Effect of L-carnitine supplementation during in vitro maturation and in vitro culture on oocyte quality and embryonic development rate of bovines
Diego F. Carrillo-González, Nélida Rodríguez-Osorio, Charles R. Long, Neil A. Vásquez-Araque,Juan G. Maldonado-Estrada
1One Health and Veterinary Innovative Research and Development Group, School of Veterinary Medicine, University of Antioquia, 050034 Medellin, Colombia
2Faculty of Agricultural Sciences, School of Zootechnics, Universidad de Sucre, Colombia
3Genomics and Bioinformatics Lab, Department of Biological Sciences, University of the Republic of Uruguay, Salto Campus, Uruguay
4Department of Veterinary Physiology and Pharmacology, College of Veterinary Medicine, Texas A&M University, College Station, Texas 77843-4466, USA
5Grupo de investigación Biotecnología Animal, Facultad de Ciencias, Universidad Nacional de Colombia, Sede Medellín, Medellín, Colombia
Keywords:Antioxidants Culture supplement Embryo development Lipid droplets Maturation Mitochondria
ABSTRACT Objective: To assess the effect of L-carnitine supplementation during in vitro oocyte maturation and in vitro culture process of bovine oocytes.Methods: L-carnitine (3.8 mM) was added to maturation medium and the effect was assessed in the quality (Experiment 1) and in the cleavage and 4-cells stage (Experiment 2). Besides,the effect of L-carnitine addition on maturation medium (3.8 mM) and culture medium(1.5 mM) on embryo rate production was assessed. In Experiment 1, bovine oocytes from abattoir were randomly separated into two groups (the control group and L-carnitine group)for in vitro maturation. Matured oocytes were examined for cumulus cells expansion as an indicator of maturation, and the content of the mitochondrial activity, the presence of lipid droplets, the reduced glutathione, and the reactive oxygen species were measured by using specific fluorochromes. In Experiment 2, oocytes were matured as performed in Experiment 1, afterward fertilized and cultured until day 3, and cleavage rate and 4-cells stage rate were determinated. In Experiment 3, in vitro maturation and fertilization were done as performed in Experiment 2, but at day 3 of culture, each group of embryos was separated into two new groups, and L-carnitine (1.5 mM) was added in culture media until day 8. The cleavage and embryo development rate were determined on the basis with the oocytes put on maturation.Hatching rate was calculated from cleaved embryos.Results: The cumulus expansion rate at grade Ⅲ and mitochondrial activity were significantly higher in the L-carnitine group in comparison with the control group (P<0.05).However, the content from the other variables tested did not significantly differ from the control group. Furthermore, the cleavage rate and 4-cells stage rate were similar in both groups (P>0.05). In addition, cleavage and the proportion of embryo development and hatching rate were similar for all groups (P>0.05).Conclusions: L-carnitine as a supplement in culture media improves the cumulus expansion and increases the mitochondrial activity during in vitro maturation process but has no apparent effect on the cleavage and development of bovine embryos. Further investigations of L-carnitine addition on in vitro culture are needed to test their effect on embryo quality.
1. Introduction
The use of biotechnologies applied to animal reproduction,including in vitro embryo production, is considered a highly relevant technique to accelerate genetic improvement in livestock, making a significant contribution to the development of competitiveness. In addition, it is a valuable tool for research in reproductive biology,and it has been used in combination with different biotechnologies such as genetic engineering, semen sexing, and animal cloning[1].However, in vitro embryo production systems present some difficulties, such as low percentages in obtaining blastocysts and low pregnancy rates, particularly for cryopreserved embryos[2].Therefore, in order to improve the efficiency of in vitro embryo production, it is necessary to increase quality blastocyst production.Modifications to traditional in vitro embryo production protocols,such as supplementation with metabolic modulators in the culture media and also in different stages of the in vitro embryo production process in vitro maturation and in vitro early embryonic development,are necessary to achieve these goals.
