Genomic dissection of gastrointestinal and lung neuroendocrine neoplasm
Haixing Wang,Li Sun,Hua Bao,Ao Wang,Panpan Zhang,Xue Wu,Xiaoling Tong,Xiaonan Wang,Jie Luo,Lin Shen,Yang W.Shao,7,Ming Lu
1Department of Endoscopy Center,The First Affiliated Hospital of Xiamen University,Xiamen 361003,China;2Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing),Department of Pathology,Peking University Cancer Hospital &Institute,Beijing 100142,China;3Translational Medicine Research Institute,Geneseeq Technology Inc.,Toronto M5G 1L7,Canada;4Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing),Department of Gastrointestinal Oncology,Peking University Cancer Hospital &Institute,Beijing 100142,China;5Medical Department,Nanjing Geneseeq Technology Inc.,Nanjing 210032,China;6Department of Pathology,China-Japan Friendship Hospital,Beijing 100029,China;7School of Public Health,Nanjing Medical University,Nanjing 210029,China
Abstract Objective:Neuroendocrine neoplasms (NENs) are relatively rare and heterogeneous malignancies with two major subtypes:low-grade neuroendocrine tumor (NET) and high-grade neuroendocrine carcinoma (NEC).Comprehensive molecular characterization of NENs is needed to refine our understanding of the biological underpinnings of different NEN subtypes and to predict disease progression more accurately.Methods:We performed whole-exome sequencing (WES) of NEN samples from 49 patients (25 NETs and 24 NECs) arising from the stomach,intestines or lung.Clinicopathologic features were assessed and associated with molecular events.Results:NENs generally harbor a low mutation burden,with TP53 being the top mutated gene found in 31% of patients.Consistent with other studies,p53 signaling pathway dysfunction is significantly enriched in NECs compared to NETs (P<0.01).Other than TP53,tissue type-specific mutation profiles of NENs were observed in our cohort compared to those reported in pancreatic NETs.Importantly,we observed significant genomic instability,with increased copy number alterations observed across the NEN genome,which was more profound in NECs and independently correlated with poor overall survival (OS) (P<0.001).NECs could be further stratified into two molecular subtypes based on OS (P<0.001) and the chromosomal instability score (CIS).Interestingly,we discovered that the gain of whole chromosome 5 occurred at the early stage of NEN development,followed by the loss of 5q exclusively in NECs (P<0.001).Conclusions:These findings provide novel insights into the molecular characteristics of NENs and highlight the association of genomic stability with clinical outcomes.
Keywords:Neuroendocrine carcinoma;whole-exome sequencing;chromosomal instability
Introduction
Neuroendocrine neoplasms (NENs) are rare,heterogeneous malignancies that are steadily rising in both prevalence and incidence (1).Due to the body-wide distribution of neuroendocrine cells,NENs can originate from various organs,with most primary cases occurring in the gastrointestinal track and the bronchopulmonary system (1,2).In 2010,the revised World Health Organization (WHO) classification of NENs defined three grades based on the mitotic count and Ki-67 index (G1,G2,and G3) (3).In 2015,the National Comprehensive Cancer Network (NCCN) recommended the addition of tumor differentiation to NEN classification (4),which classified well-differentiated neoplasms as neuroendocrine tumors (NETs,G1 or G2) and poorly differentiated neoplasms as neuroendocrine carcinomas (NECs,G3).Studies have shown that NETs and NECs have distinct prognoses and responses to treatment (3,5),with pancreatic NETs (PanNETs) responding better to traditional chemotherapy than NECs (6).However,the histopathologic classification can be challenging due to the lack of well-defined histological criteria (7),and this dichotomization is far from perfect since some NETs are shown to behave like NECs (8).Furthermore,although NETs are mostly G1/G2 grade,regions of excessive proliferation comparable to G3 can also be found within NETs,which is associated with reduced disease-specific survival (9).This suggests that different molecular subtypes may exist within the same histological group,and current classification methods based on histology or the Ki-67 index may not adequately capture important differences at the molecular level.
To date,most genomic profiling studies on NENs have mainly focused on PanNETs and small intestine NETs(SI-NETs).It has been shown thatDAXX/ATRX,MEN1and mTOR pathway genes are frequently altered in PanNETs(10-12).As a result,targeted therapy with the mTOR inhibitor everolimus has been reported to improve progression-free survival in advanced PanNET patients(13).Conversely,SI-NETs harbor relatively fewer recurrent mutations,withCDKN1Bbeing the most commonly affected gene (14,15).However,less is known about the genomic landscapes in high-grade NECs or NENs arising from other anatomical sites (i.e.,the stomach and lung).Poorly differentiated NECs are aggressive cancers with few treatment options.One recent study utilizing a 50-gene panel showed thatTP53,KRAS,PIK3CA/PTENandBRAFmutations were common in these NECs (11).Nevertheless,genomic data on NENs from sites other than the pancreas [nonpancreatic NENs (NPNENs)]remain scarce,and additional comprehensive genome-wide studies are needed to fully elucidate their molecular characteristics and uncover novel therapeutic targets and prognostic markers.Here,we performed whole-exome sequencing (WES) on tumor samples and matched normal controls from 49 NP-NEN patients (29 in the stomach,10 in the intestine,and 10 in the lung) for mutation profiling and somatic copy number alteration(SCNA) analysis,aiming to discover genomic signatures among distinct NEN subtypes and to identify potential biomarkers associated with clinical outcomes.
Materials and methods
Patient and sample overview
We retrospectively analyzed formalin-fixed paraffinembedded (FFPE) tumor samples from 121 NEN patients treated at Peking University Cancer Hospital between 2012 and 2016,with consent signed by the patients or their legal guardians.Neuroendocrine features were confirmed by hematoxylin eosin (H&E) staining under light microscopy and immunohistochemistry (IHC) staining for neuroendocrine markers.Among these 121 tumor samples,63 samples without matched normal control tissues and 9 low tumor purity (<30%) samples were excluded.The remaining 49 specimens from different tissues of origin(stomach,intestines and lung) were analyzed in this study.Each sample was assessed for the Ki-67 index,annotated G grade and differentiation (NET/NEC) based on independent pathologic evaluations by two pathologists.All NECs had a Ki67 index greater than 50%,consistent with previously reported findings (16,17).The detailed clinical information of all patients is included inSupplementary Table S1.The Ethics Committee of Peking University Cancer Hospital has reviewed and approved this study.
