o C for 24 h) (Gardner et al. 2001). Soil texture was determined using a Bouyoucos soil hydrometer (Beretta et al. 2014). Soil organic carbon (SOC) was estimated through partial oxidation according to Walkley and Black 1934 and its stock derived considering bulk density and soil depth (Vanden Bygaart and Angers 2006).
Statistical analysis
Minitab, origin pro9 and SPSS (version 20) software were used to derive correlation and regression equations related for soil characteristics, including carbon pools. The differences between SOC and PFTs were derived through one way ANOVA (Deng et al. 2016). Similarly, Pearson correlation coeffi cients for above-ground biomass carbon (AGBC) with importance value index (IVI), basal area (BA) and soil organic carbon (SOC) was also carried out. These relations were examined at 0.05 level of significance for Pearson correlation and 0.01 for ANOVA. Mean ± standard error were determined.
Results
Plant distribution and composition in PFTs
The 71 identified woody flowering plants were in 38 families and 65 genera, reflecting high phyto-biodiversity of the Chandaka Wildlife Sanctuary. Angio s perm dicots were dominant compared to monocots. Based on the number of species, the major families were: Leguminosae (18 species) > Anacardiaceae (8 species) > Rubiaceae (6 species) > and Malvaceae and Combretaceae (3 species each). According to the measurements of the 71 species and 4930 individuals, stand density (individuals ha?1) and stem density (stems ha?1) for the six PFTs was in the order of: planted teak forest > semi-evergreen Forest > peninsular sal forest > moist- mixed deciduous forest > degraded thorn shrub forest (Table 1). For the bamboo brakes, 190 clumps ha?1were measured. The average stand density in five PFTs was 884 individuals/ha. Among all GBH classes, the 10–20 cm class contributed the maximum individual plants (Fig.1A). This diameter class had an average basal area and AGB of 3.2 m 2 ha?1and 28.1 Mg ha?1, respectively. Among the five PFTs, the highest average basal area and above-ground biomass were 11.4 m 2 ha?1and 53.3 Mg ha?1, respectively, in the 30–40 cm diameter class (Fig.1B, C). With the bamboo brakes, the diameter of the clumps ranged from 0.5 to 14.50 m.
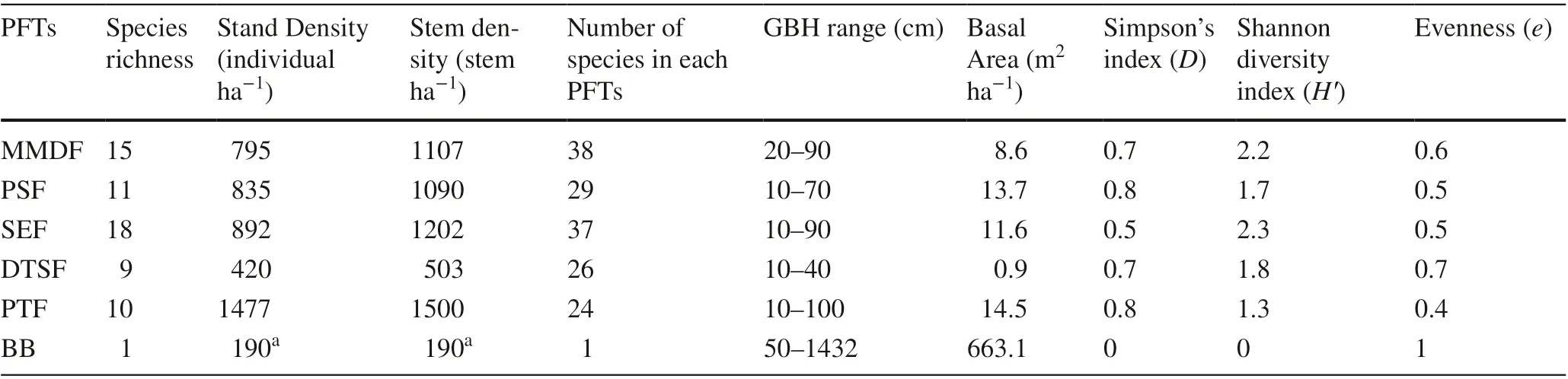
Table 1 Structure and diversity of six PFTs at CWS
Shannon-Weiner and Simpson’s diversity indices varied among the different PFTs. The Shannon-Weiner diversity index ranged from 1.32 (PTF) to 2.31 (SEF). Similarly, Simpson’s diversity index was maximum for the planted teak forest (PTF) (0.85) and minimum for the semi-evergreen forest (SEF) (0.56). Evenness value varied from 0. 40 to 0.71 (Table 1).
The importance value index (IVI) varied widely among prominent species. Overall important species among all the plant functional types were: Tectona grandis (114.9), Xylia xylocarpa (20.8), Strychnos nux- vomica (10.3), Holarrhena pubescens (12.4), Combretum roxburghii (8.7), Bambusa bambos (8.0). Lesser species were Lagerstroemia parviflora,, Polyalthia cerasoides, Careya arborea, Diospyros melanoxylon, and Syzygium cumini. Species richness among the PFTs was: Semi-evergreen forest > Moist- -mixed deciduous forest > Peninsular sal forest > Planted Teak Forest F > Degraded Shrubby Thorny Forest > Bamboo Brakes, whereas evenness was in contrast: Bamboo brakes > Degraded thorny shrubby forest > Moist-mixed deciduous forest > Semi-evergreen forest > Peninsular sal forest SF > Planted teak forest (Table 1). Simpson’s Index (species richness and evenness) was Semi-evergreen forest > Moist-mixed deciduous forest MDF > Degraded thorny shrubby forest TSF > Peninsular sal forest SF > Planted teak forest F > Bamboo brakes B. The Shannon–Wiener diversity index for the bamboo brakes was lower ( H ~ 0), reflecting low complexity of this community, whereas for the semievergreen forest it was H = 2.31, indicating high community complexity.
