Adaptability of Rhododendrons in high altitude habitats
Shruti Choudhary · Sapna Thakur · Aasim Majeed · Pankaj Bhardwaj
Abstract Tree species dominate many ecosystems throughout the world and their response to climate, in light of global warming, is a matter of primary concern. This review describes past and ongoing research in Rhododendron, an ecologically important and well-adapted genus of more than 1000 species, occupying diverse habitats. Research to date indicates survival ability and mechanisms, with an emphasis on cold tolerance. The capability of longdistance gene flow in these species increases their genetic variability which in turn enhances their adaptability to newer niches as well as to environmental gradients (mainly temperature). Attempts to explain the molecular basis of morphological and behavioural changes in Rhododendron against cold-induced damage has been made. Gradual advances in ‘omics’ have led to an enriched genomic resource dissecting the role and interaction of multiple molecular factors participating in cold adaptability. However, fewer genetic studies are available on species with an inherent or a default cold-tolerance ability. Considering this fact, understanding specific features of an adapted species can provide insights on overriding the effects of desiccation and determining phase transitions in other plants as well. We propose to integrate ecological and evolutionary studies with functional genomics to improve predictions of tree responses to their environment.
Keywords Rhododendron · Adaptation · Frost · Growth · Geographical distribution
Introduction
Rhododendron L. is a complex genus and the largest of the Ericaceae family with over 1000 species distributed worldwide and categorized into five subgenera and several sections. Some 90% species are Asian in origin and extend through the Malay Archipelago islands. East Asia is presumed as the origin of the genus with the Himalayas (1200–3700 m a.s.l.) as one of the diversification centers. In the context of growth initiation, this area is a transition between temperate and tropical climates and actively supports local adaptation and ecosystem (Bhattarai and Vetaas 2003). Vireya (Blume) H. F. Copel., one of the oldest section of Rhododendron, might have drifted north in the Cretaceous period or a younger group dispersed eastwards from India to Australia and the Solomon Islands (Brown et al. 2006). Migration to North America later occurred at a slower rate, possibly in the Tertiary or pre-Pliocene times. The subgenera Hymenanthes (Blume) K. Koch, Rhododendron L., and Tsutsusi (Sweet) Pojarkova (a subsection under Azaleastrum Planch.), are dominant among the alpine and subalpine flora of Asia (mainly in the Himalayas), and in northern Australia. Whereas Pentanthera (G. Don) Pojarkova, (a former subgenus), is mainly concentrated in eastern and western North America and western Eurasia.
From juvenile to the reproductive stage, various soil and site characteristics can affect growth and distribution pattern of a species. This relationship between abiotic factors and geographic distribution can be exemplified by R. arboreum Smith subsp. nilagiricum (Zenker) Tagg., which is endemic only to the humid forests of the Western Ghats, India (Giriraj et al. 2008); no other Indian Rhododendron spp. is found in the tropics. On the other hand, R. arboreum subsp. arboreum flourishes in the temperate Himalayas, at a distance of more than 2200 km away from the Western Ghats. According to a hypothesis, the Satpura ranges might have been a route for the migration of temperate species to the southern latitudes. Extending this concept to Rhododendron, it was hypothesized that the species gradually migrated from the eastern Himalayas (Northeast India) to relatively smaller peaks of South India (the Western Ghats). Environmental factors later contributed to the successive adaptation of the species (Kuttapetty et al. 2014). Remarkably, more than 800 Rhododendron species are widespread from tropical lowlands to subalpine sites and to polar climates. This further suggests that some species of the genus can withstand both ultralow as well as high temperatures. For instance, R. hyperythrum Hayata is native to Taiwan (900–1200 m a.s.l.), and can also grow well in the warmer climate Louisiana (USA) while the alpine R. russutum Balf. & Forr. is restricted to Yunnan, China (3400–4300 m a.s.l.; Davidian 1982; Thornton 1990). Furthermore, observations from temperature recordings and distribution patterns of four Rhododendron species in situ (1000–5000 m a.s.l.) and ex situ of the temperate Himalayas led Vetaas ( 2002) to state that extreme cold is an absolute boundary for species survival.
The plant kingdom exhibits unique anatomical features in response to environmental signals. This is supported by the underlying genes and hence, functional proteins that perform and respond to the variabilities in abiotic factors. Therefore, genotype, environment, and their interactions account for the distribution and survival of a species at distinct localities. A broad range of habitats, owing to an equally high degree of adaptability and importance, make Rhododendron an ideal model to study and interlink the influence of climate signals and genetic differentiation. Secondly, the evergreen species of the genus offer an opportunity to study the acclimation or developmental biology in over-wintering leaves without the intrusion ofendodormancy transitions, which is the case with other tissues (buds) of deciduous perennials. Elucidating the molecular basis related to protection against frost damage will help in further refining the behaviour of plants to exogenous stimuli. This review pools current molecular and morphological studies on the genus with an emphasis on the intrinsic characteristics of species reported to survive at high altitude habitats. Additionally, next-generation sequencing (NGS) technologies have improved genomic resource availability, especially of non-conventional species. In later sections of this review, high-throughput transcriptome and microRNA (miRNA) profiling on various species of the genus will be considered.
Inherent features of the genus to counteract temperate environments
High elevation habitats are characterized by cold temperatures, high radiation, and often heavy snowfalls. Temperate perennials only have a brief favorable period to grow under these conditions and may undergo extracellular iceinduced desiccation if they face extended winters of ? 10 to ? 30 °C. Rhododendrons have two major responses to desiccation induced by freezing. One strategy is to avoid or tolerate freezing stress by reducing or losing water; the second is behavioral or morphological adjustments supported by molecular factors. These will be discussed in later sections. Overwintering tissues of temperate woody species demonstrate diverse freezing behaviour or strategies under subfreezing temperatures. Extracellular freezing (in the bark), deep supercooling (of xylem ray parenchyma cells), and extra-organ freezing (of floral and vegetative buds) are determinants of cold hardiness (Sakai and Larcher 1987). Different species also vary widely in their tolerance to freezing; R. brachycarpum D. Don ex G. Don and R. maximum L. are leaf-hardy to ? 60 °C and bud-hardy to ? 30 °C, R. barbatum Wall. ex G. Don and R. griersonianum Balf. & Forr. are prone to damage at ? 18 °C, and R. ponticum L. can survive temperatures as low as ? 40 °C to ? 60 °C (Sakai et al. 1986; Marian et al. 2004).
Extracellular freezing is a common cold-tolerance strategy in hardy tissues of several Rhododendrons. Another striking feature of freezing avoidance in Rhododendron buds is super cooling. During freezing avoidance by deep supercooling in florets, no crystals form within the florets, though the other tissues of plant freeze. While in extracellular freezing, ice is formed just outside the cell (Ishikawa and Sakai 1981). They regarded supercooling in florets as ‘extraorgan’ freezing and extracellular freezing as ‘intraorgan’ or ‘intratissue’ freezing. As freezing progresses, water moves out of the florets to bud scales (an ice sink) and increases cell sap concentration causing a ‘freezing point’ depression and thus, further enhancing the supercooling ability of florets. They further noted that this ability of florets and peduncle or bud axis to lose water in response to low temperatures primarily governs the supercooled status and hardiness of buds, which in turn is determined by the degree of their desiccation as well as environmental factors such as humidity and snow coverage. Water migration within flower buds was also observed earlier in hardy Rhododendron species (Kaku et al. 1980), and later by Price et al. ( 1997) and Hacker and Neuner ( 2008). An experiment by Ishikawa et al. ( 2015) reported that the level of ice nucleation activity (INA) was responsible for establishment of freezing behaviour and its order in various tissues of flower buds. They observed that bud scales primarily freeze due to their high INA while florets, having low INA, loose water to scales. This low INA of florets also helps them to maintain an unfrozen state during deep supercooling (Ishikawa et al. 2015).
