Structure and regeneration status of woody species in the Munessa Forest, Southern Ethiopia
Mengistu Gelasso · Junqing Li
Abstract This study evaluates the structure and regeneration status of woody species in the Munessa Forest, a dry Afromontane forest in southern Ethiopia. Vegetation data were collected using a systematic sampling method. Density and distribution of seedlings, saplings and mature trees were assessed along an altitudinal gradient using quadrats of different sizes. The number of individuals, frequencies, heights and DBH of species > 1 m and DBH > 2.5 cm were recorded in altitudinal bands of 100 m. Analysis of the vegetation structure shows that the density of woody species decreases as DBH and height class increases. Basal area of stems with DBH > 2.5 cm was 53.4 m 2 ha ?1 . Population structure and regeneration patterns indicate a significant degradation of the forest due to anthropogenic disturbances. Regeneration was better for less valuable woody species than for species with economic and ecological value. This suggests a discontinuous recruitment of these species due to selective cutting of middle and higher diameter classes. Therefore, enrichment planting of high value, endangered species is necessary to maintain them as part of this forest. There is a need to develop and implement an effective forest management plan for sustainable use of these forest resources.
Keywords Woody species · Altitudinal gradient · Regeneration status · Structural distribution
Introduction
Population structure of a forest is regarded as the distribution of individuals ofeach species that provides the regeneration profile based on tree density, frequency, height, diameter at breast height, basal area and species importance value (Peters 1996; Tesfaye et al. 2002; Shibru and Balcha 2004). Understanding forest structure and composition is important for recognizing population dynamics. In addition, adequate information on species population structure is essential to understand historic disturbances, current regeneration status, ecology, and future population trends of a particular species (Clark 1991; Alvarez-Buyalla et al. 1996; Peters 1996). Quantitative analysis of forest composition and structure is also a precondition for the evaluation of its biodiversity (Singh et al. 2014).
The regeneration potential of a species may be accessed from the population structure and the dynamics of seedlings and saplings (Tesfaye et al. 2002; Duchok et al. 2005). The ratio of seedlings and saplings of a species illustrates its regeneration pattern and the future structure of the forest communities (Pala et al. 2012). If regeneration takes place continuously, then the distribution of the species cohorts shows a reverse J-shape curve, an indicator of healthy regeneration (Silvertown 1984; Teketay 1997). This type of population structure is common in natural forests where external disturbances are limited (Getachew et al. 2002; Feyera 2006).
Many studies of stand structure and regeneration assessment have been carried out in tropical Afromontane forests (Shibru and Balcha 2004; Tilahun et al. 2011; Birhanu et al. 2014; Tesfaye et al. 2017). These studies have reported the uncontrollable loss of these resources due to anthropogenic disturbances such as conversion to other land uses and commercial logging (Getachew et al. 2002). The regeneration of important woody species of Ethiopian Afromontane forests has been declining due to anthropogenic disturbances and improper forest management. Disturbances such as illegal logging and overgrazing have hindered forest regeneration (Getachew et al. 2002).
Like other dry Afromontane forests in Ethiopia, the Munessa Forest has been strongly affected by anthropogenic disturbances (Tesfaye et al. 2010). However, studies of forest structure and regeneration are of limited use for effective forest management and conservation. Therefore, this study of structural distribution and regeneration status of woody species is needed to identify native tree species that require effective conservation.
Materials and methods
The study was carried out in the Kuke Natural Forest Site of the Munessa Forest and Wildlife District of the Arsi Zone branch, located at of 38°53′E and 7°27′N, about 253 km south of Addis Ababa (Fig.1). The site is in the warm subhumid and cold humid agro-ecological zones with mean annual rainfall and temperatures of 1343 mm and 15 °C, respectively (Asferachew 2004).
The study area is 1787 ha with altitudinal gradients from 2200 to 2700 m a.s.l. The natural forests are tropical dry Afromontane (Teketay and Granstr?m 1995). Soils are developed from volcanic parent material and are rich in clay minerals and iron oxides (Fritzsche et al. 2007) and show altitudinal zonation due to the homogeneous volcanic parent material. According to the World Reference Base of Soil Resources (FAO-ISRIC-ISSS 1998), the soils are classified as Mollic Nitisols.
Sampling design and vegetation data collection
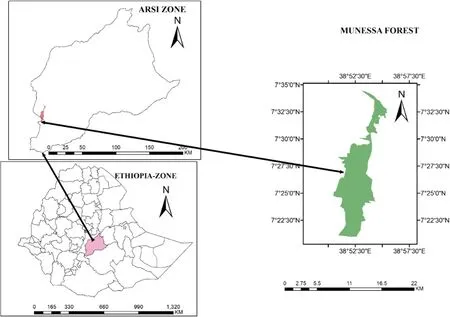
Fig.1 Location of the study area
Following a reconnaissance survey, vegetation data collection was carried out from October 20 to December 20, 2018 using systematic sampling Five line transects were established following the elevation gradient at 100 m intervals between two consecutive transect lines. On each transect, 10 quadrats (20 m × 20 m), separated by 200 m were established. During data gathering, elevation, longitude and latitude was recorded using GPS, and aspect determined with a compass. Density of seedlings and saplings were measured from 5 m × 5 m sub-quadrats demarcated at the four corners and in the center. Scientific names were identified according to Bekele ( 2007). Species that were diffi cult to identify were identified at the Addis Ababa National Herbarium.
Measurement of regeneration and population structure
Diameter at breast height (DBH) is the most common variable to measure tree size and can be a proxy of tree age. However, numerous studies use height class distribution, including seedlings and saplings, to determine the age or size structure of woody species (Geldenhuys 1993; Breckle 1997). Data on seedling and sapling density and size provides information on the distribution and regeneration status of the vegetation. In the 20 m × 20 m quadrats, all woody species were recorded and their height and DBH were measured. Heights of trees were measured using a clinometer while seedling heights were measured with a ruler. Species ≤ 1 m and DBH ≤ 2 cm were considered seedlings while saplings were > 1 m and DBH ≤ 2.5 cm. Species density was calculated by converting the number counted from all quadrats into a hectare basis, and regeneration status determined according to seedling and sapling densities (Shankar 2001). Based on this, regeneration status was classified into: good regeneration (seedlings > saplings > adults); fair regeneration, (seedlings > or ≤ saplings ≤ adults); and, poor regeneration when only saplings were present (saplings may be ≤ oradults) and no seedlings (Shankar 2001).
