Shade coffee plantations maintain woody plant diversity and structure in a cloud forest landscape of southern Mexico
Edson A. Alvarez-Alvarez · R. Carlos Almazán-Nú?ez · Fernando González-García · Marlene Brito-Millán · Alfredo Méndez-Bahena · Sergio García-Ibá?ez
Abstract Cloud forest ecosystems of the Latin American tropics are highly threatened by changes in land-use such as expanding croplands and livestock pastures that promote shifts in the structure and composition of plant communities in these forests. However, shade coffee plantations represent a forest management alternative that has been shown to maintain biodiversity in these ecosystems. In this study, we evaluated changes in the composition, diversity, and structure of Mexican cloud-forest woody species for three land use categories: cattle pastures, shade coffee plantations and advanced succession forests. For each category, fifteen 0.28-ha plots were established and the composition and diversity of vegetation was noted. Composition of species was analyzed using ordination methods, and alpha diversity was compared using Hill numbers. Seventy-seven woody species belonging to 40 families were recorded. Species richness and diversity was high in both the advanced successional forest and coffee plantations compared to cattle pastures. Vegetation composition and structure was similar between late succession forests and coffee plantations with both land uses also being more structurally complex than cattle pastures. Our results show how shade coffee cultivation is a land-use activity that maintains woody plant communities in a manner that aligns with biodiversity conservation.
Keywords Land uses · Late succession · Plant diversity · Shade coffee · Vegetation structure
Introduction
In the tropics, the conversion of forests to croplands and livestock pastures is one of the immediate causes of deforestation (Lambin et al. 2001). These expanding land uses have led to the fragmentation ofecosystems, a loss of biodiversity (Martínez et al. 2009; Mendoza-Ponce et al. 2018) and disruption ofecological processes (e.g., seed dispersal, pollination; ?ekercio?lu et al. 2004) that are key for maintaining the integrity ofecosystems (DeClerck et al. 2010). However, in large areas of the Latin American tropics, highly complex, biodiverse and multifunctional agroforestry systems have been implemented, including rustic or traditional shade coffee plantations (Bandeira et al. 2005; Perfecto et al. 2007). Coffee plantations are one of the most important crops for Central American countries and Mexico (Perfecto et al. 2007; González-Zamora et al. 2016). Coffee represents between 5 and 25% ofexports in addition to being important for local economies (Gresser and Tickell 2002; Philpott et al. 2007).
Coffee plantations in Latin America are usually established as a shade crop in forested areas. The herbaceous stratum and some shrubs are removed and the tree cover is mostly retained (Moguel and Toledo 1999; Soto-Pinto et al. 2001). Shade coffee plantations largely preserve the structure and composition of the original forest vegetation and its biodiversity (Moguel and Toledo 1999; Perfecto et al. 2007; López-Gómez et al. 2008; Macip-Ríos and Casas-Andreu 2008; Manson et al. 2008; González-Zamora et al. 2016). Therefore, shade coffee plantations are of considerable biological and ecological value for providing important environmental services, for sustaining both biodiversity and human communities (Davidson 2004; Perfecto et al. 2005; Meylan et al. 2017). These services include water capture and retention, nutrient recycling, carbon capture, river sediment regulation, prevention of landslides and flooding affecting lowlands, and mitigation of climate change (Davidson 2004; Dávalos-Sotelo et al. 2008; Geissert and Ibá?ez 2008). At the landscape level, these plantations often form biological corridors that connect fragments of natural forest vegetation (Moguel and Toledo 1999; Perfecto and Vandermeer 2002; López-Barrera and Landgrave 2008).
In Mexico, shade coffee is predominantly cultivated in humid environments, including cloud forest, an ecosystem of considerable conservation importance because of their natural resources and high biodiversity (Villase?or 2010; Gual-Díaz and Rendón-Correa 2014). The high biodiversity of cloud forest arises from the presence of both tropical and temperate floristic elements, where the canopy is dominated by species of temperate affi nity and the middle and lower layers are mostly of tropical similarity (Hamilton et al. 1995). This ecosystem also has a high rate of floral and faunal endemism (Villase?or 2010; Gual-Díaz and Rendón-Correa 2014). However, the expansion of cloud forest has been substantially reduced due to livestock ranching and agriculture. Although ranching represents an important economic base for local economies worldwide (Kelaidis 2015), this land use results in soil compaction, the elimination of native plants, and reduction in water infiltration. These processes drastically modify the composition and structure of the original vegetation that, in turn, results in negative environmental consequences, including soil degradation and biodiversity loss (Díaz et al. 2007; Martínez et al. 2009; Kikoti and Mligo 2015; Rahmanian et al. 2019). Studies on the floristic structure and composition of cloud forests have generally focused on the central-eastern region of Mexico where differences in cloud forest vegetation have been evaluated across environmental gradients (López-Gómez et al. 2008; González-Zamora et al. 2016). Natural and anthropogenic disturbances to cloud forest vegetation have been extensively documented and even related to variations in regional climate (e.g., temperature, precipitation; López-Mata et al. 2012; Williams-Linera et al. 2013) and to global climate change (Rojas-Soto et al. 2012). Overall, these studies have highlighted the modification of cloud forest communities because of disturbances or have addressed the negative implications of disturbances for floristic diversity, ecosystem function and forest regeneration. However, few studies have examined the role of shade coffee plantations in the maintenance and conservation of cloud forest flora (Bandeira et al. 2005).
This study examines a cloud forest landscape of the Sierra Madre del Sur of southern Mexico, a biologically important region characterized by complex orography and geological history that has promoted the development of a wide range ofecosystems (Luna-Vega et al. 2016). In this region, forested areas are increasingly converted to coffee plantations, croplands and ranching, giving rise to landscapes with varying degrees of disturbance and succession (Almazán-Nú?ez et al. 2016, 2018). We examine changes in the composition, diversity and structure of woody flora across three land use categories within the cloud forest landscape: (1) forests in advanced succession (hereafter “l(fā)ate forests”); (2) forests with coffee plantations (hereafter “coffee plantations”); and, (3) cattle pastures, which represent highly disturbed sites. We predict that the richness and diversity of woody species would be higher in late forests and in coffee plantations than in cattle pastures due to the maintenance of woody flora. We also predict that the composition of woody plants would differ between late forests and coffee plantations because shrub flora is continuously replaced in coffee plantations. We assumed that the structure (cover, density, foliage height diversity) of coffee plantations would imitate forests with advanced regeneration processes. The results of this study may be used as a reference to support conservation of cloud forests and agroforestry management approaches that maintain both livelihoods and the native woody flora that preserves cloud forest diversity.
Materials and methods
Study area and sampling sites
This study was carried out in Río Verde, El Paraíso and Nueva Delhi in the Atoyac de álvarez municipality in the Sierra Madre del Sur province in Guerrero state in southern Mexico (368000–374000 N; 191200–192800 W; Fig.1). Mean altitude is 1150 m a.s.l. The main vegetation type is cloud forest under different land use regimes with fragments of semi-deciduous forest and pine/oak present. The climate is warm sub-humid (Río Verde and El Paraíso) and temperate semi-warm (Nueva Delhi), with mean annual temperatures of 20–24 °C and mean annual precipitation of 1500–2000 mm (INEGI 2010).
Three distinct cloud forest land use regimes with different vegetation cover were considered: (1) late forest; (2) coffee plantations; and, (3) cattle pastures. A closed and dense canopy characterized late forest sites, once occupied by shade coffee plantations ( Coffea arabica L.), but their abandonment more than 35 years ago allowed for the regeneration of herbaceous and shrub layers, including some trees. Cambisol and luvisol are principal soil types found on late forest sites and generally found in rugged mountainous areas. Both types are intensively used as agricultural land or cattle ranching (IUSS Working Group WRB 2015). The decade-old coffee plantation sites maintained the canopy layer of cloud forests but coffee plants had replaced some shrubs. These two land uses, late forest and coffee plantations, are characterized by rugged terrain with slopes of 30°–45° and average altitudes of 1200 m a.s.l. The cattle pastures had scattered vegetation but most had been cleared for timber or for cattle ranching. Given the prevalence of this land-use type within the region, we considered it important to include it in the study as it provides a representative set of comparative land use within Mexican cloud forests. Some pasture areas had compacted soils because of cattle trampling. Their topography was less rugged (slopes of 15°–20°), with a mean altitude of 1000 m, a.s.l. Both coffee plantations and cattle pastures are on cambisol soils. Undisturbed, natural cloud forest sites were not selected because the majority of these forests are associated with coffee or banana plantations, and the majority of cloud forests are inaccessible.
