The influence of environmental factors on species composition and distribution in a community forest in Northern Thailand
Siriluck Thammanu · Dokrak Marod · Hee Han · Narayan Bhusal ·
Lamthai Asanok5 · Pipat Ketdee2 · Noppakoon Gaewsingha6 · Seunghyun Lee4 · Joosang Chung1 ,7
Abstract Understanding the environmental factors that influence tree species composition is essential for successful management of biodiversity and sustainable use of community forest resources. This study aims to assess tree species composition and distribution in the deciduous Ban Mae Chiang Rai Lum Community Forest in Northern Thailand and to analyze the influence ofenvironmental factors on tree biodiversity in the forest. We conducted a stratified systematic sampling of the forest’s total area of 3925 ha, and twenty-five 0.16 ha survey plots were established in three different stands of the deciduous forests to estimate and characterize the difference in biological diversity among the stands. Canonical correspondence analysis (CCA) was used to investigate the environment factors affecting such differences in biodiversity of the stands. The results showed a high diversity of trees in the forest as 197 species, 144 genera, and 62 plant families were recorded. The CCA ordination identified the environmental factors—the most important of which were elevation, distance to streams, soil moisture, organic matter, and distance to communities—that significantly influenced the diversity and distribution of tree species ( p < 0.05) in the community forest. Our findings indicate that the implementation of drought reduction measures such as building check dams, fire protection, and monitoring community forest-product usage would be recommended to further biodiversity conservation and the sustainable use of community forest resources.
Keywords Community forest management · Species biodiversity · Canonical correspondence analysis · Ban Mae Chiang Rai Lum community forest · Northern Thailand
Introduction
In Southeast Asia, Community Forest Management (CFM) has been recognized as an effective approach to restore degraded forests, augment the income of the poor, and help meet the needs of local residents for food and medicine (Blair and Olpadwala 1988). CFM has been applied in pursuit of sustainability and forest biodiversity conservation in Thailand (Srisutham and Kaewjampa 2010) as the Royal Forest Department (RFD) has been promoting CFM since 1987. To date, CFM has been implemented in approximately 7% of the country’s total forest area, or more than 1.2 million ha, within roughly 17,400 villages nationwide (Royal Forest Department 2014, 2020).
Thailand is one of the most bio-diverse countries in Southeast Asia. Forests cover approximately 32% of Thailand’s total land area (Royal Forest Department 2019) and are inhabited by roughly 8% of the world’s plant species (Offi ce and Natural Resources and Environmental Policy and Planning 2009 ). However, a decline of forest biodiversity has become a critical issue. There are 1442 threatened plant species in the country, 715 of which have been classified as vulnerable, 207 as endangered and 18 as critically endangered (Department of National Parks, Wildlife and Plant Conservation 2017). Participation by local people in the management of forest resources has been identified as a promising way to address the issue of biodiversity loss in the country.
Although community forest management can facilitate biodiversity conservation, there is insuffi cient information informing how CFM affects biodiversity and how environmental factors influence that biodiversity in community forests. There are numerous factors that may impact the distribution of trees in community forests.
Climatic factors can threaten the extinction of vulnerable species (e.g. atmospheric pressure, temperature, precipitation, solar radiation, etc.) (K?rner 2007; Diem et al. 2018). Climatic factors such as temperature and precipitation are key drivers to control species distribution directly when they exceed the ecophysiological tolerances of species and affect photosynthetic activity and biological processes directly (Rowe 2009). Topographic features such as slope, aspect, and elevation can impact local climate as well as soil conditions that in turn have varied effects on vegetation structure (Zhang et al. 2006; Zhang and Zhang 2007; Zhang et al. 2013, 2016). The relative distance from a water source can also affect the composition and distribution of woody vegetation because of the resulting varying amount of water available for growth (Marod et al. 1999; Scalley et al. 2009; Tavili et al. 2009; Sarvade et al. 2016; Asanok et al. 2017). Physical and chemical soil properties can inform vegetation patterns on a local scale (Oliveira-Filho et al. 1998; Hejcmanovā-Ne?erková and Hejcman 2006; Han et al. 2011; Zhao et al. 2015). For example, higher soil sand ratio lessens water-holding capacity that can lead to water stress on trees (Aranguren et al. 1982; Toledo et al. 2012; Zhang et al. 2013), and acidity levels affect the distribution of species and is linked to slope and elevation in lowland tropical forests (Nguyen et al. 2015; Vahdati et al. 2017). Soil moisture also significantly changes the growth patterns of trees in drought areas (Fu et al. 2004; Yoshifuji et al. 2006; Asanok and Marod 2016; Tilk et al. 2017).
In addition, the organic matter in the soil is relevant to an analysis ofenvironmental factors and plant communities in the forest (Sarker et al. 2014; An et al. 2015; Zhang et al. 2016). Soil nutrients such as nitrogen, potassium, phosphorus, calcium, and magnesium are correlated with the richness and distribution of plant species in tropical forests (Paulo et al. 2007; Zhang et al. 2013; Tilk et al. 2017). Further, the human impact of resource utilization, road construction, and residential development significantly affects plant diversity (Chen et al. 2014 ; M?ren and Sharma 2018; Eghdami et al. 2019).
Overall, the environment’s impact on the biodiversity in community forests is complex as it involves a multitude of factors and considerations that provide limited answers, presents additional clues and raise new questions that are crucial to a more thorough and useful understanding of the relationship. This study sought to increase awareness of CFM’s role in the furtherance of biodiversity conservation and to seek additional knowledge to support these efforts. To do so, we identified ecological characteristics, ascertained species diversity indices, and investigated pertinent environmental factors impacting three unique forest stands in the community forest. We focused on the following questions: (1) what is the diversity and composition of tree species in the community forest? (2) what are key environmental factors influencing species distribution in the different stands? and (3) what resulting information could prove to be useful in CFM to protect species diversity and its benefits to livelihoods?
Materials and methods
Study area
The Pa Mae Phrik National Forest Reserve is located in Thailand’s northern province of Lampang. Situated in the southern region of this Forest Reserve is the Ban Mae Chiang Rai Lum Community Forest (17°22′48″ N–17°27′47″ N and 99°00′47″ E–99°05′48″ E), where in this study was conducted (Fig.1).

Fig.1 Location map of the Ban Mae Chiang Rai Lum Community Forest in Northern Thailand
This community forest has a total area of 3925 ha, is at an elevational range of 140–660 m and is composed of mixed deciduous and dry dipterocarp forest subtypes. Deciduous forests occur in areas with 5–6 month dry seasons, with a mean annual rainfall of 1000–1500 mm in dry dipterocarp forests and 1000–1800 mm in mixed deciduous forest (Bunyavejchewin 1983; Santisuk 1988). Mixed deciduous forests typically grow on moderate fertile loam soil while dry dipterocarp forests have more sandy lateritic soil (Bunyavejchewin 1983, 1985; Santisuk 1988). Forest fires are a regular feature of dry season in deciduous forests (Sukwong and Dhamanittakul 1977; Bunyavejchewin 1983). A previous study identified Xylia xylocarpa, Schleichera oleosa, Sindora siamensis, Shorea obtusa, Terminalia mucronata as the dominant species in the study area (Royal Forest Department 2017). This community forest has been locally managed in collaboration with the RFD under a community forest project since 2008 and plays a vital role as a source of biodiversity in providing non-timber forest products (NTFPs) while contributing to ecosystem services beneficial to livelihoods. However, unchecked tapping into the forest resources is widespread and creating a potential existential threat to the forest.
Northern Thailand in general, and Lampang in specific, have unique climatic features (Diem et al. 2018). The climate around the study area features a wet season from April to October and a dry season from November to March. Historically, this region also experiences drought conditions. Temperatures ranged from a minimum of 31.7 °C (January and November) to a maximum of 37.1 °C (March). The mean annual temperature, relative humidity, and rainfall were 33.6 °C, 76.1%, 1129.4 mm, respectively (Thai Meteorological Department 2018).
Field survey
A field survey was conducted in the forest from July to October 2018. Results of a 2016 study conducted by the RFD in the same area were used to calculate the sampling intensity with a confidence interval of 95%. An estimate of the sampling was obtained by using the standard deviation from previous surveys (Avery and Burkhart 1983; Asrat and Tesfaye 2013). The formula was expressed as:

where n is the sampling intensity; Z is the z-value for the confidence interval of 95%;σis the density of tree species, and E is the percent standard deviation of the precision required.
A total of 25 sampling plots of 40 m × 40 m (0.16 ha) were surveyed through a systematic sampling method (Asia Network for Sustainable Agriculture and Bioresources 2010). In each plot, trees with a diameter at breast height (DBH) ≥ 4.5 cm were identified and measured in every 10 m × 10 m sub-quadrat. Within 10 m sub-quadrats, samplings with DBH < 4.5 cm and height1.30 m were recorded in 4 m × 4 m sub-quadrats, whereas seedlings were documented in 1 m × 1 m sub-quadrats within each 4 m subquadrat (Fig.2).