Mammalian oocytes of many species lipids stored as drops in the ooplasm[3]. It has been proposed that based on the consumption of oxygen, the number of lipids present in the oocyte could be able to satisfy the metabolic needs during the oocyte maturation process as the only source of energy. During cytoplasmic maturation in vivo,the oocyte can accumulate endogenous lipids; however, in vitro,during the end of oocyte maturation, a greater predisposition to the changes in lipid content in the oocyte cytoplasm may occur[3]. In the maturation media, the principal source of fatty acids is fetal bovine serum (FBS) and bovine serum albumin (BSA), which also has bound lipids. Similarly, in bovine embryos, when the early embryo development occurs in a serum-free culture media, the triglyceride account is relatively steady from two-cell phase to the blastocyst stage. Therefore, bovine oocytes can retain the non-esterified fatty acids before and during meiotic maturation. Additionally,the modulation of fatty acids content during IVM media alters the tolerance of the embryos to cryopreservation[3].
Lipid droplets are complex and dynamic intracellular storage organelles of neutral lipids (diacylglycerol, triacylglycerol,retinol esters, cholesterol esters, and free cholesterol)[4], which constitute the critical energy reserve for oocytes and embryos.Generation of adenosine triphosphate from lipids is carried out within the mitochondria through beta-oxidation of fatty acids.The mitochondrial matrix is responsible for long-chain fatty acids metabolism, and in the same way, L-carnitine acts as an essential cofactor in charge to transport of fatty acid residues into the mitochondrial matrix[3].
The process of fatty acids transport to the mitochondria matrix consists of two steps: 1) Cytosolic fatty acids in the form of fatty acyl-coenzyme A are transesterified into L-carnitine by the enzyme carnitine palmitoyl transferase 1[5]; 2) Fatty acyl carnitine passes through the outer mitochondrial membrane and is transported to the matrix by carnitine-acylcarnitine translocase[5]. Carnitine palmitoyl transferaseⅡtransesterifies fatty acids to mitochondrial coenzyme A, releasing L-carnitine to be transported by carnitine-acylcarnitine translocase through the mitochondrial membranes[5]. These reactions ensure the transport of fatty acids to the mitochondria byβ-oxidation and control the intracellular balance between free coenzyme A and acyl-coenzyme A[5]. It has been proposed that the inhibition of betaoxidation during oocyte maturation can affect the consequent early embryo development[6].
L-carnitine is synthesized from lysine and methionine[5]. However,its synthesis has not been confirmed in the early embryo[3]. In addition to its metabolic function, L-carnitine has been considered as a potent antioxidant that reduces the accumulation of reactive oxygen species (ROS) and decreases the frequency of apoptosis in animal cells[7]. Due to the capacity of L-carnitine to reduce the cellular lipid content and also to have antioxidant protection, it becomes a candidate molecule to improve the developmental competence and enhancement of cryotolerance in farm animal embryos. In the same way, L-carnitine has been used as a supplement in the in vitro maturation process in species such as mice and pig, found results as the mitochondrial activity increasing and reduction of lipid droplets content in the oocyte. Also, cryotolerance has been assessed on bovine embryos in vitro produced when L-carnitine has been used during in vitro maturation process, showing beneficial effects over the hatching rate after warming.
In this research, we evaluated the ability of L-carnitine to mobilize and reduce the relative abundance of lipid droplets, as well as to stimulate mitochondrial activity, enhance granulosa cell expansion and embryo production rate in bovine oocytes subjected to in vitro maturation and early embryonic development.
2. Materials and methods
2.1. Oocyte collection
Bovine ovaries were obtained post-mortem from cycling cows (as evidenced by the presence of corpus luteum) at Central Ganadera of Medellín slaughterhouse. Recovered ovaries were placed in bags with sterile saline phosphate buffer solution (PBS) at 34 ℃and were transported (approximately within 30 min) to Genetics laboratory in the National University of Colombia for processing.In the laboratory, under sterile conditions, the ovaries were washed three times with 1 × PBS at 37 ℃ to remove contaminating material,blood, and tissue debris. Then with a 5 mL syringe using 18G needles, follicles with a diameter of 3 to 6 mm were aspirated.Follicular fluid was collected in 15 mL conical tubes placed in a water bath at 37 ℃. Subsequently, follicular aspirates were filtered using a 70 μM pore diameter filter and oocytes were placed in a 60 mm×× 15 mm sterile Petri dish with 3 mL of saline solution 0.9%.Good quality oocyte cluster (COCs) were selected according to previously established criteria[8,9] under high-field stereomicroscope vision. Selected COCs from each replicate were washed three times in TCM 199 medium supplemented with Hank’s salts before their transfer to maturation medium.