For each land use type, three sites (nine sites in total) were chosen, and five plots randomly established per site (15 plots per land use type, 45 plots in total) to sample vegetation. Each plot had an area of 0.28 ha, i.e., 1.4 ha per site or 4.2 ha per land use type. The average distance between plots was 200 m to increase the dispersion of samples across each site.
Vegetation sampling
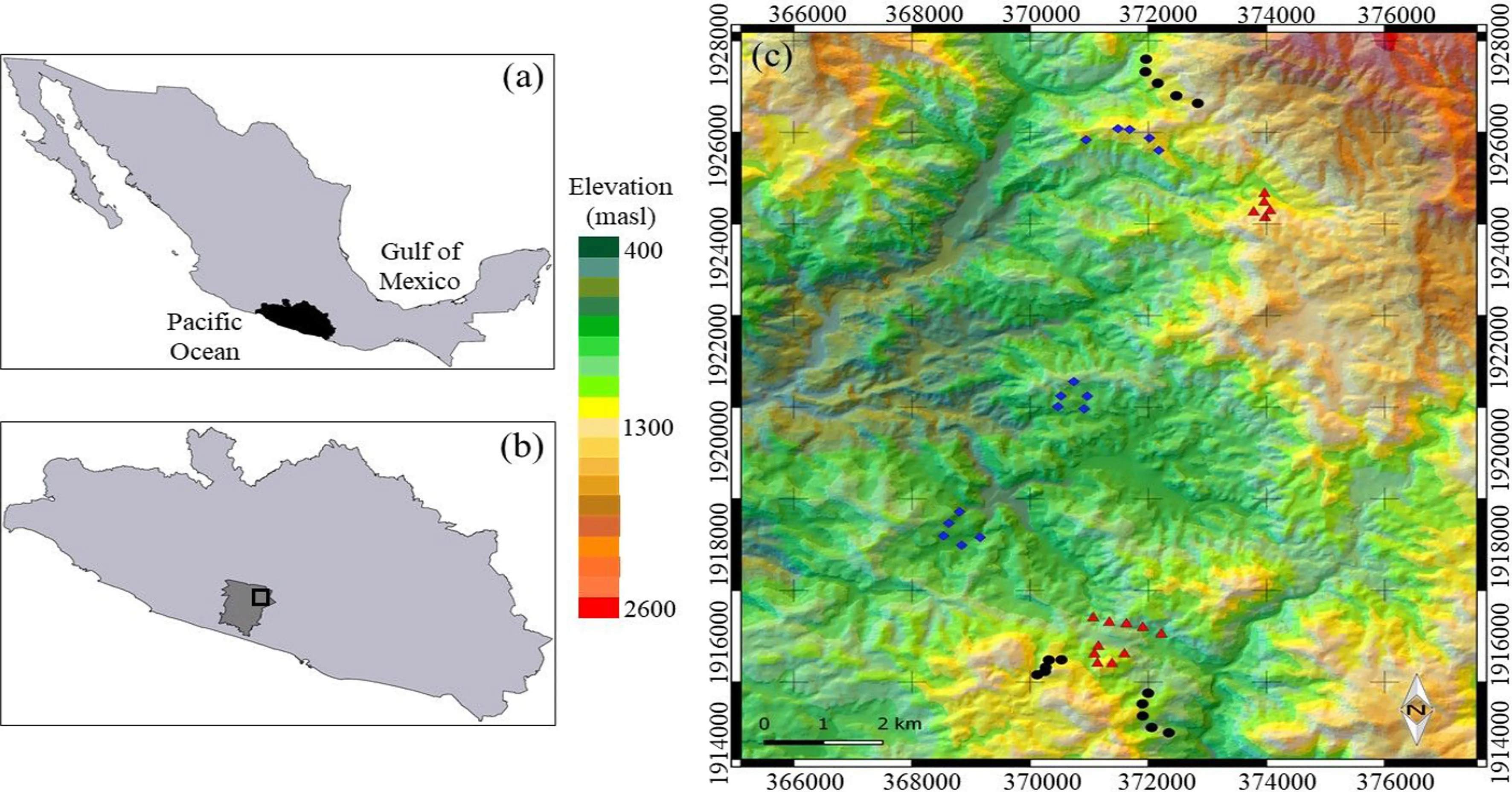
Fig.1 Geographic location of the study area including a Location of the state of Guerrero in southern Mexico, b study area in the Atoyac municipality within the state, and c vegetation sampling plots in three cloud-forest land uses: cattle pastures (blue diamonds), coffee plantations (red triangles), late forests (black circles)
To characterize the diversity and structure of the woody vegetation, two cross-section transects, where two perpendicular lines oriented to the four cardinal points demarcated with a rope, were established within each plot at each site. All tree and shrub species with a diameter at breast height (DBH) ≥ 15 cm whose branches intersected the rope were measured and identified. Foliage cover was estimated for each tree and shrub species using an ellipse formula based on maximum and minimum diameters (Muller-Dombois and Ellenberg 1974). Foliage stratification was estimated using an optical square marked with two perpendicular axes (Monta?a and Ezcurra 1980 ). The square has three mirrors arranged so that looking horizontally through the device, the height ofobjects may be determined. In each plot, foliage height and number of times that foliage touched the point of intersection of the two axes was recorded (Almazán-Nú?ez et al. 2016). This procedure was repeated every 1-m along the two transects from the center to the four cardinal points. The recorded heights were grouped into 2-m size class intervals, and the foliage height diversity was assessed using the Shannon-Wiener index. In addition, the number of tree and shrub species per unit area (density) was estimated.
For each species with flowering and/or fruiting structures, three specimens were collected for subsequent identification. Each sample was pressed and labeled for classification according to Wendt ( 1986) and deposited into the Vascular Plant Laboratory of the Sciences Department of the National Autonomous University of Mexico and the herbarium of the Autonomous University of Guerrero. Species endemism was determined based on the classification criteria of Villase?or ( 2016). Taxonomic nomenclature was assigned according to the database of the Missouri Botanical Garden (W3Tropicos 2010), while the arrangement of species systematics followed the guidelines of the Angiosperm Phylogeny Group (APG IV 2016).
Data analyses
The diversity of woody species was estimated at three levels. First-order diversity was calculated as the species richness (q = 0). Second-order diversity considered all species weighted proportionally according to their abundance in the community (q = 1). Finally, third-order diversity corresponded with Simpson’s inverse index of diversity (q = 2; Jost 2006). Diversity profiles were generated and used to compare true diversity between plant communities of the three land use categories (Jost 2006; Chao and Jost 2015). In order to compare only native vegetation and avoid skewing results, Coffea arabica was not considered for the diversity analysis profiles, as it showed high abundances in both the late forests and coffee plantations compared to native species. Differences in the diversity of woody plants (q0, q1, and q2) were considered significant based on 84% confidence intervals as recommended by MacGregor-Fors and Payton ( 2013). These analyses were performed in iNEXT (Chao et al. 2016).
The sampling effi ciency ofeach land use category and the overall study area was evaluated with the non-parametric Chao 2 richness estimator in EstimateS 9.1.0, which has been shown to a robust metric for empirical comparisons (Colwell 2013). The Chao 2 estimator is also considered a reliable measure for relatively small sampling units (i.e., the 0.28 ha circular plots used in this study; Hortal et al. 2006). In addition, this estimator is less sensitive to sampling intensity than are other estimators (Colwell and Coddington 1994; Hortal et al. 2006).