Analysis of vegetation structure
Data of individuals of selected species were grouped into height classes to illustrate forest vertical structure. Three strata were identified: upper, middle and lower layers. The upper layer included individual tree heights exceeded 2/3 of the top height, while the middle layer had heights between 1/3 and 2/3 of the top height. The lowest layer was ? 1/3 of the top height (Lamprecht 1989).
The following variables were calculated using field data:
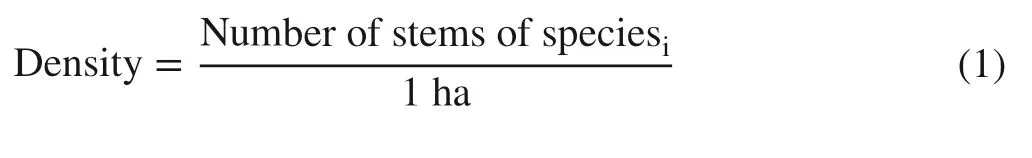
Frequency

DBH: obtained by dividing the circumference (C) ofeach tree recorded in the field byπ
Basal Area: computed for individuals with DBH > 2.5 cm and calculated as

Importance value index (IVI).
For all species with DBH > 2.5 cm, the relative density, frequency, dominance and Importance Value Indices (IVI) were calculated using the following:
Relative density (RD):
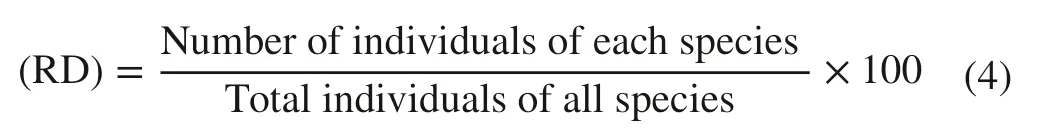
Relative frequency (RF):
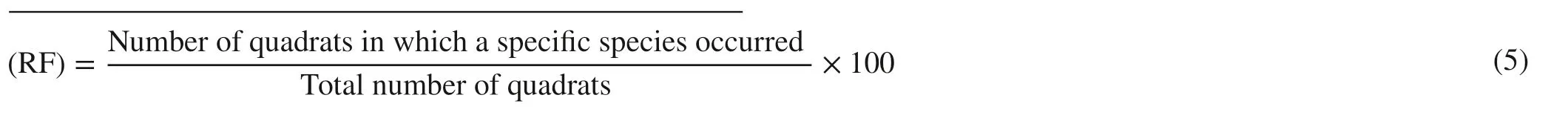
Relative dominance (RDO):

Results and discussion
Density and DBH
The density of trees and shrubs2.5 cm DBH was 445 stems ha?1. The distribution of woody species in different DBH classes was analysed and classified into 10 classes: (1) 2.5–10 cm; (2) 10.01–20 cm; (3) 20.1–40 cm; (4) 40.1–60 cm; (5) 60.1–80 cm; (6) 80.1–100 cm; (7) 100.1–120 cm; (8) 120.1–140 cm; (9) 140.1–160 cm; and, (10) > 160 cm. The majority of individuals were distributed in the 2.5–10 cm class with 139 stems ha?1(31.2%). The balance in the 2–10 DBH classes was 123 in the 2-cm class (27.5%), 113 (25.3%), 43 (9.6%), 12 (2.7%), 4 (0.9%), 2 (0.3%), 3 (0.6%), 4 (0.8%), and 5 (1.0%) in the 3-cm, 4-cm, 5-cm, 6-cm, 7-cm, 8-cm, 9-cm and 10-cm classes, respectively (Fig.2). The majority of species were in the first DBH class, and numbers decreased in the higher classes except for classes 9 and 10 in which a slight increase occurred.
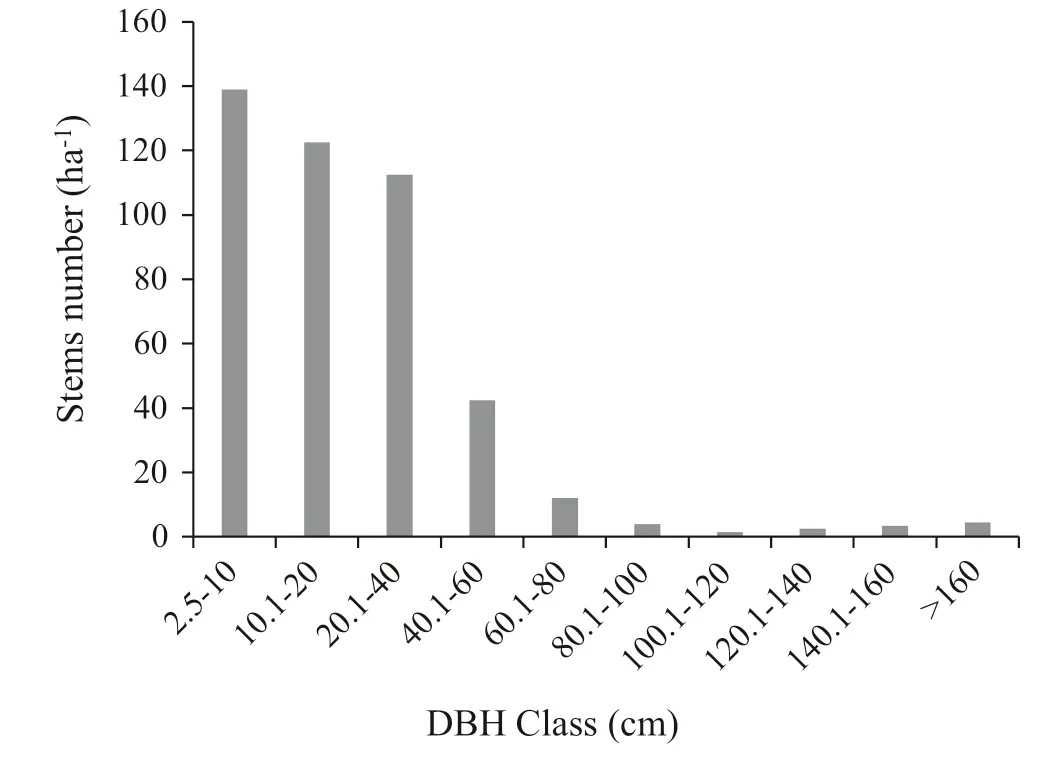
Fig.2 The general DBH distribution patterns of Munessa Forest
Species in the DBH > 120 cm class were Podocarpus falcatus Thumb, Celtis africana Burm.f, Diospyros abyssinica (Hiern) F.White, and Schefflera abyssinica (Hochst. Ex. A. Rich.) Harms. The middle DBH classes were also dominated by the above species and an additional few individuals of Maytenus arbutifolia (A.Rich.) Wilczek, Apodytes dimidiata E. Mey. Ex. Arn., Croton macrostachyus Del, Dombeya torrida, Ficus sur Forssk, Millettia ferruginea (Hochest.) Bak, Prunus africana, Hagenia abyssinica, and Bersama abyssinica Fresen. High DBH density was contributed by C. macrostachyus, C. africana, Myrsine melanophloeos, Syzygium guineense, B. abyssinica, D. torrida, M. arbutifolia, Allophylus abyssinicus, and Buddleia polystachya in lower DBH classes.