2.2. Oocyte maturation
In vitro maturation media was composed of TCM199 (12340030,Gibco Earls salts), containing 25 mM L-glutamine, 6 mg/mL fractionⅤalbumin (BSA), 3% FBS, 25 μg/mL follicle-stimulating hormone(Folltropin), 5 IU/mL luteinizing hormone (Chorulon), 1 μg/mL17B estradiol, 0.30 mM sodium pyruvate and 100 μM ascorbic acid.Media was served in seven drops of 50 μL and covered with mineral oil in a 35 mm culture dish for each treatment. Two maturation groups were designed; the first group without L-carnitine addition(the control group) and the second group with supplementation of 3.8 mM L-carnitine (Sigma-Aldrich C0158, Molecular Probes) as reported by Phongnimitr et al[10]. COCs were allocated randomly in pools of 10 COCs in each drop of 50 μL of 35 mm culture dish and conditions for in vitro maturation were 38.5 ℃, 5% CO2in the air with 90% relative humidity for 24 h.
2.3. Effect of L-carnitine addition on oocyte quality in maturation medium
To evaluate the effect of L-carnitine addition on the oocyte quality in the maturation media, cumulus expansion of maturated oocytes were observed under a stereomicroscope (Nikon SMZ 450, Nikon Instruments Inc. Melville, NY, USA; 40× magnification). The modulation of fatty acids, metabolism increase and ROS reduction were assessed trough fluorescence staining methods under an epifluorescence microscope with 200××magnification (Nikon Eclipse 80i).
2.3.1. Assessment of granulosa cell expansion
After 24 h in culture, oocytes in the control and L-carnitine groups were observed under stereoscopic vision[11,12]. A total of 436 and 408 oocytes were evaluated for L-carnitine group and control group,respectively with 13 experimental replicates (average: 30 oocytes/replicate). Depending on the expansion grade, COCs were classified as gradeⅠ, slight to no expansion; gradeⅡ, modest expansion of the outer layers of the cumulus; and gradeⅢ, full expansion[11,12].The best maturation rate of oocytes was expressed as a proportion of oocytes at gradeⅢin relation to the total number of maturated oocytes.
2.3.2. Lipid droplets assessment
A total of 140 maturated oocytes in the control group and 109 maturated oocytes in L-carnitine group were assessed to determine lipid droplets content in three replicates. The oocytes of each group were stained with Nile red. First, oocytes were denuded by pipetting and incubated at 4 ℃ for 24 h in a 1.5 mL conical tube containing 250 μL of fixation medium (Dulbecco’s PBS, 16% formaldehyde and 50% glutaraldehyde). After, oocytes were washed twice in Dulbecco’s PBS and passed to a 1.5 mL tube with 250 μL of Nile red solution (10 μg/mL) at room temperature for 24 h[13]. Subsequently,the excess dye was removed by washing twice with Dulbecco’s PBS, and oocytes were placed on a slide in a 10 μL Dulbecco’s PBS drop[14]. G-2A filter at an excitation wavelength of 510-560 nm and 580 nm emission were used with a 20× objective. The images were captured with the same exposure setting and time using a Moticam camera. The oocyte area was adjusted, and images were recorded in TIF format. Pictures were transformed into 8 bits, and the fluorescence intensity of the lipid droplets in oocytes was analyzed using ImageJ software (Version 1.41; National Institutes of Health,Bethesda, MD, USA). The mean fluorescence intensity of oocytes in the control group was used as a normalizer for the fluorescence intensity measurement of the oocyte in the L-carnitine group[15].
2.3.3. Mitochondrial activity assessment
A total of 56 and 68 oocytes in the control group and L-carnitine group, respectively were selected to evaluate the mitochondrial activity in three replicates. All matured oocytes were denuded by pipetting and incubated at room temperature for 25 min in Dulbecco’s PBS with 125 nM Mitotraker according to the manufacturer’s recommendations[16]. After this time, the excess dye was removed by washing twice with Dulbecco’s PBS. Oocytes were passed in groups of 3 structures to 10 μL Dulbecco’s PBS drops, on a slide[14]. B-2E/C filter at an excitation length and emission of 480 nm and 520 nm, respectively was used with a 20××objective.The images were captured with the same exposure setting and time using a Moticam camera. The oocyte area was adjusted, and images were recorded in TIF format. Pictures were transformed into 8 bits,and the fluorescence intensity was assessed as previously described for lipid droplets on mitochondria, which was the same as for the lipid droplets assessment[15].