For each species, a relative importance value (RIV) was calculated based on three measures: (1) relative density, (2) frequency, and (3) foliage cover. Relative density is the proportion of the number of individuals of a species to the total number of species in the sample. Frequency represents the proportion of the number of plots with species present to the total number of plots per site, and foliage cover was calculated as the ratio of a species cover to the total cover of all species per site. To compare the mean values of foliage cover, plant density and foliage height diversity across sites and land uses, we employed two-way analysis of variance (ANOVA; factor 1: land use, factor 2: site) following a priori analyses of normality and homoscedasticity. The comparisons were analyzed a posteriori with Tukey’s HSD test. To fulfill the assumptions of normality and homogeneity of variance, the data were log transformed (x + 1). The values of the structural variables (foliar cover, plant density, foliage height diversity) were executed in SPSS 20.0 (SPSS 2011) and presented as averages with corresponding ± standard error (SEs).
The distribution of RIVs and composition of plant communities among land uses was evaluated according to a non-metric multidimensional scaling analysis (NMDS). This method graphically shows similarities in the composition and abundance of species among sampling plots (Minchin 1987). Kruskal’s stress metric was calculated to determine fit, which generated values ranging from 0 (perfect fit) to 0.20 (poor fit; de Leeuw and Mair 2009).
In addition, a one-way analysis of similarity (ANOSIM), followed by a correspondence analysis (CA) were executed to assess differences in species composition. In both NMDS and CA, the RIV ofeach species was used. ANOSIM is a non-parametric test that uses similarity matrices (Clarke 1993). We used the chord dissimilarity coeffi cient in NMDS because it achieved a better representation of the relationships between communities compared to other measures (Orlóci 1978; Legendre and Gallagher 2001). Finally, a correspondence analysis was carried out to segregate species according to environmental gradients (i.e., land use categories). Species with a low RIV were not included due to their low representativeness in the study sites. All multivariate statistical analyses were performed in Past 2.17 (Hammer et al. 2001), CANOCO 4.5 and CANODRAW 4.0 (ter Braak and ?milauer 2002).
Results
Floristic composition
Seventy-seven woody species belonging to 40 families were identified (Table S1). Based on the Chao 2 estimator, 74% of the total expected species for the study area were recorded. The Melastomataceae family was most numerous (8), followed by Fabaceae (6), and Rubiaceae and Asteraceae (4 each; Table S1). A total of nine species were endemic to Mexico ( Clethra fragrans, Cymbopetalum gracile, Ocotea candidovillosa, Populus simaroa, Quercus splendens, Saurauia serrata, Verbesina fastigiata, Vernonia cordata, Zinowiewia concinna) and four under the ‘a(chǎn)t risk category’ according to the Mexican government ( Pinus chiapensis, P. simaroa, S. serrata, Z. concinna; SEMARNAT 2010). The species with the highest RIV were Pinus maximinoi, C. fragans and Q. crispifolia in the late forests; Coffea arabica, Q. crispifolia and Inga vera in the coffee plantations; and, Conostegia xalapensis, Q. splendens, and Chromolaena odorata in the cattle pastures (Fig.2).
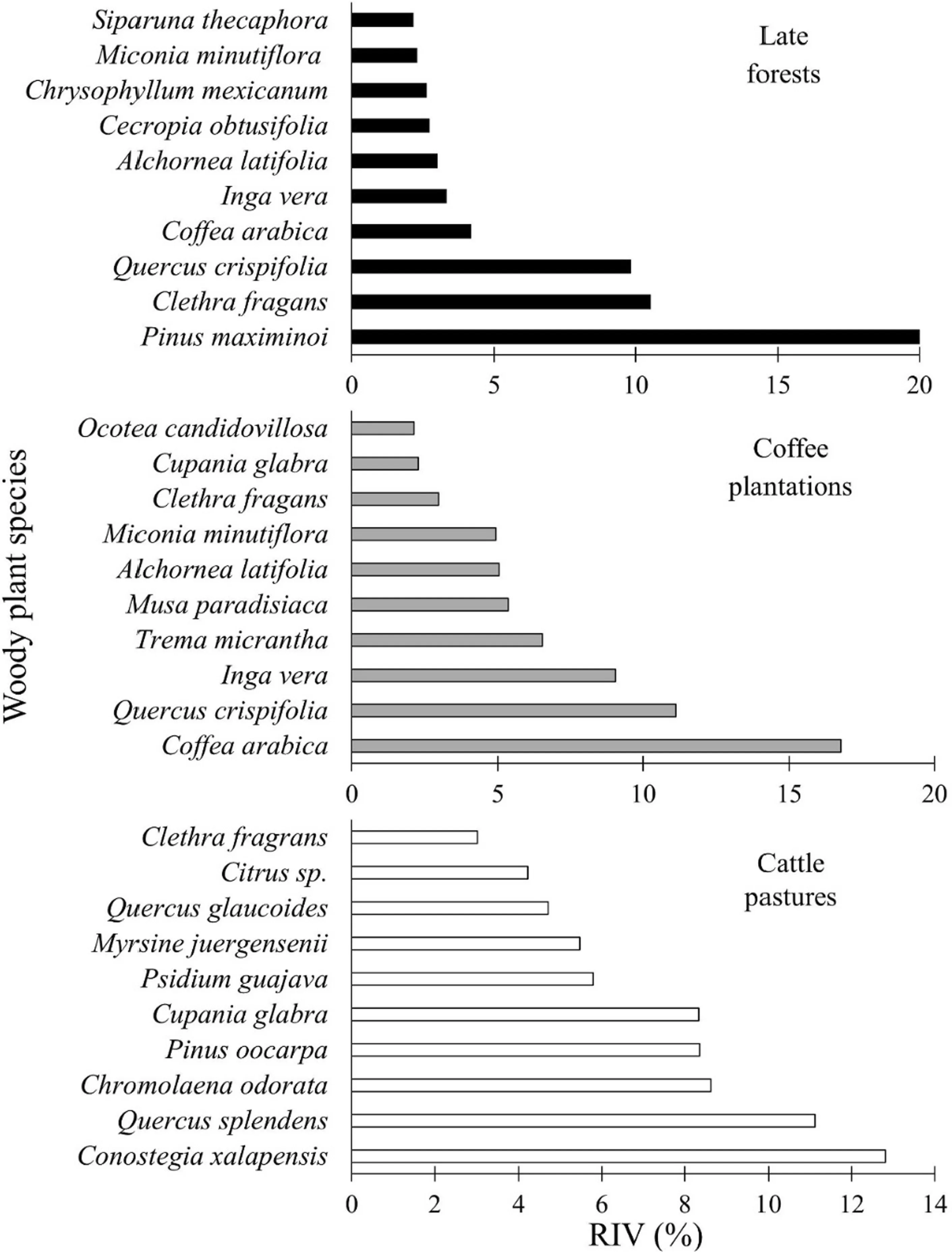
Fig.2 Mexican cloud forest woody species with their corresponding upper-bound relative importance values (RIV) in three land use categories
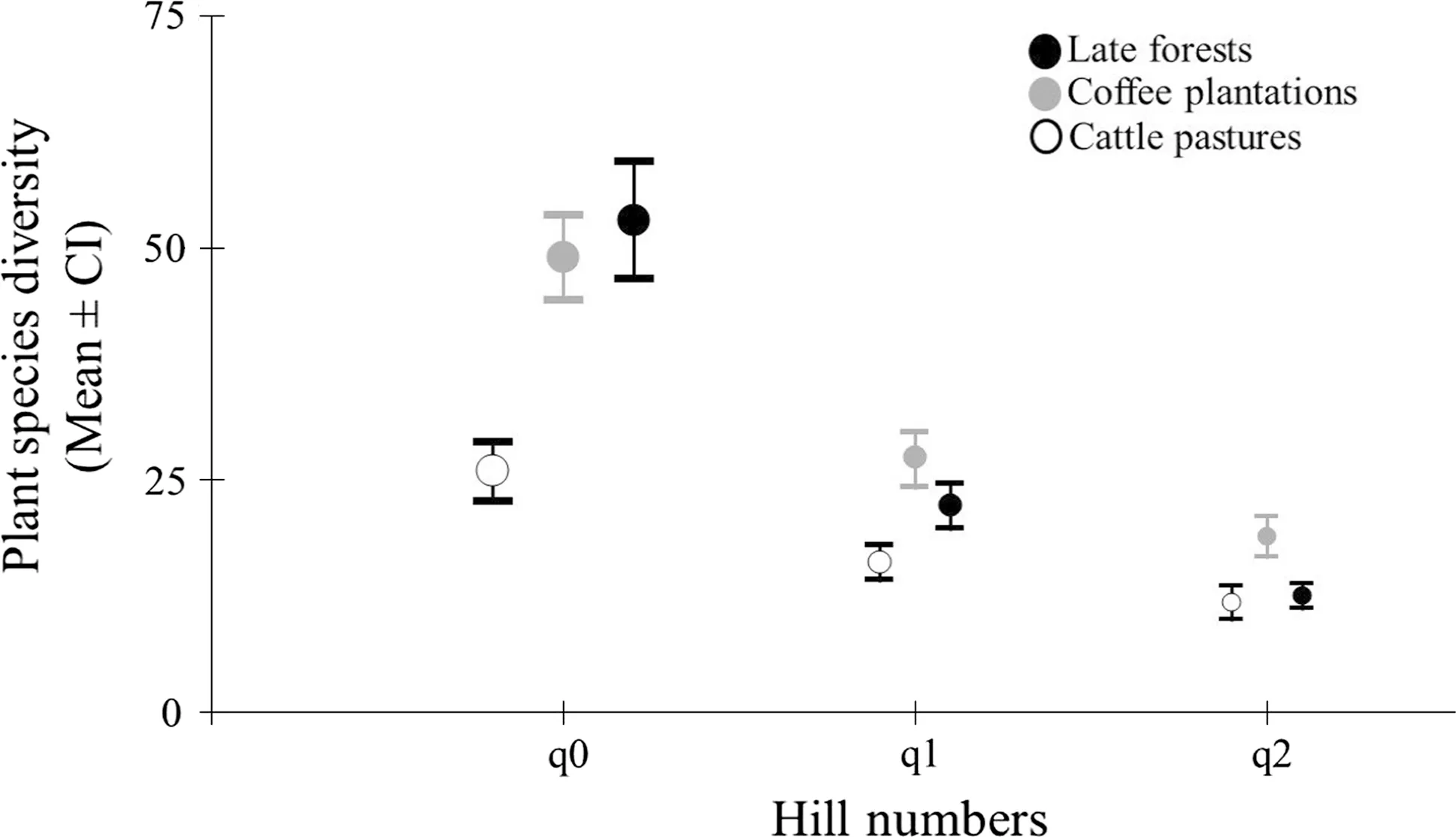
Fig.3 Mean values of richness (q0) and diversity (q1 and q2) of woody species in three land uses in a cloud forest of southern Mexico
Species richness and diversity among different land uses
According to the Chao 2 estimator, 89%, 83% and 81% of woody species in late forests, coffee plantations and cattle pastures, respectively, were recorded. Late forests and coffee plantations had the highest species richness (q0 = 53 and 49 species, respectively) compared to the cattle pastures (26 species; Fig.3). Similarly, late forests and coffee plantations had the highest species diversity (q1, exponential of Shannon entropy; Fig.3). According to the inverse Simpson concentration (q2), the coffee plantations had the highest diversity values compared to the other two land uses (Fig.3).
Vegetation structure among different land uses
The late forests and coffee plantations had the highest mean foliage cover (F2,44= 3.52, p = 0.004), plant density (F2,44= 24.06, p < 0.001) and foliage height diversity (F2,44= 13.41, p < 0.001) compared to cattle pastures (Fig.4 a–c). The interaction between land use and site was only significant for plant density (F2,4= 2.81, p = 0.02).
Distribution of species by land use