Data collection on environmental factors
Climate data were generated by Hobo U23-001 and UA-002-64 which operated in six stations located in plots 3, 10, 13, 15, 17, and 24. The monitoring equipment was spread out and placed in these representative plots to provide the optimal coverage to accumulate climate data from throughout the study area. Historically, relevant climatic data did not vary significantly during the dry season. In addition, the dry season often featured forest fires. As such, data on temperature, relative humidity and light intensity were collected for 92 days during the wet season from July 11 to October 11, 2018.
Data regarding 15 environmental factors were collected from the 25 sampling plots. Those factors were broadly classified into topographic, edaphic, and anthropogenic. The topographic factors were elevation (EL), slope (SL), and aspect (AS), and the data were extracted out of 1:50,000 topographic maps produced by 20 m contour lines using the ArcGIS. The edaphic factors were investigated from samples collected at a depth of 15–20 cm in three random locations within each 40 m × 40 m plot during the rainy season from June to July 2018. Acidity (pH), organic matter (OM), soil texture [sand (SA), silt (SI), clay (CL)], available phosphorus (P), exchange potassium (K), exchange calcium (Ca), and soil moisture (SM) in the samples were analyzed. Soil pH was measured by 1:1 soil/water mol?1KCL (National Soil Survey Center 1996). OM was estimated by wet digestion and titration using Walkley–Black method (Nelson and Sommers 1996). Soil texture was determined by Pipette method (Gee and Bauder 1986). P was measured by Bray II (Bray and Kurtz 1945), while K and Ca were extracted with 1 M NH4OAc at pH 7.0 (Thomas 1982), respectively. The soil moisture analysis was performed by oven drying at 105 °C (National Soil Survey Center 1996). ArcGIS’s Euclidean distance tool was used to calculate the distance to streams (DS), distance to roads (DR), and distance to communities (DC) from the sampling plot.
Data analysis
The data analysis consisted ofexamining the biological diversity of the community forest and exploring environmental factors that affect tree species composition and distribution.
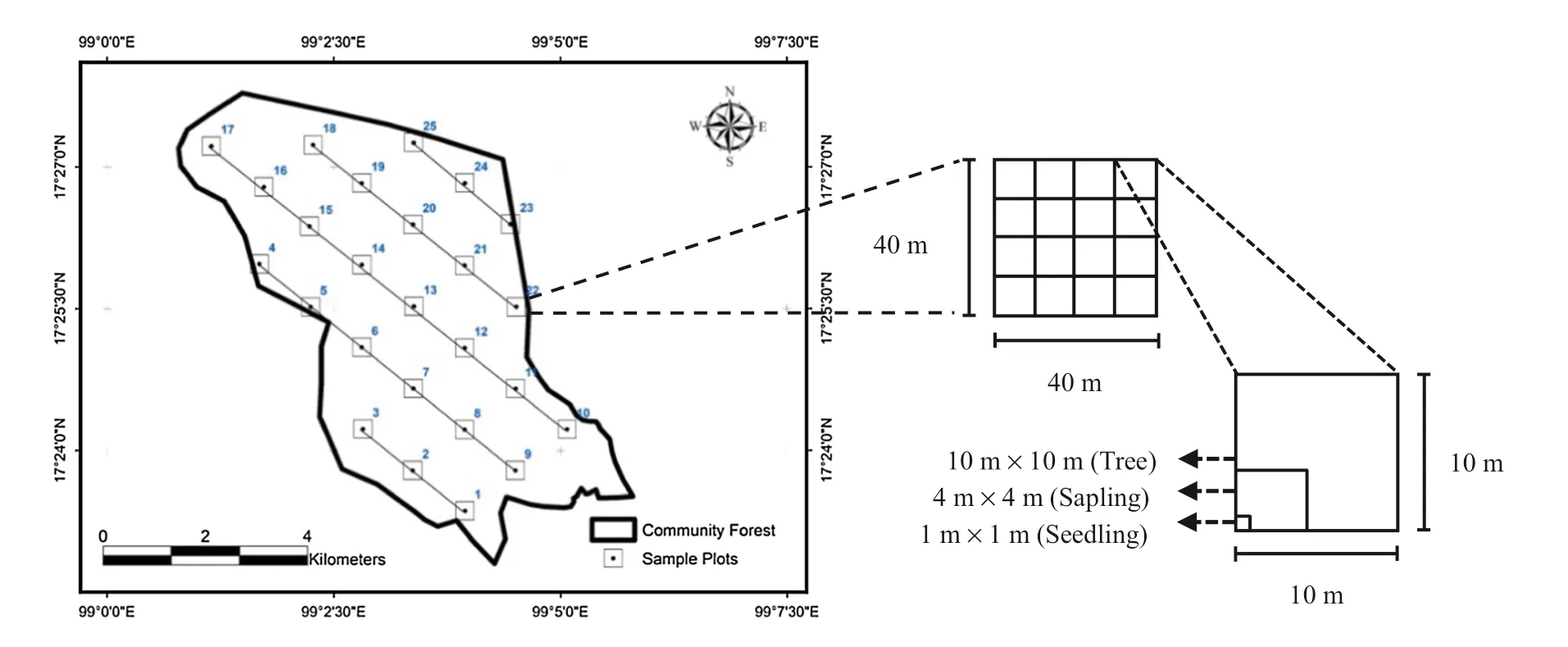
Fig.2 Sampling plots and sub-quadrats for identification and measurement
The forest stands in the community forest were identified by cluster analysis employing importance value index (IVI) matrices in each sampling plot. A cluster analysis requires pruning of the dendrogram at a level representing a compromise between the group and the number of groups. Optimum pruning for the dendrogram was selected by applying the Relative Sorensen Distance and Ward’s Linkage Method (McCune and Grace 2002).
To analyze the ecological characteristics of the three stands, we identified mature trees (DBH ≥ 4.5 cm) and calculated the stand density and basal area of individual tree species in each stand type.
Tree species diversity in each individual stand was analyzed, and a comparison of stand-to stand diversity was examined. A rarefaction approach was implemented in order to compare the diversity from the three different data-source stands (Colwell et al. 2012). Individual based interpolation and extrapolation of Hill numbers (q = 1, 2, 3) (Hill 1973) were carried out for each stand and the species diversity among the three forest stands was estimated as follows: species richness (q = 0), Shannon diversity (the exponential of Shannon entropy, q = 1), and Simpson diversity (the inverse of Simpson concentration, q = 2).
The ecological importance of the tree species in each stand was quantified through the IVI using the equation:
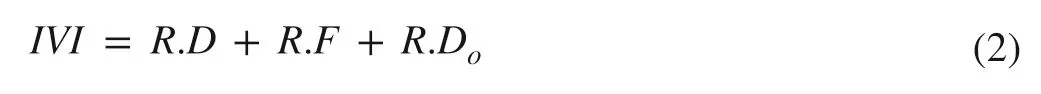
where R.D is the relative density of the tree species; R.F is their relative frequency, and R.Dois their relative dominance. They were calculated as R.D = number of individuals of the species × 100/total number of quadrate studies, R.F = number of quadrates in which species occurred × 100/total number of quadrate studies, and R.Do= total basal area of species × 100/total basal area of all the species (Curtis and McIntosh 1951).
To investigate the environmental factors that affect tree species in the community forest, a dataset of 15 factors was tested for normality using the Shapiro–Wilk test for an alpha level of 0.05. The abnormal data was transformed to a normal distribution to improve the linearity. In this study, AS, SI, SM, DR, and DC were normally distributed ( p > 0.05). Ten factors had values less than the chosen alpha level; log10(y) was used to transform SL, pH, OM, CL, K, and Ca, and (y)^3, 1/(y), sqrt(y), and ? 1/sqrt(y) were used to transform SA, EL, DS, and P, respectively.
The differences in stand characteristics, such as stand density, basal area, and the environmental factors among the three stand types were subjected to a one-way analysis of variance (ANOVA) with a post hoc Tukey’s honestly significant difference (HSD) test (Zhang et al. 2016). Species compositions in the three forest stands were compared using the Jaccard similarity index (Magurran 1988). The correlations between environmental factors were determined through Pearson correlation analysis. In case of a high correlation in which a matrix of two independent variables has correlation coeffi cients (r) > 0.7 across all environmental factors, only one of the factors was selected for the ordination analysis (Sarker et al. 2014).
The relationship between tree species distribution and environmental factors was finally assessed using the canonical correspondence analysis (CCA) (McCune and Grace 2002). The matrices between the IVI of mature trees (DBH ≥ 4.5 cm) in each stand and the environmental factors were analyzed in the CCA ordination. The correlation significance between the matrices was determined using the Monte Carlo test with 999 permutations. Only the environmental factors significantly related (Monte Carlo permutation test, p < 0.05) to the mature tree species were retained. All the statistical calculations were performed using PC.ORD version 5.10 (McCune and Mefford 2006) and the R program version 3.5.1 for Windows software (R Development Core Team 2019).
Results
Forest stand classification
The resulting dendrogram reflected 5.83% chaining and was cut with 25% of the remaining information explained by three stand types: Millettia leucantha- Lagerstroemia duperreana stand (MLS), Shorea siamensis- Shora obtusa stand (SSS), and S. obtusa- S. siamensis stand (SOS) (Fig.3).