Above-ground biomass (AGB) and AGB carbon
The AGB of the six PFTs ranged from 4.3 to 145.5 Mg ha?1, and highest for the bamboo brakes (BB) and lowest for the degraded thorn shrub forest (DTSF). Corresponding to the values of AGB, basal areas were also highest for the BB and lowest for DTSF. Similarly, the value of AGB carbon ranged from 2.2 to 72.8 Mg C ha?1in proportion to the results of the AGB (Table 2). The percentage of AGB carbon contribution by prominent woody species were X. xylocarpa (6.0%), S. nux- vomica (2.6%), L. parviflora (1.6%), S. robusta (9.3%), H. pubescens (1.7%, T. grandis (24.3%) and B. bambos (34.5%). The AGB carbon of five important species in each PFT is noted in Fig.2, excluding the bamboo brakes and the planted teak forest. Among the six PFTs, the bamboo brake BB was dominated by the Indian thorny bamboo, ( Bambusa bambos) and obviously, the planted teak forest was dominated by Tectona grandis. In moist-mixed deciduous forest (MMDF) and the semi-evergreen forest (SEF), the important species were X. xylocarpa, T. grandis, S. nux- vomica, S. cumini, C. arborea and H. pubescens. However, in the peninsular sal forest (PSF), sal, Shorea Robusta, is important, as is Holarrhena pubescens in the degraded thorn shrub forest.
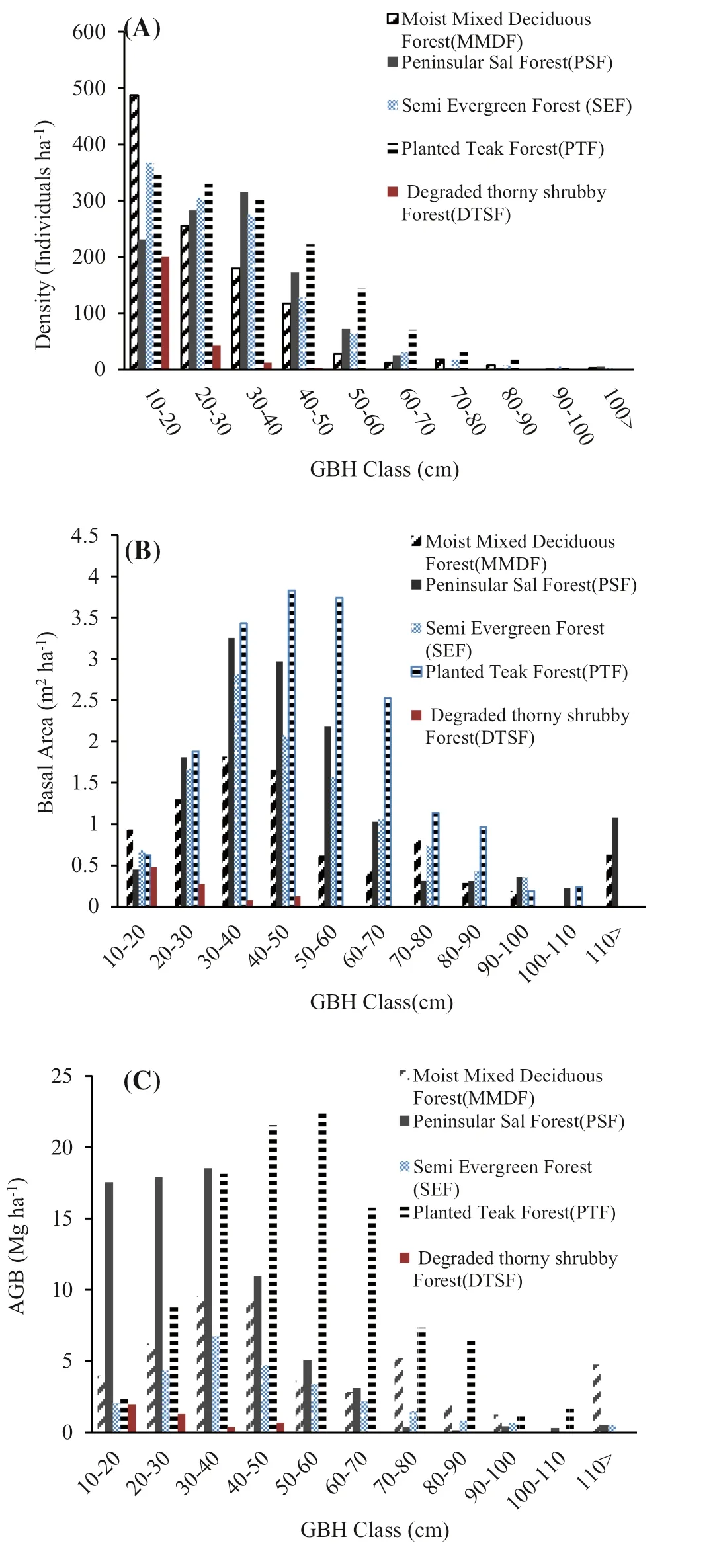
Fig.1 Stem density ( a), basal area ( b) and AGB ( c) by diameter class in five PFTs
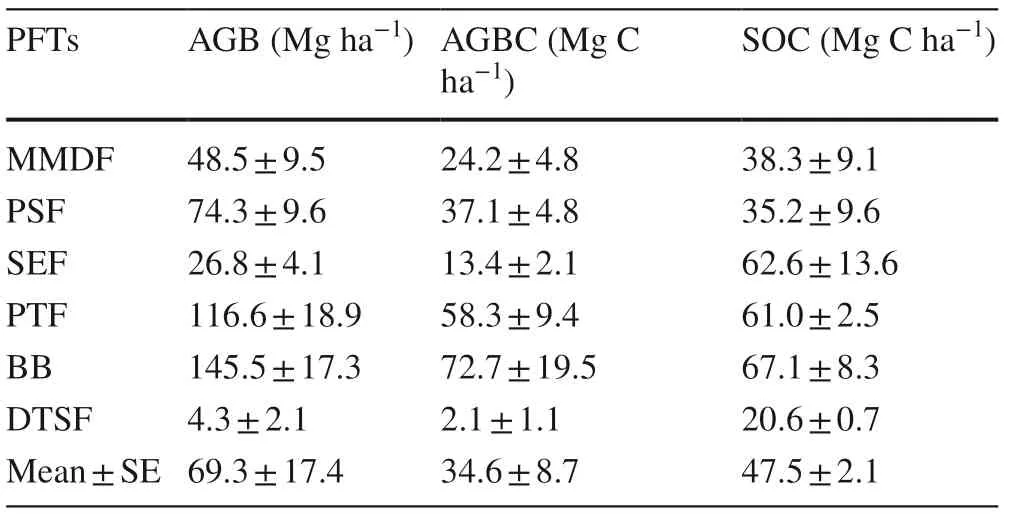
Table 2 Mean (± SE) AGB, AGBC and SOC stock for the six PFTs
Soil analysis
Physicochemical parameters of 240 soil samples were evaluated to provide average values (Table 3). Soil pH varied from 5.0 to 5.7, with variable electrical conductivity (EC) from 86.6 to 934.2 μs/cm and moisture content (6.8–11.8%). Soils of the Sanctuary were composed of 94.1% sand, 4.5% silt, and 1.2% clay with a fine sand category. Soil texture was predominantly sandy with a clay fraction of 0.6–1.9%. The sample from the degraded thorn shrub forest (DTSF) showed some variation in pH and EC. However, all the six PFTs soils had similar texture and moisture content capacity. The SOC content ranged from 5.2 to 17.1 gm/kg, and in different had similar results as above-ground biomass, i.e., lowest for DTSF (20.7 ± 0.8 Mg ha?1) and highest for the bamboo brakes (67.1 ± 8.4 Mg ha?1) (Table 3).