For many Rhododendron spp., snow cover favors survival in winters. Low thermal conductivity and light transmission of snow offer a shield from low temperatures and excessive irradiation, respectively. A suffi cient snow cover allows for simultaneous winter desiccation during late winter and spring or re-hardening in response to high temperatures in early spring (Neuner et al. 1999a, b; Palacio et al. 2015). V?in?l? and Repo ( 2000 ) reported that frost hardiness was related to extra and intracellular resistance of non-frozen leaves during acclimation. For another section of Rhododendrons, flowering time and fruit setting depend on snowmelt time and temperature, particularly along alpine gradients. Kudo and Suzuki ( 2004) demonstrated that the induction of flowering and fruit production varied among sympatric species, R. buxifolium H. Low ex Hook. f., R. ericoides H. Low ex Hook. f., and three other alpine dwarf species. Mountain inclinations cause minute variability in abiotic features and consequently, interrupt flowering time within different populations of a single species. Flowering phenology in four sub-species of R. arboreum, representing temperate to subalpine habitats, was recorded in situ (Ranjitkar et al. 2013). It was suggested that an expanding distribution range may be attributed to the evergreen nature and bloom advancement of the test species in response to global warming or even during water or chilling deficits (Sharp et al. 2009; Ranjitkar et al. 2013). Similarly, fruit set was significantly higher in R. aureum Georgi populations at sites of the latest snowmelt (Kudo 1993). There was reduced pollinator activity during snowfall. Flowering at later time periods promoted crosspollination but fruiting was not observed due to the onset of autumn frost before maturation (Kudo 1993). Hence, seasonal snowfall patterns are connected to the diversity as well as response of species to climate change (Hirao et al. 2006; Palacio et al. 2015).
A few mechanisms have been proposed for Rhododendrons through which the species overcome or recover from cold and photoinhibition. Different species employ various anatomical, physiological, and molecular strategies to tolerate/resist cellular dehydration due to cold. Ranging from structural alterations in leaf anatomy and behavior, the adaptation encompasses regulation of photosynthesis, adjustment in osmotic/water content, and modulation in the synthesis of metabolites having a distinct role as a cryo/osmo/oxidation-protectant (Nilsen 1991; Neuner et al. 1999b; Wang et al. 2009). The cold-regulated genes and accompanying networks are master modulators of cellular membranes, macromolecules, and metabolism. A simplified depiction of perception and response strategies available in the genus, especially under low temperatures, has been summarized in Fig.1.
Physiology and phenology of Rhododendron spp. under high-altitude conditions
Frosts, strong winds, and poor light quality or quantity in a temperate environment lower the overall productivity of a plant. Temperate plants often have long life cycles of slow growth; well-developed root systems; short extremities; specific leaf, shoot, and reproductive growth habits; and extended meristem development periods (McGraw 1989; Kudo and Suzuki 2004). Additionally, a deliberate allocation in timing and complimentary utility ofendo/exogenous nutrient resources, (also including water content), is advantageous, especially in cold environments. Here a temporal change in resource-availability is evident, parallel to shorter growth and reproduction periods (Dhyani et al. 1988). For example, a flux of nutrients such as phosphorus, potassium, and nitrogen becomes significant to increase leaf area, photosynthate levels, and light-harvesting processes as well as to promote development (Jonasson 1995). Besides organized nutrient cycling, modulations in foliar traits like photosynthetic capacity, growth rate, transpiration, respiration, and light perception are taken as adaptive measures (Karlsson 1994; Nakano and Ishida 1994; Johnson and Smith 2008). Several Rhododendron species exhibit either a simultaneous emergence or multiple foliage growth. Leaf biomass and longevity in evergreen species of the genus help to conserve a seasonal carbon balance (Negi 2006). These species have a leaf lifespan of more than 1 year, permitting year-round photosynthesis and growth (Vetaas 2002; Negi 2006; Marty et al. 2010).
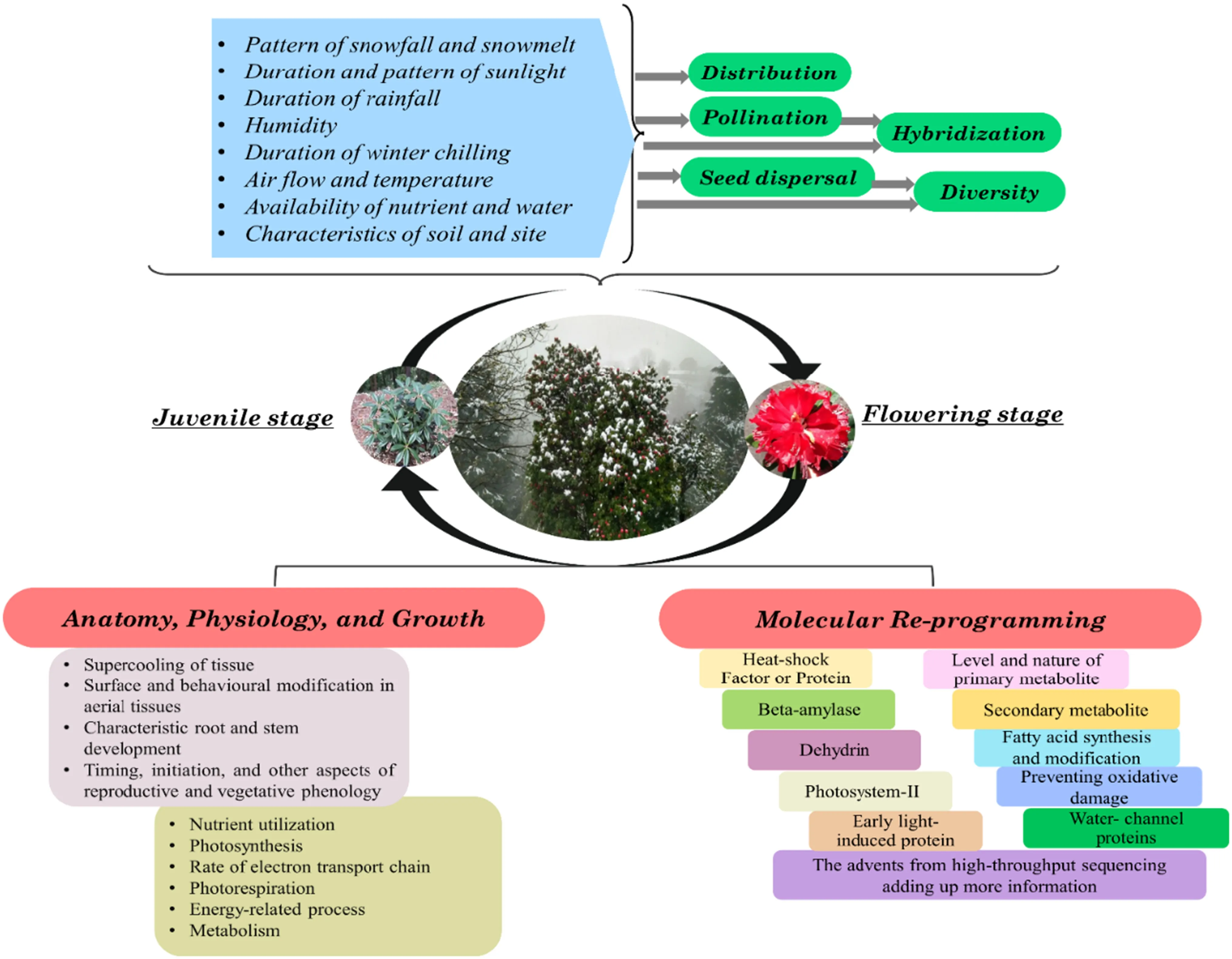
Fig.1 Perception of abiotic signals, including landscape features, elicits a cascade of molecular, genetic, and developmental changes in the plant. The aspects reported in Rhododendron genus and as discussed in the review are indicated
Besides supercooling, altering leaf morphology, behavior, and anatomy by an additional layer of the upper epidermis, palisade, and cuticle or through thermonasty (which is characterized by leaf drooping or curling at subfreezing temperatures) are other adaptive features towards desiccation-avoidance and photoprotection. The variation in leaf-freezing tolerance (LFT) is usually taken as a measure of cold hardiness (CH) and reflects the difference in cold acclimation (CA) ability. LFT was measured in terms ofelectrolyte leakage in five cultivars of R. ponticum. It increased during fall, reached a maximum in December, and decreased thereafter. These parameters are comparable to those of bud hardiness (Lim et al. 1998a). With regards to behavioral changes, the more winter-hardy R. catawbiense Michx. (LFT50 ≈ ? 35 °C), exhibits leaf curling at subfreezing temperatures, whereas the less hardy, R. ponticum (LFT50 ≈ ? 16 °C), is non-thermonastic or unresponsive (Wang et al. 2008). Similarly, a significant correlation between temperature and leaf curling, and between leaf water potential and leaf angle was observed for R. maximum and R. catawbiense under the influence of varying cell water content (Nilsen 1987). Foliar, epidermal, and cuticular thickenings, or outgrowths like trichomes, can reflect or absorb harmful radiation (Ruhland and Day 1996; Lipp and Nilsen 1997). Other tissues exhibit different mechanisms to withstand temperate environments– supercooling of xylem and buds, equilibrium freezing in the bark, and simultaneous dormancy or CA transitions in buds. In addition to growth and vegetation features, the method of seed dispersal and pollination were proposed to be behind cold tolerance in R. ponticum (Thomson et al. 1993; Milne 2004; Stout 2007). Additionally, interpretations based on flowering and fruiting phenologies led Pornon et al. ( 1997) to consider that a phenotypic response to variable microhabitats made the difference of a species adaptive ability. Similarly, the adoption of an alternative mode of seed dispersal (e.g., hydrochory, i.e., dispersal by water) and pollination (ornithophily or via sphingidae moths and butterflies) was considered to be the survival success of two Rhododendron species (Stevens 1976; Kondo et al. 2009).