DBH class distribution showed an inverted J-shaped (Fig.2), indicating good regeneration and recruitment. However, this structure does not describe the general trends of population dynamics and the recruitment process of individual tree species in this forest.
The analysis of population structure for each tree and shrub species could give more relevant information for forest management and conservation (Kelbessa and Soromessa 2008; Didita et al. 2010; Dibaba et al. 2014). DBH classes of selected woody species with higher importance value were analyzed to predict the future of these individuals (Fig.3 a–f). The first pattern was represented by M. arbutifolia (Fig.3 e) and shows a higher number of individuals in the lowest diameter class with gradually declining numbers with increasing diameter. Although it represents an inverted J-shape distribution, there are no individuals in higher diameter classes, which might be due to species to not grow into higher diameter classes by nature. B. abyssinica also has same structural distribution. This type of population pattern indicates good reproduction and recruitment status of the species (Fisaha et al. 2013; Zegeye et al. 2011). A similar distribution was also reported by Haile et al. ( 2008) from Bale Mountain National park.
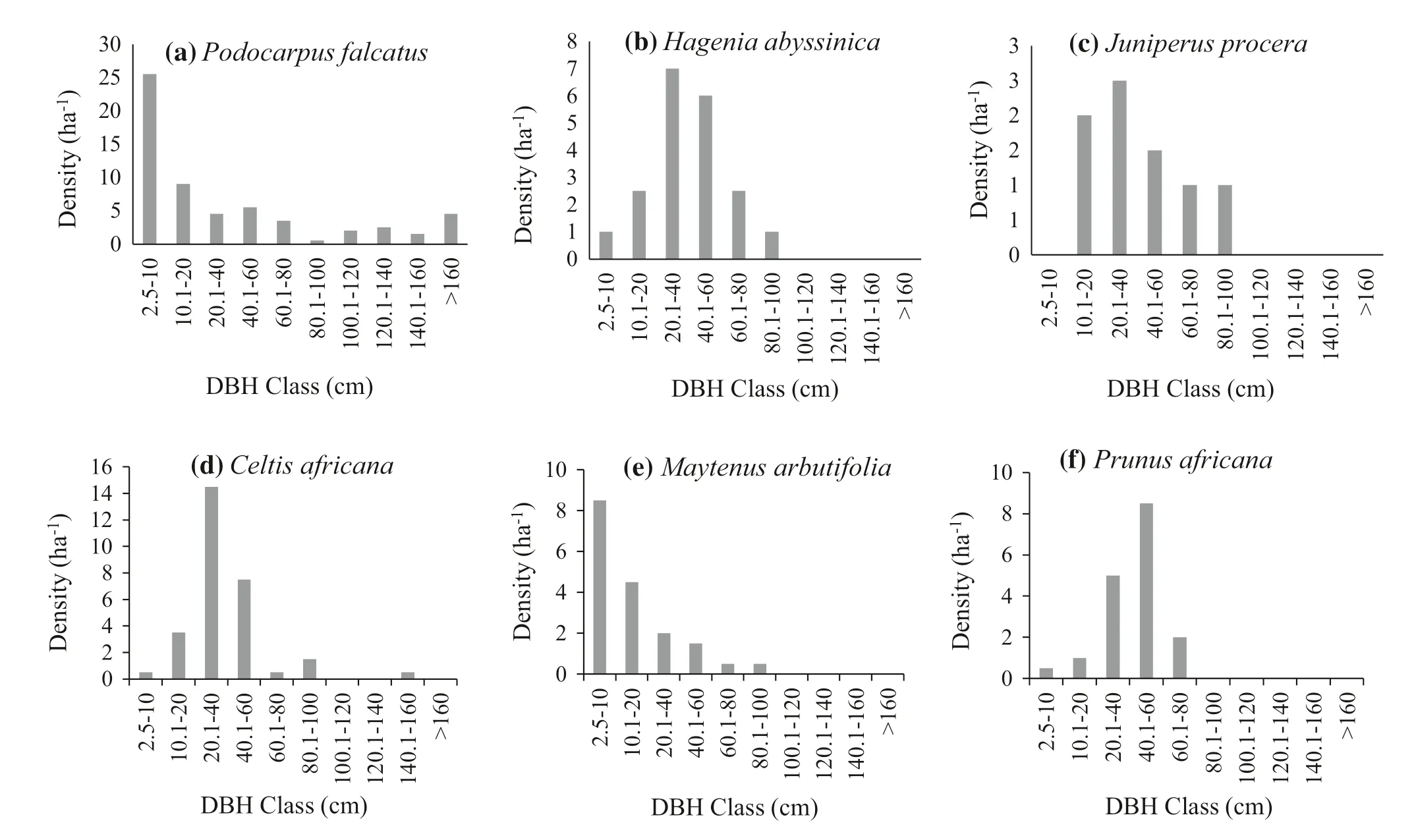
Fig.3 DBH class distribution of selected trees of the Munessa- Forest
The second pattern, exemplified by H. abyssinica, Juniperus procera and P. africana, is a reflection of the absence or presence of very few stems in lower diameter classes, an increasing number of individuals in the next two classes, and a sharp decline in the next higher classes (Fig.3 b, c, and f). This illustrates hampered or impeded regeneration which could be attributed to poor recruitment combined with the selective cutting of individuals in higher diameter classes. The local population use large diameters of these species for lumber and other construction purposes. As a result, the density of these classes was lower in higher diameters. Senbeta and Teketay ( 2003) also reported similar population patterns in the Kimphe Forest by species including Olea welwitschii Knobl and A. dimidiata E.Mey. Ex Arn.