The species communities varied significantly by land use (ANOSIM global R = 0.48, p < 0.001; Fig.5). The NMDS showed overlapping of the minimum convex polygons that grouped the late forest and coffee plantation plots but separated the cattle pasture plots (Fig.5). Kruskal’s stress value denoted a good fit ( stress = 0.15; Fig.5). Additionally, the first axis (eigenvalue = 0.83; 29% cumulative variance) in CA analysis separated cattle pastures from the other two land uses, and the second axis (eigenvalue = 0.54; 48% cumulative variance) grouped late forests and coffee plantations (Fig.6). The CA analysis also showed a marked segregation among late forest sites (Fig.6). Inga sapindoides, Pinus maximinoi, Pouteria campechiana and Drimys granadensis were associated with late forests; Coffea arabica, Miconia mexicana, Musa paradisiaca and Coccoloba barbadensis with coffee plantations; and Chromolaena odorata, Gonzalagunia panamensis, Quercus splendens and Conostegia xalapensis with cattle pastures (Fig.6).
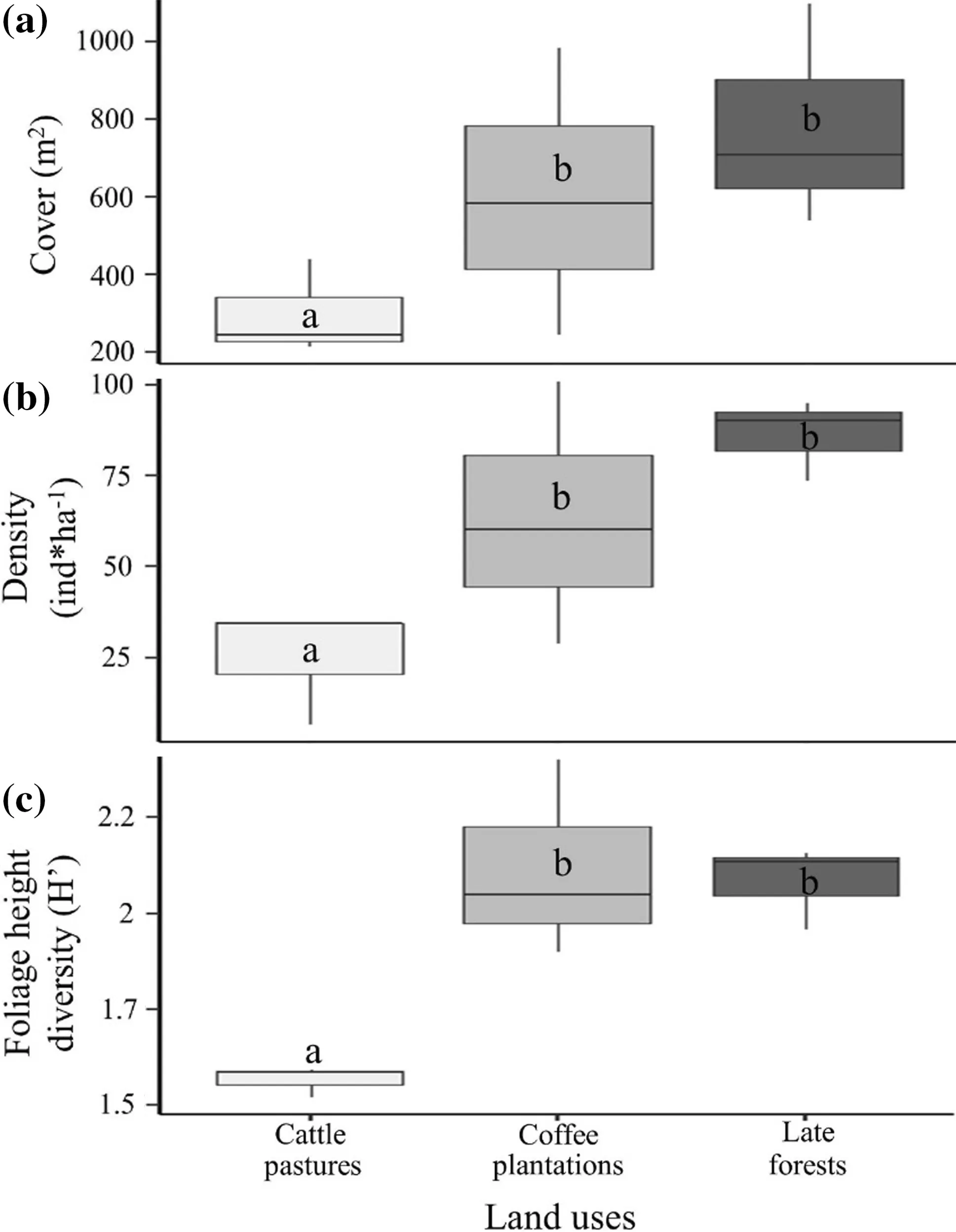
Fig.4 Mean values (± SEs) of the structural parameters of woody species of three land uses in a cloud forest of southern Mexico; letters indicate significant differences ( p < 0.05) according to Tukey’s HSD test
Discussion
Richness and diversity of species in three cloud forest land uses
Species richness and diversity were higher in late forests and coffee plantations as expected. The slight differences in the number of species between coffee plantations and late forests is explained by the partial replacement of the understory in coffee plantations (Perfecto et al. 1996, 2007). In the late forests, the period of abandonment of these sites (approximately 35 years) allowed the regeneration of some species, mainly within the shrub layer (Marcano-Vega et al. 2002; Lozada et al. 2007; Zdravko and Shingo 2014). For example, species such as Drimys granadensis, Parathesis serrulata, Vismia baccifera and Zanthoxylum melanostictum were present inthe late forests but not in coffee plantations, as they were likely eliminated from the understory to avoid or reduce competition with coffee plants (Méndez et al. 2007; Juárez-Lopez et al. 2017). Although some species were removed within the shade coffee plantations, our results suggest that this did not significantly affect species composition. Several tree species such as Cecropia obtusifolia, Verbesina fastigiata, Miconia mirabilis and Trema micrantha were present in both late forests and coffee plantations. Of these, the first two are characteristic of secondary post-forest vegetation (Carvajal and González-Villarreal 2005). M. mirabilis is characteristic of regenerating forests (Martini and dos Santos 2007), while T. micrantha is common in coffee plantations and forests in advanced succession (López-Gómez et al. 2008). This suggests that the management of coffee plantations does not diminish the potential microhabitats used by the plant communities in abandoned coffee plantations, but instead allows these communities to undergo advanced succession processes (Bandeira et al. 2005; Soto-Pinto et al. 2007; López-Gómez et al. 2008). Moreover, our results of both diversity and structure of woody vegetation in coffee plantations capture how this type of land use may enhance diversity to maintain the ecological integrity of cloud forests. Observations by Philpott et al. ( 2008) show how the intensity of coffee management (e.g., amount of canopy pruning) for floral and faunal diversity enhancement, support our findings that shade coffee plantations (which have minimal canopy intervention in Mexico) can be biodiversity promoting systems. Similar diversity enhancement patterns have also been observed in agro-silvo-pastoral systems in the Mediterranean (Bagella et al. 2016) and in other agroforestry systems across temperate and tropical regions (Jose 2012).

Fig.5 Non-metric multidimensional scaling of three land uses in a cloud forest of southern Mexico based on the relative importance values and composition of species; sampling plots ofeach land use are grouped by minimum convex polygons
On the other hand, the low number of species in the cattle pastures highlighted the effect of high-disturbance land use on plant species. Plant communities in cattle pastures are exposed to several intrinsic and extrinsic factors that limit the survival of most species. For example, species may be subject to livestock grazing, changes in soil physical properties and nutrients, and inadequate microclimates, especially in open areas, which can slow or even stop the regeneration of plant communities. Seed dispersal also may be lower or absent, leading to a reduction in seed banks (Holl et al. 2000; Cubi?a and Aide 2001; Hooper et al. 2005; Mu?iz-Castro et al. 2006; Ortega-Pieck et al. 2011). In contrast, in late forests and in coffee plantations, environmental conditions of high humidity, precipitation, and uptake and retention of water and soil nutrients (Davidson 2004; Tscharntke et al. 2011) favor greater richness and diversity of plant species.
Vegetation structure in three cloud forest land uses