Managing plant–water relations in cold environments
A series of changes in tissue–water relations following water stress indicate that freezing is accompanied by dehydration. Cold induces desiccation by inhibiting root hydraulic conductivity and extracellular freezing. Anisko and Lindstrom ( 1996) suggested that cold stress may reduce hydration at subfreezing temperatures. Meanwhile, cell wall rigidity increases to develop more and more negative turgor pressure that boosts resistance to freeze-induced dehydration. Channel proteins such as aquaporins (AQPs) facilitate water transport across membranes. As temperatures dropped from ? 7 to ? 50 °C, the downregulation of AQPs was proportional to resistance against freeze-induced dehydration, while no AQP expression could be detected during acclimation (Wei et al. 2005, 2006). AQP-mediated water redistribution due to extracellular freezing can cause thermonasty or nastic movements in plants. Furthermore, antisense suppression and T-DNA insertion studies with AQPs demonstrated that the ability to recover from water deficit was associated with changes in root hydraulic conductivity, transpiration rates, and osmotic potential. Whereas the gene for gated outward rectifying potassium channel was also upregulated and was associated with the protection ofoverwintering leaves from dehydration (Wei et al. 2006). In R. catawbiense, PIP2 or the plasma-membrane intrinsic protein2, (a subfamily of AQPs), was down-regulated and subsequent leaf curling was observed at cooling beyond the range of the ice-nucleation activity (INA) temperature. On the other hand, R. ponticum, a non-curling species, exhibited an up-regulation of PIP2 (Chen et al. 2013). A contrasting PIP2 expression and leaf behaviour reflected a differential responses of the two species towards freezing. Arabidopsis plants overexpressing the Rhododendron PIP2 gene had higher dehydration rates and susceptibility to quick rehydration but less constitutive tolerance and cold acclimation (CA) ability (Peng et al. 2008a).
Modulating photoacclimation, photosynthesis, and metabolism at high-altitudes
Rhododendrons are exposed to frost and high light intensities under the canopy of deciduous forests. Strong light; timing and duration of snowfall; radiation; and ambient temperatures can induce photoinhibition by affecting the photosystem II (PSII) effi ciency (Jin and Ke 2004). If the excessive solar energy is not dissipated as heat or fluorescence, photooxidative damage to the PSII complex may also occur. Other than chronic photoinhibition, a prolonged reduction of the maximal photochemical effi ciency of PSII results in increased internal carbon dioxide concentrations due to chloroplast dysfunctions (Larcher and Siegwolf 1985). Simultaneously, reduced photosynthetic activity can make plants more susceptible to desiccation. A study on the recovery pattern of micropropagated R. ponticum subsp. baeticum after exposure to high irradiance provided an answer to the photoacclimation response of the species (Osório et al. 2010). A fluorescence analysis assessed that the response of photochemical quenching was proportional to the irradiance levels. Photoprotection involved a decreased investment in light-harvesting complexes while increasing the electron transport rate and pool size of xanthophyll pigments and PSII reaction centers. This would also maximize photosynthetic gain and protect its components from excess energy, which otherwise can impair photosynthesis (Osório et al. 2010).
Extending the concept of photoacclimation with simultaneous regulation of photosynthesis, a nuclear-encoded protein of the thylakoid membranes, early light-induced protein (ELIPs), was found upregulated in the winter-adapted Rhododendron (Peng et al. 2008b). During high light intensities and suboptimal temperatures, ELIP functions as a pigment carrier or chlorophyll exchange protein to protect chloroplasts by dissipating excessive light energy. Similarly, several cold, light, and photosynthesis-related genes were affected in cold-acclimated R. catawbiense (Wei et al. 2005). Additionally, the downregulation of photorespiration and energy-related processes would most likely maintain the carbon resource during adaptation. Carbon assimilation, calcium, and magnesium in leaves were low, while weight, sulphur, and phosphate levels increased during winter acclimation, and later resumed normal levels under favourable temperatures (Harris et al. 2006). Some other stress-related expressed sequence tags (ESTs) were identified to prevent desiccation including upregulation of: (1) beta-amylase (generates carbohydrates to serve as osmoprotectants); (2) acyl-CoA synthetase, CTP: choline phosphate cytidylyltransferase, and delta-12 fatty acid desaturase (involved in phospholipid biosynthesis and desaturation); (3) coumarate 3-hydroxylase (in cell wall thickening); (4) plastidic NADPmalic enzyme (produces malate for fatty acid biosynthesis); and (5) downregulation of NADH dehydrogenase subunits (lessen the risk of ROS-induced damage and metabolic dysfunction (Wei et al. 2006).
Specialized proteins during desiccation-induced tolerance under low temperatures
The major manifestation of cold temperatures is shown in dehydration and arises after the intracellular crystallization of water. Dehydrins (DHNs) were initially proposed proteins to counter against such situations during frequent frosts. Induced by cold temperatures, these proteins are very responsive to temperate climates. As cryoprotectants, ionsequesters or molecular chaperones, DHNs either associate with cold-labile proteins and nucleic acids or stabilize cell membranes via hydrophobic interactions, lipid demixing or unintended separation of substances during dehydration. DHNs belong to the late embryogenesis abundant (LEA) family of proteins having a heat-stable amphipathic α-helix that share 15 residues consensus sequence or K-segment. The RcDhn5 gene, as characterized in the R. catawbiense leaf, encodes an acidic SK2 type DHN with exceptional four cysteine residues. At least nine forms of DHNs have been reported in Rhododendrons, some of which may not be associated with cold or dehydration tolerance. A change from juvenile to maturity increased the levels of a 25-kiloDalton (kDa) DHN in leaves, making it a good genetic and physiological indicator to distinguish between super to less cold-hardy species viz, R. catawbiense, R. maximum, and R. fortunei Lindl., and their hybrids (Lim et al. 1999). Additionally, profiling 20 other species demonstrated that the abundance of 11 DHNs (25–73 kDa) varied in acclimatized compared to non-acclimatized tissues but to a lesser extent (Marian et al. 2004). However, some cultivars lack DHN-like proteins during the transition to spring (Harris et al. 2006). It was further noted that, although the overexpression of DHNs can improve ‘constitutive’ tolerance in transgenic Arabidopsis, there was no effect on cold acclimation capacity (Peng et al. 2008c). As a conclusion, it was the abundance or the type of cellular function, rather than the intraspecific diversity of DHN, that affected the potential for cold hardiness.