The third pattern, represented by P. falcatus (Fig.3 a), reveals a high number of individuals in the lowest diameter class with gradually declining numbers with increasing diameters but a slight increase after DBH class 7. This species revealed an interrupted inverted J curve indicating good regeneration but discontinuous recruitment into larger size classes due to selective cutting of middle diameter classes. The fourth distribution pattern was illustrated by C. africana (Fig.3 d), showing few individuals in the first diameter class followed by a slight increase in the second, a large number in the third class, and no trees in middle and higher classes. This species shows an interrupted Gaussian curve which indicates poor regeneration and recruitment. This could be attributed to the free grazing and browsing of domestic animals which is very common in the study area, and would affect the establishment and survival of seedlings (Bekele 2000).
Variations in population structure of woody species may be due to environmental factors that can influence regeneration, causing dissimilarities in regeneration performance, human interferences, and changes in other natural and artificial factors.
Height class distribution and vertical structure
Twenty-four species, totaling 2200 individuals/ha, with heights > 1 m, were selected to describe the height structure of the Munessa-Forest. Heights were classified into eight classes (Table 1). The highest species density was in the lowest height class, decreasing gradually towards the middle and highest classes, height class, decreasing gradually towards the middle and highest classes, indicatinga continuous representation of individuals in all height classes. There was a higher number of stems in height classes 1 and 2, accounting for 88.5% of the total number of species, while the other classes (heights > 10 m) accounted for 11.5% (Table 1).
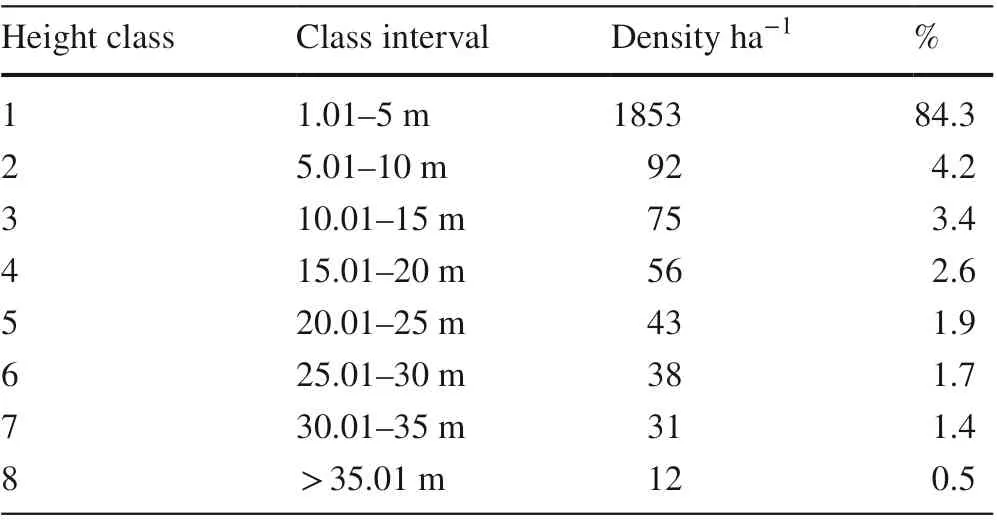
Table 1 Number of individuals by height class (m)
Most individuals that contributed to the first height class were P. falcatus, B. abyssinica, Albizia gummifera, M. arbutifolia and C. macrostachyus. The second and third height classes consisted mainly of Maesa lanceolata, Galiniera saxifraga, P. falcatus and C. macrostachyus. P. falcatus represented 66.7% of the upper canopy, while P. africana and C. africana made up the balance (33.3%).
The greatest height, an emergent tree, was P. falcatus at 42 m. The vertical structure may be categorized into upper, middle and lower stories. Two-thirds of the upper story is > 28 m, the middle story is between 14–28 m, and the lower story is < 14 m. The highest density of 1981 ha?1species was in the lower story, accounting for 90.0% followed by the middle (7.4%) and upper story (2.6%). The general pattern of height class distribution showed distinct inverted J-shaped curves, indicating that the forest was dominated by smaller individuals.
The high number of stems in the lowest height class indicates that the forest has been heavily disturbed by anthropogenic activities of local people through selective cutting, mainly for fuelwood and construction, and by illegal logging of timber. The forest is currently in different stages of secondary development. The dominance of small-sized individuals indicates good regeneration but low recruitment, likely as a result of human factors. Similar results were reported for the Asabot Dry Afromontane Forest (Tura et al. 2017), the Menagesha Amba Mariam Forest (Tilahun 2009), the Berbere Forest (Tesfaye et al. 2017), and the Sanka Meda Forest (Shambel 2010).
Basal area (BA)
The basal area of the Munessa Forest was 53.4 m 2 ha?1for species > 2.5 cm DBH. Approximately 47.1 m 2 ha?1or 88.1% of the basal area was contributed by nine species. P. falcatus contributed 28.0 m 2 ha?1followed by C. africana with 4.8 m 2 ha?1. The lowest BA, ? 0.01 m 2 ha?1, was for species such as Carissa edulis (Forssk.) Vahl, Calpurnia aurea (Ait.) Benth and Vernonia amygdalina Delile. Species with the highest BA may not necessarily have the highest density, indicating size variation among species (Bekele 1994). Some species such as S. abyssinica, J. procera, and D. abyssinica had high basal areas because of their size although they had low density and frequency. On the other hand, M. melanophloeos, Galiniera saxifrage (Hochst.) Bridson, B. abyssinica, and S. guineense (Willd.) DC. had high density and frequency but their basal areas were low due to the nature of the species to not grow into higher diameter classes.
Basal area provides a better measure of the relative importance of a species compared to a simple stem count (Kebede 2010). Therefore, the species with the highest basal area could be considered as the most important species in this Forest.
Lamprecht ( 1989) suggested that according to Dawkins ( 1959), the average basal area of many tropical forests in Africa is 23–37m 2 ha?1. Based on this study, the basal area of the Munesa Forest is relatively high. However, the basal area of the Munesa Forest compared with other dry Afromontane forests in Ethiopia indicates that the Munesa Forest has a lower basal area compared with those of the Dodola, Kimphe Lafa, and Berbere forests, while the Chilimo and Sanka Meda forests have lower basal area than the Munesa Forest. The reason for such variation is that forests with higher basal areas have older, larger trees, whereas the Munessa forest is dominated by small trees and shrubs. Old trees of large size are infrequent in the forest. Table 2 compares the basal area of the Munessa Forest with basal areas of nine other dry Afromontane forests in Ethiopia.