Climate data
Relative humidity and light intensity data did not vary significantly plot-to-plot, but temperature data was significantly different ( p < 0.001). The average temperature during the 92-day collection period varied between 25.09 °C (plot 17) and 27.37 °C (plot 10). The average relative humidity ranged from 79.19% (plot 15) to 85.10% (plot 17), and the average light intensity ranged from 3011.67 l × (plot 15) to 4698.48 l × (plot 17). The overall average temperature, relative humidity, and light intensity throughout the study area were 26.85 °C, 82.23%, and 3549.09 l ×, respectively (Fig.4).
Species composition and diversity
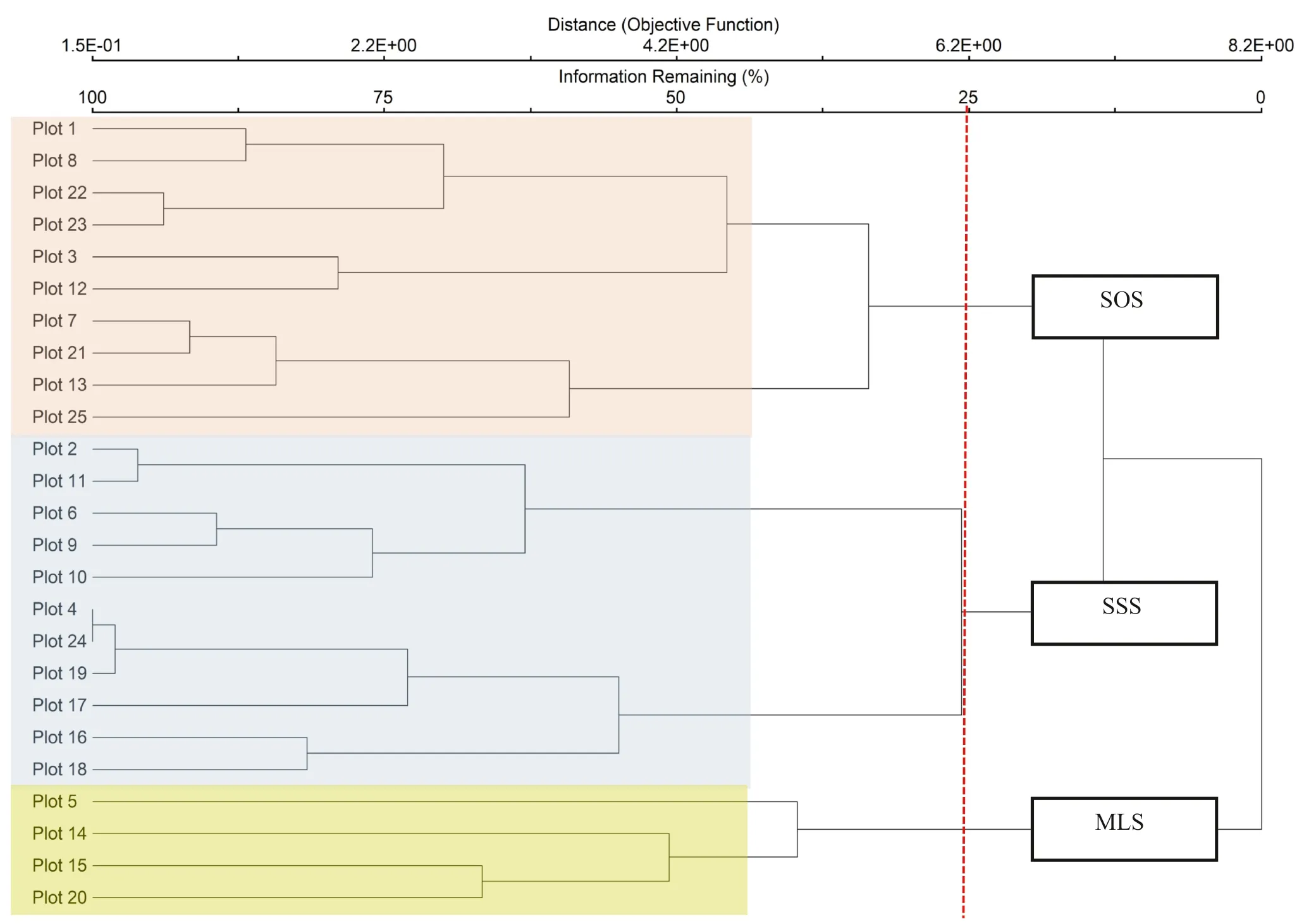
Fig.3 Classification of the stand types in the deciduous area of the Ban Mae Chiang Rai Lum Community Forest in Northern Thailand. MLS = Millettia leucantha- Lagerstroemia duperreana, SSS = Shorea siamensis- Shora obtusa, SOS = Shorea obtusa- Sindora siamensis
The field survey recorded 18,567 trees comprising 197 species (129 tree, 99 sapling, and 141 seedling species), 144 genera, and 62 plant families. The Jaccard index showed a 21.29% similarity (33 species) between the MLS and SSS, a 36.30% similarity (53 species) between the SSS and SOS, and a 17.78% similarity (24 species) between the MLS and SOS. The forest had an average density of 966 trees h?1and an average basal area of 16.74 m 2 h?1(Table S1). The one-way ANOVA test showed that the average density were significantly different stand-to-stand ( p < 0.01), though average basal area did not vary significantly.
The five species with the highest IVI in the MLS were M. leucantha, L. duperreana, M. brandisiana, Antidesma sootepense, and Pterocarpus macrocarpus. The most dominant tree species in terms of its IVI in the SSS was S. siamensis, followed by S. obtusa, X. xylocarpa, T. mucronata, and Dipterocarpus tuberculatus. S. obtusa was the most common species in the SOS, and it included S. siamensis, X. xylocarpa, Canarium subulatum, and Ellipanthus tomentosus (Table S2).
The rarefaction-produced tree species diversity values in different sample sizes of three forest stands are shown in Table S3. A comparison ofextrapolated species diversity shows that species richness was estimated to be the highest in the MLS, followed by the SSS and the SOS, respectively. The Shannon diversity and Simpson diversity also estimates are highest in the MLS, the SSS, and the SOS, in descending order (Fig.5).
Relationship between environmental factors and species diversity
The mean values and standard deviation of the 15 environmental factors analyzed in the Ban Mae Chiang Rai Lum Community Forest are shown in Table S4. The degree of soil acidity (pH), organic matter (OM), silt (SI), clay (CL), exchange potassium (K), soil moisture (SM), distance to roads (DR), and distance to communities (DC) were greater in the MLS than in the SSS and SOS. The values ofelevation (EL), slope (SL), available phosphorus (P), and exchange calcium (Ca) were highest in the SSS, and in decreasing order in the MLS and SOS. The value of aspect (AS), distance to streams (DS), and sand ratio (SA) were greater in the SOS than in the SSS and MLS.