Statistical analysis
The data of AGB carbon and SOC distribution among the six PFTs were analysed through one- way ANOVA. Correlation and regression analysis were conducted for AGB carbon,basal area, importance value index (IVI) and soil physicochemical parameters.
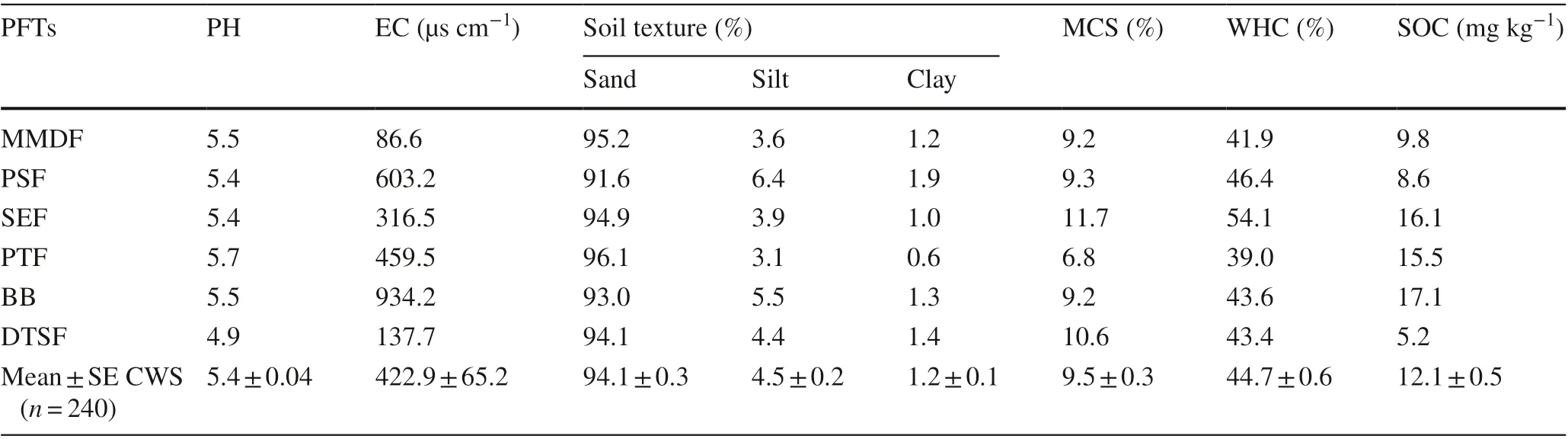
Table 3 Soil physicochemical parameters of the six PFTs
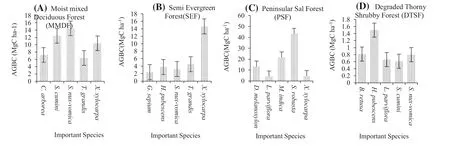
Fig.2 Above- ground biomass carbon (AGBC) of the five most important species in four PFTs
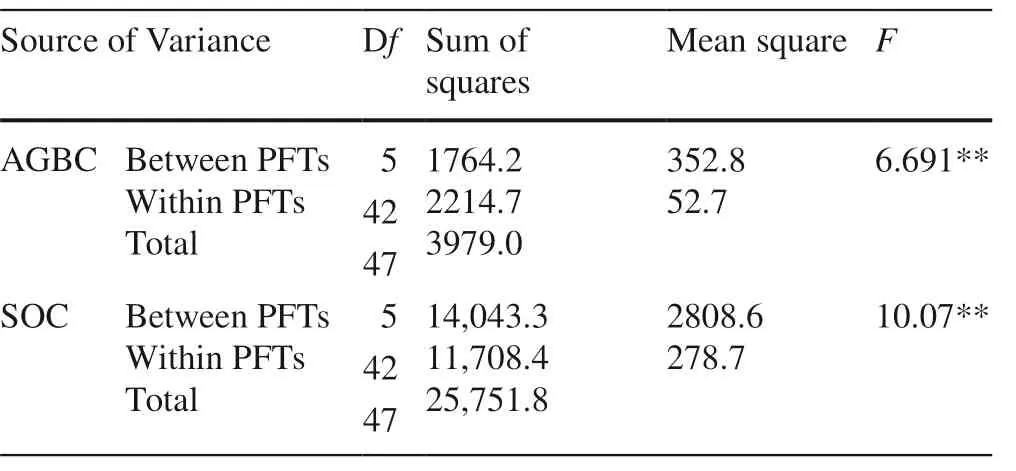
Table 4 Summary ofone-way ANOVA of AGBC and SOC among six PFTs
Comparing the results of AGBC and SOC (Table 4), f-values were significant at 0.01 levels, and the null hypothesis was rejected. The mean scores of AGBC and SOC differed significantly. The measure ofeffect through Etasquared value indicates that 44.3% of the variability of AGBC and 54.5% of the variability of SOC for PFTs. Thus the difference in variability is less in above-ground biomass carbon compared to the soil organic carbon.