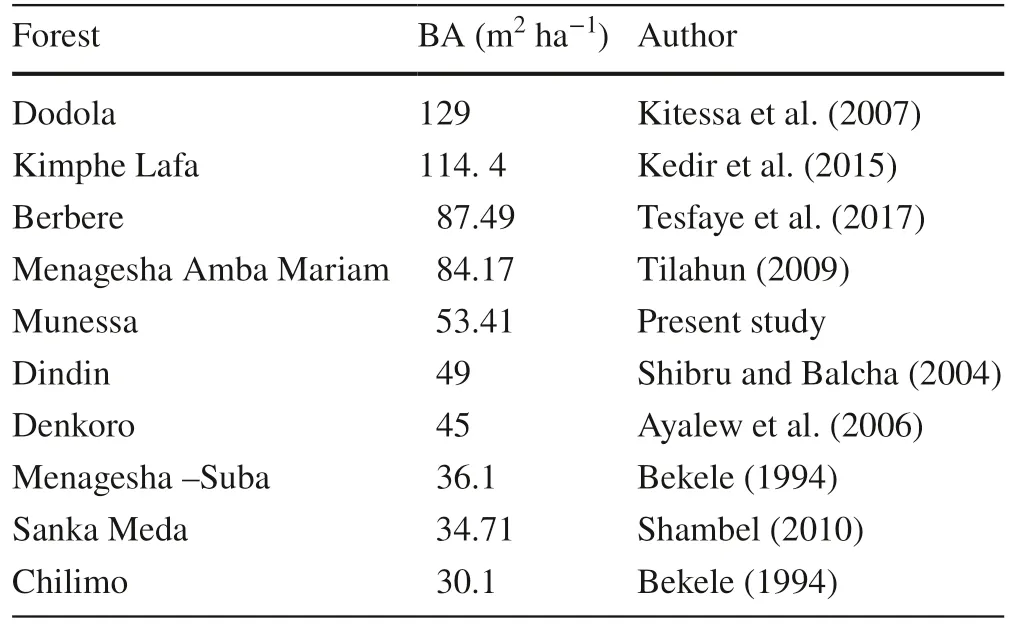
Table 2 Comparison of the basal area of the Munessa Forest with basal areas ofother forests in Ethiopia
Frequency
Woody species were classified into five frequency classes based on appearance in the plots. These classes were: (1) 0–20%, (2) 21–40%, (3) 41–60%, (4) 61–80%, and (5) > 80%. Two species, ( P. falcatus and C. macrostachyus), had the highest frequency as 45 and 35 out of 50, respectively). Species with > 50% frequency were M. arbutifolia, C. africana, M. lanceolata, and A. abyssinicus. Species with the lowest occurrence were A. dimidiata, Acanthus eminens, and Myrsine africana. Most species (66.0%) were in frequency class 1, while the remaining were in frequency classes 2, 3, 4 and 5 in descending order, containing 22.6, 7.5, 1.9, and 1.9%, respectively (Fig.4).
Frequency indicates homogeneity and heterogeneity of vegetation. Lamprecht ( 1989) indicated that a high percentage of species in high frequency and low percentage of species in low frequency classes points out similar composition. In contrast, high values in low-frequency classes and low values in high-frequency classes reveal a high diversity (Shibru and Balcha 2004). Based on this reasoning, Munessa Forest has high vegetation diversity.
Importance value index (IVI)
The IVI is important for comparing the ecological importance of species and for setting conservation priorities (Lamprecht 1989). Species with high IVI values are dominant in particular ecosystems (Shibru and Balcha 2004). The IVIs of tree species in the Munessa Forest is shown in Table 3. According to IVI values, P. falcatus, C. africana, M. lanceolata, C. macrostachyus, P. africana, M. arbutifolia, and H. abyssinica were the seven most dominant species, constituting 53.4% of the total importance value, while the rest of the species (86.8%) had IVI values < 8 (Table 3).