DNA isolation and library preparations
Genomic DNAs from FFPE sections were extracted with a QIAamp DNA FFPE Tissue Kit and quantified by Qubit 3.0 using the dsDNA HS Assay Kit (Thermo Fisher Scientific,Waltham,USA).Library preparations were performed with a KAPA Hyper Prep Kit (KAPA Biosystems,Wilmington,USA).Target enrichment was performed using the xGen Exome Research Panel v1.0 and Hybridization and Wash Reagents Kit (Integrated DNA Technology,Coralville,USA) according to the manufacturer’s protocol.Sequencing was performed on an Illumina HiSeq 4000 platform using PE150 sequencing chemistry (Illumina,San Diego,USA) to a mean coverage depth of 150× for tumor samples and 50× for matched normal control samples.
Sequencing data processing
Trimmomatic (18) was used for FASTQ file quality control.Leading/trailing low-quality reads (quality reading below 15) or N bases were removed.The high-quality reads were then aligned to the human reference genome(hg19) by BWA-MEM (19) with default parameters.Local realignment around indels and base quality score recalibration were performed with the Genome Analysis Toolkit (GATK 3.4.0).
Since loss of heterozygosity (LOH) is a common occurrence in cancer (20-22),allele-specific copy number(ASCN) analysis was performed with Fraction and Allele-Specific Copy Number Estimates from Tumor Sequencing(FACETS) (23) for the precise detection and separation of amplifications,deletions and LOH.The segmented ASCN results were then mapped to human genes based on their genomic coordinates.The ASCNs of major and minor alleles from the normal control sample were assumed as one copy.For each gene,the Euclidean distance of ASCN between paired tumor and normal samples was calculated as follows:
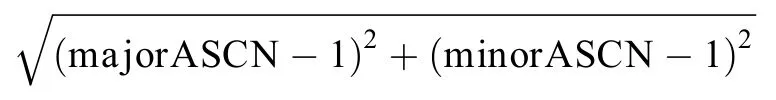
The overall chromosomal instability score (CIS) for each tumor sample was defined as the arithmetic average of Euclidean distances across all genes.Stomach samples were then clustered into two groups with the K-means algorithm,and the grouping criteria were extrapolated to categorize intestine and lung samples.
Genomic Identification of Significant Targets in Cancer(GISTIC) 2.0 (24) was used to identify significantly amplified and deleted focal-and arm-level landscape of somatic copy number alterations (SCNAs).Arm-level gain and loss were defined as log2 depth ratios >0.2 and <-0.2,respectively.Focal-level amplification and deletion were defined as log2 depth ratios >0.4 and <-0.4,respectively.Segments with 0 minor copy number were defined as LOH.
Somatic single-nucleotide variants (SNVs) were identified using MuTect (25),and small insertions and deletions (indels) were detected using SCALPEL (26).SNV and indel annotation was performed by ANNOVAR(Version 20180603,http://annovar.openbioinformatics.org/en/latest/).Common single nucleotide polymorphisms(SNPs) were removed if they were found in public databases (Exome Variant Server,1,000 Genomes Project and Exome Aggregation Consortium) at a frequency >1%.In the somatic analysis,we considered only nonsynonymous,stop-gain SNVs and frameshift/nonframeshift indels.A mutation significance analysis was carried out using the MutSig2CV algorithm (27),as elaborated in The Cancer Genome Atlas (TCGA) project.Significantly mutated genes were identified based on a P<0.01.
Statistical analysis
Fisher’s exact test was used to analyze categorical variables in the contingency table.The Wilcoxon rank-sum test was used to find associations between categorical and numerical variables.The association between overall survival (OS)and chromosomal instability (CIN) was analyzed using the Kaplan-Meier method and compared by log-rank tests with the R packagessurvivalandsurvminer.The multivariate analysis was performed using Cox regression,controlling for age,sex,and histology subtypes.Oncoplots were drawn withComplexheatmap.Two-sided P<0.05 were considered significant.All statistical analyses were performed with R software (Version 3.3.3;R Foundation for Statistical Computing,Vienna,Austria).
Results
Patient overview and clinical characteristics
The patients’ clinical information and tumor characteristics involved in this study are summarized inTable 1,with detailed information listed inSupplementary Table S1.The patients’ ages ranged from 27 to 81 years old,with a median of 61 years old.The male to female ratio was 1.45:1,with significantly more male patients having NECs(83.3%) than NETs (36.0%) (P=0.001,Fisher’s exact test).Stage I-IV NENs diagnosed at different anatomical sites,including the stomach (n=29),lung (n=10) and intestines(n=10),were collectively termed NP-NENs.Notably,all lung NENs were histologically classified as small cell NECs in our cohort,while intestinal NENs were exclusively NET phenotypes.Equal distributions of NETs(n=15) and NECs (n=14),as well as small cell (n=6) and large cell (n=7) phenotypes within NECs,were observed at the stomach.All NEC samples were G3 grade,with a Ki67 index >50%,while NETs contained samples from G1 to G3.We separated the lung and intestine samples from the stomach samples when testing for associations between genetic alterations and clinical characteristics or survival.