Vegetation on cattle pastures was simpler than that of late forests and coffee plantations. Spatial cover, density, and height diversity of species on the three land uses were influenced by the intensity ofeach land use. The most representative species (with the highest relative importance values) in the cattle pastures were Conostegia xalapensis and Chromolaena odorata, demonstrating the capacity of these species to adapt to conditions of disturbance and to persist for many years in areas under secondary succession (Almeda 1993). Some species such as Cupania glabra and Quercus splendens had high RIV’s in the cattle pastures, mainly because of their high frequency in plots rather than because of their small crowns. Many of these individuals (approximately 50%) were shorter (< 12 m) than in primary forests (García et al. 2000; Rangel et al. 2002; Vandermeer and de la Cerda 2004), which also helps to explain the lower structural parameter values in the cattle pastures. This is related to livestock activities that result in soil compaction that may prevent the regeneration of species.
The structural parameters (cover, density, foliage height diversity) did not differ between late forests and coffee plantations. In late forests, the recovery of herbaceous and shrubby vegetation can occur after site abandonment by passive restoration such as seed dispersal by animals, which has been observed in other cloud forests (Lugo and Helmer 2004; Hernández-Ladrón et al. 2012). In the present study, despite the abandonment of the sites for more than 35 years, both the late forest and coffee plantation sites had comparable cover percentage. This is similar to reports for rustic coffee systems in Mexico (Soto-Pinto et al. 2001; López-Gómez et al. 2008). The maintenance ofexisting canopies, including trees taller than 20 m, confirms that the management of rustic or traditional shade coffee systems, particularly in the study area, does not affect mature trees. Some of these trees have important ecological functions such as Alchornea latifolia, which is useful for erosion control and the restoration of degraded soils (Vázquez-Yanez et al. 1999). Other species such as C. glabra, Inga vera, Miconia minutiflora and Musa paradisiaca provide optimum levels of shade for growing coffee plants (Perfecto et al. 1996; Moguel and Toledo 1999; Staver et al. 2013; Meylan et al. 2017). Shade coffee plantations, as similar to natural plantations, can play important roles in the restoration and conservation of plant and animal communities (Philpott et al. 2008). The structure of coffee plantations also serves to minimize the indirect effects of fragmentation at the local and landscape level (Perfecto and Vandermeer 2002; Williams-Linera et al. 2002; Williams-Linera and López-Gómez 2008; Soto-Pinto et al. 2012; González-Zamora et al. 2016).
Distribution of vegetation in three cloud forest land uses
The species composition of late forests and coffee plantations was similar, which does not support our original prediction that the composition of woody plants from both land uses would differ due to shrubby flora being partially and continuously replaced in coffee plantations. In fact, the non-metric multidimensional scaling or NMDS analysis showed an overlap among plots of late forest and coffee plantation sites as having the highest similarity in terms of species composition and RIV values, whereas the cattle pastures were distinctly separate. These differences in species assemblages reflect the higher intensity of land use and human disturbances in cattle pastures (González-Zamora et al. 2016). Cattle pastures had species such as C. xalapensi s, Gonzalagunia panamensis and C. odorata in addition to several exotic or introduced plants such as Psidium guajava and Spathodea campanulata, which are considered indicators of disturbance (Somarriba 1988; Pascarella et al. 2000; Miceli-Méndez et al. 2008). On the other hand, the similarity between late forests and coffee plantations reflects their similar vegetation structure and composition (mostly trees), which is also demonstrated in previous studies (e.g., Moguel and Toledo 1999; Soto-Pinto et al. 2001). Therefore, our results indicate that shade coffee plantations may provide similar environmental services as late succession forests for maintaining biodiversity and human wellbeing (Davidson 2004; Bandeira et al. 2005; Perfecto et al. 2005; Meylan et al. 2017). In addition, both the late forests and coffee plantations provided better microclimatic (humidity, precipitation) and soil conditions (higher soil organic matter) than cattle pastures, which in turn influenced plant species composition within land uses.
Conclusions
Woody plant composition, diversity and structure of shade coffee plantations are similar to those of forests in advanced succession. Our results confirm previous findings on the importance of rustic or traditional coffee plantations for maintaining and conserving biodiversity and environmental services (Moguel and Toledo 1999; Soto-Pinto et al. 2001; Davidson 2004; Manson et al. 2008). This is important, considering that the temperate humid forests of the Sierra Madre del Sur are mostly fragmented and distributed across landscapes with varying levels of disturbance and succession (Almazán-Nú?ez et al. 2016, 2018). Such disturbances, including selective logging, to the original structure and composition of many plant communities have contributed to the listing of several species to ‘a(chǎn)t risk’ categories.