Another well-studied family of genes in Rhododendron is the one encoding for heat-shock proteins (HSPs). Their expression is controlled by heat shock factors (HSF) that in turn are regulated by DREB (dehydration-responsive element binding) and specific ubiquitin ligases (negative inducers). Although widely linked to heat stress, HSPs are also induced at low temperatures to allow rapid adaptation to continually changing temperatures, light intensities, and oxidative stress. These molecular chaperones prevent intracellular proteins from degradation and improper folding in an ATP-independent manner. Functional conservation in HSP domains was suggested as an ecological adaptation of Rhododendron (Wu et al. 2007; Liao et al. 2009). Sequence variations, on the other hand, could diversify the function of chaperons ranging from- proteases, 14-3-3, anti-freeze, or INA proteins, depending largely upon the physiological condition of the plant.
Inferences from marker-based genetic diversity studies
Genetic variability determines the extent of adaptation towards long-term survival of a species in changing climates. Hybridization played an important role in evolution and speciation, particularly in the Sino-Himalayan region where interfertile Rhododendron spp. are often located sympatrically, i.e., occur in the same geographical area. A weak reproductive barrier within the genus has allowed extensive hybridization and subsequent gene introgression. Likewise, the mode of pollination, seed propagation, and evolutionary history influence gene flow and genetic drift and thereby, genetic diversity within and among populations (Kameyama et al. 2000; Wolfet al. 2004; Hirao et al. 2006). An exogenous selection from genome-habitat interaction can also affect hybridization and hence reproductive output. Habitatmediated survival was observed for Rhododendron hybrids growing in Tiryal Dag, northeast Turkey (Milne et al. 2003) and also for natural hybrids between R. delavayi Franch. × R. cyanocarpum (Franch.) Franch. ex W.W. Sm. (Ma et al. 2010). The maintenance of reproductive isolating barriers due to ecological, habitat or adaptation differentiation lead to speciation. For example, colonization around ancient and modern-day rivers influenced the genetic structure of R. ripense Makino (Kondo et al. 2009). Overall, inherent genetic diversity has made the Rhododendron genus taxonomically more complex to understand (Zha et al. 2009 ). At the same time however, a knowledge of genetic diversity can lay the foundation for plant improvement.
Determination of the spatial patterns of genetic variation has been employed in landscape or adaptation genomics whereby markers are utilized. From divergence studies across various sections of the genus, morphological, biochemical, and molecular markers have been developed and applied to cladistics (biological classification system based on common ancestry between the organisms), genetic variation and characterization, and ecological and evolutionary studies (Pornon et al. 2000; Kurashige et al. 2001; Goetsch et al. 2005; Chung et al. 2007; De Keyser et al. 2010; Liu et al. 2012; Choudhary et al. 2014; Li et al. 2016). Markerassisted selection of genes can help to understand genotype to phenotype relationships in the context of a selected trait like an environmental response and can help in the management of tree populations. Lim et al. ( 1998b) traced the inheritance of cold-hardiness in the F2 generation and backcrosses derived from R. catawbiense × R. fortunei, and suggested that a few genes with strong additive effects controlled the variation; the trait evolved through natural selection acting on existing genetic variability. Further, earlier procedures of marker isolation and genotyping were labourintensive, expensive, time-consuming, and sensitive to clone biases with limited transferability. In-depth coverage, high resolution, and reduced costs of next-generation sequencing (NGS) platforms have replaced conventional protocols, opening new realms in genomics (Wang et al. 2017; Xing et al. 2017; Zhang et al. 2017b; Choudhary et al. 2018a; Xiao et al. 2018).
High-throughput sequencing studies in the genus
NGS platforms have revolutionized DNA sequencing procedures, producing considerable data, and resulted in an expedient approach to discover novel genes and molecular markers. With this technology, many plant genomes were sequenced successfully which had been diffi cult to produce in the case of non-model species. Libraries of cDNA prepared from different tissues of various Rhododendron species and a genome of a single species have been sequenced to date (Fang et al. 2017; Xing et al. 2017; Zhang et al. 2017a, b; Choudhary et al. 2018a; Xiao et al. 2018). Phenotypes, chlorophyll fluorescence kinetics, and transcriptome data of a heat- tolerant cultivar, R. obtusum cv. “Yanzhimi”, illustrated enhanced regulation of photosynthesis as well as expression ofearly heat response genes, including HSF, DREB, ZAT (Zinc-Finger Transcription Factor), and NAC factors [comprising of genes having NAC domain that stands for NAM (no apical meristem), ATAF (Arabidopsis transcription activation factor), and CUC (cupshaped cotyledon)] (Fang et al. 2017). While in R. rex Lévl., responses to temperature stimuli, photosynthesis, osmotic stress, metabolic regulation, growth, and photoinhibition tolerance were vital for cold adaptation (Zhang et al. 2017a). Similar responses, in addition to flowering regulation and development, were observed for R. arboreum when comparing transcriptomes of flowering and vegetative season tissues (Choudhary et al. 2019). To summarize, these species express ‘cell rescue’, including genes involved in the interaction, response, and protection from environmental factors. Another important conclusion was that the genetic features of tolerance to cold mimic that of dehydration during drought, salinity, and metal-rich soils, and in arctic, sub-arctic and tropical climates, as all of these factors lead to disturbed plant–water relationships resulting in specific adaptations.
Heat-shock proteins (HSP) and dehydrin (DHN) family of proteins play a crucial role in physiological stresses, including cold. To classify the functional and evolutionary relationships of the corresponding genes in the recently sequenced species of Rhododendron, a phylogenetic analysis was undertaken. The data were retrieved from the NCBI (National Center for Biotechnology Information, Maryland, USA) with accessions SRP092027 ( R. arboreum), SRP108445 ( R. camtschaticum Pall.), SRP108011 ( R. rex), ERP023948 ( R. tomentosum Harmaja), ERP023948 ( R. scopulorum Hutch.), SRP095380 ( R. delavayi), SRP115374 [ R. obtusum (Lindl.) Planch.], SRP064996 ( R. fortunei), and DRP003715 ( R. dauricum L.). The short reads were assembled using Trinity, a software package that processes large sets of RNA-Seq data for de novo reconstruction of transcriptomes. The output of Trinity is in the form of contigs that are termed as unigenes. The function to these genes were then assigned based on sequence (by blastp) and domain homology (by InterPro scan). The transcripts annotated to HSP and DHN were extracted from de novo assembly of Rhododendron species. For genes with splicing variants, the longest transcript was selected to represent the gene. Additionally, the protein sequences of cytosolic small HSP classes I and II (cysHSP1 and cysHSP2), chloroplast HSP (cpHSP), and DNHs of the Rhododendron genus retrieved from GenBank were also used to construct a phylogenetic tree. Finally, a consensus was generated by aligning the sequences of all the Rhododendron species using MUSCLE v.3.8.31 (Edgar 2004). Consensus sequences were then translated into amino acid sequences and used in a blastp search at an E-value threshold of 1e?6. Overall, a phylogenetic tree based on 76 sequences representing Rhododendron HSPs and DHNs was constructed using the maximum likelihood estimation by iqtree (Hoang et al. 2018) and visualized using Archaeopteryx (Han and Zmasek 2009). The identifiers of unigenes of recently sequenced Rhododendron spp. are labeled with different colors (Fig.2). The phylogenetic tree based on HSP and DHN has further strengthened the output of homology search as well as validated the function of corresponding unigenes in the species. As observed in Fig.2, different types of HSP and DHN homologs were grouped according to the taxonomic classification of the species as per the Germplasm Resources Information Network (GRIN) maintained by the United States Department of Agriculture. However, a few taxonomic differences were also noted. For instance, the cpHSP homolog in R. tomentosum and R. dauricum of the subgenus Rhododendron were grouped together. Similarly, the dehydrin homolog in two of the hardiest species, R. catawbiense and R. rex (both of the Hymenanthes subgenus), were closely grouped with one another as well as to R. tomentosum (of the Rhododendron subgenus), but distantly to R. arboreum of the Hymenanthes subgenus. These proteins are encoded by a multigene family that can vary among taxa and the present finding is evidence of intrageneric or interspecific variability similar to those reported in other plants. With a diverse habitat-regime, Rhododendrons exhibit a high degree of adaptation, which in turn, is the reflection of genetic variability underlying a trait. Diversity within the sequence of HSPs and DHNs was observed between different species of Rhododendron. Selection pressure on a gene during the course ofevolution is the determinant of its performance and function under a physiological state. Consequently, tracing the evolutionary history and interspecific diversity can shed light on the importance of functional divergence of a gene in relation to the adaptive advantages of a species over others.