The AGBC was influenced by basal area (BA; r = 0.67**, r 2 = 0.44) and the importance value index (IVI; r = 0.98**, r2= 0.45) (Table 5). The higher the BA and IVI, the higher the AGBC among the six PFTs. The Pearson correlation coeffi cient between AGBC and SOC ( r = 0.48*, p = 0.01) presented a consistent relation and hence the community structural parameters were also positively correlated with above-ground biomass. The regression of AGBC versus SOC confirmed the predicted r 2 (0.23) ≥ adjusted r 2 (0.20) and the new observations of the models closely fit the dataset. However, lower regression values represent an imprecise dataset. Furthermore, the prediction was not specific for r 2 = 0.006 for SOC versus clay content. The correlation study was also negative for this. The bivariate correlations between SOC with pH and electrical conductivity (EC) were low ( r = 0.34 and r = 0.20). The r-value of SOC with the moisture content in soil (MCS) and water holding capacity (WHC) (0.22 and 0.13, respectively) indicate the acceptance of the null hypothesis. In addition, the correlation was negative ( r = ? 0.076, p < 0.05) between percentages oforganic carbon and clay content of the soil, but positively correlated with sand content (Table 6).
Allocation of carbon in two pools
The pattern of carbon allocation in different PFTs of the sanctuary is shown in Fig.3. Maximum carbon allocation was in the SOC pool, contributing 82.2% and 90.5% in the semi-evergreen (SEF) and degraded thorn shrub (DTSF) forests, followed by the above-ground biomass carbon (AGBC) pool in each, of 17.8% and 9.5%, respectively. However, in case of the bamboo brakes (BB) and the peninsular sal forest (PSF), maximum carbon allocation was 52.0% and 51.4% in the AGBC pools, respectively, followed by the SOC pools at 48.0% and 48.6%, respectively. The moist-mixed deciduous forest (MMDF) and planted teak forest (PTF) had also higher carbon in SOC pools as compared to the AGBC. For the overall study, maximum carbon allocation was observed in the SOC pool (51–91%), followed by above- ground biomass pool (9–52%), indicating that soils are a major carbon sink in secondary dry forests.
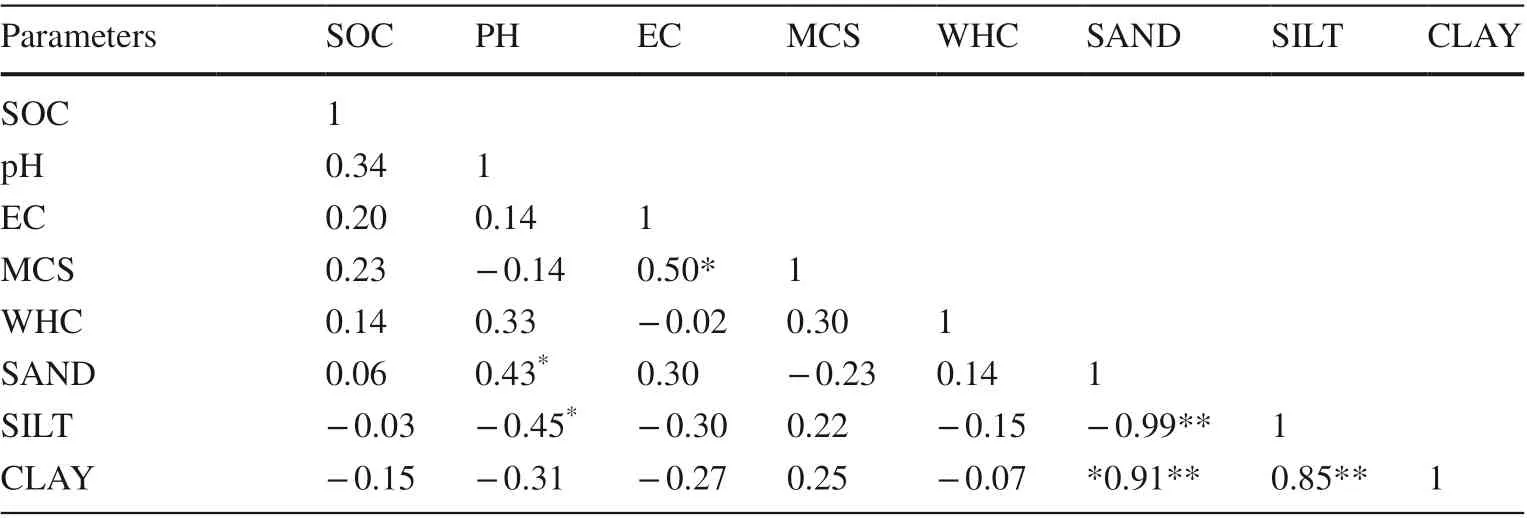
Table 6 Pearson correlation matrix of soil physico-chemical parameters
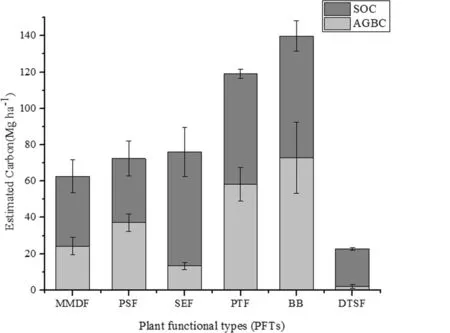
Fig.3 Bar chart for AGBC and SOC in the six PFTs (MMDF -Moist Mixed-Deciduous Forest; PSF-Peninsular Sal Forest; SEF-Semi-Evergreen Forest-; PTF-Planted Teak Forest; DTSF-Degraded Thorn Shrub Forest; BB-Bamboo Brakes)
Discussion
Topography, habitats and disturbances factors influence species diversity and distribution of plant functional types in tropical forests (Padalia et al. 2004). The species richness and composition of different forest types are consequences of factors such as plant productivity, competition, environmental variables, and biotic interference (Criddle et al. 2003). Average tree density in this study was 884 individuals/ha (range 420–1477 trees ha?1). This result is higher compared to different forests of India (Parthasarathy 1999; Kadavul and Parthasarathy 2006; Sahu et al. 2010; Reddy et al. 2011; Naidu and Kumar 2016; Dar and Sundarapandian 2016). In this study, the Shannon–Wiener diversity index value) ranged between [0.81 and 4.1], indicating moderate community complexity (Sundarapandian and Swamy 2000; Sahu et al. 2012). Similarly, Simpson’s index [0.56 0.85] falls within the range in other forests (Lalfakawma et al. 2010). Forests with high tree species diversity have higher diversity indices (Adekunle et al. 2013).