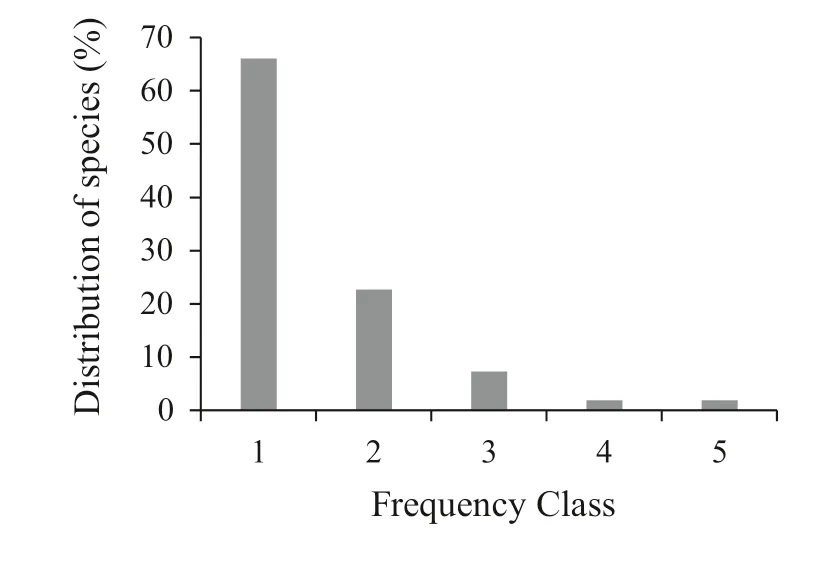
Fig.4 Frequency distribution for woody species in the Munessa Forest. Classes are: (1) 1–20%, (2) 21–40%, (3) 41–60%, (4) 61–80%, and (5) > 80%
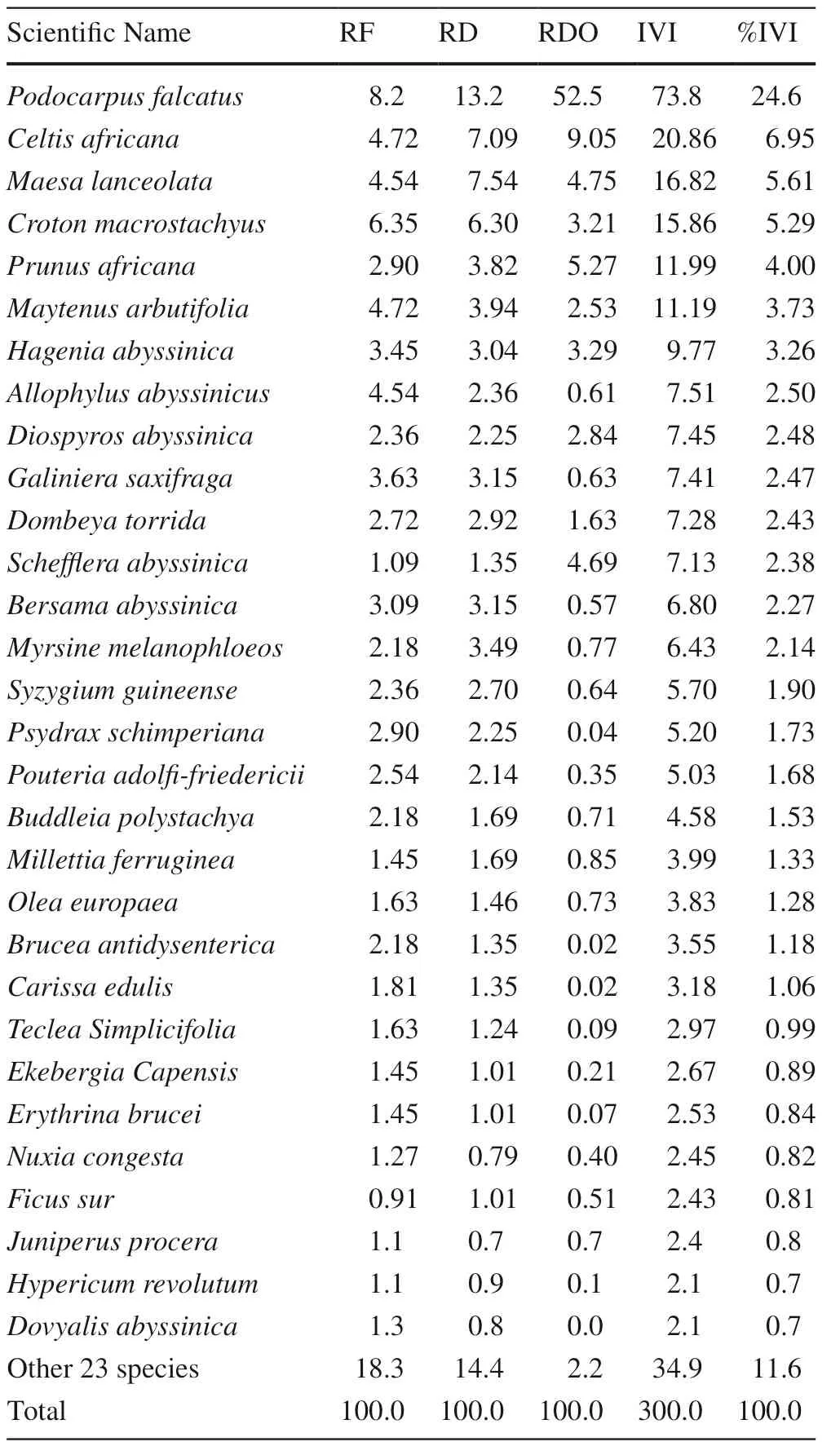
Table 3 Importance Value Index of the dominant tree species (RD = relative density, RF = relative frequency; RDO = relative dominance, IVI = important value index)
The importance value index was grouped into six classes based on their conservation priority as follows: (1) > 20; (2) 15.01–20; (3) 10.01–15; (4) 5.01–10; (5) 1.01–5; (6) < 1. Percentages of species in the IVI classes were 3.8, 3.8, 3.8, 20.7; 60.4; and 7.5 from classes 1–6 respectively (Fig.5). Species that exhibited lower IVI values need high conservation efforts, while those with higher IVI values require continuous monitoring (Gemedo and Simon 2007). Therefore, species that have high IVI values, including P. falcatus, C. africana, C. macrostachyus, M. lanceolata, H. abyssinica, P. africana and M. arbutifolia, need monitoring while high valuable species with lower IVI values are considered to be threatened in Ethiopia, and also at risk of local extinction require immediate protection and conservation to assist their natural regeneration. The remaining species in intermediate priority classes also need conservation.
Regeneration of woody species
The density and composition of seedlings and saplings show the regeneration patterns of the forest species. Overall density of seedlings, saplings and trees was 3474, 2302 and 445 ha?1, respectively. The ratio of seedlings to saplings was 1.5, and saplings to mature trees 5.1, and seedlings to mature trees 7.8. (This indicates that the density of seedlings > saplings > mature trees.) According to the distribution of seedlings, saplings and mature trees, 58% exhibited good regeneration, 34% fair regeneration and 8% poor regeneration (Fig.6).