Economic and modernity-driven anthropogenic disturbances to natural environments, including intensive land use regimes, result in negative consequences for biodiversity and ecological integrity (Lambin et al. 2001; Fjelds? and Burgess 2008). Unfortunately, these disturbances are currently affecting most of Mexico’s ecosystems (Ponce-Reyes et al. 2012; Rojas-Soto et al. 2012; Mendoza-Ponce et al. 2018). Given this context, it is significant that shade coffee plantations have been shown to be important reservoirs of biodiversity, and may represent one strategy for the conservation and sustainable use of tropical humid forest ecosystems compared to other intensive land uses. Rustic coffee plantations are a biodiversity-enhancing alternative to maintain and conserve cloud forests while simultaneously providing economic benefits. However, across the tropics this traditional agroforestry system is being abandoned due to pest outbreaks and ineffective pest control measures, e.g. coffee rust, Hemileia vastatrix. Although, more recently additional biological control agents are being studied to help mitigate this fungal pest and thereby support a land use approach that maintains biodiversity in cloud forests (Vandermeer et al. 2009; Jackson et al. 2012). In addition, the often low prices at which coffee is purchased from producers has resulted in economic losses for farmers, with some increasingly pursuing ‘fair trade’ opportunities (Avelino et al. 2015). Although our study highlights the biological and ecological importance of shade coffee plantations, it is important to build and consolidate integral forest management strategies that both preserve biological resources and diversity ofecosystems, and work in partnership with local communities. Collaborative strategies for conserving tropical humid forests of southern Mexico is especially important, considering the accelerated pace of globally driven deforestation and land use changes in this region.
AcknowledgementsThe first author thanks CONACYT (#858464) for the scholarship awarded for his Master’s degree and to the Universidad Autónoma de Guerrero for allowing him to complete his Master’s degree in Natural Resources and Ecology. We are thankful to Diana Ruiz, Rosalba Rodríguez and Pablo Sierra who helped with the fieldwork, as well as to Lucio Lozada and Francisco Maradiaga for identifying plant specimens.
References
Almazán-Nú?ez RC, Corcuera P, Parra-Juárez L, Jiménez-Hernández J, Charre GM (2016) Changes in structure and diversity of woody plants in a secondary mixed pine-oak in the Sierra Madre del Sur of Mexico. Forests 7(4):90
Almazán-Nú?ez RC, Charre GM, Pineda-López R, Corcuera P, Rodríguez-Godínez R, Alvarez-Alvarez EA, Bahena-Méndez A (2018) Relationship between bird diversity and habitat along a pine-oak successional forest in southern Mexico. In: dos Santos-Viana HF, García-Morote FA (eds) New perspectives in forest science. IntechOpen, London, UK, pp 185–201
Almeda F (1993) Melastomataceae. In: Rzedowski J, Rzedowski GC (eds) Flora del bajío y regiones adyacentes. Fascículo 10. Instituto de Ecología AC, Veracruz, pp 1–36
APG IV (Angiosperm Phylogeny Group) (2016) An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot J Linn Soc 181(1):1–20
Avelino J, Cristancho M, Georgiou S, Imbach P, Aguilar L, Bornemann G, L?derach P, Anzueto F, Hruska AJ, Morales C (2015) The coffee rust crises in Colombia and Central America (2008–2013): impacts, plausible causes and proposed solutions. Food Secur 7(2):303–321
Bagella S, Caria MC, Farris E, Rossetti I, Filigheddu R (2016) Traditional land uses enhanced plant biodiversity in a Mediterranean agro-silvo-pastoral system. Plant Biosyst 150(2):201–207
Bandeira FP, Martorell C, Meave JA, Caballero J (2005) The role of rustic coffee plantations in the conservation of wild tree diversity in the Chinantec region of Mexico. Biodivers Conserv 14(5):1225–1240
Carvajal S, González-Villarreal LM (2005) La familia Cecropiaceae en el estado de Jalisco, Mexico. Universidad de Guadalajara, Jalisco, p 25
Chao A, Jost L (2015) Estimating diversity and entropy profiles via discovery rates of new species. Methods Ecol Evol 6(8):873–882
Chao A, Ma KH, Hsieh TC (2016). iNEXT (iNterpolation and
EXTrapolation) online: software for interpolation and extrapolation of species diversity. https://chao.shiny apps.io/iNEXT Onlin e/. Accessed 15 Oct 2019
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust J Ecol 18(1):117–143
Colwell RK (2013) EstimateS: statistical estimation of species richness and shared species from samples. Version 9.1.0. Storrs, USA: University of Connecticut. http://vicer oy.eeb.uconn.edu/estim ates/index.html. Accessed 15 Oct 2019
Colwell RK, Coddington JA (1994) Estimating terrestrial biodiversity through extrapolation. Philos Trans R Soc B 345(1311):101–118
Cubi?a A, Aide TM (2001) The effect of distance from forest edge on seed rain and soil seed bank in a tropical pasture. Biotropica 33(2):260–267
Dávalos-Sotelo R, Morato MI, Pinillos-Cueto EM (2008) Almacenamiento de carbono. In: Manson RH, Hernández-Ortiz V, Gallina S, Mehltreter K (eds) Agroecosistemas cafetaleros de Veracruz: biodiversidad, manejo y conservación. Instituto de Ecología ACInstituto Nacional de Ecología, Mexico, pp 223–234
Davidson S (2004) Shade coffee agro-ecosystems in Mexico. J Sustain For 21(1):81–95
de Leeuw J, Mair P (2009) Multidimensional scaling using majorization: SMACOF in R. J Stat Softw 31(3):1–30
DeClerck FAJ, Chazdon R, Holl KD, Milder JC, Finegan B, Martinez-Salinas A, Imbach P, Canet L, Ramos Z (2010) Biodiversity conservation in human-modified landscapes of Mesoamerica: past, present and future. Biol Conserv 143(10):2301–2313
Díaz S, Lavorel S, McIntyre S, Falczuk V, Casanoves F, Milchunas DG, Skarpe C, Rusch G, Sternberg M, Noy-Meir I, Landsberg J, Zhang W, Clark H, Campbell BD (2007) Plant trait responses to grazing—a global synthesis. Glob Chang Biol 13:313–341
Fjelds? J, Burgess ND (2008) The coincidence of biodiversity patterns and human settlement in Africa. Afr J Ecol 46(1):33–42
García CM, Guevara-Féver F, Rodríguez MAM, Silva-Sáenz P, Chávez-Carbajal MA, García-Ruíz I (2000) Estudio florístico en el área de la comunidad indígena de Nuevo San Juan Parangaricutiro, Michoacán, México. Acta Bot Mex 52:5–41
Geissert D, Ibá?ez A (2008) Calidad y ambiente físicoquímico de los suelos. In: Manson RH, Hernández-Ortiz V, Gallina S, Mehltreter K (eds) Agroecosistemas cafetaleros de Veracruz: biodiversidad, manejo y conservación. Instituto de Ecología AC-Instituto Nacional de Ecología, México, pp 213–222
González-Zamora A, Esperón-Rodríguez M, Barradas VL (2016) Mountain cloud forest and grown-shade coffee plantations: a comparison of tree biodiversity in central Veracruz, Mexico. For Syst 25(1):1–11
Gresser C, Tickell S (2002) Mugged: poverty in your cup. Oxfam International, Washington, p 54
Gual-Díaz M, Rendón-Correa A (2014) Bosques mesófilos de monta?a de México: diversidad, ecología y manejo. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, Mexico, p 351
Hamilton LS, Juvik JO, Scatena FN (1995) Tropical montane cloud forest. Springer, New York, p 352
Hammer O, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Version 2.17. Norway: University of Oslo. https://palae o-elect ronic a.org/2001_1/past/issue 1_01.htm. Accessed 15 Oct 2019