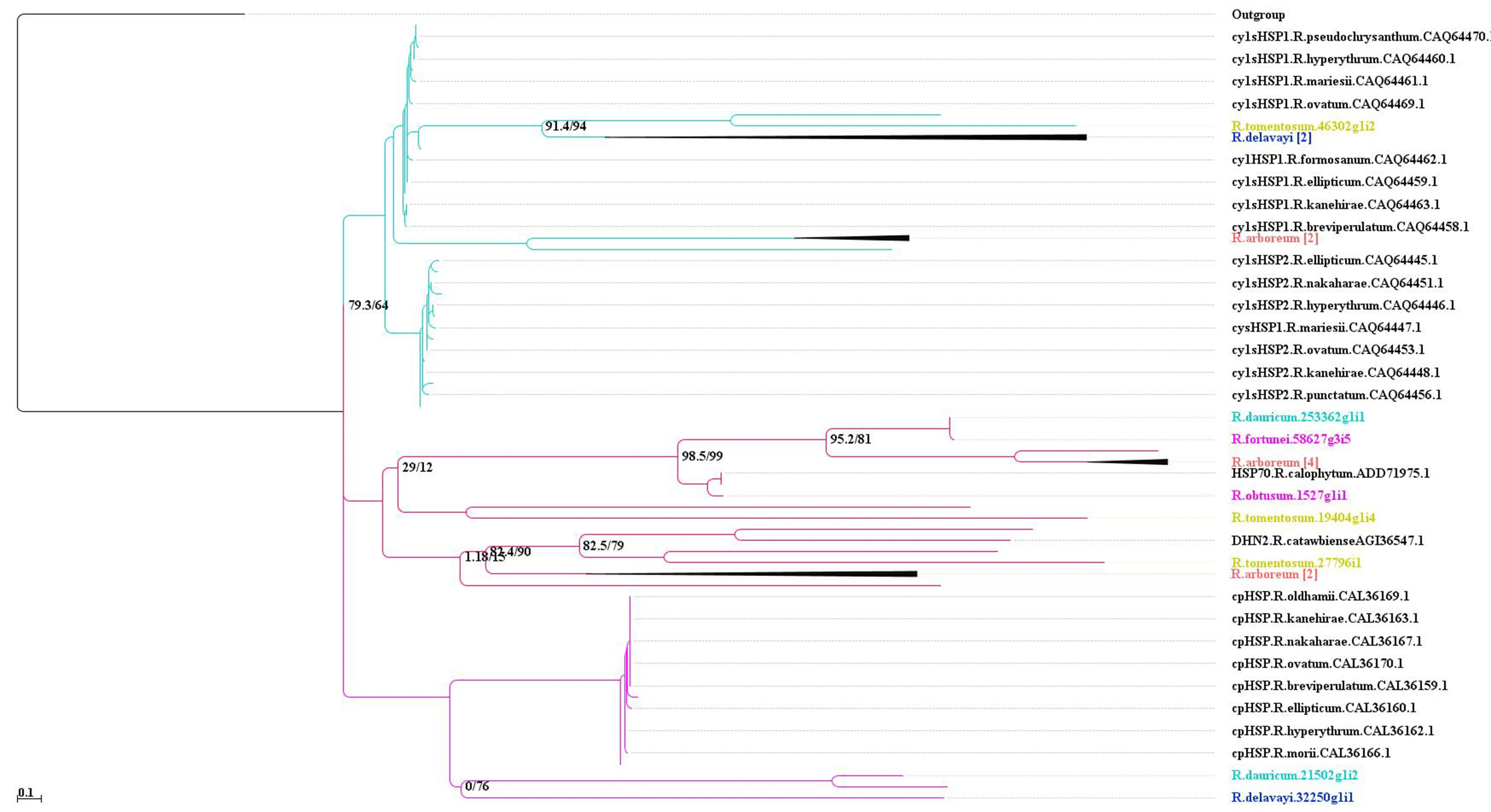
Fig.2 A phylogenetic tree based on the homologs of DHNs and HSPs in different species of the genus Rhododendron
Other than genes, plants can maintain their homeostasis under various environmental conditions by utilizing additional post-transcriptional regulations through a ‘master switch’ called small RNA. The ambient environment can affect plant physiology whereby small RNAs act as intermediary signals. The microRNAs are 20–22 nucleotide long class ofendogenous, non-coding small RNAs that can affect gene expression related to metabolic and biological processes crucial to growth, development, signalling, and stress responses. There is two-way coordination between miRNA accumulation levels and external factors (light and temperature). However, miRNA data available for Rhododendron is negligible. Choudhary et al. ( 2018b) predicted 466 novel and conserved miRNAs expressed during flowering and vegetative growth in R. arboreum using deep small RNA sequencing. The raw small RNA and transcriptome sequence data are available from the NCBI accessions- SRP148106 and SRP092027, respectively. The miRNA targets located based on the R. arboreum transcriptome sequenced earlier (Choudhary et al. 2018a), were involved with metabolism, reproduction and response to abiotic stimuli (Choudhary et al. 2018b). The expression patterns of miRNAs between the two seasons representing two different temperatures was then compared. The temporal expression exhibited by specific isoforms of the same miRNA family, i.e., targeting different genes in the two seasons, can be linked with the adaptability of the species. Furthermore, miRNAs targeting the circadian clock in R. arboreum were discussed with the purpose to demonstrate the molecular factors regulating flowering development and environmental responses. We extended the earlier work of miRNA-prediction in R. arboreum (Choudhary et al. 2018b) by determining the targets of these miRNAs in the transcriptomes ofother Rhododendron species, which will prove useful for further functional and comparative studies in the genus. A GO-term distribution for these targets is presented in Fig.3.
Metabolite attributes towards cold temperatures
The metabolite profile can vary between different tissues and physio-developmental stages of a plant as well as within a genus. The modulation in the level of metabolites related to photosynthesis, carbohydrates, amino-acid, lipids, hormones, reactive oxygen species (ROS), and secondary metabolism are typical during cold treatment in plants. Anthocyanins protect leaves from excessive radiation and so do flavonoids, hemicellulose, and lignin or their precursors that accumulate during cold acclimation. Swiderski et al. ( 2004) analyzed flavonoids and their glycosides in various taxons of Polish Rhododendrons and compared them with the extent of injury at sub-zero temperatures. They proposed that flavonoid concentration in leaf tissues could be related to frost resistance and used as a breeding criterion. Phenolics, phenylalanine ammonia lyase, and leaf pubescence and cell wall thickness materials like hemicellulose and lignins, are also considered as ice barriers during cold-hardiness (Filella and Pe?uelas 1999). Being perennials of high altitude, there must be a survival strategy not only against cold temperatures but towards multiple and simultaneous stress conditions, for example, drought or UV radiation. In this regard, physio-morphological and metabolic similarities are displayed by the tissues when exposed to UV-B and low temperatures. Research has shown that exposure to elevated UV-B (280–320 nm), coupled with decreasing day length and night temperatures, induced a greater freezing tolerance (< ? 8 °C) in some cultivars (Dunning et al. 1994; Chalker-Scott and Scott 2004). A deposition or activity of acylated anthocyanins, flavonoids, hydroxycinnamates, lignin, tannins, and cuticular substances and enzymes catalyzing their synthesis was recognized in the UV-resistance mechanism of a species when examined along a gradient of increasing UV radiation in Catalonia, north-eastern region of Spain (200–2200 m a.s.l.; Filella and Pe?uelas 1999). Similarly, a higher concentration of sugars in buds collected at shallow snow depths was suggestive of a strong physiological hardening (Palacio et al. 2015).