The mean basal area ofour study of 9.3 ± 1.7 m 2 ha?1was lower than those reported in previous studies of Indian forests (Cannon et al. 1994; Sist and Nguyen-The 2002; Borchsenius et al. 2004; Tripathi and Singh 2009; Gairola et al. 2012; Dar and Sundarapandian 2016). However, our results are comparable to the dry tropical forests of the Vindhyan region of 3.8–10.4 m 2 ha?1(Singh and Singh 1991), 6.6–23.2 m 2 ha?1(Jha and Singh 1990) and miombo woodlands of Zambia, 16.5 m 2 ha?1(Kalaba et al. 2013). Different altitudes, species density and age, diameter class distribution, regulate basal area (Rao et al. 1990). In the present study, basal area was positively correlated with above-ground biomass carbon ( r = 0.67, p < 0.05), which is in agreement with other studies (Murali et al. 2005; Gandhi and Sundarapandian 2017). This may be because of the allometric equations developed used the parameter of basal area or diameter at breast height (DBH) as prime factors.
Above-ground biomass (AGB) and carbon stocks of forests are influenced by basal area, stand density, species richness and importance value index (IVI) (Poorter 2015; Behera et al. 2017). In this study, there was a positive correlation between carbon stock with basal area and IVI ( r = 0.67, p < 0.05). Basal area, AGB and IVI were considered for identifying potential carbon amassing species. Accounting for these parameters, Bambusa bambos had relatively higher C stocks in both AGBC and SOC pools. In addition, species such as Tectona grandis, Xylia xylocarpa, Shorea robusta, Madhuca indica, and Diospyros melanoxylon and shrubs like Cipadessa baccifera, Naringi crenulata, and Xantolis tomentosa offered supplementary AGBC and SOC to the pools.
The correlation of large vegetative biomass and increased net primary productivity resulted from the higher input of carbon to the stock (Malhi et al. 2011), whereas soil topography, moisture contents, pH, and conductivity showed less variations in the different PFTs (Schrumpfet al. 2011; Aizat et al. 2014). Among the plant functional types, the bamboo brakes had higher AGBC and SOC and act as growing carbon sinks. Some deviations found in the semi-evergreen and degraded thorny shruby forests were due to the seasonal effects of sampling. Low silt and clay percentages [4.5 and 1.2% respectively may retain low organic carbon in soil content] as clay particles retain about 50–75% and silt 20–40% of the soil organic content (Christensen 2001). SOC reflects the amount oforganic matter present in the soil (Rawls et al. 2003). Hence, the high percentage of sand, low percentage of clay and moisture content limited microbial activities and the decomposition oforganic matter, which reduced SOC levels (Yue et al. 2018). The heterogeneity of forest soils, vegetation types, and plant growth conditions in the different PFTs of the sanctuary result in a wide range oforganic carbon levels. Soil organic carbon was positively correlated with pH, electrical conductivity and soil moisture content. The derivation of correlation and linear regression data was significant and affected by extraneous variables. The C/N ratio (51.4 ± 13.8) and low clay content reflect low carbonate humification as well as recent additions of above- ground and below- ground plant residues (Freixo et al. 2002). The forests types like moist mixed deciduous forest, Semi-evergreen forest, peninsular sal forest, planted teak forest of the Chandaka Wildlife Sanctuary needs protection for the long- term to enhance carbon sequestration and storage. This study provides information for the selection of important species for gap- filling and in plantations increase forest productivity.
Above-ground biomass is an important factor to assess the productivity of forest ecosystems. Data deficits in current biomass inventories in tropical forests and particularly in Indian forest ecosystems have been observed. Hence, the present information on above-ground biomass should be a useful addition to a national carbon inventory of India, and amount of carbon sequestered in secondary forests may contribute to the global carbon cycle. The estimated aboveground biomass carbon values at a PFT level has implications in carbon flux modelling at the ecosystem level. The current field measurements of above-ground biomass carbon and soil organic carbon at the PFT level in secondary deciduous forests may be useful in parameterization of different eco-physiological input variables for expressing the productivity in biome models (Running and Coughlan 1988). This information may be helpful for validation of productivity data obtained from the Moderate Resolution Imaging Spectrodiameter (MODIS) Net primary productivity Project (Neumann et al., 2016). Further, this study provides options for adaptation and management of dry secondary forests in a changing climate.
Conclusion
This study assessed the carbon stock in different plant functional types of the Chandaka Wildlife Sanctuary and established correlations between species diversity, important value index, basal area, above-ground biomass and soil organic carbon. Soil organic carbon and the importance value index (IVI) were positively correlated with above- ground biomass carbon. Our study estimated the maximum carbon storage in the soil (51–91%) followed by above- ground biomass (9–52%), indicating that soils are a major carbon sinks in secondary dry forests. The low carbon stock values of the Chandaka Wildlife Sanctuary may be attributed to the large number of small diameter, immature trees in the various forests. The bamboo brakes cover a major area (40%) and had the highest carbon stock, followed the planted teak forest (PTF), > peninsular sal forest (PSF) > moist- mixed deciduous forest (MMDF) > semi-evergreen forest (SEF) > degraded thorn shrub forest (DTSF). The important species occupying larger diameter classes would be greater carbon sinks. The larger biomass and their soil organic carbon resulted in higher input of carbon from vegetation to the soil carbon stock. Thus gap- filling planting of dominant species would increase forest productivity and amounts of carbon sequestered. The present above-ground biomass carbon levels of secondary tropical deciduous forests may be implemented in the flux of tentative CO2with seasonal variations at the ecosystem level.