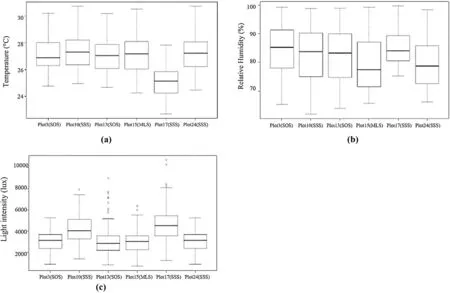
Fig.4 Average a temperature (°C), b relative humidity (%), and c light intensity (lux)
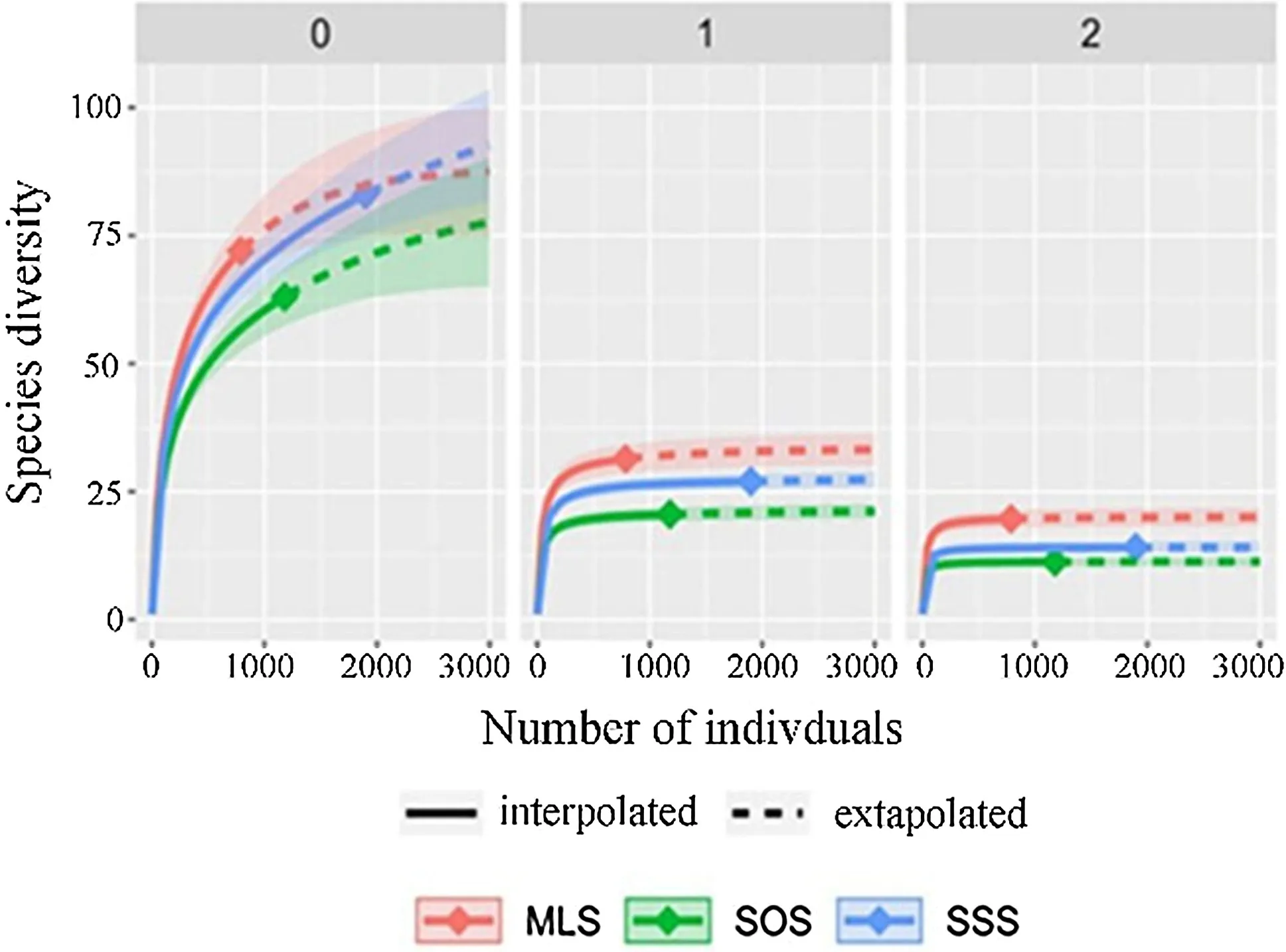
Fig.5 Comparison of individual-based rarefaction and extrapolation of species diversity for Hill numbers among three forest stands: species richness (q = 0), Shannon diversity (q = 1), and Simpson diversity (q = 2)
Five factors differed significantly among the three stand types; SL, OM, SA, CL, and K differed from one stand type to another ( p < 0.05). There was no significant difference in EL, AS, DS, pH, SI, P, Ca, SM, DR, and DC among the stands. The post hoc Tukey’s HSD test identified that the CL value was different between the MLS and the SOS, between the SOS and the SSS, and between the SSS and the MLS. OM and SA differed substantially between the MLS and the SOS, but they did not differ in the SSS. Similarly, SL differed between the SOS and the SSS, but it did not differ in the MLS. K varied when comparing the MLS with the SOS and the SSS with the SOS.
A matrix of two independent environmental factors showed that there were high correlation coefficients (r > 0.7) between the factors (Table S5). OM was positively correlated with K (r = 0.932). SA was positively associated with EL (r = 0.704), but negatively associated with SI (r = ? 0.890), CL (r = ? 0.929), DR (r = ? 0.746), and K (r = ? 0.724). CL positively correlated to DR (r = 0.710), but negatively correlated to EL (r = ? 0.707). EL negatively related to SL (r = ? 0.740). DC was positively related with DR (r = 0.853). Considering those correlations between the environmental factors, the ten variables, EL, AS, DS, pH, OM, P, Ca, SM, SI, and DC, were selected for further analysis using CCA. In the preliminary CCA, two poorly correlated factors, Ca and SI, were eliminated; the remaining eight factors were used in the final CCA.
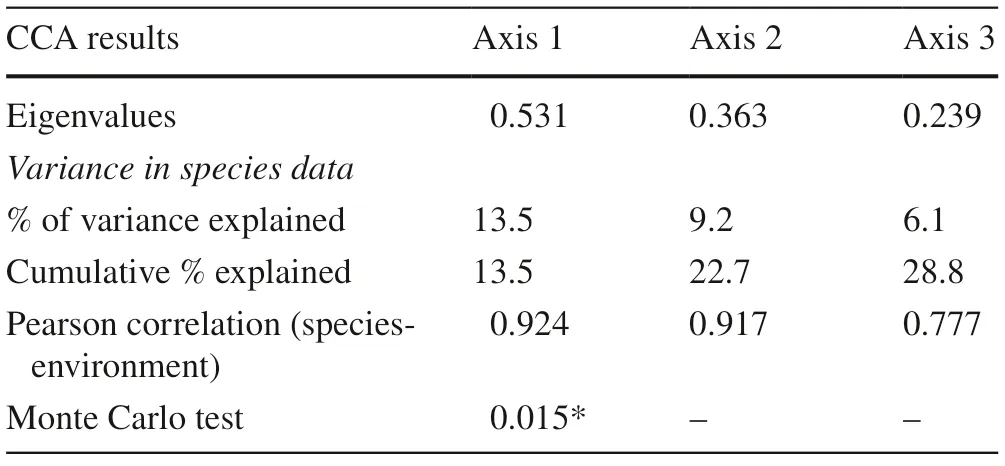
Table 1 Results of the canonical correspondence analysis (CCA) on the relationship between the distribution of tree species and the environmental factors
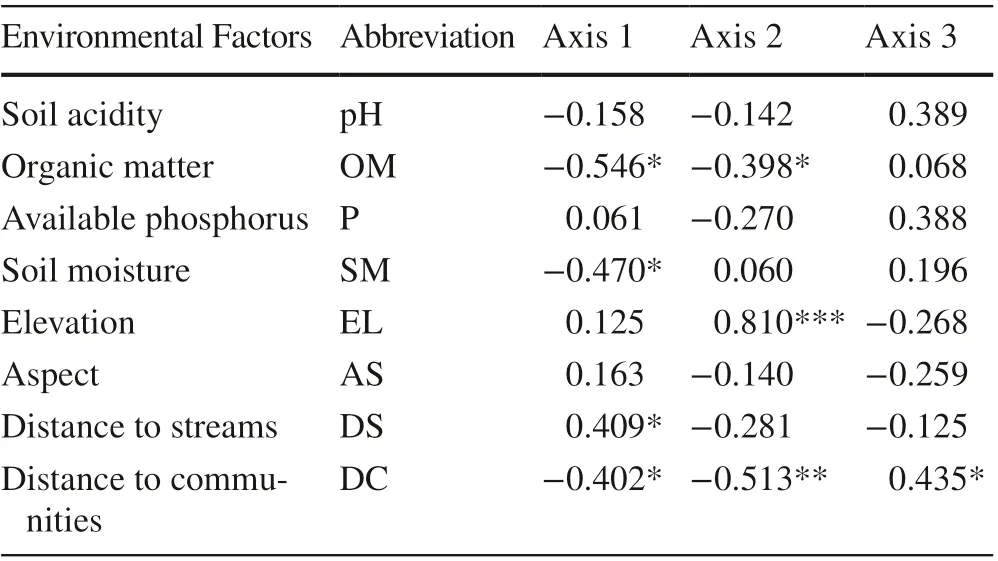
Table 2 Correlation coeffi cients between the eight environmental factors with the first three axes of the CCA
The IVI values (i.e. R.D, R.F, and RDo) ofeach of the 129 mature tree species and the eight explanatory environmental factors (pH, OM, P, SM, EL, AS, DS, and DC) in the 25 sampling plots were subjected to the CCA. The results of the CCA showed strong correlations between the distribution of tree species and the environmental factors (Table 1). The eigenvalues obtained for the first and second axes were 0.531 and 0.363, respectively. The Pearson correlation showed that the environmental factors had a high correlation to the species for axis 1 (0.924) and axis 2 (0.917). Therefore, these axes were considered as good indicators of relationships between the environmental factors and the species distribution in the community forest. The Monte Carlo permutation test confirmed that the first axis explained the significant variation in the community forest ( p < 0.05). The significance of the second and third axes was not reported for the reason that a simple randomization test for these axes could bias the p values (McCune and Mefford 2006).
The correlations between the environmental factors and the CCA axes are listed in Table 2. OM (r = ? 0.546) had the highest negative correlation coeffi cient with the first axis, and SM (r = ? 0.470), DC (r = ? 0.402), and pH (r = ? 0.158) were also negatively correlated with it. Conversely, DS (r = 0.409) had a positive correlation, followed by AS (r = 0.163), EL (r = 0.125), and P (r = 0.061). The second axis revealed a negative correlation with DC (r = ? 0.513), OM (r = ? 0.398), DS (r = ? 0.281), pH (r = ? 0.142), and AS (r = ? 0.140), but it displayed a positive correlation with EL (r = 0.810) and SM (r = 0.060). The comparison among the environmental factors showed that pH was positively correlated with OM (r = 0.563) and P (r = 0.547) as was OM with P (r = 0.604) (Table S5).