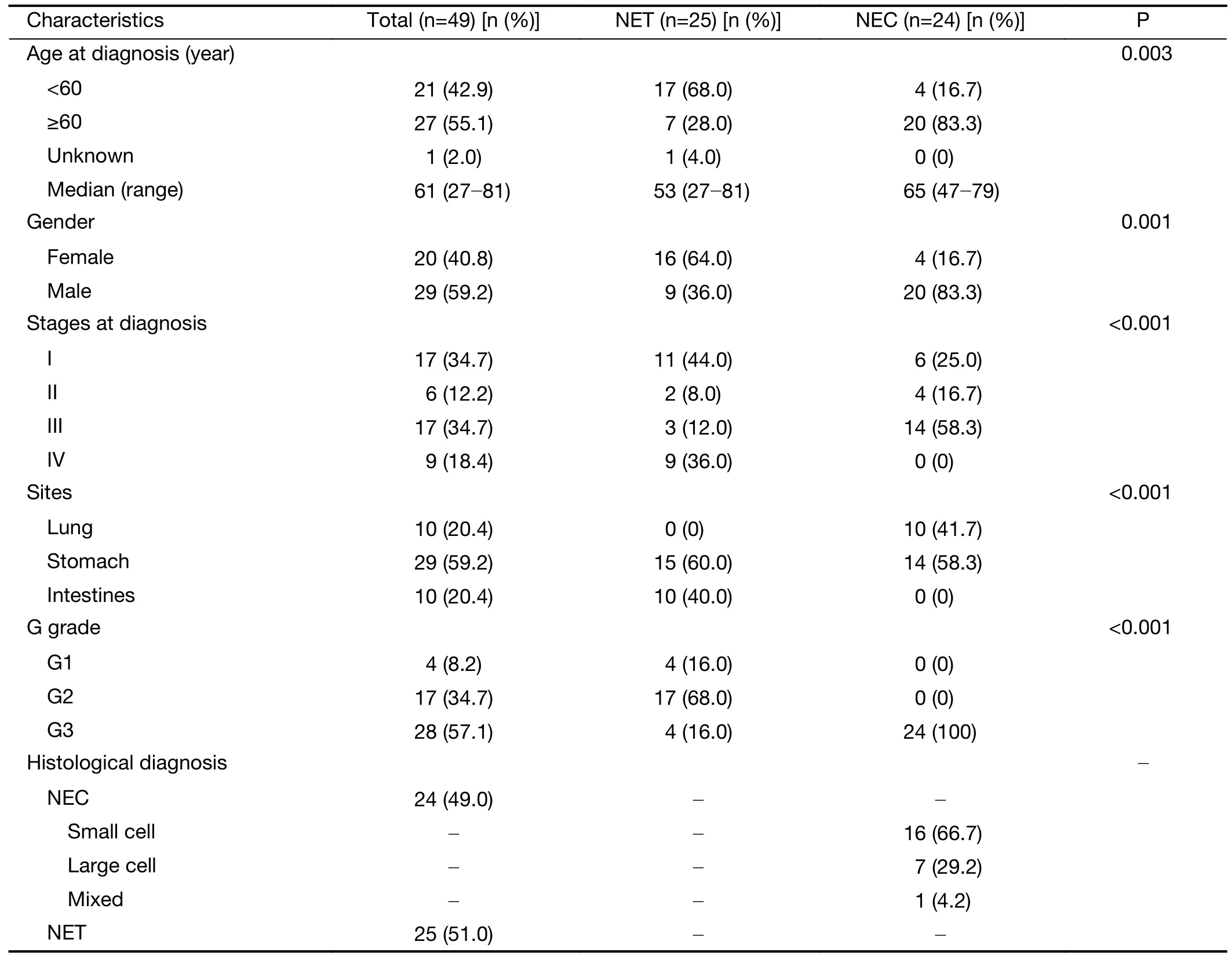
Table 1 Patients’ clinical information and tumor characteristics
Mutational signature profile
In this cohort of NP-NENs,the most frequent nucleotide substitutions were the C:G > T:A transition (46.7%),the C:G > A:T transversion (18.0%),and the T:A > C:G transition (14.2%).In PanNENs (12),the C:G > T:A transition (42.3%),the T:A > C:G transition (16.3%),and the C:G > A:T transversion (15.8%) were the top 3 substitution types.Therefore,NP-NENs and PanNENs displayed similar patterns of single nucleotide substitution.Among the 30 mutational signatures classified on Catalogue Of Somatic Mutations In Cancer (COSMIC),the most prominent ones in this NP-NEN cohort were signature 1 (34.6%) which was associated with aging,and signature 3 (15.4%) which was associated with DNA double-strand break-repair dysfunction.Notably,signature 3 was also linked with germline and somatic mutations in BRAC1 and BRAC2.Similarly,these two signatures were the dominant types in PanNENs but occurred with reduced frequencies of 16.0% and 11.4%,respectively.On the other hand,notably increased signatures in PanNENs included signature 13 (4.1%vs.2.0%),signature 16 (3.9%vs.0.2%),and signature 19 (3.6%vs.0.2%).The average proportions of each signature in NP-NENs and PanNENs are summarized inSupplementary Table S2.
Somatic mutations in NP-NENs
Frequently somatically mutated genes reoccurring in more than three patients in this NP-NEN cohort as well as previously reported PanNEN-associated genes [TSC2,MEN1,ATRX,RB1,CDKN2A,BRAF,SETD2,MUTYH,PTEN,TSC1,VHLandDEPDC5(12)]are shown inFigure 1.In total,we observed different mutation profiles of NP-NENs compared to PanNENs.Somatic mutations inMEN1have been reported in~35% of PanNENs (12,28).However,only three patients (6%;two NETs and one NEC from the stomach) in this cohort hadMEN1somatic mutations.Other frequently mutated PanNEN-related genes (10) also occurred at a much lower frequency in our cohort,includingATRX(n=3),SETD2(n=2),PTEN(n=1),andDEPDC5(n=1).On the other hand,consistent with previous reports (11),the tumor suppressor geneTP53was the most frequently mutated gene (31% of patients) and was specifically enriched in NECs (58.3%vs.4.0%,P<0.001,Fisher’s exact test) (Figure 1).Considering that all intestinal samples were NETs and all lung samples were NECs,we further analyzed the distribution ofTP53mutations in stomach samples and confirmed that it is indeed enriched in NECs (64.0%vs.7.0%,P=0.002,Fisher’s exact test).The retinoblastoma (RB) pathway is often inactivated in high-grade small cell NENs,although a variable prevalence of theRB1mutation has been reported (29,30).We identifiedRB1loss-of-function mutations in two small cell NEC samples of the lung and in one stomach small cell NEC within this cohort.Additional frequently mutated genes in this cohort includedFTH1(a potential prognostic marker in breast cancer) (31),POTEC(a tumor subtype-specific cancer-testis antigen) (32),andNOS2(dual roles reported in tumorigenesis) (33).FTH1mutations also had a trend of higher prevalence in NEC samples.Furthermore,the mTOR pathway genesTSC1/TSC2were collectively mutated in 8% of the tumors.BRAFV600Ewas previously reported in colorectal/gastroenteropancreatic NENs (34,35),and here we identified this mutation in two intestinal NET patients(Figure 1).The frequencies of common somatic mutations and LOH reported in PanNENs and in our NP-NEN cohort are summarized inSupplementary Table S3.