AcknowledgementsThe authors are indebted to the Principal Chief Conservator of Forests, Government of Odisha for their financial support. The team wishes to convey a deep sense of gratitude to the Director, CSIR-IMMT, Bhubaneswar for laboratory facilities. We are also thankful to the Head, Department of Botany, North Orissa University, Baripada for co-operation to do this research work. We are pleased to place on record the help rendered by the Division Forest Offi cer of Chandaka Wildlife Sanctuary, Rangers of Chandaka and Bhubaneswar divisions and forest offi cials during our various survey trips.
References
Adekunle VAJ, Olagoke A, Akindele SO (2013) Tree species diversity and structure of a nigerian strict nature reserve. Trop Ecol 54(3):275–288
Aizat M, Mohamad-Roslan AMK, Sulaiman WNA, Karam DS (2014) The relationship between soil pH and selected soil properties in 48 years logged-over forest. Int J Environ Sci 4:1129–1140
Behera SK, Sahu N, Mishra AK, Bargali SS, Behera MD, Tuli R (2017) Aboveground biomass and carbon stock assessment in Indian tropical deciduous forest and relationship with stand structural attributes. Ecol Eng 99:513–524
So how are you meant to behave? Western audiences sit quietly, listening intently3 to the music and immersing4 themselves in the dance, then everyone claps loudly at the end. But Martins is convinced you should enjoy ballet in your own way. It s not necessary to follow convention5() .
Beretta AN, Silbermann AV, Paladino L, Torres D, Bassahun D, Musselli R, García-Lamohte A (2014) Soil texture analyses using a hydrometer: modification of the Bouyoucos method. Cien Inv Agrar 41:263–271
Berhongaray G, Cotrufo FM, Janssens IA, Ceulemans R (2018) Belowground carbon inputs contribute more than above-ground inputs to soil carbon accrual in a bioenergy poplar plantation. Plant Soil 434(1–2):363–378
Biswal AK, Thatoi HN, Sahu D (2005) Floral diversity in Chandaka wildlife sanctuary, Orissa. J Econ Taxon Bot 29:385–402
Borchsenius F, Nielsen PK, Lawesson JE (2004) Vegetation structure and diversity of an ancient temperate deciduous forest in SW Denmark. Plant Ecol 175:121–135
Brown S, Lugo AE (1982) The storage and production oforganic matter in tropical forests and their role in the global carbon cycle. Biotropica 14:161–187
Cannon CH, Peart DR, Leighton M, Kartawinata K (1994) The structure of lowland rainforest after selective logging in West Kalimantan, Indonesia. For Ecol Manag 67:49–68
Champion SH, Seth SK (1968) A revised survey of the forest types of India. A revised survey of the forest types of India. Manager of Publications, Delhi, pp 403–404
Chaturvedi RK, Raghubanshi AS, Singh JS (2011) Carbon density and accumulation in woody species of tropical dry forest in India. For Ecol Manag 262:1576–1588
Chave J, Andalo C, Brown S, Cairns MA, Chambers JQ, Eamus D, Lescure JP (2005) Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecolog 145:87–99 Chhabra A, Palria S, Dadhwal VK (2002) Spatial distribution of phytomass carbon in Indian forests. Glob Change Biol 8:1230–1239 Christensen B (2001) Physical fractionation of soil structural and functional complexity in organic matter turnover. Eur J Soil Sci 52:345–353
Condit R, Hubbell SP, Lafrankie JV, Sukumar R, Manokaran N, Foster RB, Ashton PS (1996) Species-area and species-individual relationships for tropical trees: a comparison of three 50-ha plots. J Ecol 84(4):549–562
Criddle RS, Church JN, Smith BN (2003) Fundamental causes of the global patterns of species range and richness. Russ J Plant Physiol 50:192–199
Dar JA, Sundarapandian S (2016) Patterns of plant diversity in seven temperate forest types of Western Himalaya, India. J Asia-Pac Biodivers 9:280–292
De Deyn GB, Cornelissen HC, Bardgett RD (2008) Plant functional traits and soil carbon sequestration in contrasting biomes. Ecol lett 11(5):516–531
Deng L, Zhu GY, Tang ZS, Shangguan ZP (2016) Global patterns of the effects of land-use changes on soil carbon stocks. Glob Ecol Conserv 5:127–138
Dixon RK, Solomon AM, Brown S, Houghton RA, Trexier MC, Wisniewski J (1994) Carbon pools and flux of global forest ecosystems. Science 263(5144):185–190
Ekoungoulou R, Liu X, Ifo SA, Loumeto JJ, Folega F (2014) Carbon stock estimation in secondary forest and gallery forest of Congo using allometric equations. Int J Sci Technol Res 3(3):465–474
FAO (2010) (Food and Agriculture Organization of the United Nations)
Global forest resources assessment 2010. FAO Forestry Paper 163 Rome, 49–63. http://www.fao.org/fores try/fra/fra20 10/en/. Accessed Mar 2018
Freixo AA, de A Machado PLO, dos Santos HP, Silva CA, de S Fadigas F (2002) Soil organic carbon and fractions of a Rhodic Ferralsol under the influence of tillage and crop rotation systems in southern Brazil. Soil Tillage Res 64(3–4):221–230
Gairola S, Sharma CM, Ghildiyal SK (2012) Chemical properties of soils in relation to forest composition in moist temperate valley slopes of Garhwal Himalaya, India. The Environmentalist 32(4):512–523
Galley RE (2014) Ecology of tropical rain forests. In: Monson RK (ed) Ecology and the environment. Springer, New York, pp 247–272
Gamble JS, Fischer CEC (1935) Flora of the presidency of madras 1915–1935. Adlard & Co., London, 2(1):51–55
Gandhi DS, Sundarapandian S (2017) Large-scale carbon stock assessment of woody vegetation in tropical dry deciduous forest of Sathanur reserve forest, Eastern Ghats, India. Environ Monit Asses 189(4):187