The CCA results demonstrate the relationships between environmental factors and species distribution (Figs. 6 and 7). In Grouping 1, the EL was an important factor in species distribution in plots 2, 9, and 11 of the SSS and in plots 1, 7, 8, and 12 of the SOS. The abundant species in this grouping were Atalantia monophylla (ATMO), Antidesma ghaesembilla (ANGH), Gluta usitata (GLUS), Bauhinia bracteata (BABR), Flacourtia indica (FLIN), Memecylon edule (MEED), and Flacourtia rukam (FLRU). In Grouping 2, DS was linked to Ochna integerrima (OCIN), Syzygium cumini (SYCU), Madhuca dongnaiensis (MADO), Morinda elliptica (MOEL), and Tectona grandis (TEGR) in plots 6 and 10 of the SSS and 3, 13, 21, and 23 of the SOS. This suggests that these species can be impacted by their distance from streams. Regarding Grouping 3, SM, OM, and DC were closely related to Polyalthia cerasoides (POCE), Zollingeria dongnaiensis (ZODO), Hymenodictyon orixense (HYOR), Zizyphus oenoplia (ZIOE), P. macrocarpus (PTMA), Mitragyna rotundifolia (MIRO), Lannea coromandelica (LACO), Bridelia retusa (BRRE), and Wendlandia tinctoria (WETI) in plots 14, 15, and 20 of the MLS and in plot 24 of the SSS. This indicates that they were restricted by moisture content, limited by OM, and closely correlated to human disturbance.
Discussion
The Ban Mae Chiang Rai Lum Community Forest was divided into three forest stands: the mixed deciduous MLS and the dry dipterocarp SSS and SOS (Fig.3). A rarefaction prediction of species diversity (Fig.5 and Table S3) and the wide array of species, genera, and families (Table S1) recorded via the field survey indicate remarkable diversity, similar to other forests of Northern Thailand (Popradit et al. 2015; Khamyong et al. 2018). There were no noteworthy differences in the average basal areas, but average density varied significantly among the three stand types. There was a degree of species similarity among the stands whereas the forest structures and species compositions were distinct (Table S2), In addition, the importance of the environment factors varied stand-to-stand (Table S4) as did their degree of correlation (Table S5).
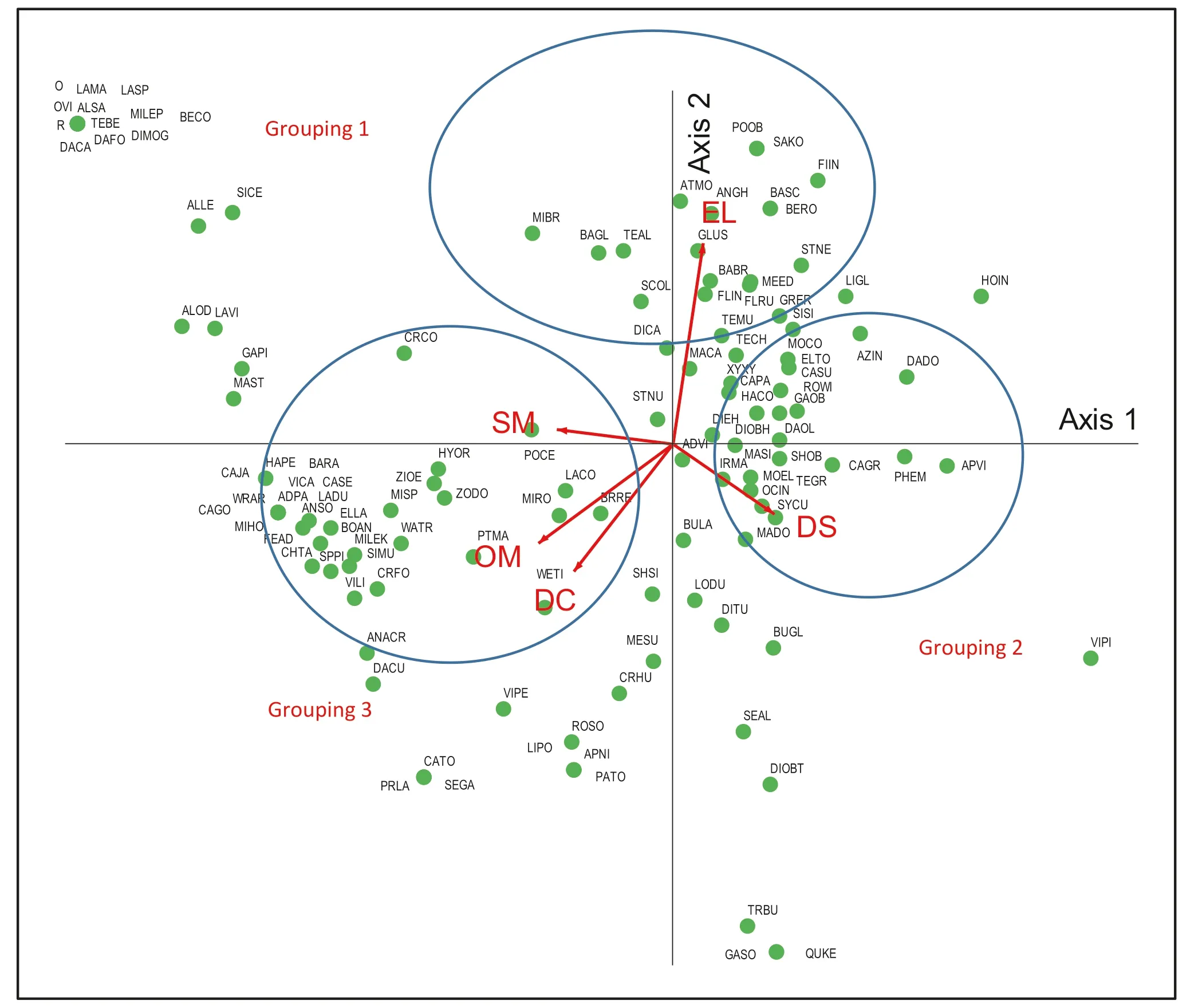
Fig.6 The CCA ordination diagram representing the relationship between the distribution of tree species and the environmental factors. Full names of tree species by abbreviation are listed in Table S6
Our results represent that the heterogeneity of species distribution patterns among the different forest stand types could be a response to a variation in the environmental factors; distinct environmental conditions in forest stands can be effective indicators of species distribution in the Ban Mae Chiang Rai Community Forest in Northern Thailand.
The results of the CCA demonstrated that species composition and distribution of the mature trees were related to topographic, edaphic, and anthropogenic factors (Table 1). The environmental factors showed both positive and negative impacts on species diversity. Specifically, EL, DS, SM, OM, and DC were the most evidently linked to species distribution (Table 2). Moreover, environmental factors had different effects on species distribution between stand types (Figs. 6 and 7). This indicates that species composition and distribution can be limited by a combination ofenvironmental factors across distinct forests.
Among the five significant factors (i.e. EL, DS, SM, OM, and DC), EL appeared to be the most influential on species distribution. The IVI of mature trees was positively related to elevation in the SSS and SOS; elevational gradients promote not only species diversification but also soil differentiation (Zhang and Zhang 2007; Zhang et al. 2013, 2016). Though, climatic factors can vary with elevation (Zhang et al. 2006; K?rner 2007), the elevational features of dry dipterocarp in Thailand occur mainly in elevations up to 900 m (Bunyavejchewin et al. 2011). At this elevation, there is no drastic change in lowland vegetation similar to that found in most tropical zones at elevations above 1000 m, including in the North of Thailand (Küchler and Sawyer 1967). Thus,the various soil properties unique to elevation could be key to the composition and distribution of tree species in the dipterocarp forest stands this study area (Bunyavejchewin et al. 2011).
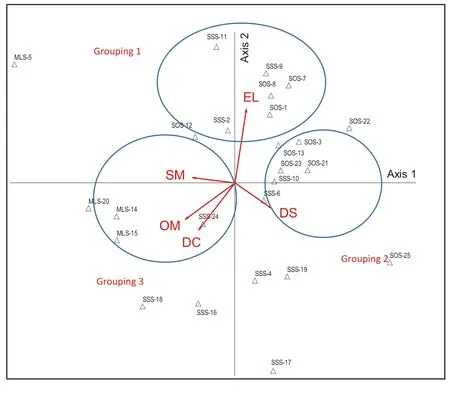
Fig.7 The five most important environmental factors explaining the characteristics of different stand types in the CCA ordination: elevation (EL), distance to streams (DS), soil moisture (SM), organic matter (OM), and distance to communities (DC) affecting 25 plots in the three stands (Δ): Millettia leucantha- Lagerstroemia duperreana stand (MLS), Shorea siamensis- Shora obtusa stand (SSS), and Shorea obtusa- Sindora siamensis stand (SOS)
Other tree species associated with dominant species were also found in the dry dipterocarp stand. This suggests that higher elevation promotes species diversity in dry dipterocarp forests. Teejuntuk et al. ( 2003) reported that the diversity of tree species in Doi Inthanon National Park, Northern Thailand increased up to an elevation of about 1800 m. Also, it has been found that tree species in dry dipterocarp forests can move into higher land to grow with other tree species in an ecotone zone (Kutintara 1975; Marod et al. 2015) which may be related to a more favorable environment for growth at slightly higher elevation (Ivanov et al. 2008).
Elevational gradients often limit the distribution of tree species by decreasing soil moisture and nutrient levels (Bridge and Johnson 2000). Several studies have reported that an increase in species diversity may have an elevational limit above which diversity would become negatively correlated (Liu et al. 2018). For instance, the resources necessary for plant growth are limited at extremely high elevations because of strong winds and shallow soils (Zhang et al. 2013).