2.3.4. Determination of reduced glutathione (GSH)
After in vitro maturation, a total of 98 oocytes in the control group and 107 oocytes in L-carnitine group were selected to determine the level of reduced glutathione in three replicates (Lγ-glutamyl-L-cysteinyl-glycine, GSH). Matured oocytes were stained with monochlorobimane (Invitrogen M1381MP, Molecular Probes). Staining process was conducted in clusters of 10 matured and denuded oocytes. They were incubated for 20 min at room temperature in 50 μL drops of the dye (250 μM); after this time, the excess dye was removed by washing twice with Dulbecco’s PBS,and they were place in small drops (10 μL) of PBS on slides[14]. To facilitate visualization, each cluster was separated into three small drops (three oocytes per drop). Filter C-FL UV-2E/C was used at an emission and excitation length of 490 nm and 394 nm, respectively.The images were captured with the same exposure setting and time using a Moticam camera. The oocyte area was adjusted, and images were recorded in TIF format. Pictures were transformed into 8 bits,and the GSH fluorescence intensity was analyzed using ImageJ software as previously described for lipid droplets[14].
2.3.5. Quantification of ROS
To determine the amount of ROS, 50 oocytes in the control group and 59 oocytes in L-carnitine group were matured and stained with dihydrofluorescein diacetate (Sigma-Aldrich D6883, Molecular Probes). All groups of matured oocytes were placed in groups of 10 oocytes and were incubated in 50 μL drops of dihydrofluorescein diacetate (10 μM) for 20 min at room temperature, then oocytes were washed in PBS and passed in groups of 3 oocytes, in a 10 μL drop of PBS, put onto a slide[14]. FITC/ FLUO-3 filter was used (B-4A)with an emission length at 514 nm and excitation at 490 nm. The images were captured with the same exposure setting and time using a Moticam camera. The oocyte area was adjusted, and images were recorded in TIF format. Pictures were transformed into 8 bits, and the ROS fluorescence intensity in oocytes was analyzed by using ImageJ software as previously described for lipid droplets[15].
2.4. Effect of L-carnitine addition on cleavage and 4-cells stage of bovine embryos in the in vitro maturation medium
To evaluate the effect of the addition of L-carnitine on the cleavage rate and 4-cells stage rate of bovine embryos in the in vitro maturation medium, COCs were previously maturated and randomly divided into two groups, the first group without L-carnitine addition(the control group) and the second group with supplementation 3.8 mM L-carnitine (Sigma-Aldrich C0158, Molecular Probes)as reported by Phongnimitr et al[10], and as was described above.Afterward, matured oocytes were in vitro fertilized and cultured for 48 h.
2.4.1. In vitro fertilization
A total of 513 matured oocytes in the control group and 652 matured oocytes in L-carnitine group were fertilized using the same frozen-thawed bovine sperm, which were previously evaluated and used with satisfactory result in our laboratory. Oocytes were transferred to 50 μL fertilization- Tyrode’s albumin lactate pyruvate media (IVL02, Caisson Laboratories, 836 South 100 East Smithfield,UT 84335) supplemented with 10 mg/mL heparin (Sigma H0519),1 mM hypotaurine (Sigma H1384), 250 mM epinephrine (Sigma E4642), 2 mM penicillamine (Sigma P4875), 1 × antibiotic solution(ICN 1670049 MP Biomedicals). Semen was thawed at 36 ℃ for 1 min,and motile spermatozoa were separated using Percoll gradients(Allgrad? 90% y 45% LifeGlobal group, 393 Soundview Rd,Guilford, CT 06437) by centrifugation at 780×g for 5 min at room temperature. The pellet was diluted with 300 μL of Fertilization-Talp media, and the final concentration was calculated (2×106sperm/mL)and added in each drop containing COCs for 18 to 20 h at 38.5 ℃ in a humidified atmosphere of 5% CO2in the air[17,18].