Gardner CMK, Robinson DA, Blyth K, Cooper JD (2001) Soil water content. In: Smith KA, Mullins CE (eds) Soil and environmental analysis: physical methods. CRC Press, New York, pp 1–64
Gove AD, Majer JD, Rico-Gray V (2005) Methods for conservation outside of formal reserve systems: the case of ants in the seasonally dry tropics of Veracruz, Mexico. Biodivers Conserv 126:328–338
Gray JM, Bishop TFA, Wilson BR (2015) Factors controlling soil organic carbon stocks with depth in eastern Australia. Soil Sci Soc Am J 79:1741–1751
ISFR (2017) (Indian State of Forest Report) Forest survey of India, Ministry of Environment and Forests, Government of India, India, pp 121–135
Jha CS, Singh JS (1990) Composition and dynamics of dry tropical forest in relation to soil texture. J Veg Sci 1:609–614
Jobbagy EG, Jackson RB (2000) The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol Appl 10:423–436
Kadavul K, Parthasarathy (2006) Structure and composition of woody species in tropical semi-evergreen forest of Kalrayan hills, Eastern Ghats, India. Trop Ecol 40:247–260
Kalaba FK, Quinn CH, Dougill AJ (2013) The role of forest provisioning ecosystem services in coping with household stresses and shocks in Miombo woodlands, Zambia. Ecosyst Serv 5:143–148
Kao D, Iida S (2006) Structural characteristics of logged evergreen forests in Preah Vihear, Cambodia, 3 years after logging. For Ecol Manag 225:62–73
Kasischke ES, Amiro BD, Barger NN, French NH, Goetz SJ, Grosse G, Masek JG (2013) Impacts of disturbance on the terrestrial carbon budget of North America. J Geophys Res Biogeosci 118:303–316
Ketterings QM, Coe R, Van Noordwijk M, Palm CA (2001) Reducing uncertainty in the use of allometric biomass equations for predicting above-ground tree biomass in mixed secondary forests. For Ecol Manag 146:199–209
K?hl M, Neupane PR, Lotfiomran N (2017) The impact of tree age on biomass growth and carbon accumulation capacity: a retrospective analysis using tree ring data of three tropical tree species grown in natural forests of Suriname. PLoS ONE 12(8):181–187
Kumar BM, Rajesh G, Sudheesh KG (2006) Aboveground biomass production and nutrient uptake of thorny bamboo [ Bambusa bambos (L.) Voss] in the homegardens of Thrissur, Kerala. J Trop Agric 43:51–56
Lalfakawma Sahoo UK, Roy S, Vanlalhriatpuia K, Vanalalhluna PC (2010) Community composition and tree population structure in undisturbed and disturbed tropical semi-evergreen forest stands of North-East India. Appl Ecol Envion Res 7(4):303–318
Lasco RD, Guillermo IQ, Cruz RVO, Bantayan NC, Pulhin FB (2004) Carbon stocks assessment of a secondary forest in mount Makiling forest reserve, Philippines. J Trop For Sci 16(1):35–45
Laurance WF, Oliveira AA, Laurance SG, Condit R, Nascimento HE, Sanchez-Thorin AC, Lovejoy TE, Andrade A, Dangelo S, Ribeiro JE, Dick CW (2004) Pervasive alteration of tree communities in undisturbed Amazonian forests. Nature 428:171–175
Laurance WF, Useche DC, Rendeiro J, Kalka M, Bradshaw CJ, Sloan SP, Arroyo-Rodriguez V (2012) Averting biodiversity collapse in tropical forest protected areas. Nature 489:290–294
Madugundu R, Nizalapur V, Jha CS (2008) Estimation of LAI and aboveground biomass in deciduous forests: western Ghats of Karnataka, India. Int J Appl Earth Obs Geoinf 10(2):211–219
Malhi Y, Doughty C, Galbraith D (2011) The allocation ofecosystem net primary productivity in tropical forests. Phil Trans R Soc B 366(1582):3225–3245
Margalef R (1958) Temporal succession and spatial heterogeneity in phytoplankton. Perspectives in marine biology. University of California Press, Berkeley, pp 323–349
Marimon BS, Felfili JM, Lima ES (2002) Floristics and phytosociology of the gallery forest of the Bacaba stream, Nova Xavantina, Mato Grosso, Brazil. Edinb J Bot 59:15–32
Mills AJ, Oconnor TG, Donaldson JS, Fey MV, Skowno AL, Sigwela AM, Lechmere-Oertel RG, Bosenberg JD (2005) Ecosystem carbon storage under different land uses in three semi-arid shrublands and a mesic grassland in South Africa. S Afr J Sci 22:183–190
Mishra R (1968) Ecology Work Book. Oxford and IBH Publishing Company, New Delhi
Mishra BP, Tripathi OP, Laloo RC (2005) Community characteristics of a climax subtropical humid forest of Meghalaya and population structure of ten important tree species. Trop Ecol 46:241–251
Mohandass D, Davidar P (2009) Floristic structure and diversity of a tropical montane evergreen forest (shola) of the Nilgiri mountains, southern India. Trop Ecol 50:219–229
Mohanraj R, Saravanan J, Dhanakumar S (2011) Carbon stock in Kolli forests, Eastern Ghats (India) with emphasis on aboveground biomass, litter, woody debris and soils. Biogeosci For 4:61–65
Mueller-Dombois D, Ellenberg H (1974) Aims and methods of vegetation ecology. Wiley, New York, pp 45–66
Murali KS, Bhatt DM, Ravindranath NH (2005) Biomass estimation equations for tropical deciduous and evergreen forests. Int J Agri Resour Gover and Eco 4(1):81–92
Naidu MT, Kumar OA (2016) Tree diversity, stand structure, and community composition of tropical forests in Eastern Ghats of Andhra Pradesh. India J Asia-Pac Biodivers 9:328–334
Neumann M, Moreno A, Thurnher C, Mues V, Harkonens S, Mura M, Bouriaud O, Lang M, Cardellini G, Thivolle-Cazat A, Bronisz K, Merganic J, Alberdi I, Astrup R, Mohren F, Zhao M, Hasenauer H (2016) Creating a regional MODIS satellite-driven net primary production dataset for European forests. Remote Sens 8(7):554
