Is it better to choose immediate dialysis treatment for renal transplant patients after PCI?
Qian-Yun GUO, Xun-Xun FENG, Zhi-Jian WANG, Yue-Ping LI, Jing QI, Yu-Jie ZHOU
Department of Cardiology, Beijing Anzhen Hospital, Capital Medical University, Beijing Institute of Heart Lung and Blood Vessel Disease, Beijing Key Laboratory of Precision Medicine of Coronary Atherosclerotic Disease, Clinical Center for Coronary Heart Disease, Capital Medical University, Beijing, China
Keywords: Contrast-induced encephalopathy; Hemodialysis; Kidney transplantation; Percutaneous coronary intervention
Administration of iodinated contrast agents can give rise to allergic/hypersensitivity reactions and contrast-induced nephropathy. In extremely rare cases, intravascularly administered iodinated contrast agents can trigger contrast-induced encephalopathy (CIE) in patients undergoing percutaneous coronary intervention (PCI).[1]Here, we describe the case of a male patient diagnosed with CIE after undergoing PCI with a non-ionic contrast agent, iodixanol. This is the first such case to be reported in a patient undergoing hemodialysis after kidney transplantation.
The patient, a 54-year-old male with a history of kidney transplantation, hypertension, and diabetes, had one stent inserted in the left anterior descending artery, and two stents inserted in the right coronary artery 10 years ago. Three weeks prior to presentation, the patient had undergone routine hemodialysis due to a decline in renal function. Upon admission, the patient exhibited stable vitals. Laboratory tests documented that the creatinine level was 293.1 μmol/L (normal range: 57-111 μmol/L), fasting glucose level was 4.86 mmol/L (normal range: 3.9-6.1 mmol/L), blood pressure was 136/82 mmHg, B-type natriuretic peptide was 657 pg/mL (normal range: 0-100 pg/mL), and left ventricular ejection fraction was 62%. During the PCI, the patient received 120 mL of iodixanol (Visipaque 320, GE Healthcare, Cork, Ireland). A stent was inserted because of the symptomatic nature of the lesion (Figure 1). There were no complications during the procedure.
Three hours after the procedure, the patient developed right-sided weakness and partial seizures. Initially, he was diagnosed with embolic cerebral infarction, but computed tomography (CT) scans did not reveal any evidence of intracranial hemorrhaging or obstruction of the cerebral vasculature (Figure 2), and the only evident symptom was brain edema. Considering the absence of signs of ischemic stroke, thrombolytic medications were not administered. Instead, the patient received intravenous normal saline, underwent hemodialysis, and was given antiepileptic medications. Under this regimen, an electroencephalogram and a subsequent CT scan of the brain were nearly normal after 24 h (Figure 2). Within 48 h, no more neurological deficits became evident, and the patient was discharged on 7thday after admission (Figure 3). The patient recovered uneventfully from the CIE, and neurological and psychometric tests were negative at 120 days after the procedure.
In extremely rare cases, patients undergoing PCI with intravascularly delivered contrast agents can suffer from CIE. The first known case of contrast agent-induced CIE was reported in 1970.[2]To date, approximately 60 cases of CIE consequent to coronary angiography (CAG) have been described, with variations in rates depending on the contrast agent used.[3]In addition to triggering focal neurological and visual defects, neurotoxic side effects of contrast agents include encephalopathy-induced seizures. Most patients with CIE have a favorable prognosis and improve rapidly within 72 h. This condition is generally self-limiting, although in some cases it can result in permanent neurological impairment or death.[3]In many cases, the distinction between CIE and other potential conditions including cerebral infarction, hyperperfusion syndrome, or subarachnoid hemorrhage is challenging. In the absence of other causes explaining a sudden onset of neurological symptoms after the administration of a contrast agent, CT scans or magnetic resonance imaging can aid in obtaining an accurate differential diagnosis.[4,5]In the reported case, the correct diagnosis of CIE prevented the exposure of the patient to any risks that might result from unnecessary treatment.