In this study area, elevation ranged between 140 and 660 m. Higher elevation and SI, CL, and K were negatively correlated whereas SA was positively correlated (Table S5). Poor sandy soil is an important driver for tree species in dry dipterocarp forests (Bunyavejchewin 1983, 1985; Santisuk 1988). Therefore, some tree species in the SSS and the SOS may be able to grow at higher elevation because of more favorable soil conditions without the limiting climatic conditions found at extremely high elevation.
In this study, it appears that DS and SM influenced species diversity and distribution. The positive correlation between species diversity in the SSS and SOS with the DS revealed that diversity is related to water availability. This indicates that dry conditions may negatively impact species diversity and distribution. Several species in the dry dipterocarp forests are strongly associated with proximity to water sources. This suggests that tree species adapt to drought areas that have low soil moisture, high aridity, and fire disturbance. These are important factors that determine the species occurrence in dry dipterocarp forests in Thailand (Rundel and Boonprakob 1995; Marod et al. 2002; Bunyavejchewin et al. 2011).
Similarly, the negative correlation of species diversity in the MLS and SSS with SM substantiated the occurrence of these forests in dry regions (Marod et al. 1999). Tree species such as P. macrocarpus (PTMA), P. cerasoides (POCE), Z. dongnaiensis (ZODO), H. orixense (HYOR), and L. coromandelica (LACO) were closely associated with SM, which suggests that they were restricted by moisture levels and sensitive to drought conditions. Generally, these species had a higher water demand than other tree species in deciduous forests that could adapt and survive in arid areas (Marod et al. 1999). Previous findings indicate that SM decreased as the distance from a reservoir increased (Sarvade et al. 2016), suggesting that the species in MLS and SSS were also related to distance from a water source.
Deciduous forests often occur in arid areas with sandy soil; 53% of the soils in mixed deciduous forests and 70% of those in deciduous dipterocarp forests consist of sand (Myo et al. 2016). Sandy soils are more susceptible to leaching which can lead to drought (Aranguren et al. 1982; Zhang et al. 2013). Soils with higher sand content have lower waterholding capacity, thereby causing plants to compete more for soil moisture (Toledo et al. 2012). Contrarily, trees growing in clay soil have more effi cient root system and are less susceptible to drought stress because of the greater capacity of the soil for water retention. Trees with deeper roots in sandy soil have a greater chance of surviving as they adapt to reduce drought stress (Wessel 1971). Hence, a deep root system enables plants to adapt to and survive in extremely dry conditions, such as being distant from a water supply or being in an area with limited soil moisture. In this study, our results imply that trees survive and grow well despite the dry conditions in the SSS and SOS; the suffi cient depth of root system of trees in the both stands has enabled them to adapt to drought stress. However, the existence some species in the MLS and SSS may be limited by the level of moisture in soil for their growth.
The negative correlation of the dominant species in the MLS and SSS with OM and DC, explains that both environmental factors constrain tree growth and diversity in their corresponding forest stands. The growth, species distribution, and composition of trees in tropical areas are influenced by soil nutrients (John et al. 2007; Santiago et al. 2012). Many studies have shown that OM is a key environmental factor in plant communities (Zhang and Zhang 2007; Slik et al. 2009; Sarker et al. 2014), and that it contributes to the availability of nutrients and water by improving soil structure and physical conditions, increasing the water holding capacity of the soil, and providing a habitat for plant roots and soil organisms (Carter 2002; Meng et al. 2014). Thus, soils with more OM are more fertile and more favorable for the optimal growth of trees (Vahdati et al. 2017).
In the study forest, however, there were tree species in the MLS and SSS that were inversely associated with OM, indicating that this environmental factor was restricting their growth. As explained by Grime ( 1977), if nutrients are scarcely available in the soil, then plants will have to adapt to low-resource and nutrient-poor conditions, making natural selection predominant in the process.
Plant productivity is positively linked with OM, as reported by Bauer and Black ( 1994), thus, more OM should be made available for plants to increase their productivity. Our findings also revealed that OM had a positive correlation with pH and P (Table S5), indicating that trees are not only affected by OM content but that pH and P are major soil properties involved in plant growth. In a previous study conducted in a tropical forest in Ben En National Park, Vietnam, it was reported that an increase in OM could augment P and pH, thereby enhancing species composition and distribution of plants in the area (Hoang et al. 2011).
The dominant species were closely correlated with DC, supporting a previous report that human disturbance is a threat to plant diversity loss (Millennium Ecosystem Assessment 2005). Not only were dominant species found distributed in the stands, but shrub species such as W. tinctorial (WETI), M. rotundifolia (MIRO), B. retusa and Z. oenoplia (ZIOE) were also distributed near the communities. These species are often used for firewood and construction. As such, there is a high risk of them being harvested thereby affecting their distribution. Furthermore, they are pioneer species that play a vital role in ecological succession after disturbances (Department of National Parks, Wildlife and Plant Conservation 2007; Asanok et al. 2020). Utilization by communities may have an impact on the natural restoration of forest ecosystems in the community forest.
Many studies have shown that the impact on tree species in forests could be worse if they were situated closer to a community (Thapa and Chapman 2010; Hoang et al. 2011; Chen et al. 2014; Asanok et al. 2017; Martínez-Camilo et al. 2018). These studies reported that trees would be more abundant in less disturbed plots located far from human settlements. Furthermore, Teejuntuk et al. ( 2003) discussed that at an elevation less than 1800 m in Northern Thailand, the occurrence ofecologically similar species increased at higher elevation since forests at higher elevation are relatively far and inaccessible to man. Lowland forests, conversely, have become significantly more fragmented and degraded because of deforestation through illegal logging, burning, and grazing. Human disturbance is more rampant in areas with easy access, poor monitoring and weak enforcement of regulations (M?ren and Sharma 2018). The apathy of locals to forest management also aggravates the problem and contributes to the decline of forest biodiversity.
Conclusions
Our study suggests that elevation, distance to streams, soil moisture, organic matter, and distance to communities are the most important factors influencing species composition and distribution in the deciduous forests of the Ban Mae Chiang Rai Lum Community Forest in Northern Thailand. Even through tree species in deciduous forests are able to shift to higher elevation to find more favorable conditions and adapt themselves to grow in drought areas, the limitation oforganic matter, water availability, and soil moisture can still impact tree species diversity. This could prove to be important and useful information for community forest management. We recommend that management practices for drought reduction such as building check dams and fire protection be implemented to protect biodiversity, especially in the MLS and SSS.
In addition, biodiversity conservation would be better served by monitoring the utilization of forest resources by nearby communities. Species propagation, silviculture, and transplantation should be initiated and/or expanded, particularly in neighboring villages or private farms, to enhance the productivity and viability of the community forest. However, future studies are needed to investigate the threat that community utilization of trees poses to tree species’ very survival as this was not an area of investigation in this study.
AcknowledgementsWe thank the Royal Forest Department (RFD) and the Asian Forest Cooperation Organization (AFoCO) for providing such a valuable opportunity for us to pursue this project. We wish to express our sincere gratitude to the village leaders and people of Ban Mae Chiang Rai Lum for their assistance during our fieldwork.
Author’s contributionsConception and design ofexperiments: Siriluck Thammanu, Joosang Chung, Dokrak Marod, Lamthai Asanok, and Seunghyun Lee. Field forest survey: Siriluck Thammanu, Lamthai Asanok, Pipat Ketdee, and Noppakoon Gaewsingha. Data analysis: Siriluck Thammanu, Dokrak Marod, and Hee Han. Wrote the paper: Siriluck Thammanu, Joosang Chung, Hee Han, and Narayan Bhusal. All authors read and approved the final manuscript.
Open AccessThis article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creat iveco mmons.org/licen ses/by/4.0/.