2.4.2. In vitro culture and cleavage and 4-cells stage assessment
At 18 h post-insemination, 513 presumptive zygotes in the control group and 652 presumptive zygotes in L-carnitine group were stripped and in vitro cultured in drops of 70 μL of KSOMaa (IVL04,Caisson Laboratories, 836 South 100 East Smithfield, UT 84335)supplemented with 6 mg/mL BSA-fatty acid free, 1× antibiotic solution, and 3% FBS, at 38.5 ℃, 5 % CO2and 90 % relative humidity. Forty-eight hours after initiation of the culture (day 2), the cleavage oocytes and oocytes of 4-cells stage were recorded under a stereomicroscope in eight replicates, and the rates were calculated as number of cleavage oocytes/ total number of insemination oocytes,number of 4-cells stage oocytes/ total number of insemination oocytes ×100, respectively.
2.5. Effect of L-carnitine supplementation on embryo rate production during in vitro maturation and in vitro culture
To evaluate the effect of the addition of L-carnitine in two stages of in vitro embryo production, 461 COCs were allocated to the control group (without L-carnitine addition) and 518 COCs were allocated to treatment group with supplementation 3.8 mM L-carnitine(Sigma-Aldrich C0158, Molecular Probes)[10] for in vitro maturation.Afterward, matured oocytes were in vitro fertilized and cultured until day 8. Besides, on day 3 of culture, embryos from the control group were separated into two new groups (group 1 and group 4) and embryos from treatment group were separated in group 2 and group 3. The culture media for groups 3 and 4 were supplemented with 1.5 mM L-carnitine as reported by Ghanem et al[16] (Sigma-Aldrich C0158, Molecular Probes) from day 3 to day 8[7,16]. Cleavage rate,embryo production rate, and hatching rate were recorded under a stereomicroscope (40× magnification).
2.5.1. In vitro fertilization
All COCs were fertilized in seven replicates using the same frozenthawed bovine sperm, which were previously evaluated and used with satisfactory result in our laboratory. Matured oocytes were fertilized using a final sperm concentration of 2×106sperm/mL in each drop and co-cultured for 18 to 20 h at 38.5 ℃ in a humidified atmosphere of 5% CO2in the air[17,18].
2.5.2. In vitro culture and embryo development assessment
After insemination, all presumptive zygotes were stripped from cumulus cells and in vitro cultured in drops of 70 μL of KSOMaa(as described in Experiment 2) at 38.5 ℃, 5 % CO2and 90 %relative humidity. Forty-eight hours after initiation of the culture(day 2), the rate of cleavage rate and 4-cells stage were determined under a stereomicroscope as mentioned above. Then embryos were separated into four groups and cultured until day 8 as previously reported[18]. These four groups were as follows: group 1 without L-carnitine; group 2 with L-carnitine during maturation (3.8 mM L-carnitine during in vitro maturation); group 3 with L-carnitine during maturation and culture (3.8 mM L-carnitine during in vitro maturation and 1.5 mM L-carnitine during in vitro culture) and group 4 with L-carnitine during culture (1.5 mM L-carnitine during in vitro culture)[7,16]. A total of 224, 246, 272 and 237 zygotes were allocated into groups 1, 2, 3 and 4, respectively. To determinate the in vitro production rate and the development kinetics, data were collected on day 7 and 8 of culture and rates were determined based on total inseminated oocytes or total embryos cleaved[19]. Embryos that were partially or fully extruded from the zona pellucida were considered as hatching, and the hatching rate was calculated as follows: hatching embryos/cleaved embryos××100[19].
2.6. Ethics
This study was approved by the Ethics Committee for Animal Experimentation from the University of Antioquia (certificate No.115 on February 6, 2018).
2.7. Statistical analysis
All data were analyzed by one-way analysis of variance, and the differences between the treatment groups were compared using the Tukey test. The data without a normal distribution were analyzed using the Mann-Whitney U Test. Statistica, version 10.0 (StatSoft Inc. Tulsa OK, USA) was used, and the differences were statistically significant at P-value < 0.05.