Hernández-Ladrón IG, Rojas-Soto OR, López-Barrera F, Puebla-Olivares F, Díaz-Castelazo C (2012) Dispersión de semillas por aves en un paisaje de bosque mesófilo en el centro de Veracruz, México: su papel en la restauración pasiva. Rev Chil Hist Nat 85(1):89–100
Holl KD, Loik ME, Lin EHV, Samuels IA (2000) Tropical montane forest restoration in Costa Rica: overcoming barriers to dispersal and establishment. Restor Ecol 8(4):339–349
Hooper E, Legendre P, Condit R (2005) Barriers to forest regeneration of deforested and abandoned land in Panama. J Appl Ecol 42(6):1165–1174
Hortal J, Borges PA, Gaspar C (2006) Evaluating the performance of species richness estimators: sensitivity to simple grain size. J Anim Ecol 75(1):274–287
INEGI (2010) (Instituto Nacional de Estadística, Geografía e Informática). Página electrónica institucional. https://www.inegi .org.mx/. Accessed 15 Oct 2019
IUSS Working Group WRB (2015) Base referencial mundial del recurso suelo 2014, actualización 2015: sistema internacional de clasificación de suelos para la nomenclatura de suelos y la creación de leyendas de mapas de suelos. Informes sobre recursos mundiales de suelos 106. FAO, Roma, Italia, p 218
Jackson D, Skillman J, Vandermeer J (2012) Indirect biological control of the coffee leaf rust, Hemileia vastatrix, by the entomogenous fungus Lecanicillium lecanii in a complex coffee agroecosystem. Biol Control 61:89–97
Jose S (2012) Agroforestry for conserving and enhancing biodiversity. Agrofor Syst 85(1):1–8
Jost L (2006) Entropy and diversity. Oikos 113(2):363–375
Juárez-Lopez BM, Velázquez-Rosas N, López-Binnqüist C (2017) Tree diversity and uses in coffee plantations of a Mixe community in Oaxaca, Mexico. J Ethnobiol 37(4):765–778
Kelaidis P (2015) Introduction: principal steppe regions. In: Bone M, Johnson D, Kelaidis P, Kintgen M, Vickerman LG (eds) Steppes: the plants and ecology of the world’s semiarid regions. Timber Press, Portland, p 360
Kikoti IA, Mligo C (2015) Impacts of livestock grazing on plant species composition in montane forests on the northern slope of Mount Kilimanjaro, Tanzania. Int J Biodivers Sci Ecosyst Serv Manag 11(2):114–127
Lambin E, Turner B, Geist H, Agbola S, Angelsen A, Bruce J, Coomes O, Dirzo R, Fischer G, Folke C, George P, Homewood K, Imbernon J, Leemans R, Li X, Moran E, Mortimore M, Ramakrishnan P, Richards J, Skanes H, Steffen W, Stone G, Svedin U, Veldkamp T, Vogel C, Xu J (2001) The causes of land-use and landcover change: moving beyond the myths. Glob Environ Chang 11(4):261–269
Legendre P, Gallagher E (2001) Ecologically meaningful transformations for ordination of species data. Oecologia 129:271–280
López-Barrera F, Landgrave R (2008) Variación de la biodiversidad a nivel paisaje. In: Manson RH, Hernández-Ortiz V, Gallina S, Mehltreter K (eds) Agroecosistemas cafetaleros de Veracruz: biodiversidad, manejo y conservación. Instituto de Ecología ACInstituto Nacional de Ecología, Mexico, pp 259–270
López-Gómez AM, Williams-Linera G, Manson RH (2008) Tree species diversity and vegetation structure in shade coffee farms in Veracruz, Mexico. Agric Ecosyst Environ 124:160–172
López-Mata L, Villase?or JL, Cruz-Cárdenas G, Ortiz E, Ortiz-Solorio C (2012) Predictores ambientales de la riqueza de especies de plantas del bosque húmedo de monta?a de México. Bot Sci 90(1):27–36
Lozada T, de Koning GHJ, Marché R, Klein A-M, Tscharntke T (2007) Tree recovery and seed dispersal by birds: comparing forests, agroforestry and abandoned agroforestry in coastal Ecuador. Perspect Plant Ecol Evol Syst 8(3):131–140
Lugo A, Herlmer E (2004) Emerging forests on abandoned land: Puerto Rico’s new forests. For Ecol Manag 190:145–161
Luna-Vega I, Espinosa D, Contreras-Medina R (2016) Biodiversidad de la Sierra Madre del Sur: una síntesis preliminar. Universidad Nacional Autónoma de México, Mexico, p 528
MacGregor-Fors I, Payton ME (2013) Contrasting diversity values: statistical inferences based on overlapping confidence intervals. PLoS ONE 8(2):e56794
Macip-Ríos R, Casas-Andreu G (2008) Los cafetales en México y su importancia para la conservación de los anfibios y reptiles. Acta Zool Mex 24(2):143–159
Manson RH, Hernández-Ortiz V, Gallina S, Mehltreter K (2008) Agroecosistemas cafetaleros de Veracruz: biodiversidad, manejo y conservación. Instituto de Ecología AC-Instituto Nacional de Ecología, Mexico, p 348
Marcano-Vega H, Aide TH, Báez D (2002) Forest regeneration in abandoned coffee plantations and pastures in the cordillera central of Puerto Rico. Plant Ecol 161(1):75–87
Martínez ML, Pérez-Maqueo O, Vázquez G, Castillo-Campos G, García-Franco J, Mehltreter K, Equihua M, Landgrave R (2009) Effects of land use change on biodiversity and ecosystem services in tropical montane cloud forests of Mexico. For Ecol Manag 258(9):1856–1863
Martini AMZ, dos Santos FAM (2007) Effects of distinct types of disturbance on seed rain in the Atlantic forest of NE Brazil. Plant Ecol 190(1):81–95
Méndez VE, Gliessman SR, Gilbert GS (2007) Tree biodiversity in farmer cooperatives of a shade coffee landscape in western El Salvador. Agric Ecosyst Environ 119:145–159
Mendoza-Ponce A, Corona-Nú?ez R, Kraxner F, Leduc S, Patrizio P (2018) Identifying effects of land use cover changes and climate change on terrestrial ecosystems and carbon stocks in Mexico. Glob Environ Chang 53:12–23
Meylan L, Gary C, Allinne C, Ortiz J, Jackson L, Rapidel B (2017) Evaluating the effect of shade trees on provision ofecosystem services in intensively managed coffee plantations. Agric Ecosyst Environ 245:32–42
Miceli-Méndez CL, Ferguson BG, Ramírez-Marcial N (2008) Seed dispersal by cattle: natural history and applications to Neotropical forest restoration and agroforestry. In: Myster RW (ed) Postagricultural succession in the Neotropics. Springer, New York, pp 165–191
Minchin P (1987) An evaluation of the relative robustness of techniques for ecological ordination. Vegetation 69:89–107
Moguel P, Toledo VM (1999) Biodiversity conservation in traditional coffee systems of Mexico: a review. Conserv Biol 13(1):1–11
Monta?a C, Ezcurra E (1980) Simple instrument for quick measurement of crown projections. J For 78:699
Muller-Dombois D, Ellenberg H (1974) Aims and methods of vegetation ecology. Wiley, New York, p 580
Mu?iz-Castro MA, Williams-Linera G, Benayas JM (2006) Distance effect from cloud forest fragments on plant community structure in abandoned pastures in Veracruz, Mexico. J Trop Ecol 22(4):431–440
Orlóci L (1978) Multivariate analysis in vegetation research. Springer, Basel, p 452
Ortega-Pieck A, López-Barrera F, Ramírez-Marcial N, García-Franco JG (2011) Early seedling establishment of two tropical montane cloud forest tree species: the role of native and exotic grasses. For Ecol Manag 261(7):1336–1343
Pascarella JB, Aide TM, Serrano MI, Zimmerman JK (2000) Landuse history and forest regeneration in the Cayey Mountains, Puerto Rico. Ecosystems 3(3):217–228
Perfecto I, Vandermeer J (2002) Quality of agroecological matrix in a tropical montane landscape: ants in coffee plantations in southern Mexico. Conserv Biol 16(1):174–182
Perfecto I, Rice RA, Greenberg R, van der Voort ME (1996) Shade coffee: a disappearing refuge for biodiversity. Bioscience 46(8):598–608
Perfecto I, Vandermeer J, Mas A, Soto-Pinto L (2005) Biodiversity, yield, and shade coffee certification. Ecol Econ 54(4):435–446