Padalia H, Chauhan N, Porwal MC, Roy PS (2004) Phytosociological observations on tree species diversity of Andaman Islands, India. Curr Sci 87:799–806
Parthasarathy N (1999) Tree diversity and distribution in undisturbed and human impacted sites of tropical wet evergreen forest in southern Western Ghats, India. Biodivers Conserv 8:1365–1381
Pielou EC (1966) The measurement of diversity in different types of biological collections. J Theoretic Biol 13:131–144
Poorter L (2015) Diversity enhances carbon storage in tropical forests. Glob Ecol Biogeogr 24(11):1314–1328
Ramachandran A, Jayakumar S, Haroon RM, Bhaskaran A, Arockiasamy DI (2007) Carbon sequestration: estimation of carbon stock in natural forests using geospatial technology in the Eastern Ghats of Tamil Nadu, India. Curr Sci 92:323–331
Rao P, Barik SK, Pandey HN, Tripathi RS (1990) Community composition and tree population structure in a sub-tropical broad-leaved forest along a disturbance gradient. Vegetatio 88:151–162
Ravindranath NH, Somashekhar BS, Gadgil M (1997) Carbon flow in India forests. Cli. Change 35:297–320
Rawls WJ, Pachepsky YA, Ritchie JC, Sobecki TM, Bloodworth H (2003) Effect of soil organic carbon on soil water retention. Geoder 116:61–76
Reddy CS, Babar S, Amarnath G, Pattanaik C (2011) Structure and floristic composition of tree stand in tropical forest in the Eastern Ghats of Andhra Pradesh, India. J For Res 22:491–500
Running SW, Coughlan JC (1988) A general model of forest ecosystem processes for regional applications: i-hydrologic balance, canopy gas exchange and primary production processes. Ecol Model 42:125–154
Sagar R, Raghubanshi AS, Singh JS (2003) Tree species composition, dispersion and diversity along a disturbance gradient in a dry tropical forest region of India. For Ecol Manag 186:61–71
Sahu SC, Dhal NK, Bhadra AK (2010) Arboreal taxa diversity of tropical forest of Gandhamaran hill range, eastern ghats, India: an approach to sustainable biodiversity conservation. Taiwania 55:208–215
Sahu SC, Dhal NK, Mohanty RC (2012) Tree species diversity and soil nutrient status in a tropical sacred forest ecosystem on Niyamgiri hill range, Eastern Ghats, India. J Mt Sci 9:492–500
Sahu SC, Sharma J, Ravindranath NH (2015) Carbon stocks and fluxes for forests in Odisha (India). Trop Ecol 56(1):77–85
Saxena HO, Brahmam M (1996) The flora of Orissa 1994–1996, vol Vol-I–IV. Regional Research Laboratory (CSIR), Bhubaneswar and Orissa Forest Development Corporation ltd., Bhubaneswar, pp 100–1500
Schrumpf M, Schulze ED, Kaiser K, Schumacher J (2011) How accurately can soil organic carbon stocks and stock changes be quantified by soil inventories? Biogeosci 8:1193–1212
Singh L, Singh JS (1991) Species structure, dry matter dynamics and carbon flux of a dry tropical forest in India. Ann Bot 68:263–273
Sist P, Nguyen-The N (2002) Logging damage and the subsequent dynamics of a dipterocarp forest in East Kalimantan (1990–1996). For Ecol Manag 165:85–103
Sundarapandian SM, Swamy PS (2000) Forest ecosystem structure and composition along an altitudinal gradient in the Western Ghats, South India. J Trop For Sci 12:104–123
Tchouto MGP, De Boer WF, De Wilde JJFE, Van der Maesen LJG (2006) Diversity patterns in the flora of the Campo-Maan rain forest, Cameroon: do tree species tell it all? For Divers Manag 2:293–314
Tripathi KP, Singh B (2009) Species diversity and vegetation structure across various strata in natural and plantation forest in Katerniaghat Wildlife Sanctuary, North India. Trop Ecol 50:191–200
Tripathi PO, Upadhaya K, Tripathi KS, Pandey HN (2010) Diversity, dominance and population structure of tree species along fragment size gradient of subtropical humid forest of Northeast India. Res J Environ Earth Sci 2:97–105
Vanden Bygaart AJ, Angers DA (2006) Towards accurate measurements of soil organic carbon stock change in agreecosystem. Cana J Soil Sci 86(3):465–471
Walkley A, Black IA (1934) An examination of Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci 37:29–37
Wei Y, Li M, Chen H, Lewis BJ, Yu D, Zhou W, Fang X, Zhao W, Dai L (2013) Variation in carbon storage and its distribution by stand age and forest type in boreal and temperate forests in Northeast China. Plos one 8(8):e72201
Woodward FL, Lomas MR, Kelly CK (2004) Global climate and distribution of plant biomes. Philos Trans R Soc Lond Ser B Bio Sci 359:1465–1476
Yue J, Guan J, Deng L, Zhang J, Li G, Du S (2018) Allocation pattern and accumulation potential of carbon stock in natural spruce forests in Northwest China. Peer J 6:e4859
 Journal of Forestry Research2021年2期
Journal of Forestry Research2021年2期
- Journal of Forestry Research的其它文章
- Stem taper functions for Betula platyphylla in the Daxing’an Mountains, northeast China
- Leaf phenotypic variation of endangered plant Tetracentron sinense Oliv. and influence of geographical and climatic factors
- Ecological variations of woody species along an altitudinal gradient in the Qinling Mountains of Central China: area-based versus mass-based expression of leaf traits
- Variations in stem radii of Larix principis-rupprechtii to environmental factors at two slope locations in the Liupan Mountains, northwest China
- A review of ecological mechanisms for management practices of protective forests
- Variation of basic density, calorific value and volumetric shrinkage within tree height and tree age of Ugandan grown Eucalyptus grandis wood