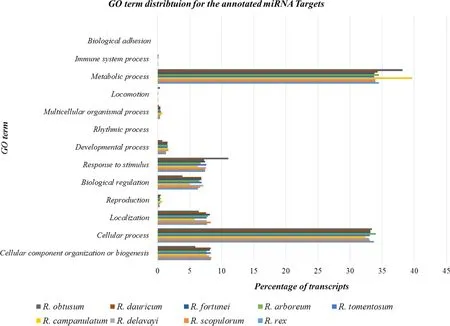
Fig.3 A GO term distribution for miRNA targets predicted for nine Rhododendron species
There is a close regulation ofone or the other components of metabolism by genes and their interaction with environment. These processes can be studied and correlated in detail by employing the robust ‘omics’ techniques. Several Rhododendron species contain useful phytochemicals, yet information on their synthesis is scarce. Genes, enzymes, and pathways of specific relevance across diverse non-model species can be predicted from high-throughput RNA-Seq data. Pathway analysis predicted genes related to carotenoid biosynthesis during different stages of flower development in R. molle (Blume) G. Don (Xiao et al. 2018). Integration of transcriptomics and metabolomics can resolve a biologically or economically significant pathway to provide a firm base for future genomics and functional studies. For example, the cold-dependent regulation of a crucial gene of the phenylpropanoid biosynthetic pathway, phenylalanine ammonia lyase, is seen in response to UV-B exposure, drought, and salinity. Similarly, the non-targeted metabolome profiling in R. arboreum revealed crucial biosynthetic pathways in the tissues of two growing seasons, supported well by the transcriptome data (Choudhary et al. 2019). Exploring gene networks is beneficial, not only from a pharmacological point of view, but also provide useful insights into the metabolic features that confer adaptive advantages to a species. However, an understanding of the genetic and metabolic control of freezing tolerance is still limited and has hampered an estimation of cold hardiness needed to in plant breeding efforts. A study on differential molecular responses in species of diverse origins during various physiological or developmental stages can help investigate adaptation mechanisms in detail.
Conclusion
The plant kingdom, being largely immobile, has evolved more than animal systems in terms of withstanding various degrees of abiotic pressures by exhibiting unique and diverse adaptive mechanisms. The geographical expansion of Rhododendron from the tropics to polar climates is evidence of their persistence in environmental extremes that makes them ideal for ecological, landscape, developmental, and functional studies. Local adaptations accumulate to allow a species to gradually cope with the microclimate and to endure fluctuations in the new condition as well (adaptive evolution). As inferred from hybridization studies, genetic differentiation determines the variations in morpho-physiology and enables the hybrids to tolerate different environments. Earlier studies were mainly focused on the determination of morphology, population structure, and targeted metabolites. With the recent advent of NGS technologies or the ‘omics’ era, the genome and transcriptome of some Rhododendron spp. have been made available. A molecular assessment via high-throughput sequencing data can be further coupled with metabolomics for an in-depth acclimatization study and for modeling the response of the populations under changing environments. These findings may have application in breeding studies as temperature is a limiting factor that restricts the location, suitability, and overall productivity of a plant, especially of crops growing in a specific climate.
AcknowledgementsS. Thakur thank the Indian Council of Medical Research, New Delhi for the fellowships towards their Ph.Ds.
Author’s contributionsPB conceived and designed the research work. SC did the data analysis and wrote the draft. ST and AM further discussed and improved the review. All authors have read and approved the final manuscript.
References
Anisko T, Lindstrom OM (1996) Cold hardiness and water relations parameters in Rhododendron cv. catawbiense Boursault. subjected to drought episodes. Physiol Plant 98(1):147–155
Bhattarai KR, Vetaas OR (2003) Variation in plant species richness of different life forms along a subtropical elevation gradient in the Himalayas, east Nepal. Global Ecol Biogeogr 12(4):327–340
Brown GK, Nelson G, Ladiges PY (2006) Historical biogeography of Rhododendron section Vireya and the Malesian Archipelago. J Biogeogr 33(11):1929–1944
Chalker-Scott L, Scott JD (2004) Elevated ultraviolet B radiation induces cross protection to cold in leaves of Rhododendron under field conditions. Photochem Photobiol 79(2):199–204
Chen KT, Wang X, Fessehaie A, Yin YH, Wang XL, Arora R (2013) Is expression of aquaporins (plasma membrane intrinsic protein 2s, PIP2s) associated with thermonasty (leaf-curling) in Rhododendron. J Plant Physiol 170(16):1447–1454
Choudhary S, Thakur S, Saini RG, Bhardwaj P (2014) Development and characterization of genomic microsatellite markers in Rhododendron arboreum. Conserv Genet Res 6(4):937–940
Choudhary S, Thakur S, Najar RA, Majeed A, Singh A, Bhardwaj P (2018a) Transcriptome characterization and screening of molecular markers in ecologically important Himalayan species ( Rhododendron arboreum). Genome 61(6):417–428
Choudhary S, Thakur S, Majeed A, Bhardwaj P (2018b) Exploring microRNA profiles for circadian clock and flowering development regulation in Himalayan Rhododendron. Genomics. https://doi.org/10.1016/j.ygeno.2018.09.019
Choudhary S, Thakur S, Jaitak V, Bhardwaj P (2019) Gene and metabolite profiling reveals flowering and survival strategies in Himalayan Rhododendron arboreum. Gene 690:1–10
Chung JD, Lin TP, Chen YL, Cheng YP, Hwang SY (2007) Phylogeographic study reveals the origin and evolutionary history of a Rhododendron species complex in Taiwan. Mol Phylogenet Evol 42(1):14–24
Davidian HH (1982) Rhododendron species-1. Lepidotes. Timber Press, Portland
De Keyser E, Shu QY, van Bockstaele E, de Riek J (2010) Multipoint-likelihood maximization mapping on 4 segregating populations to achieve an integrated framework map for QTL analysis in pot azalea ( Rhododendron simsii hybrids). BMC Mol Biol 1:11
Dhyani PP, Purohit AN, Negi DCS (1988) Variations in energy budget and water vapour transfer processes in some broadleaf timberline tree species at different altitudes. Plant Physiol Biochem 15:64–74
Dunning CA, Chalker-Scott L, Scott JD (1994) Exposure to ultraviolet-B radiation increases cold hardiness in Rhododendron. Physiol Plant 92(3):516–520
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucl Acids Res 32(5):1792–1797
Fang LC, Tong J, Dong YF, Xu DY, Mao J, Zhou Y (2017) De novo RNA sequencing transcriptome of Rhododendron obtusum identified the early heat response genes involved in the transcriptional regulation of photosynthesis. PLoS ONE 12(10):e0186376
Filella I, Pe?uelas J (1999) Altitudinal differences in UV absorbance, UV reflectance and related morphological traits of Quercus ilex and Rhododendron ferrugineum in the Mediterranean region. Plant Ecol 145(1):157–165
Giriraj A, Irfan-Ullah M, Ramesh B, Karunakaran P, Jentsch A, Murthy M (2008) Mapping the potential distribution of Rhododendron arboreum Sm. ssp. nilagiricum (Zenker) Tagg (Ericaceae), an endemic plant using ecological niche modelling. Curr Sci 94(12):1605–1612