Perfecto I, Armbrecht I, Philpott SM, Soto-Pinto L, Dietsch TM (2007) Shaded coffee and the stability of rainforest margins in northern Latin America. In: Tscharntke T, Leuschner C, Zeller M, Guhadja E, Bidin A (eds) The stability of tropical rainforest margins: linking ecological, economic and social constraints of land use and conservation. Springer, Basel, pp 227–264
Philpott SM, Bichier P, Rice R, Greenberg R (2007) Field-testing ecological and economic benefits of coffee certifications programs. Conserv Biol 21(4):975–985
Philpott SM, Arendt WJ, Armbrecht I, Bichier P, Diestch TV, Gordon C, Greenberg R, Perfecto I, Reynoso-Santos R, Soto-Pinto L, Tejeda-Cruz C, Williams-Linera G, Valenzuela J, Zolotoff JM (2008) Biodiversity loss in Latin American coffee landscapes: review of the evidence on ants, birds, and trees. Conserv Biol 22(5):1093–1105
Ponce-Reyes R, Reynoso-Rosales VH, Watson JEM, VanDerWal J, Fuller RA, Pressey RL, Possingham HP (2012) Vulnerability of cloud forest reserves in Mexico to climate change. Nat Clim Chang 2:448–452
Rahmanian S, Hejda M, Ejtehadi H, Farzam M, Memariani F, Py?ek P (2019) Effects of livestock grazing on soil, plant functional diversity, and ecological traits vary between regions with different climates in northeastern Iran. Ecol Evol 9(14):8225–8237
Rangel SR, Carlos E, Zenteno R, de Lourdes M, Enriquez A (2002) El género Quercus (Fagaceae) en el estado de Mexico. Ann Mo Bot Gard 89(4):551–593
Rojas-Soto O, Sosa V, Ornelas JF (2012) Forecasting cloud forest in eastern and southern Mexico: conservation insights under future climate change scenarios. Biodivers Conserv 21(10):2671–2690
?ekercio?lu CH, Daily GC, Ehrlich PR (2004) Ecosystem consequences of bird declines. Proc Nat Acad Sci USA 101(52):18042–18047
SEMARNAT (2010) (Secretaría de Medio Ambiente y Recursos Naturales). Norma Oficial Mexicana NOM-059-SEMARNAT-2010, Protección ambiental-Especies nativas de México de flora y fauna silvestres, Categoría de riesgo y especificaciones para su inclusión, exclusión o cambio-Lista de especies en riesgo. Diario Oficial de la Federación, Diciembre 30, 2010. Ciudad de México, México. http://bibli oteca .semar nat.gob.mx/janiu m/Docum entos /Ciga/agend a/DOFsr /DO245 4.pdf. Accessed 16 Oct 19
Somarriba E (1988) Pasture growth and floristic composition under the shade of guava ( Psidium guajava L.) trees in Costa Rica. Agrofor Syst 6:153–162
Soto-Pinto L, Romero-Alvarado Y, Caballero-Nieto J, Segura G (2001) Woody plant diversity and structure of shade-growncoffee plantations in Northern Chiapas, Mexico. Rev Biol Trop 49:977–987
Soto-Pinto L, Villalvazo-Lopez V, Jimenez-Ferrer G, Ramirez-Marcial N, Montoya G, Sinclair F (2007) The role of local knowledge in determining shade composition of multistrata coffee systems in Chiapas, Mexico. Biodivers Conserv 16(2):419–436
Soto-Pinto L, Castillo-Santiago MA, Jiménez-Ferrer G (2012) Agroforestry systems and local institutional development for preventing deforestation in Chiapas, Mexico. In: Moutinho P (ed) Deforestation around the world. IntechOpen, London, pp 333–350
SPSS (2011) SPSS Statistics for Windows. Version 20.0. SPSS Institute Inc, Chicago, USA. https://www.ibm.com/mx-es/analy tics/spss-stati stics-softw are. Accessed 15 Oct 19
Staver C, Bustamante O, Siles P, Aguilar C, Quinde K, Castellón J, Somarriba F, Tapia A, Brenes S, Deras M, Matute N (2013) Intercropping bananas with coffee and trees: prototyping agroecological intensification by farmers and scientists. Acta Hortic 986:79–85
ter Braak CJF, ?milauer P (2002) CANOCO Reference manual and CanoDraw for Windows user’s guide: software for canonical community ordination (version 4.5). Microcomputer Power, Ithaca, New York, USA. www.canoc o.com. Accessed 15 Oct 19
Tscharntke T, Clough Y, Bhagwat SA, Buchori D, Faust H, Hertel D, H?lscher D, Juhrbandt J, Kessler M, Perfecto I, Scherber C, Schroth G, Veldkamp E, Wanger TC (2011) Multifunctional shade-tree management in tropical agroforestry landscapes: a review. J Appl Ecol 48(3):619–629
Vandermeer J, de la Cerda IG (2004) Height dynamics of the thinning canopy of a tropical rain forest: 14 years of succession in a post-hurricane forest in Nicaragua. For Ecol Manag 199(1):125–135
Vandermeer J, Perfecto I, Liere H (2009) Evidence for hyperparasitism of coffe rust ( Hemileia vastatrix) by the entomogeneous fungus, Lecanicillium lecanii, through a complex ecological web. Plant Pathol 58(4):636–641
Vázquez-Yanez C, Batis-Mu?oz AI, Alcocer-Silva MI, Gual-Díaz M, Sánchez-Dirzo C (1999) árboles y arbustos potencialmente valiosos para la restauración ecológica y la reforestación. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad-Instituto de Ecología AC-Universidad Nacional Autónoma de México, México, DF, p 15
Villase?or JL (2010) El bosque húmedo de monta?a en México y sus plantas vasculares: catálogo florístico-taxonómico. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad-Universidad Nacional Autónoma de México, México, DF, p 38
Villase?or JL (2016) Checklist of the native vascular plants of Mexico. Rev Mex Biodivers 87:559–902
W3Tropicos (2010) Missouri Botanical Garden’s VAST nomenclatural database and associated authority files. Missouri Botanical Garden, Saint Louis, Missouri. http://tropi cos.org/. Accessed 16 Oct 19
Wendt T (1986) árboles. In: Lot A, Chiang F (eds) Manual de herbario: administración y manejo de colecciones, técnicas de recolección y preparación de ejemplares botánicos. Consejo Nacional de la Flora de México AC, México, pp 133–142
Williams-Linera G, López-Gómez A (2008) Estructura y diversidad de la vegetación le?osa. In: Manson RH, Hernández-Ortiz V, Gallina S, Mehltreter K (eds) Agroecosistemas cafetaleros de Veracruz: biodiversidad, manejo y conservación. Instituto de Ecología AC-Instituto Nacional de Ecología, Mexico, pp 55–68
Williams-Linera G, Manson RH, Vera EI (2002) La fragmentación del bosque mesófilo de monta?a y patrones de uso del suelo en la región oeste de Xalapa, Veracruz, México. Madera y Bosques 8(1):69–85
Williams-Linera G, Toledo-Garibaldi M, Hernández CG (2013) How heterogeneous are the cloud forest communities in the mountains of central Veracruz, Mexico? Plant Ecol 214(5):685–701
Zdravko B, Shingo N (2014) Abandoned coffee plantations: biodiversity conservation or path for nonnative species? Case study in a Neotropical montane forest. Interciencia 39(8):554–561
 Journal of Forestry Research2021年2期
Journal of Forestry Research2021年2期
- Journal of Forestry Research的其它文章
- Stem taper functions for Betula platyphylla in the Daxing’an Mountains, northeast China
- Leaf phenotypic variation of endangered plant Tetracentron sinense Oliv. and influence of geographical and climatic factors
- Ecological variations of woody species along an altitudinal gradient in the Qinling Mountains of Central China: area-based versus mass-based expression of leaf traits
- Variations in stem radii of Larix principis-rupprechtii to environmental factors at two slope locations in the Liupan Mountains, northwest China
- A review of ecological mechanisms for management practices of protective forests
- Variation of basic density, calorific value and volumetric shrinkage within tree height and tree age of Ugandan grown Eucalyptus grandis wood