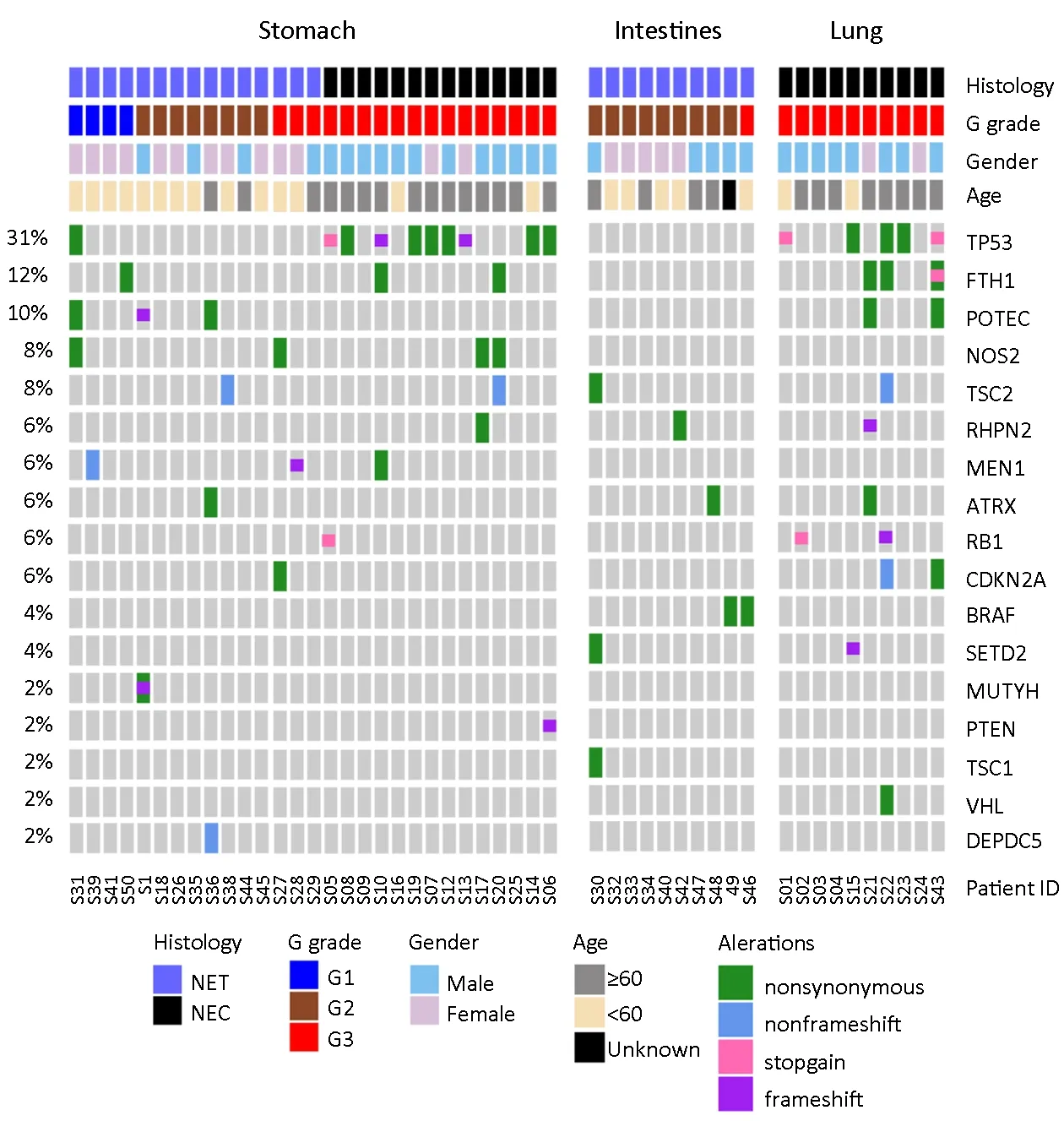
Figure 1 Somatic mutations identified in neuroendocrine neoplasms (NENs) of stomach,intestine and lung.Somatic mutation profiles of 49 NEN patients characterized by whole-exome sequencing (WES) analyses.The top panel indicates five different clinical characteristics of each patient,with the patient’s ID and color coding labeled at the bottom.Samples were grouped based on anatomic sites (stomach,intestine and lung),histology [neuroendocrine tumor (NET)/neuroendocrine carcinoma (NEC)],and G grade (G1/G2/G3).The comutation matrix represents significantly mutated genes identified by MutSig2CV and previously reported pancreatic NEN-associated genes.