3. Results
3.1. Effect of L-carnitine on oocyte maturation and cell expansion of granulosa
Percentage of oocytes at cumulus expansion grade Ⅲ after 24 h of maturation and mitochondrial activity in L-carnitine group were significantly higher (P<0.05) than that in the control group (Table 1). However, there was no significant difference in lipid droplets content, GSH activity or ROS production (all P>0.05) between oocytes treated with L-carnitine and the control group (Table 1). The results of fluorescent microscopy also showed similar fluorescence intensities between the two groups (Figure 1).
3.2. Effect of L-carnitine addition during in vitro maturation on cleavage and 4-cells stage
On day 3, there was no significant difference in the cleavage rate or 4-cells stage rate between the control group [(69.59 ± 16.20)%and (73.88 ± 14.30)%, respectively] and L-carnitine group[(63.96 ±11.76)% and (63.68 ± 17.30)%, respectively] (P>0.05).
3.3. Embryo development assessment
When evaluating the effect of L-carnitine (1.5 mM) addition on the culture medium over the embryo development rate, we found that all groups had a similar in vitro embryo production rate (P>0.05).Likewise, no significant differences (P>0.05) in the hatching rate on day 8 were found for all four groups of culture (Table 2).
4. Discussion
To produce high-quality bovine embryos in vitro capable of implanting and continuing development to term, it is necessary to improve several in vitro conditions including temperature, gases concentration, and media components. Despite great advances, in vitro produced embryos are still of lower quality than them in vivo produced counterparts.
The collection of COCs from the heterogeneous ovary population of slaughterhouse cows has resulted in a wide variation in bovine in vitro embryo production rate and quality[20]. It has been wellestablished that oocyte quality is the key determinant of blastocyst rate, while culture conditions can additionally affect embryo quality[18,21,22]. In our study, we found that gradeⅢcumulus expansion after in vitro maturation was significantly increased with 3.8 mM L-carnitine supplementation of in vitro maturation media.In bovine oocytes, the cumulus expansion occurs in response to different events such as gonadotropins variation, steroids,factors secreted by the oocyte, growth factors, and other unknownmolecules[23]. In the same way, the cumulus expansion is induced by the preovulatory cascade initiated after luteinizing hormone surge,which has been associated with a massive production of mucoid extracellular matrix proteins by cumulus cells, creating the expanded cumulus cells[24]. Additionally, Aaderma et al[25] found that a good cumulus expansion is a good predictor of blastocysts production[25].

Table 1. Effect of L-carnitine on in vitro maturation of bovine oocytes.
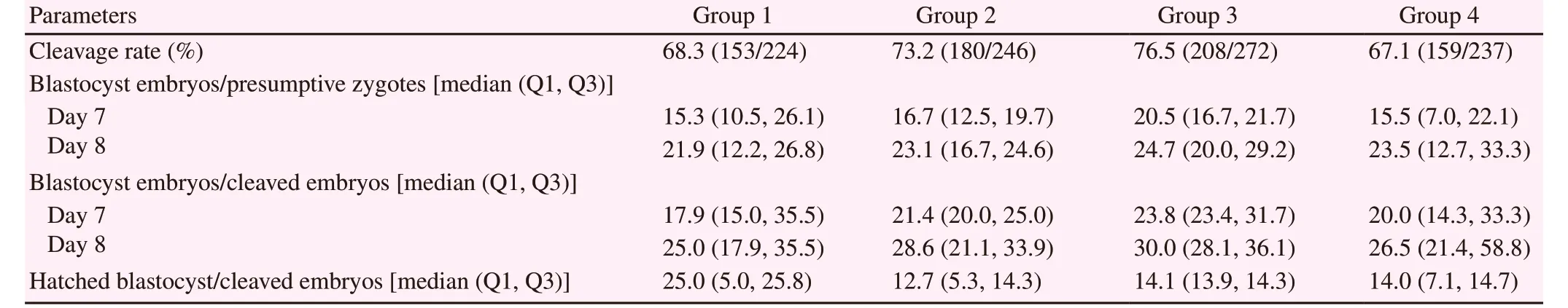
Table 2. Effect of L-carnitine during on embryo production and hatching.