References
An P, Li XJ, Zheng YR, Eneji AE, Qiman Y, Zheng MQ, Inanaga S (2015) Distribution of plant species and species-soil relationship in the East Central Gurbantunggut Desert, China. J Geogr Sci 25:101–112. https://doi.org/10.1007/s1144 2-015-1156-0
Aranguren J, Escalante G, Herrera R (1982) Nitrogen cycle of tropical perennial crops under shade trees. Plant Soil 67:247–258. https://doi.org/10.1007/BF021 82772
Asanok L, Marod D (2016) Environmental factors influencing tree species regeneration in different forest stands growing on a limestone hill in Phrae Province, Northern Thailand. JFES 32:237–252. https://doi.org/10.7747/JFES.2016.32.3.237
Asanok L, Kamyo T, Norsaengsri M, Salinla-um P, Rodrungruang K, Karnasuta N, Navakam S, Pattanakiat S, Marod D, Duengkae P, Kutintara U (2017) Vegetation community and factors that affect the woody species composition of riparian forests growing in an urbanizing landscape along the Chao Phraya River, Central Thailand. Urban For Urban Green 28:138–149. https://doi.org/10.1016/j.ufug.2017.10.013
Asanok L, Taweesuk R, Papakjan K (2020) Woody species colonization along edge-interior gradients of deciduous forest remnants in the Mae Khum Mee Watershed Northern Thailand. Int J For Res. https://doi.org/10.1155/2020/58673 76
Asia Network for Sustainable Agriculture and Bioresources (2010) Participatory inventory of non-timber forest products. ANSAB, Kathmandu, p 64
Asrat Z, Tesfaye Y (2013) Training manual on: forest inventory and management in the context of SFM and REDD+. Hawassa University, Wondo Genet, p 69
Avery TE, Burkhart HE (1983) Forest measurements, 3rd edn. McGraw-Hill Publishing Company, New York, p 331
Bauer A, Black AL (1994) Quantification of the effect of soil organic matter content on soil productivity. Soil Sci Soc Am J 58:185–193. https://doi.org/10.2136/sssaj 1994.03615 99500 58000 10027 x
Blair HW, Olpadwala PD (1988) Forestry in development planning: Lessons from the rural experience. Westview Press, London, p 205
Bray R, Kurtz L (1945) Determination of total, organic, and available forms of phosphorus in soils. Soil Sci 59:39–46
Bridge SRJ, Johnson EA (2000) Geomorphic principles of terrain organization and vegetation gradients. J Veg Sci 11:57–70. https ://doi.org/10.2307/32367 76
Bunyavejchewin S (1983) Analysis of tropical dry deciduous forest of Thailand. I. Characteristics of dominance types. Nat Hist Bull Siam Soc 31:109–122
Bunyavejchewin S (1985) Analysis of the tropical dry deciduous forest of Thailand. II. Vegetation in relation to topographic and soil gradients. Nat Hist Bull Siam Soc 33:3–20
Bunyavejchewin SC, Baker P, Davis SJ (2011) Seasonally dry tropical forests in continental Southeast Asia-structure, composition, and dynamics. In: McShea WJ, Davis SJ, Bhumpakphan N (eds) The ecology and conservation of seasonally dry forests in Asia. Smithsonian Institution Scholarly Press, Washington, pp 9–35
Carter MR (2002) Soil quality for sustainable land management: organic matter and aggregation interactions that maintain soil functions. Agron J 94:38–47. https://doi.org/10.2134/agron j2002 .0038
Chen YK, Yang XB, Yang Q, Li DH, Long WX, Luo WQ (2014) Factors affecting the distribution pattern of wild plants with extremely small populations in Hainan Island, China. PLOS ONE 9:e97751. https://doi.org/10.1371/journ al.pone.00977 51
Colwell RK, Chao A, Gotelli NJ, Lin SY, Mao CX, Chazdon RL, Longino JT (2012) Models and estimators linking individual-based and sample-based rarefaction, extrapolation and compare of assemblages. J Plant Ecol 5:3–21. https://doi.org/10.1093/jpe/rtr04 4
Curtis JT, McIntosh RP (1951) An upland forest continuum in the prairie-forest border region of Wisconsin. Ecology 32:476–796. https://doi.org/10.2307/19317 25
Department of National Parks, Wildlife and Plant Conservation (2007) Manual for studies of wild plants. The Agricultural Cooperative Federation of Thailand Ltd., Bangkok, p 195
Department of National Parks, Wildlife and Plant Conservation (2017) Threatened plants in Thailand. Omega Printing Co., Ltd., Bangkok, p 224
Diem PK, Pimple U, Sitthi A, Varnakovida P, Tanaka K, Pungkul S, Leadprathom K, LeClerc MY, Chidthaisong A (2018) Shifts in growing season of tropical deciduous forests as driven by El Ni?o and La Ni?a during 2001–2016. Forests 9:448. https://doi.org/10.3390/f9080 448
Eghdami H, Azhdari G, Lebailly P, Azadi H (2019) Impact of land use changes on soil and vegetation characteristics in Fereydan, Iran. Agriculture 9:58. https ://doi.org/10.3390/agric ultur e9030 058
Fu BJ, Liu SL, Ma KM, Zhu YG (2004) Relationships between soil characteristics, topography and plant diversity in a heterogeneous deciduous broad-leaved forest near Beijing, China. Plant Soil 261:47–54. https://doi.org/10.1023/B:PLSO.00000 35567.97093 .48
Gee GW, Bauder JW (1986) Particle-size analysis. In: Klute A (ed) Methods of soil analysis, 2nd edn. ASA and SSSA, Madison, pp 383–411
Grime JP (1977) Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am Nat 111:1169–1194. https://doi.org/10.1086/28324 4?mobil eUi=0
Han H, Jang K, Song J, Seol A, Chung W, Chung J (2011) The effects of site factors on herb species diversity in Kwangneung forest stands. For Sci Technol 7:1–7. https ://doi.org/10.1080/21580 103.2011.55994 2
Hejcmanovā-Ne?erková P, Hejcman M (2006) A canonical correspondence analysis (CCA) of the vegetation-environment relationships in Sudanese savannah, Senegal. S Afr J Bot 72:256–262. https://doi.org/10.1016/j.sajb.2005.09.002
Hill MO (1973) Diversity and evenness: a unifying notation and its consequence. Ecology 54:427–432. https ://doi.org/10.2307/19343 52
Hoang V, Baas P, Ke?ler P, Slik J, Steege HT, Raes N (2011) Human and environmental influences on plant diversity and composition in Ben En National Park, Vietnam. J Trop For Sci 23:328–337
Ivanov VY, Bras RL, Vivoni ER (2008) Vegetation-hydrology dynamics in complex terrain of semiarid areas: 2. Energy-water controls of vegetation spatiotemporal dynamics and topographic niches of favorability. Water Resour Res 44:W03430. https://doi.org/10.1029/2006W R0055 95
John R, Dalling JW, Harms KE, Yavitt JB, Stallard RF, Mirabello M, Hubbell SP, Valencia R, Navarrete H, Vallejo M, Foster RB (2007) Soil nutrients influence spatial distributions of tropical tree species. PNAS 104:864–869. https://doi.org/10.1073/pnas.06046 66104
Khamyong N, Wangpakapattanawong P, Chairuangsri S, Inta A, Tiansawat P (2018) Tree species composition and height-diameter allometry of three forest types in Northern Thailand. CMU J Nat Sci 17:289–306. https://doi.org/10.12982/CMUJN S.2018.0021
K?rner C (2007) The use of ‘a(chǎn)ltitude’ in ecological research. Trends Ecol Evol 22:569–574. https://doi.org/10.1016/j.tree.2007.09.006
Küchler AW, Sawyer JO (1967) A study of the vegetation near Chiengmai, Thailand. Trans Kans Acad Sci 70:281–348
Kutintara U (1975) Structure of the dry dipterocarp forest. Dissertation, Colorado State University
Liu H, Zheng L, Yin SJ (2018) Multi-perspective analysis of vegetation cover changes and driving factors of long time series based on climate and terrain data in Hanjiang River Basin, China. Arab J Geosci 11:1–16. https://doi.org/10.1007/s1251 7-018-3756-3
Magurran AE (1988) Ecological diversity and its measurement. Groom Helm Ltd, London, p 179
M?ren IE, Sharma LN (2018) Managing biodiversity: impacts of legal protection in mountain forests of the Himalayas. Forests 9:476. https://doi.org/10.3390/f9080 476
Marod D, Kutintara U, Chanchai Y, Hiroshi T, Tohru N (1999) Structural dynamics of a natural mixed deciduous forest in Western Thailand. J Veg Sci 10:777–786. https://doi.org/10.2307/32373 02
Marod D, Kutintara U, Tanaka H, Nakashizuka T (2002) The effects of drought and fire on seed and seedling dynamics in a tropical seasonal forest in Thailand. Plant Ecol 61:41–57
Marod D, Hermhuk S, Thinkamphaeng S, Panmongkol A (2015) Tree distribution across the forest ecotone of lower montane forest at Doi Suthep-Pui National Park, Chiang Mai Province. Thai J For 34:99–108
Martínez-Camilo R, González-Espinosa M, Ramírez-Marcial N, Cayuela L, Pérez-Farrera Má (2018) Tropical tree species diversity in a mountain system in Southern Mexico: local and regional patterns and determinant factors. Biotropica 50:499–509. https://doi.org/10.1111/btp.12535
McCune B, Grace JB (2002) Analysis ofecological communities. MjM Software Design, Oregon, p 300
McCune B, Mefford MJ (2006) PC-ORD: multivariate analysis ofecological data. Version 5.10. MjM Software Design, Oregon
Meng QF, Sun YT, Zhao J, Zhou LR, Ma XF, Zhou M, Gao W, Wang GC (2014) Distribution of carbon and nitrogen in water-stable aggregates and soil stability under long-term manure application in solonetzic soils of the Songnen plain, Northeast China. J Soil Sediment 14:1041–1049. https ://doi.org/10.1007/s1136 8-014-0859-7