Landscape of SCNAs
SCNA events were frequently observed in NETs of different organ-specific origins (36,37).To further refine the landscape of SCNAs in NP-NENs and identify common patterns of genomic alterations among different organ types,we examined SCNAs in all 49 samples.Overall,NP-NEN samples,especially NECs,displayed complex SCNAs,with numerous gains,losses,and LOHs observed in almost all chromosomes (Figure 2A).GISTIC2 analysis identified 4 significant recurrent (Q<0.01) focallevel SCNA events (Figure 2B,Figure 3A,top panel):amplifications of 19q12 (Q<0.01,27% of patients,containing theCCNE1gene),deletions of 13q14.2(Q<0.001,35% of patients,containing theRB1gene),10q23.2 (Q<0.001,31% of patients,containing thePTENgene),and 9q21.11 (Q<0.01).At the chromosomal arm level,five gains and eight losses were found as significantly recurrent SCNAs (GISTIC2 Q<0.05,Figure 3A,middle panel).Importantly,SCNAs in these regions encompass well-known tumor-related genes such asEGFR(7p),NRAS(1q),TP53(17p),APC(5q),BRCA1andRB1(13q).Among these arm-level events,10q loss,20q gain and 7p gain were also reported in PanNENs (38) (Supplementary Table S4).However,other high frequency events in PanNENs,such as 11p and 11q loss as well as 7q and 17q gain,occurred in fewer NP-NEN patients.
Association between SCNAs and patients’ clinicopathologic characteristics
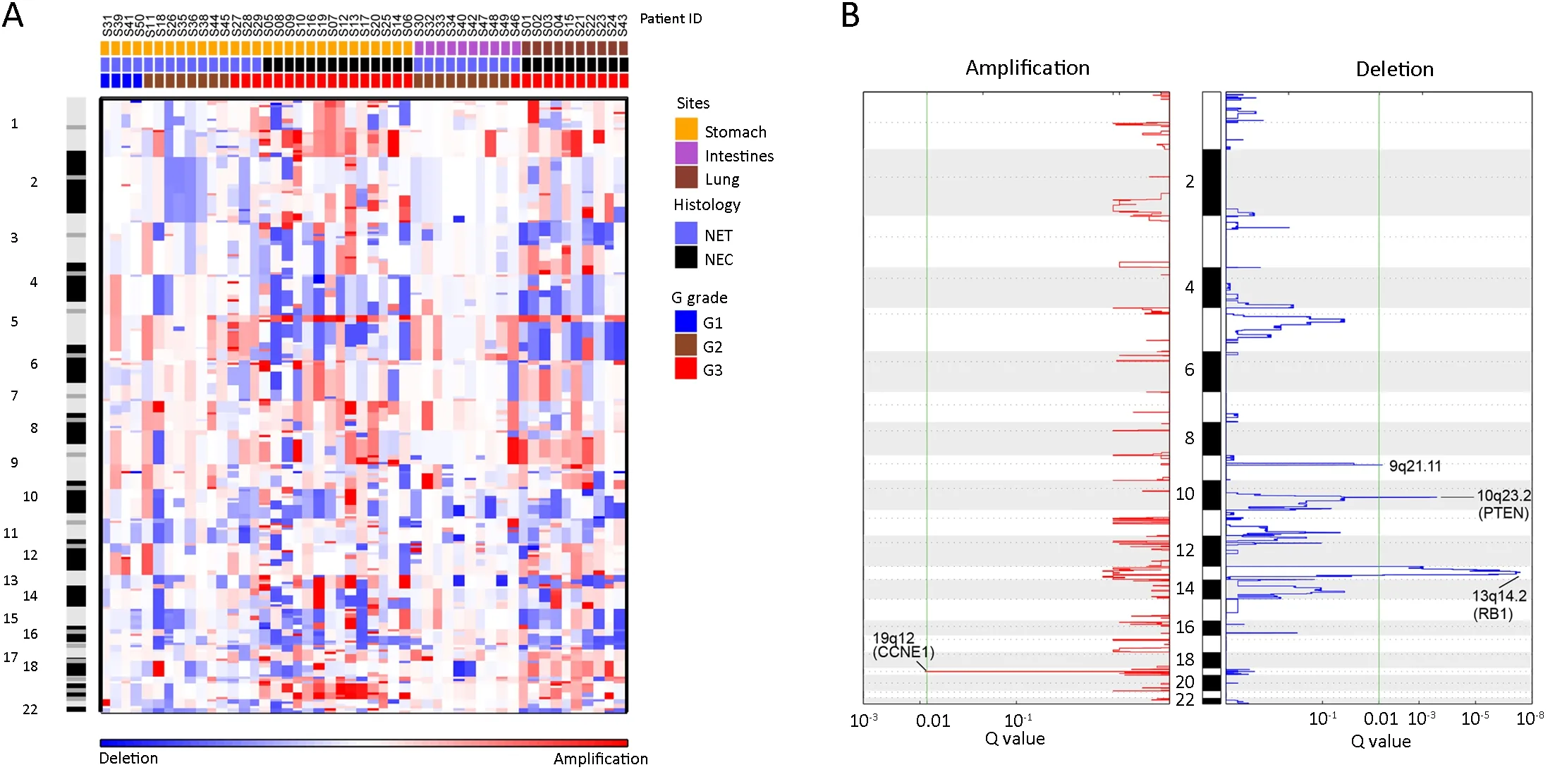
Figure 2 Landscape of arm-and focal-level somatic copy number alterations (SCNAs) in neuroendocrine neoplasms (NENs).(A) Heat map of arm-level copy number gains (red) and losses (blue) of 49 NEN tumor samples across the whole genome,ordered by site,histology,and G grade.Each column represents one patient.Chromosome numbers were labeled from top to bottom;(B) Significant recurrent focal-level copy number gains (red) and losses (blue) identified by Genomic Identification of Significant Targets in Cancer (GISTIC) 2.0.The false discovery rate (Q value) is plotted at the bottom.A Q value less than 0.01 (green line) is considered significant,and significantly altered focal loci are labeled.