Figure 1. Stained oocytes after maturation with and without 3.8 mM L-carnitine. Lipid content is stained with Nile red and oocytes are examined by fluorescent microscopy (20×) with a G-2A filter at an excitation length of 510-560 nm and emission at 580 nm. (A) Oocyte matured in the control medium; (B) oocyte matured in medium supplemented with 3.8 mM L-carnitine. Mitochondrial activity is stained with Mitotracker green fluorescent dye and oocytes are examined by fluorescent microscopy (20×) with a B-2E/C filter at an excitation length at 480 nm and emission at 520 nm;(C) Oocyte matured in the control medium; (D) oocyte matured in medium supplemented with 3.8 mM L-carnitine. The amount of glutathione in the matured oocytes were stained with mBCI and oocytes are examined by fluorescent microscopy (20×) with C-FL UV-2E/C filter at an emission length at 490 nm and excitation at 394 nm; (E) Oocyte matured in the control medium; (F) oocyte matured in medium supplemented with 3.8 mM L-carnitine. The amount of reactive oxygen species, the oocytes are stained with FDA and oocytes were examined by fluorescent microscopy (20×) with FITC/FLUO-3 filter (B-4A) with an emission length at 514 nm and excitation at 490 nm; (G) Oocyte matured in control medium; (H) oocyte matured in medium supplemented with 3.8 mM L-carnitine stained with FDA to test the amount of reactive oxygen species.
Some researches reported that L-carnitine supplementation affects the redistribution of intracellular lipid droplets in bovine oocytes[26]and a significant increase in oocyte mitochondrial activity in bovine oocytes[27,28]. We did not find a significant difference in the lipid droplet content, this result is similar wit the work reported by Chankitisakul et al[26], who found no significant reduction in lipid droplet density. However, we found a significant difference in the mitochondrial activity (P<0.05), where the maturated oocytes in presence of L-carnitine had an increase in the fluorescence in comparison with the control group, associated with an increase in the mitochondrial activity.
L-carnitine works as an essential co-factor necessary for the mobilization of long-chain fatty acids into the mitochondrion[28]. It appears that its addition to the culture medium significantly increases β-oxidation in mouse COCs maturing in vitro and in follicles grown in vitro[6,28,29]. In addition, an increment in theβ-oxidation level has been associated with an improvement in the oocyte quality, demonstrated by the ability to continue in development until the blastocyst stage after fertilization[6,28,29] and higher number cells in the inner cell mass[6]. In the present study we found a significant effect of L-carnitine on mitochondrial activity of bovine oocytes. In the other hand, the addition of 3.8 mM L-carnitine did not significantly affect GSH activity nor ROS level in bovine oocytes. Contradictory results are reported by Mishra et al[30], who found that 10 mM L-carnitine significantly increased GSH level and decreased ROS level. This controversial results could be partially explained because, in the present study, it was used 3.8 mM instead of 10 mM used by Mishra et al[30]. However, the Mishra’s research was conducted in sheep oocytes, in contrast to ours that was made with bovine oocytes. The above could be associated with a metabolism difference between species in order to number and distribution of mitochondria[31], mitochondrial activity, and presence of ROS, evidenced in a different outcome when the L-carnitine was used as a supplement during in the in vitro embryo production process.
The lack of effect of L-carnitine on cleavage rate and the 4-cells embryo stage is in agreement with previous reports by Phongnimitr et al[10], Takahashi et al[7], Ghanem[18], and Knitlova et al[32], despite slight differences in concentrations used and development stage evaluated by these authors. Controversial results are reported on the effect of L-carnitine on cleavage rates of porcine oocytes[33] or development of porcine embryos[34]. Taken together, this evidence suggests that L-carnitine did not affect quality parameters nor developmental efficiency of bovine oocytes cultured and fertilized in vitro. However, no effect of L-carnitine was observed in the early embryo development rates during in vitro embryo culture when the L-carnitine was used at 1.5 mM in the in vitro culture media. These findings are different from previous reports where L-carnitine was used at the same concentration during in vitro culture and showed that the embryo production rate increasedsignificantly in comparison with their respective control group[16,18]. Nonetheless, most authors just use the L-carnitine during in vitro maturation process, but they do not add L-carnitine again during in vitro culture[7,10,18,32]. When we assessed the embryo production rate, the control group and in vitro culture group did not show a statistically significant difference;however, Phongnimitr et al[10] found that 0.6 mg/mL of L-carnitine improved