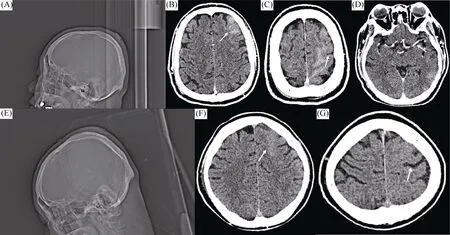
Figure 2. Skull CT scanning before and after the occurrence of CIE.
The molecular mechanisms governing the development of CIE remain uncertain. Results obtained in animal models support the hypothesis that the physiochemical properties of contrast agents can disrupt the osmotic homeostasis of the blood-brain barrier (BBB), and the resulting hyperosmolality drives the progressive cerebral edema.[6]
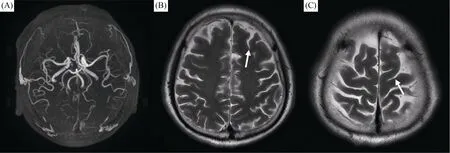
Figure 3. Following-up MRI.
Several reports suggest that higher individual or cumulative doses of contrast agents can increase the risk of CIE.[7,8]For example, Uchiyama and coworkers found that patients suffering from CIE had been administered an average of 250 mL of contrast agents; this dose was lower, 220 mL, in patients undergoing hemodialysis, and higher, 285 mL, in patients not on hemodialysis.[8]In the present case, the patient received 120 mL of iodixanol to enable stent placement. This was a relatively high dose for a single CAG procedure, particularly in a patient with a history of kidney transplantation and hypertension, and currently subjected to regular dialysis. Both the present and past reports indicate that even isosmolar non-ionic contrast compounds, including iodixanol, can mediate the development of CIE. In a previous animal study, Wilcox and collaborators documented that both iopamidol and iodixanol were able to readily cross the BBB.[9]In the presented case, the decision was made to utilize an isosmolar non-ionic contrast agent, and to use it in the smallest quantity possible. At the same time, this case showed that the CIE could not be prevented by routine hemodialysis in patients with the deterioration of renal function after renal transplantation, raising the possibility that in order to prevent CIE, the patient should undergo dialysis immediately after PCI.
Risk factors for CIE include a history of kidney failure and hypertension, with the latter being the most important risk factor. Approximately half of the patients developing CIE suffer from chronic hypertension.[10,11]The permeability of the BBB is believed to be adversely impacted by the decreased vascular autoregulation in chronic hypertension patients, potentially facilitating CIE. Impaired kidney metabolism and function may also facilitate CIE due to a delayed excretion of contrast agents.
While there is no effective means of preventing the development of CIE, it is recommended that patients receive intravascular saline infusion while undergoing CAG, and are adequately hydrated. The intravascular administration of steroids or mannitol may help to control the symptoms of edema in some patients, but the effectiveness of these compounds is limited in subjects undergoing hemodialysis as a consequence of anuria or oliguria resulting from impaired renal function.[4,7,10-13]Mannitol can enhance BBB permeability, potentially increasing the penetration of the contrast agent into the brain due to the delayed elimination of these agents from circulation. Therefore, the present patient was not administered mannitol, and instead received intravenous fluids and underwent dialysis, which yielded satisfactory results.
The development of CIE is challenging to predict. The presented patient had a history of previous successfully performed PCI without experiencing any side effects of the contrast agent. Most patients suffering from CIE achieve a good prognosis with supportive care, with symptoms resolving completely in a short period of time. While CIE typically does not result in long term complications, in the present case, the patient was unable to undergo revascularization therapy as a result of this condition, suggesting the presence of other potential complications of CIE.
Going forward, it is critical that clinicians weigh the potential for the development of CIE by the patient against the necessity of a contrast-based diagnostic procedure. To ensure ethical and optimal patient care, cardiologists must be aware of the likelihood of CIE. If the patient exhibits symptoms consistent with CIE, the most common differential diagnoses, such as meningitis or ischemic/hemorrhagic stroke, must be excluded.