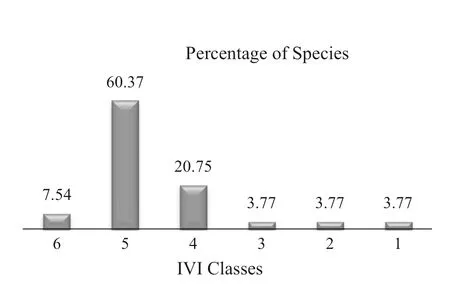
Fig.5 IVI classes and percentages of woody plant species in Munessa Forest
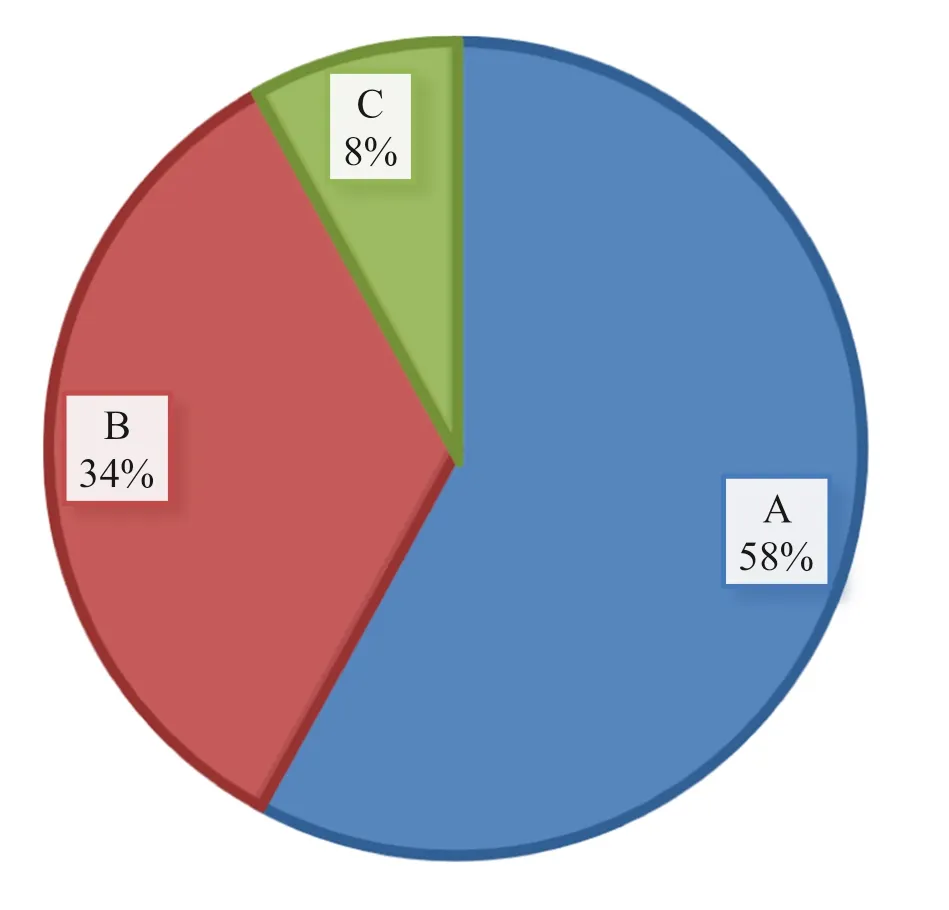
Fig.6 Density percentage of seedlings ( a), saplings ( b) and mature tree/shrub ( c) species
Some of the species that exhibited good regeneration were B. abyssinica, A.gummifera (J.F.Gmel.) C.A.Sm, M. arbutifolia, M. melanophloeos, P. africana, C. africana, S. guineense and D. abyssinica. The fair regenerating species were P. falcatus, C. macrostachyus, Psydrax schimperiana (A. Rich.) Bridson, Teclea Simplicifolia, M. ferruginea and C. edulis. H. abyssinica, J. procera, A. dimidiata, and Schefflera volkensii were poorly regenerated. Species which have economic, medicinal and/or ecological values, including H. abyssinica and J. procera show poor regeneration. This is because most trees are over mature and not producing seeds, as well as unfavourable environmental conditions such as poorly developed soil and livestock browsing. Good regeneration was more common for less valuable trees/shrubs than for species with high importance values, revealing discontinuous recruitment due to selective cutting of middle and higher DBH classes.
Conclusion
The assessment of forest structure and its regeneration status is essential for its management, conservation and sustainable utilization. The analysis of diameter at breast height and height class distribution patterns revealed an inverted J-shaped distribution, which is a reflection of good regeneration. The dominance of small-sized individuals also indicates that the forest is in a secondary stage of development. The regeneration of less valuable species was greater than that of more valuable ones in which several economically and ecologically important species had population structures that exhibited abnormal recruitment structures with poor reproduction potential mainly due to anthropogenic disturbance. Therefore, to sustain biological diversity of this particular forest, this study recommends the preservation of indigenous species, especially those with low importance value, using both in-situ and ex-situ conservation and enrichment planting ofeconomically valuable species, including P. falcatus, J. procera, and H. abyssinica. It is essential to develop and implement effective forest conservation measures to sustainably use biodiversity resources.
References
Alvarez-Buyalla ER, Garcia-Barrios R, Lara-Moreno C, Martinez-Ramos M (1996) Demographic and genetic models in conservation biology: application and perspectives for tropical rain forest tree species. Annu Rev Ecol Syst 27:387–421
Asferachew A (2004) Biomass and nutrient studies of selected tree species of natural and plantation forests: implications for a sustainable management of the Munessa-Shashemene forest, Ethiopia. Ph.D. thesis, University of Bayreuth, Bayreuth, Germany.
Ayalew A, Bekele T, Demissew S (2006) The undifferentiated Afromontane Forest of Denkoro in the central highlands of Ethiopia: a floristic and structural analysis. SINET Ethiop J Sci 29:45–56
Bekele T (1994) Phytosociology and ecology of humid Afromontane Forest on the Central Plateau of Ethiopia. J Veg Sci 5:87–98
Bekele T (2000) Plant population dynamics of Dodonaea angustifolia and Olea europaea subsp. cuspidata in dry Afromontane forests of Ethiopia. Ph.D. thesis, Uppsala University, Uppsala.
Bekele T (2007) Useful Trees and Shrubs for Ethiopia: Identification, Propagation, and Management for 17 agroclimatic zones. Technical manual. 550 pp.
Birhanu K, Teshome S, Ensermu K (2014) Structure and regeneration status of Gedo Dry Evergreen Montane Forest, West Shewa Zone of Oromia National Regional State, Central Ethiopia. Sci Technol Arts Res J 3(2):119–131
Breckle S (1997) Population studies on dominant and abundant tree species in the montane tropical rainforests of the biological reserve North of San Ramon, Costa Rica. Trop Ecol 38:259–272
Clark JS (1991) Disturbance and population structure on the shifting mosaic landscape. Ecology 72:1119–1137
Dawkins HC (1959) The volume increment of natural tropical high-forest and limitations on its improvements. Emp For Rev 38(2):175–180
Dibaba A, Soromessa T, Kelbessa E, Tilahun A (2014) Diversity, structure and regeneration status of the woodland and riverine vegetation of sire Beggo in Gololcha District, eastern Ethiopia. Momona Ethiop J Sci 6:70–96
Didita M, Nemomissa S, Woldemariam T (2010) Floristic and structural analysis of the woodland vegetation around Dello Menna, Southeast Ethiopia. J For Res 21:395–408. https://doi.org/10.1007/s1167 6-010-0089-9
Duchok RK, Kent AD, Khumbongmayum A (2005) Population structure and regeneration status of medicinal tree Illicium griffi thii in relation to disturbance gradients in temperate broad-leaved forest of Arunachal Pradesh. Curr Sci 89:673–676
FAO-ISRIC-ISSS (1998) World Reference Base for Soil Resources. World Soil Resources Report Nr 84, FAO, Rome
Feyera S (2006) Biodiversity and Ecology of Afromontane Rainforests with Wild Coffee Arabica L. Populations in Ethiopia. Ecology and Development Series No. 38, 144 p. Center for Development Research, University of Bonn.