Goetsch L, Eckert AJ, Hall BD (2005) Molecular systematics of Rhododendron (Ericaceae): a phylogeny based upon RPB2 gene sequences. Syst Bot 30(3):616–626
Hacker J, Neuner G (2008) Ice propagation in dehardened alpine plant species studied by infrared differential thermal analysis (IDTA). Arct Antarct Alp Res 40(4):660–670
Han MV, Zmasek CM (2009) phyloXML: XML for evolutionary biology and comparative genomics. BMC Bioinform 10(1):356–361
Harris GC, Antoine V, Chan M, Nevidomskyte D, K?niger M (2006) Seasonal changes in photosynthesis, protein composition and mineral content in Rhododendron leaves. Plant Sci 170(2):314–325
Hirao A, Kameyama Y, Ohara M, Isagi Y, Kudo G (2006) Seasonal changes in pollinator activity influence pollen dispersal and seed production of the alpine shrub Rhododendron aureum (Ericaceae). Mol Ecol 15(4):1165–1173
Hoang DT, Chernomor O, von Haeseler A, Minh BQ, Vinh LS (2018) UFBoot2: improving the ultrafast bootstrap approximation. Mol Biol Evol 35(2):518–522
Ishikawa M, Sakai A (1981) Freezing avoidance mechanisms by supercooling in some rhododendron flower buds with reference to water relations. Plant Cell Physiol 22(6):953–967
Ishikawa M, Ishikawa M, Toyomasu T, Aoki T, Price WS (2015) Ice nucleation activity in various tissues of Rhododendron flower buds: their relevance to extraorgan freezing. Front Plant Sci 6:1–12
Jin ZX, Ke SS (2004) The diurnal variation of photosynthesis in leaves of Rhododendron fortunei. Bull Bot Res 4:20
Johnson DM, Smith WK (2008) Cloud immersion alters microclimate, photosynthesis and water relations in Rhododendron catawbiense and Abies fraseri seedlings in the southern Appalachian Mountains, USA. Tree Physiol 28(3):385–392
Jonasson S (1995) Resource allocation in relation to leaf retention time of the wintergreen Rhododendron lapponicum. Ecology 76(2):475–485
Kaku S, Iwaya M, Kunishige M (1980) Supercooling ability of Rhododendron flower buds in relation to cooling rate and cold hardiness. Plant Cell Physiol 21(8):1205–1216
Kameyama Y, Isagi Y, Naito K, Nakagoshi N (2000) Microsatellite analysis of pollen flow in Rhododendron metternichii var. hondoense. Ecol Res 15(3):263–269
Karlsson P (1994) Photosynthetic capacity and photosynthetic nutrient-use effi ciency of Rhododendron lapponicum leaves as related to leaf nutrient status, leaf age and branch reproductive status. Funct Ecol 8(6):694–700
Kondo T, Nakagoshi N, Isagi Y (2009) Shaping of genetic structure along Pleistocene and modern river systems in the hydrochorous riparian azalea, Rhododendron ripense (Ericaceae). Am J Bot 96(8):1532–1543
Kudo G (1993) Relationships between flowering time and fruit set of the entomophilous alpine shrub, Rhododendron aureum (Ericaceae), inhabiting snow patches. Am J Bot 80(11):1300–1304
Kudo G, Suzuki S (2004) Flowering phenology of tropicalalpine dwarf trees on Mount Kinabalu, Borneo. J Trop Ecol 20(5):563–571
Kurashige Y, Etoh JI, Handa T, Takayanagi K, Yukawa T (2001) Sectional relationships in the genus Rhododendron (Ericaceae): evidence from mat K and trn K intron sequences. Plant Syst Evol 228(1):1–14
Kuttapetty M, Pillai P, Varghese R, Seeni S (2014) Genetic diversity analysis in disjunct populations of Rhododendron arboreum from the temperate and tropical forests of Indian subcontinent corroborate Satpura hypothesis of species migration. Biologia 69(3):311–322
Larcher W, Siegwolf R (1985) Development of acute frost drought in Rhododendron ferrugineum at the alpine timberline. Oecologia 67(2):298–300
Li ZL, Cheng C, Zhang GW, Fang YP, Jin WB, Wang SZ (2016) ESTSSR marker-based genetic diversity analysis of Rhododendron simsii germplasm in Guifeng mountain. J Agric Sci Technol 17(5):1073–1076
Liao PC, Lin TP, Lan WC, Chung JD, Hwang SY (2009) Duplication of the class I cytosolic small heat shock protein gene and potential functional divergence revealed by sequence variations flanking the α-crystallin domain in the genus Rhododendron (Ericaceae). Ann Bot 105(1):57–69
Lim CC, Arora R, Krebs SL (1998a) Genetic study of freezing tolerance in Rhododendron populations: implications for cold hardiness breeding. J Am Rhod Soc 52:143–148
Lim CC, Arora R, Townsend EC (1998b) Comparing Gompertz and Richards functions to estimate freezing injury in Rhododendron using electrolyte leakage. J Am Soc Hort Sci 123(2):246–252
Lim CC, Krebs S, Arora R (1999) 25-kDa dehydrin associated with genotype-and age-dependent leaf freezing-tolerance in Rhododendron: a genetic marker for cold hardiness? Theor Appl Genet 99(5):912–920
Lipp C, Nilsen E (1997) Impact of subcanopy light environment on the hydraulic vulnerability of Rhododendron maximum to freeze-thaw cycles and drought. Plant Cell Environ 20(10):1264–1272
Liu YM, Zhang LH, Liu Z, Luo K, Chen SL, Chen KL (2012) Species identification of Rhododendron (Ericaceae) using the chloroplast deoxyribonucleic acid psbA-trnH genetic marker. Pharmacog Mag 8(29):29
Ma YP, Zhang CQ, Zhang JL, Yang JB (2010) Natural hybridization between Rhododendron delavayi and R. cyanocarpum (Ericaceae), from morphological, molecular and reproductive evidence. J Integr Plant Biol 52(9):844–851
Marian CO, Krebs SL, Arora R (2004) Dehydrin variability among rhododendron species: a 25 kDa dehydrin is conserved and associated with cold acclimation across diverse species. New Phytol 161(3):773–780
Marty C, Lamaze T, Pornon A (2010) Leaf life span optimizes annual biomass production rather than plant photosynthetic capacity in an evergreen shrub. New Phytol 187(2):407–416
McGraw JB (1989) Effects of age and size on life histories and population growth of Rhododendron maximum shoots. Am J Bot 76(1):113–123
Milne RI (2004) Phylogeny and biogeography of Rhododendron subsection Pontica, a group with a tertiary relict distribution. Mol Phylogenet Evol 33(2):389–401
Milne RI, Terzioglu S, Abbott R (2003) A hybrid zone dominated by fertile F1s: maintenance of species barriers in Rhododendron. Mol Ecol 12(10):2719–2729
Nakano T, Ishida A (1994) Diurnal variations of photosynthetic rates and xylem pressure potentials in four dwarf shrubs (Ericaceae) in an alpine zone. Proc NIPR Symp Polar Biol 7:243–255
Negi G (2006) Leaf and bud demography and shoot growth in evergreen and deciduous trees of central Himalaya, India. Trees 20(4):416–429
Neuner G, Ambach D, Aichner K (1999a) Impact of snow cover on photoinhibition and winter desiccation in evergreen Rhododendron ferrugineum leaves during subalpine winter. Tree Physiol 19(11):725–732
Neuner G, Ambach D, Buchner O (1999b) Readiness to frost harden during the dehardening period measured in situ in leaves of Rhododendron ferrugineum L. at the alpine timberline. Flora 194(3):289–296
Nilsen ET (1987) Influence of water relations and temperature on leaf movements of Rhododendron species. Plant Physiol 83:607–612