Millennium Ecosystem Assessment (2005) Ecosystems and human well-being: biodiversity synthesis. World Resource Institute, Washingtion, p 137
Myo KK, Thwin S, Khaing N (2016) Floristic composition, structure and soil properties of mixed deciduous forest and deciduous dipterocarp forest: case study in Madan Watershed, Myanmar. Am J Plant Sci 7:279–287. https://doi.org/10.4236/ajps.2016.72027
National Soil Survey Center (1996) Soil survey laboratory methods manual. Natural Resources Conservation Service, Washington, p 693
Nelson DW, Sommers LE (1996) Toal carbon, organic carbon, and organic matter. In: Sparks DL et al (eds) Methods of soil analysis, 2nd edn. ASA and SSSA, Madison, pp 961–1010
Nguyen TV, Mitl?hner R, Bich NV, Do TV (2015) Environmental factors affecting the abundance and presence of tree species in a tropical lowland limestone and non-limestone forest in Ben En -National Park, Vietnam. JFES 31:177–191. https ://doi.org/10.7747/JFES.2015.31.3.177
Offi ce of Natural Resources and Environmental Policy and Planning (2009) Thailand: National report on the implementation of the convention on biological diversity. ONEP, Bangkok, p 76
Oliveira-Filho AT, Curi N, Vilela EA, Carvalho DA (1998) Effects of canopy gaps, topography, and soils on the distribution of woody species in a Central Brazilian deciduous dry forest. Biotropica 30:362–375. https://doi.org/10.1111/j.1744-7429.1998.tb000 71.x
Paulo J, Souza D, Araújo GM, Haridasan M (2007) Influence of soil fertility on the distribution of tree species in a deciduous forest in the Triangulo Mineiro region of Brazil. Plant Ecol 191:253–263. https://doi.org/10.1007/s1125 8-006-9240-2
Popradit A, Srisatit T, Kiratiprayoon S, Yoshimura J, Ishida A, Shiyomi M, Murayama T, Chantaranothai P, Outtaranakorn S, Phromma I (2015) Anthropogenic effects on a tropical forest according to the distance from human settlements. Sci Rep 5:14689. https://doi.org/10.1038/srep1 4689
R Development Core Team (2019) R: a language and environment for statistical computing
Rowe RJ (2009) Environmental and geometric drivers of small mammal diversity along elevational gradients in Utah. Ecography 32:411–422. https://doi.org/10.1111/j.1600-0587.2008.05538.x
Royal Forest Department (2014) Implementation guidlines for communit forest projects of the Royal Forest Department. RFD, Bangkok, p 23 (in Thai)
Royal Forest Department (2017) Study of carbon sequestration and biodiversity in the Ban Mae Chiang Rai Community Forest, Northern Thailand. RFD, Bangkok, p 26 (in Thai)
Royal Forest Department (2019) Executive summary. RFD, Bangkok, p 22 (in Thai)
Royal Forest Department (2020) Community forest project approval between 2000-Present. RFD. http://www.fores t.go.th/commu nityexten sion/2017/02/02/. Accessed 30 Jan 2020
Rundel PW, Boonprakob K (1995) Forest ecosystems of Thailand. In: Bullock SH, Mooney H, Medina E (eds) Seasonal dry tropical forests. Cambridge University Press, New York, pp 93–123
Santiago LS, Wright SJ, Harms KE, Yavitt JB, Korine C, Garcia MN, Turner BL (2012) Tropical tree seedling growth responses to nitrogen, phosphorus and potassium addition. J Ecol 100:309–316. https://doi.org/10.1111/j.1365-2745.2011.01904.x
Santisuk T (1988) An account of the vegetation of Northern Thailand. Franz Steiner Verlag Wiesbaden, Stuttgart, p 101
Sarker SK, Rashid S, Sharmin M, Haque MM, Sonet SS, Nur-Un-Nabi M (2014) Environmental correlates of vegetation distribution in tropical Juri forest, Bangladesh. Trop Ecol 55:177–193
Sarvade S, Gupta B, Singh M (2016) Composition, diversity and distribution of tree species in response to changing soil properties with increasing distance from water source—a case study of Gobind Sagar Reservoir in India. J Mt Sci 13:522–533. https://doi.org/10.1007/s1162 9-015-3493-y
Scalley TH, Crowl TA, Thompson J (2009) Tree species distributions in relation to stream distance in a mid-montane wet forest, Puerto Rico. Caribb J Sci 45:52–63. https://doi.org/10.18475/cjos.v45i1 .a8
Slik JWF, Raes N, Aiba SI, Brearley FQ, Cannon CH, Meijaard E, Nagamasu H, Nilus R, Paoli G, Poulsen AD, Sheil D, Suzuki E, van Valkenburg JLCH, Webb CO, Wilkie P, Wulff raat S (2009) Environmental correlates for tropical tree diversity and distribution patterns in Borneo. Divers Distrib 15:523–532. https://doi.org/10.1111/j.1472-4642.2009.00557.x
Srisutham M, Kaewjampa N (2010) Indigenous community forest management in northeastern Thailand: biodiversity conservation through rural development. Int J Environ Rur Dev 1:28–32
Sukwong S, Dhamanittakul P (1977) Fire ecology investigations in dry dipterocarp forest. In: Proceedings of the National Forestry Conference. Royal Forestry Department, Bangkok, pp 41–56
Tavili A, Rostampour M, Chahouki MAZ, Farzadmehr J (2009) CCA application for vegetation—environment relationships evaluation in arid environments (Southern Khorasan rangelands). Desert 14:101–111. https://doi.org/10.22059/JDESE RT.2010.21752
Teejuntuk S, Sahunalu P, Sakura K, Sungpalee W (2003) Forest structure and tree species diversity along an altitudinal gradient in Inthanon National Park, Northern Thailand. Tropics 12:85–102. https://doi.org/10.3759/tropi cs.12.85
Thai Meteorological Department (2018) Daily meteorology. Northern Meteorological Center, Bangkok
Thapa S, Chapman DS (2010) Impacts of resource extraction on forest struture and diversity in Bardia National Park, Nepal. For Ecol Manag 259:641–649. https://doi.org/10.1016/j.forec o.2009.11.023
Thomas GW (1982) Exchangeable cations. In: Page AL et al (eds) Methods of soil analysis, 2nd edn. ASA and SSSA, Madison, pp 159–165
Tilk M, Tullus T, Ots K (2017) Effects ofenvironmental factors on the species richness, composition and community horizontal structure of vascular plants in Scots pine forests on fixed sand dunes. Silva Fenn 1:2. https://doi.org/10.14214/sf.6986
Toledo M, Pe?a-Claros M, Bongers F, Alarcón A, Balcázar J, Chuvi?a J, Lea?o C, Licona JC, Poorter L (2012) Distribution patterns of tropical woody species inresponse to climatic and edaphic gradients. J Ecol 100:253–263. https ://doi.org/10.111 1/j.1365-2745.2011.01890.x
Vahdati FB, Mehrvarz SS, Dey DC, Naqinezhad A (2017) Environmental factors–ecological species group relationships in the Surash lowland-mountain forests in Northern Iran. Nord J Bot 35:240–250. https://doi.org/10.1111/njb.01221
Wessel M (1971) Fertilizer requirements of cacao ( Theobroma cacao L.) in South-Western Nigeria. Koninklijk Instituut voor de Tropen, Amsterdam, p 104
Yoshifuji N, Kumagai T, Tanaka K, Tanaka N, Komatsu H, Suzuki M, Tantasirin C (2006) Inter-annual variation in growing season length of a tropical seasonal forest in Northern Thailand. For Ecol Manag 229:333–339. https://doi.org/10.1016/j.forec o.2006.04.013
Zhang JT, Zhang F (2007) Diversity and composition of plant functional groups in mountain forests of the Lishan Nature Reserve, North China. Bot Stud 48:339–348
Zhang XP, Wang MB, She B, Xiao Y (2006) Quantitative classification and ordination of forest communities in Pangquangou National Nature Reserve. Acta Ecol Sin 26:754–761. https://doi.org/10.1016/S1872-2032(06)60013-9
Zhang ZH, Hu G, Ni J (2013) Effects of topographical and edaphic factors on the distribution of plant communities in two subtropical karst forests, Southwestern China. J Mt Sci 10:95–104. https://doi.org/10.1007/s1162 9-013-2429-7
Zhang CS, Li XY, Chen L, Xie GD, Liu CL, Pei S (2016) Effects of topographical and edaphic factors on tree community structure and diversity of subtropical mountain forests in the Lower Lancang River Basin. Forests 7:222. https ://doi.org/10.3390/f7100 222
Zhao LJ, Xiang WH, Li JX, Lei PF, Deng XW, Fang X, Peng CH (2015) Effects of topographic and soil factors on woody species assembly in a Chinese Subtropical Evergreen Broadleaved Forest. Forests 6:650–669. https://doi.org/10.3390/f6030 650
 Journal of Forestry Research2021年2期
Journal of Forestry Research2021年2期
- Journal of Forestry Research的其它文章
- Stem taper functions for Betula platyphylla in the Daxing’an Mountains, northeast China
- Leaf phenotypic variation of endangered plant Tetracentron sinense Oliv. and influence of geographical and climatic factors
- Ecological variations of woody species along an altitudinal gradient in the Qinling Mountains of Central China: area-based versus mass-based expression of leaf traits
- Variations in stem radii of Larix principis-rupprechtii to environmental factors at two slope locations in the Liupan Mountains, northwest China
- A review of ecological mechanisms for management practices of protective forests
- Variation of basic density, calorific value and volumetric shrinkage within tree height and tree age of Ugandan grown Eucalyptus grandis wood