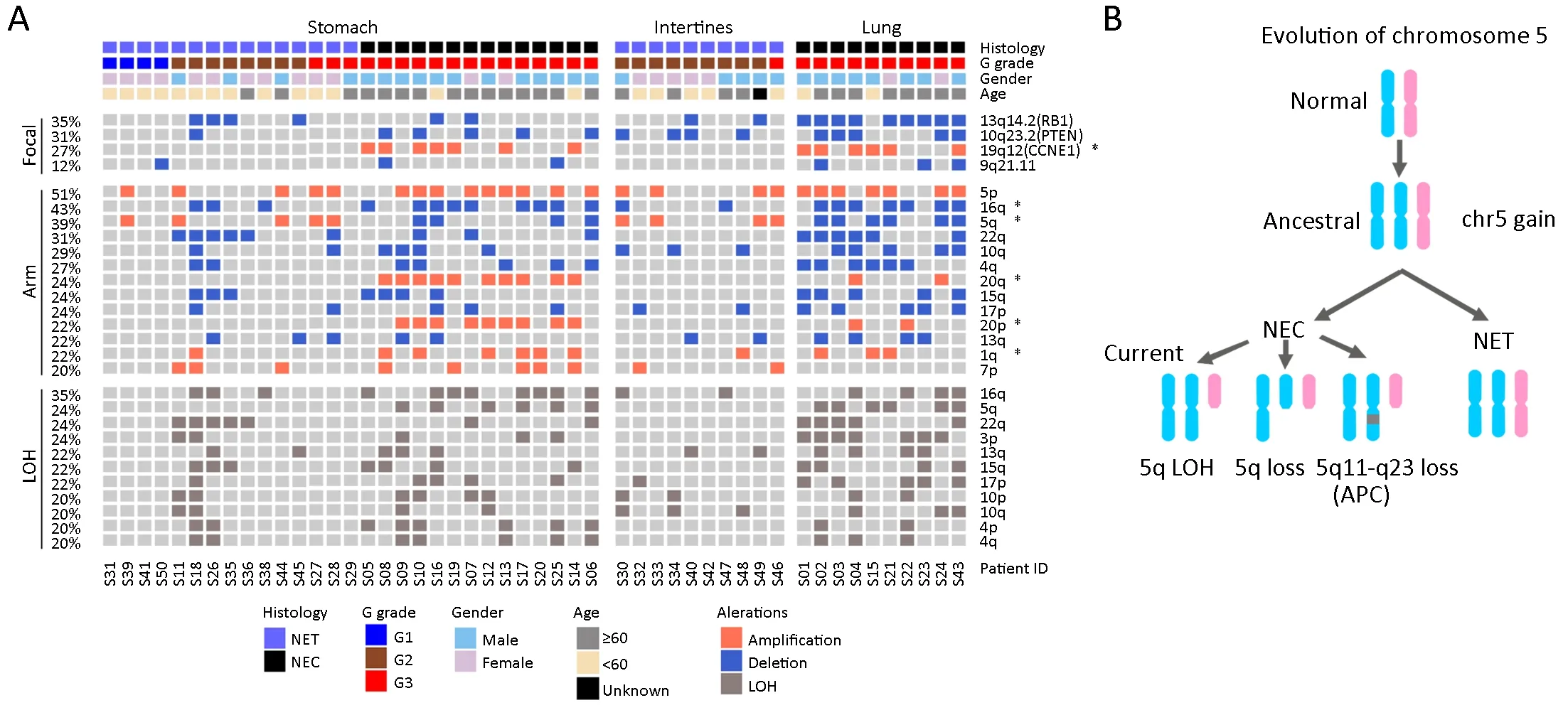
Figure 3 Recurrent arm-and focal-level somatic copy number alteration (SCNA) events in neuroendocrine neoplasms (NENs).(A)Distribution of recurrent arm-and focal-level SCNA events.Clinical characteristics were labeled for each patient,as indicated on the top.Top panel:significant focal-level (GISTIC2,Q<0.01) gains (orange) and losses (blue).Middle panel:significant arm-level (GISTIC2,Q<0.05) gains (orange) and losses (blue).Bottom panel:recurrent (≥20% of patients) arm-level losses of heterozygosity (LOH) (dark gray).*denotes significantly enriched events in neuroendocrine carcinomas (NECs) within the stomach cohort and the entire cohort;(B) A flowchart outlines the evolutionary pattern of chromosome 5 during NEN tumorigenesis.Two homologous chromosomes are represented as different colors,and the dark gray box indicates focal-level loss.
First,we examined the association between recurrent SCNAs and clinicopathologic characteristics in the stomach cohort.Amplifications in chromosome 19q12(CCNE1),20q,20p,and 1q as well as deletion in 16q were significantly associated with NECs,while amplification in 5q was found exclusively within NETs (P<0.05,Fisher’s exact test,Table 2,Figure 3A).These patterns were reaffirmed in the intestine and lung samples,as chromosome 19q12 (CCNE1),20q and 20p amplifications were exclusively found in lung NECs,while 5q amplification was observed only in intestine NETs.In addition,CCNE1belongs to the p53 signaling pathway and is frequently overexpressed in various cancers (39,40).In the entire cohort,23/49 (47%) patients had either aTP53mutation orCCNE1amplification,and only one patient had NET histology.Together,these results indicate that p53 signaling pathway dysfunction is a potential molecular characteristic of the NEC subtype of NENs.
Interestingly,in stomach samples,chromosome 5q amplification was exclusively associated with NETs (5/5),while 5q deletion was found only in NECs (4/4).Further examination showed that all 5q alterations cooccurred with 5p amplification.The same pattern was observed in the intestine and lung samples (Figure 3A).Furthermore,although some NEC samples showed 5p gain but not 5q loss,they all harbored a significant segment loss of 5q11-q23 (containing tumor suppressor genes such asAPCandMAP3K1,data not shown).Therefore,there is a strong correlation between 5p and 5q arm abnormalities.One possible evolutionary route that may have led to the observed chromosome 5 alteration pattern is that copy number gain of the entire chromosome occurred first during the early stage of NEN tumorigenesis,followed by the loss of 5q or segments of 5q containing tumor suppressor genes that induced the transition from NEN to NEC (Figure 3B).