In summary, we are reporting a rare case of CIE in a patient with a history of diabetes, kidney transplantation, and hypertension, after he underwent PCI. The patient was free of symptoms within 48 h and did not suffer any permanent neurological impairment. Thus, a proper CIE diagnosis is essential to avoid unnecessary exposure of patients to risks associated with unneeded treatments.
Acknowledgments
This study was supported by the China Postdoctoral Science Foundation (2019M650032), and the China Scholarship Council (CSC) award (201706210415). All authors had no conflicts of interest to disclose.
References
1 Dukkipati S, O’Neill WW, Harjai KJ, et al. Characteristics of cerebrovascular accidents after percutaneous coronary interventions. J Am Coll Cardiol 2004; 43: 1161-1167.
2 Riahi L, Mediouni M, Messelmani M, et al. A singular manifestation of contrast-induced encephalopathy following coronary angiography. Neurol India 2019; 67: 1525-1527.
3 Spina R, Simon N, Markus R, et al. Contrast-induced encephalopathy following cardiac catheterization. Catheter Cardiovasc Interv 2017; 90: 257-268.
4 Dattani A, Au L, Tay KH, et al. Contrast-induced encephalopathy following coronary angiography with no radiological features: a case report and literature review. Cardiology 2018; 139: 197-201.
5 Pagani-Estévez GL, Nasr DM, Brinjikji W, et al. Dual-energy CT to diagnose pseudoedema in contrast-induced encephalopathy following cerebral angiography. Neurocrit Care 2017; 27: 261-264.
6 Wang H, Li T, Zhao L, et al. Dynamic effects of ioversol on the permeability of the blood-brain barrier and the expression of ZO-1/Occludin in rats. J Mol Neurosci 2019; 68: 295-303.
7 Pokersnik JA, Liu L, Simon EL. Contrast-induced encephalopathy presenting as acute subarachnoid hemorrhage. Am J Emerg Med 2018; 36: 1122.e3-1122.e4.
8 Senthilkumaran S, Balamurugan N, Jena NN, et al. Contrast- induced encephalopathy and diagnostic modalities-Can it make a difference? Am J Emerg Med 2018; 36: 2328.
9 Donepudi B, Trottier S. A seizure and hemiplegia following contrast exposure: understanding contrast-induced encephalopathy. Case Rep Med 2018; 2018: 9278526.
10 Leong S, Fanning NF. Persistent neurological deficit from iodinated contrast encephalopathy following intracranial aneurysm coiling. A case report and review of the literature. Interv Neuroradiol 2012; 18: 33-41.
11 Zhang G, Wang H, Zhao L, et al. Contrast-induced encephalopathy resulting from use of ioversol and iopromide. Clin Neuropharmacol 2020; 43: 15-19.
12 Montejo C, Rodríguez A, Pascual-Vicente M, et al. Transient contrast-induced encephalopathy after internal carotid artery embolization prior to surgery for nasopharyngeal carcinoma. Neurologia. Published Online First: 9 March 2018. DOI: 10.1016/j.nrl.2018.01.009.
13 de Falco A, De Simone M, d’Onofrio F, et al. Posterior reversible encephalopathy syndrome overlapping contrast-induced encephalopathy after coronary angiography. Neurol Sci 2019; 40: 1951-1953.
 Journal of Geriatric Cardiology2020年2期
Journal of Geriatric Cardiology2020年2期
- Journal of Geriatric Cardiology的其它文章
- Diagnostic chest X-ray in atrial septal defects
- Short-term efficacy of unibody single-branched stent in the treatment of lesions involving the left subclavian artery: two-year follow-up outcomes
- A novel treatment of refractory coronary embolism: thrombus aspiration catheter-assisted twisting wire technique
- MicroRNA-29a attenuates angiotensin-II induced-left ventricular remodeling by inhibiting collagen, TGF-β and SMAD2/3 expression
- Beneficial effects of moderate to vigorous physical activity on cardiovascular disease among Chinese adults
- Atrial fibrillation among Russian men and women aged 55 years and older: prevalence, mortality, and associations with biomarkers in a population-based study