Fisaha G, Hundera K, Dalle G (2013) Woody plants diversity, structural analysis and regeneration status of Wof Washa Natural forest, North-east Ethiopia. Afr J Ecol 51:599–608
Fritzsche F, Zech W, Guggenberger G (2007) Soils of Ethiopian rift valley escarpment: a transect study. CATENA 70:209–219
Geldenhuys C (1993) Reproductive biology and population structures of Podocarpus falcatus and P. latifolius in Southern Cape forests. Bot J Linn Soc 112:59–74
Gemedo D, Simon S (2007) Species richness and conservation status of some ecologically and economically important species in Sheko Forest, Institute of Biodiversity Conservation, Forest Genetic Resources Conservation Department, Ethiopia. https://www.trope ntag.de/2007/proce eding s/node3 09.html.
Getachew T, Teketay D, Fetene M (2002) Regeneration of 14 tree species in Harenna Forest, Southeast Ethiopia. Flora 197:461–474
Haile Y, Ensermu K, Tamrat B, Ermias L (2008) Floristic composition and structure of the Dry Afromontane Forest at Bale Mountains National Park, Ethiopia. SINET: Ethiop J Sci 31(2):103–120
Kebede B (2010) Floristic composition and structural analysis of Gedo dry evergreen montane forest, West Shewa Zone of Oromia National Regional State, Ethiopia. M.Sc.Thesis, Addis Ababa University
Kedir A, Kitessa H, Gemedo D (2015) Floristic composition, vegetation structure and regeneration status of Kimphe Lafa Natural Forest, Oromia Regional State, West Arsi, Ethiopia. Res Rev A J Life Sci 5:19–32
Kelbessa E, Soromessa T (2008) Interfaces of regeneration, structure, diversity, and uses of some plant species in Bonga Forest: a reservoir for Wild Coffee Gene Pool. SINET: Ethiop J Sci 31:121–134
Kitessa H, Tamrat B, Ensermu K (2007) Floristic and phytogeographic synopsis of dry Afromontane Coniferous Forest in the Bale Mountains (Ethiopia) the implication to biodiversity conservations. SINET: Ethiop J Sci 30:1–12
Lamprecht H (1989) Silviculture in the tropics: tropical forest ecosystem and their tree species possibilities and methods for their long-term utilization. Federal Republic of Germany, GTZ
Pala NA, Negi AK, Gokhale Y, Bhat JA, Todaria NP (2012) Diversity and regeneration status of Sarkot Van Panchyat in Garhwal Himalaya. India J For Res 23(3):399–404
Peters CM (1996) The ecology and management of non-timber forest resources. Washington DC: World Bank Technical Paper. https://doi.org/10.1596/0-8213-3619-3.
Phillips EA (1959) Methods of vegetation study. Henry Holt and Co., Inc, New York, p 6
Senbeta F, Teketay D (2003) Diversity, community types and population structure of woody plants in Kimphee Forest, a virgin natural reserve in southern Ethiopia. Ethiop J Biol Sci 2:169–187
Shambel B (2010) Floristic composition, structure, and regeneration status of plant species in Sanka Meda Forest, Guna District, Arsi Zone of Oromia Region, Southeast Ethiopia. M.Sc. Thesis, Addis Ababa University, Ethiopia
Shankar U (2001) A case study of high tree diversity in sal ( Shorea robusta) dominated lowland forest of Eastern Himalaya: floristic composition, regeneration, and conservation. Curr Sci 81(7):776–786
Shibru S, Balcha G (2004) Composition, structure and regeneration status of woody plant species in Dindin Natural Forest, Southeast Ethiopia: an implication for conservation. Ethiop J Biol Sci 3:15–55
Silvertown J (1984) Introduction to plant population ecology. Vegetation 56:86
Singh N, Tamta K, Tewari A, Ram J (2014) Studies on vegetational analysis and regeneration status of Pinus roxburghii Roxb. and Quercus leucotrichophora forests of Nainital Forest Division. GJSFR 14:41–47
Teketay D (1997) Seedling populations and regeneration of woody species in dry Afromontane forests of Ethiopia. For Ecol Manage 98:149–165
Teketay D, Granstr?m A (1995) Soil seed banks in dry Afromontane forests of Ethiopia. J Veg Sci 6:777–786
Tesfaye G, Teketay D, Fetene M (2002) Regeneration of 14 tree species in Harenna Forest, Southeast Ethiopia. Flora 197:461–474
Tesfaye G, Teketay D, Fetene M, Beck E (2010) Regeneration of seven indigenous tree species in a dry Afromontane forest, southern Ethiopia. Flora 205:135–143
Tesfaye B, Demeke D, Shiferew B (2017) Structure and natural regeneration status of woody plants of Berbere Afromontane Moist Forest, Bale Zone, South East Ethiopia Implication to Biodiversity Conservation. Open J For 7:352–371
Tilahun A (2009) Floristic composition, structure and regeneration status of Menagesha Amba Mariam Forest Central Highland of Shewa. M.Sc. Thesis, Addis Ababa University, Addis Ababa
Tilahun A, Soromessa T, Kelbessa E, Dibaba A (2011) Floristic composition and community analysis of Menagesha Amba Mariam Forest (Egdu Forest) in Central Shewa, Ethiopia. Ethiop J Biol Sci 10:111–136
Tura TT, Soromessa T, Leta S, Argaw M (2017) Plant community composition and structure of Asabot Dry Afromontane Forest, West Harare Zone, Ethiopia. J Biodiv Endanger Species 5:202. https://doi.org/10.4172/2332-2543.10002 02
Zegeye H, Teketay D, Kelbessa E (2011) Diversity and regeneration status of woody species in Tara Gedam and Abbaye forests, northwestern Ethiopia. J For Res 22(3):315–328
 Journal of Forestry Research2021年2期
Journal of Forestry Research2021年2期
- Journal of Forestry Research的其它文章
- Stem taper functions for Betula platyphylla in the Daxing’an Mountains, northeast China
- Leaf phenotypic variation of endangered plant Tetracentron sinense Oliv. and influence of geographical and climatic factors
- Ecological variations of woody species along an altitudinal gradient in the Qinling Mountains of Central China: area-based versus mass-based expression of leaf traits
- Variations in stem radii of Larix principis-rupprechtii to environmental factors at two slope locations in the Liupan Mountains, northwest China
- A review of ecological mechanisms for management practices of protective forests
- Variation of basic density, calorific value and volumetric shrinkage within tree height and tree age of Ugandan grown Eucalyptus grandis wood