CIN and patients’ clinical outcomes
In addition to individual SCNA events,the analysis of genome-wide chromosomal alterations and aneuploidy provides a holistic view of tumor genomic instability.To quantitatively assess the extent of whole-genome copy number abnormalities,we calculated the CIS for each tumor sample,which is defined as the average ASCN distance from all sequenced genes between paired tumor and normal control samples.A bimodal distribution of the CIS among all 49 patients was observed,suggesting the existence of two distinct clusters (Supplementary Figure S1A).In the stomach cohort,the K-means classifying algorithm produced two clusters,with 20 (69%) samples asthe chromosomal stable (CS) group and 9 (31%) samples as the CIN group (Supplementary Figure S1B).The cut-off CIS value was 1.1.The same cut-off value was applied to the overall cohort,which generated very similar proportions between CS and CIN (67%vs.33%).Overall,NECs consisted of both CS and CIN tumors (10vs.14),while all NETs were of the CS status (Figure 4A,B).The ASCN plots from samples representative of the CS and CIN groups are visualized at the genomic scale inSupplementary Figure S2A,B,respectively.Notably,tumor ploidy and tumor mutation burden (TMB) were also significantly higher in CIN tumors compared to CS tumors(P<0.01,Wilcoxon rank-sum test;Supplementary Figure S2C,D).Together,these results suggest that the development of NEC took distinct evolutionary trajectories,illustrated by the significantly more altered genome in NECs,with dysfunction in the p53 pathway,chromosome 5 amplification and 5q deletion (Figure 4C).
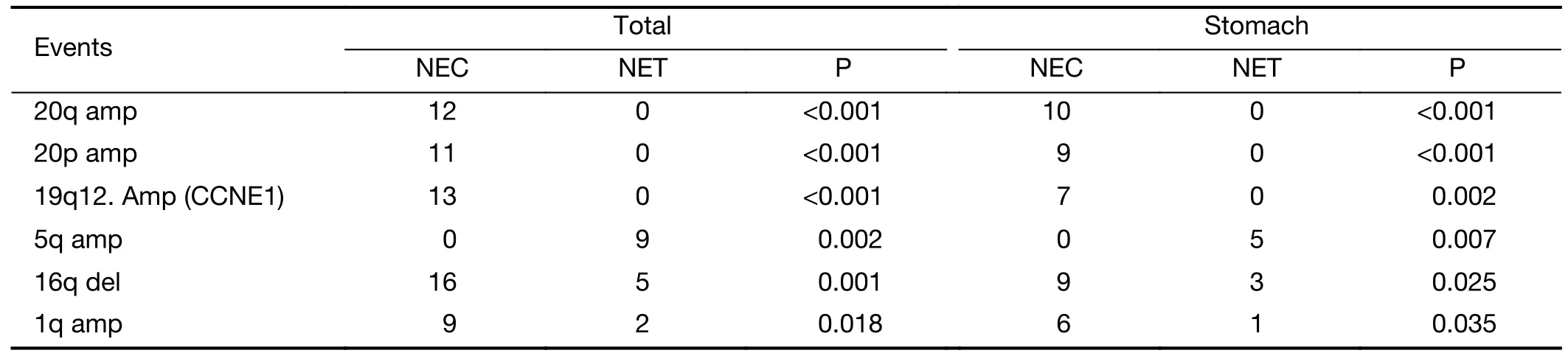
Table 2 SCNAs events significantly associated with NET or NEC
Next,we tested the potential association between genomic instability and patients’ clinical outcomes.To reduce the bias of newly diagnosed patients with a limited follow-up,only noncensored patients or patients with at least 24 months of follow-up (12 stomach,6 lung,and 5 intestine samples) were included in the analysis.Among the 12 stomach NEN patients,the CIN group showed significantly worse OS compared to the CS group(P=0.003,log-rank test,Figure 5A).In all tissue types of 23 patients,the same result was observed (P<0.001,logrank test,Figure 5B).Although the difference was not significant when examined only in the lung cohort(P=0.162,log-rank test),the median OS of the CIN group was only 8 months compared to that of the CS group (not reached).A larger sample size will likely produce a significant correlation.When patients were further stratified by both CIN and histology,CIN-NEC patients had significantly reduced OS compared to both CS-NET and CS-NEC patients (P=0.013 in the stomach only;P<0.001 in all tissue types of 23 patients,log-rank test,Figure 5C,D).Furthermore,when the cohort was stratified by gender,age,grade,or stage,CIN tumors were associated with reduced OS in all groups (P<0.05,log-rank test,Supplementary Figure S3).After controlling for histology,gender,and age,CIN was the only factor associated with OS in all 23 patients (Supplementary Table S5).
Discussion
To date,all categorization methods of NENs are based on proliferative marker measurements or a histological evaluation by a pathologist.However,it is increasingly evident that neither method can fully distinguish clinically favorable tumors from aggressive carcinomas.Therefore,the genomic profiling of NENs can supplement the current categorization system to better predict survival and guide treatment decisions.Most previous genomic studies of NENs have focused on the relatively more frequent PanNETs or SI-NETs and have often profiled a small set of genes (11).Data on clinically unfavorable NECs and NENs originating from other organs (NP-NENs) are limited.Thus,there is an urgent need to uncover new molecular drivers and subtypes within NP-NENs that correlate with the patient’s clinicopathologic characteristics or survival.