Nilsen ET (1991) Relationship between freezing tolerance and thermotropic leaf movement in five Rhododendron species. Oecologia 87(1):63–71
Osório ML, Osório J, Romano A (2010) Chlorophyll fluorescence in micropropagated Rhododendron ponticum subsp. baeticum plants in response to different irradiances. Biol Plant 54(3):415–422
Palacio S, Lenz A, Wipf S, Hoch G, Rixen C (2015) Bud freezing resistance in alpine shrubs across snow depth gradients. Environ Exp Bot 118:95–101
Peng YH, Arora R, Li GW, Wang X, Fessehaie A (2008a) Rhododendron catawbiense plasma membrane intrinsic proteins are aquaporins, and their over-expression compromises constitutive freezing tolerance and cold acclimation ability of transgenic Arabidopsis plants. Plant Cell Environ 31(9):1275–1289
Peng YH, Lin WL, Wei H, Krebs SL, Arora R (2008b) Phylogenetic analysis and seasonal cold acclimation-associated expression of early light-induced protein genes of Rhododendron catawbiense. Physiol Plant 132(1):44–52
Peng YH, Reyes JL, Wei H, Yang YI, Karlson D, Covarrubias AA, Krebs SL, Fessehaie A, Arora R (2008c) RcDhn5, a cold acclimation-responsive dehydrin from Rhododendron catawbiense rescues enzyme activity from dehydration effects in vitro and enhances freezing tolerance in RcDhn5 overexpressing Arabidopsis plants. Physiol Plant 134(4):583–597
Pornon A, Escaravage N, Till-Bottraud I, Doche B (1997) Variation of reproductive traits in Rhododendron ferrugineum L. (Ericaceae) populations along a successional gradient. Plant Ecol 130(1):1–11
Pornon A, Escaravage N, Thomas P, Taberlet P (2000) Dynamics of genotypic structure in clonal Rhododendron ferrugineum (Ericaceae) populations. Mol Ecol 9(8):1099–1111
Price WS, Ide H, Arata Y, Ishikawa M (1997) Visualisation of freezing behaviours in flower bud tissues of cold-hardy Rhododendron japonicum by nuclear magnetic resonance micro-imaging. Funct Plant Biol 24:599–605
Ranjitkar S, Luedeling E, Shrestha KK, Guan KY, Xu JC (2013) Flowering phenology of tree rhododendron along an elevation gradient in two sites in the Eastern Himalayas. Int J Biometeorol 57(2):225–240
Ruhland C, Day T (1996) Changes in UV B radiation screening ef fectiveness w ith leaf age in Rhododendron maximum. Plant Cell Environ 19(6):740–746
Sakai A, Larcher W (1987) Frost survival of plants-responses and adaptation to freezing stress. Springer, Berlin, p 62
Sakai A, Fuchigam i L, Weiser CJ (1986) Cold hardiness in the genus Rhododendron. J Am Soc Hort Sci 111(2):273–280
Sharp R, Else M, Cameron R, Davies W (2009) Water def icits promote f lowering in Rhododendron via regulation of pre and post initiation development. Sci Hort 120(4):511–517
Stevens P (1976) A ltitudinal and geographical distributions of f lower types in Rhododendron section Vireya, especially in the Papuasian species, and their signif icance. Bot J Linnean Soc 72(1):1–33
Stout JC (2007) Reproductive biology of the invasive exotic shrub, Rhododendron ponticum L. (Ericaceae). Bot J Linnean Soc 155(3):373–381
Sw iderski A, Muras P, Koloczek H (2004) Flavonoid composition in frost-resistant Rhododendron cultivars grown in Poland. Sci Hort 100(1):139–151
Thomson A, Radford G, Norris D, Good J (1993) Factors af fecting the distribution and spread of Rhododendron in North Wales. J Environ Manag 39(3):199–212
Thornton JT (1990) Breeding rhododendrons for the Gulf South. J Am Rhododendron Soc 44:91–93
V?in?l? A, Repo T (2000) Impedance spectroscopy in frost hardiness evaluation of Rhododendron leaves. Ann Bot 86(4):799–805
Vetaas OR (2002) Realized and potential climate niches: a comparison of four Rhododendron tree species. J Biogeogr 29(4):545–554
Wang X, Arora R, Horner HT, Krebs SL (2008) Structural adaptations in overw intering leaves of thermonastic and nonthermonastic Rhododendron species. J Am Soc Hort Sci 133(6):768–776
Wang X, Peng Y, Singer JW, Fessehaie A, Krebs SL, Arora R (2009) Seasonal changes in photosynthesis, antioxidant systems and ELIP expression in a thermonastic and non-thermonastic Rhododendron species: a comparison of photoprotective strategies in overw intering plants. Plant Sci 177(6):607–617
Wang SZ, Li ZL, Jin WB, Xiang F, Xiang J, Fang YP (2017) Development and characterization of polymorphic m icrosatellite markers in Rhododendron simsii (Ericaceae). Plant Sp Biol 32(1):100–103
Wei H, Dhanaraj AL, Row land LJ, Fu Y, Krebs SL, Arora R (2005) Comparative analysis of expressed sequence tags from cold-acclimated and non-acclimated leaves of Rhododendron catawbiense M ichx. Planta 221(3):406–416
Wei H, Dhanaraj AL, Arora R, Row land LJ, Fu Y, Sun L (2006) Identif ication of cold acclimation-responsive Rhododendron genes for lipid metabolism, membrane transport and lignin biosynthesis: importance of moderately abundant ESTs in genom ic studies. Plant Cell Environ 29(4):558–570
Wolf PG, Doche B, Gielly L, Taberlet P (2004) Genetic structure of Rhododendron ferrugineum at a w ide range of spatial scales. J Hered 95(4):301–308
Wu ML, Lin TP, Lin MY, Cheng YP, Hwang SY (2007) Divergent evolution of the chloroplast small heat shock protein gene in the genera Rhododendron (Ericaceae) and Machilus (Lauraceae). Ann Bot 99(3):461–475
Xiao Z, Su JL, Sun XB, Li C, He LS, Cheng SP, Liu XQ (2018) De novo transcriptome analysis of Rhododendron molle G. Don f lowers by Illum ina sequencing. Genes Genomics 46(6):591–601
Xing W, Liao JY, Cai MY, Xia QF, Liu Y, Zeng W, Jin XL (2017) De novo assembly of transcriptome from Rhododendron latoucheae Franch. using Illum ina sequencing and development of new ESTSSR markers for genetic diversity analysis in Rhododendron. Tree Genet Genomes 13(3):53
Zha HG, M ilne RI, Sun H (2009) Asymmetric hybridization in Rhododendron agastum: a hybrid taxon comprising mainly F1s in Yunnan, China. Ann Bot 105(1):89–100
Zhang L, Xu PW, Cai YF, Ma LL, Li SF, Li SF, Xie WJ, Song J, Peng LC, Yan HJ, Zou L, Ma YP, Zhang CJ, Gao Q, Wang JH (2017a) Draft genome assembly of. Giga Rhododendron delavayi Franch. var. delavayi. Science 6(10):1–11
Zhang Y, Zhang X, Wang YH, Shen SK (2017b) De novo assembly of transcriptome and development of novel EST-SSR markers in Rhododendron rex Lévl. through Illumina sequencing. Front Plant Sci 8:1664
 Journal of Forestry Research2021年2期
Journal of Forestry Research2021年2期
- Journal of Forestry Research的其它文章
- Stem taper functions for Betula platyphylla in the Daxing’an Mountains, northeast China
- Leaf phenotypic variation of endangered plant Tetracentron sinense Oliv. and influence of geographical and climatic factors
- Ecological variations of woody species along an altitudinal gradient in the Qinling Mountains of Central China: area-based versus mass-based expression of leaf traits
- Variations in stem radii of Larix principis-rupprechtii to environmental factors at two slope locations in the Liupan Mountains, northwest China
- A review of ecological mechanisms for management practices of protective forests
- Variation of basic density, calorific value and volumetric shrinkage within tree height and tree age of Ugandan grown Eucalyptus grandis wood