the blastocyst production rate from 23.4% (the control group) to 29.7% (L-carnitine group). In the same way, Knitlova et al[32] found that L-carnitine (2.5 mM) improved the embryo production rate from meiotically less competent oocytes (33.3%)in comparison with the control group (25.83%); however, they did not find a difference when L-carnitine was used in meiotically more competent oocytes (45.4% vs. 39.7%, respectively). Although studies in mice showed that supplementation with L-carnitine improved developmental competence of mouse oocytes in terms of hatching rates in blastocysts by enhancingβ-oxidation of fatty acids[6,18,32]and reduction of lipid content[35], these effects were not observed in our study. Discrepancies between results using either bovine or mouse oocytes might be attributed to: 1) The source of L-carnitine,because some authors have used L-carnitine hydrochloride[32] and other L-carnitine inner salts[26]; 2) Regarding the differences in quality of oocytes, Knitlova et al[32] found that 2.5 mM of L-carnitine improved the embryo production rate in those oocytes from small follicles (<5 mm), considered meiotically less competent[32].However, their counterparts oocytes derived from medium follicles(6-10 mm) and classified as meiotically more competent, did not show a difference[32]. In our study, the inclusion criteria to follicle diameter were from 3 to 6 mm, similar to the Knitlova’s group(<5 mm). However, we did not find differences between treatments,using 3.8 mM of L-carnitine during in vitro maturation process. The above could be explained because the source of L-carnitine used in our study is different in comparison with that used by Knitlova et al[32].
On the other hand, when we compared the blastocyst rate in the control group (28.61%, calculated from the total numbers of cleaved oocytes) with the results by Ghanem (22.5%)[18], Phongnimitr et al (25.1%)[10], and Knitolava et al (group <5 mm = 25.8%)[32], we found that all results are similar (without taking into account the different laboratory conditions).
Probably, L-carnitine treatment could exert a positive effect on oocytes with compromised developmental competence (e.g., vitrified oocytes), but did not improve oocytes with an apparently high competence (the control group). Thus, further research is needed to clarify the precise cellular processes modulated by the addition of L-carnitine treatment during oocyte maturation[26]. Further studies could evaluate the effect of L-carnitine on the quality of the oocytes throughout the process, such as gene expression, cryo-tolerance, and implantation rate of in vitro production post-transfer into recipient cows.
Despite the fact that the results of this research cannot determine a specific L-carnitine treatment for the in vitro maturation and in vitro culture of bovine embryos, we can conclude that under our laboratory conditions, the L-carnitine supplementation during in vitro oocyte maturation improves the cumulus expansion and increases the mitochondrial activity, with no overt effects on the development rate of bovine embryos. Besides, this study supports the concept that L-carnitine supplementation could be used in oocytes from follicles with a small diameter to improve the in vitro maturation process.Further researches could be directed to assess the embryo quality in terms of total cell number, cryo-tolerance, and implantation rate of transferred embryos, using the different L-carnitine concentrations in a different stage of in vitro embryo production.
Conflict of interest statement
We declare that we do not have a conflict of interest with any financial organization regarding the material discussed in the manuscript.
Acknowledgments
The authors thank the Central Ganadera of Medellín abattoir for providing the ovaries. Particular thanks to the Genetic Laboratory,Universidad Nacional de Colombia, Medellín, and other collaborators who facilitated the execution of this research work.
Foundation project
This study was funded by the Administrative Department of Science Technology and Innovation (COLCIENCIAS) (Grant No.727, 2015).
 Asian Pacific Journal of Reproduction2019年6期
Asian Pacific Journal of Reproduction2019年6期
- Asian Pacific Journal of Reproduction的其它文章
- Stable state of serum inflammatory cytokines during induction of benign prostate hyperplasia in dogs
- Effects of Crassocephalum bauchiense (Hutch) leaf aqueous extract on toxicity indicators and reproductive characteristics in Oryctolagus cuniculus exposed to potassium dichromate
- Protective effect of Citrus reticulata peel extract against potassium dichromateinduced reproductive toxicity in rats
- Prolonged post-thaw culture of embryos does not improve outcomes of frozen human embryo transfer cycles: A prospective randomized study
- Effect of cognitive behavioral therapy on anxiety and depression of infertile women:A meta-analysis
- Human blastomere rotation in early cleavage embryos is not associated with reduced implantation: Evidence from time-lapse videography