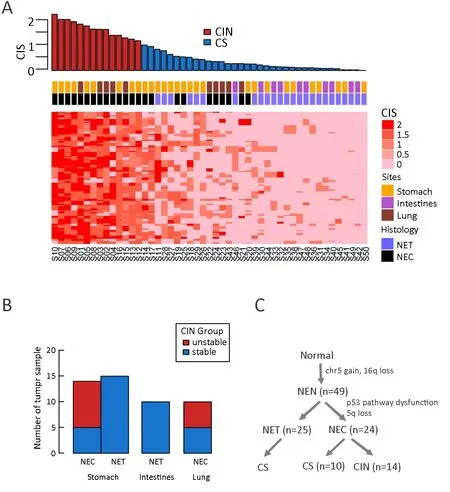
Figure 4 Chromosomal instability subtypes in nonpancreatic neuroendocrine neoplasms (NP-NENs).(A) Genome-wide chromosomal instability score (CIS) based on all protein-coding genes.The top panel shows the distribution of the CIS among 49 patients in descending order.Patients were classified as chromosomal stable (CS,blue) or chromosomal instability (CIN,red) using k-means in the R package.The CIS matrix was constructed by presenting the CIS of each individual gene;(B) Distribution of the CS and CIN subtypes across three clinical characteristics (histology,tissue site and G grade);(C) A flowchart outlines how NEN tumors were classified into different molecular subtypes.NET,neuroendocrine tumor;NEC,neuroendocrine carcinoma.
In this study,we performed WES profiling of 49 NPNENs from three different organ types for genomic profiling.NP-NENs and Pan-NENs had similar mutational signature patterns,which are closely associated with aging and dysfunction in the DNA double-strand break-repair mechanism.However,NP-NENs exhibited a distinct mutational landscape from that of PanNETs.The most common PanNEN-associatedMEN1mutation (28)was found at a surprisingly low frequency in NP-NENs.On the other hand,the mTOR pathway was frequently altered in PanNETs (12).Here,we also observed a considerable degree of aberration in genes of this pathway in NP-NENs.RB1 loss of function is commonly reported in small cell NENs (30),and we observed both mutation and copy number loss in RB1 in patients with small cell NEC of the lung.Additionally,we discovered that genes not previously linked to NENs occurred at considerable frequencies in our cohort,such asFTH1,POTEC,andNOS2.Finally,consistent with previous reports,TP53was the most frequently mutated gene and was associated with poorly differentiated NECs (11,30,41).These results suggest that NP-NENs and PanNENs have different mechanisms of tumorigenesis.
Compared to the low mutation burden in NP-NENs,our study revealed extensive focal-and arm-level SCNAs in NENs,especially in NECs.The gain of chromosome 5 was previously identified as a common feature in gastrointestinal NETs (42),and the loss of 5q was reported in lung NECs (43).Here,we observed chromosome 5 gain in both NETs and NECs,but only NECs underwent subsequent 5q deletion.This is potentially an important evolutionary mechanism of NEN tumorigenesis and a prerequisite of transformation from a clinically favorable NET subtype to a malignant NEC subtype (Figure 4C).Another distinct feature of NECs is chromosome 20 gain,which was also found to be associated with reduced survival in other studies (36).
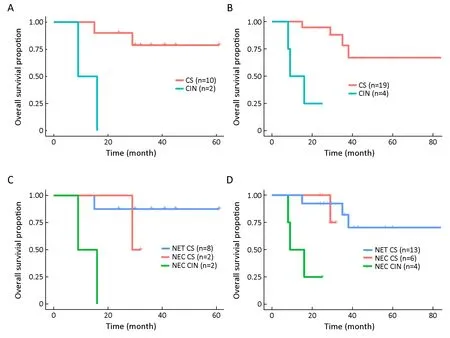
Figure 5 Distinction of overall survival (OS) between chromosomal instability (CIN) and chromosomal stable (CS) patients.Patients were included in the analysis if the follow-up time was longer than 24 months or if death occurred.Kaplan-Meier plot of OS between CIN and CS patients in the stomach neuroendocrine neoplasm (NEN) cohort (P=0.003) (A) and in all tissue types (P<0.001) (B);Kaplan-Meier plot when patients were stratified by both the chromosomal instability score (CIS) and histology type in the stomach only (P=0.013) (C) and in all tissue types (P<0.001) (D).NET,neuroendocrine tumor;NEC,neuroendocrine carcinoma.
CIS subtypes have been well characterized in colorectal and gastric cancers;however,they have not been reported in NENs.Our study investigated genome-wide chromosomal alterations in NENs and found that a proportion of NECs displayed extensive alterations in chromosome copy numbers.Specifically,NECs were further stratified into a CS group,which had comparable survival outcomes with NETs,and a CIN group,which had significantly reduced survival.However,our conclusion could be limited by the small sample size of patients with long-term survival data,which was further reduced in the stratified cohort analysis.Nonetheless,the diminished survival in the CIN group was observed across different clinical strata,suggesting its prognostic value.Future studies with large sample sizes are needed to fully validate the utility of CIS in NEN classification in conjunction with the Ki67 index and histology.
Conclusions
Our findings provide insights into the molecular and evolutionary characteristics of NENs and highlight the importance of differentiation between CIS subtypes and their associations with survival outcomes.We demonstrated that despite originating from different organs,NECs share remarkably similar genetic signatures.Future studies involving larger cohorts and diverse organ types are warranted to estimate the distribution of CIS in NENs and validate its prognostic value.
Acknowledgements
None.
Footnote
Conflicts of Interest:Hua Bao,Xue Wu,Ao Wang,Xiaoling Tong and Yang W.Shao are employees of Geneseeq Technology,Inc.Xiaonan Wang is an employee of Nanjing Geneseeq Technology,Inc.The authors have no conflicts of interest to declare.
 Chinese Journal of Cancer Research2019年6期
Chinese Journal of Cancer Research2019年6期
- Chinese Journal of Cancer Research的其它文章
- Prediction of pathological nodal stage of locally advanced rectal cancer by collective features of multiple lymph nodes in magnetic resonance images before and after neoadjuvant chemoradiotherapy
- Economic evaluation of cervical cancer screening strategies in urban China
- An elevated preoperative serum calcium level is a significant predictor for positive peritoneal cytology in endometrial carcinoma
- Relationship between inter-α-trypsin inhibitor heavy chain 4 and ovarian cancer
- Diagnostic value of negative enrichment and immune fluorescence in situ hybridization for intraperitoneal free cancer cells of gastric cancer
- Stomatin plays a suppressor role in non-small cell lung cancer metastasis
