Stable state of serum inflammatory cytokines during induction of benign prostate hyperplasia in dogs
Kamran Golchin-Rad, Asghar Mogheiseh, Fahimeh Heidari, Saeed Nazifi, Nooshin Derakhshandeh,Mohammad Abbaszadeh Hasiri
Department of Clinical Sciences, School of Veterinary Medicine, Shiraz University, Shiraz, Fars, Iran
Keywords:Benign prostate hyperplasia Cytokines Dog Interleukin Tumor necrosis factor
ABSTRACT Objective: To monitor serum inflammatory cytokines during induction of benign prostate hyperplasia in dogs.Methods: This research was designed as a case-control study. There were 20 adult mixedbreed intact male dogs, which were divided into the normal group (n=10) and the benign prostate hyperplasia group (n=10). In the benign prostate hyperplasia group, benign prostate hyperplasia was induced by injection of testosterone (75.00 mg/dog, i.m.) and estrogen(0.75 mg/dog, i.m.) on day 0 (day of the first injection), day 21, day 42, and day 63. The doses of testosterone were doubled on days 21, 42, and 63. The normal group did not receive any injection. Blood sampling was performed from the jugular vein at days 0, 21, 42, and 63. The concentrations of interleukin-8, interleukin-10, and tumor necrosis factor-α (TNF-α) were assayed by enzyme-linked immunosorbent assay.Results: The levels of interleukin-8, interleukin-10 and TNF-αwere not significantly different between the normal group and the benign prostate hyperplasia group. Also, concentrations of the pro-inflammatory cutokines were not significantly different between the normal group and the BPH group in each day of sampling.Conclusions: In spite of the induction of benign prostate hyperplasia, changes in the concentration of blood serum inflammatory cytokines were not significantly different with that of the normal group and between the days of induction of benign prostate hyperplasia during two months. It reveals that there is a stable state of serum inflammatory cytokines during induction of benign prostate hyperplasia in dogs.
1. Introduction
Animal models have been used to investigate the physiopathology of benign prostate hyperplasia (BPH) and its various medical treatments[1]. Like humans, spontaneous BPH occurred in the chimpanzees and dogs[2,3]. One of the most common canine prostate disorders is BPH. Spontaneous BPH in male dogs is age-dependent,with low prevalence by age 2, and high prevalence by age 5-8 years old[4]. Many of BPH affected dogs did not show clinical signs[5].As there is a resemblance between the characteristics of BPH in old dogs and men, researchers use old dogs as a model for the study of BPH and its treatment protocols[6]. However, because of low access to old dogs with BPH, various experimentally-induced BPH models have been developed by transgenic, hormonal, and xenografting methods[6].
Hyperproliferation of stromal and glandular cells in the transition zone and periurethral areas observed in the prostate gland are thought to be affected by BPH[7]. The physiopathology of BPH has not been explained exactly and several theories have been suggested to account for the progressive hyperplastic processes underlying BPH. Some evidence has indicated that inflammation contributes to the development and progression of prostate hyperplasia[8].
The presence of inflammation as a component of BPH has been confirmed in the canine model of BPH and human prostate tissues.Significant inflammation was observed in hormonally induced BPH in dogs. The common suggestion is that chronic inflammation and endocrine changes disturb homeostasis and leads to tissue damage or, even, abnormal stem cell expansion. Damaged homeostasis can cause chronic inflammation and endocrine changes. A “defective cycle” is started, which leads to hyperplasia with fibrosis and conversions in the prostate tissue composition[9].
Histopathological evidence has confirmed the presence of chronic inflammation in BPH-induced dogs. To the best of our knowledge and searches in literature, there have been no reports about the changes in serum inflammatory cytokines during experimental induction of BPH. Therefore, this study aimed to monitor the concentration changes in serum inflammatory cytokines [interleukin(IL)-8, IL-10, and tumor necrosis factor-α(TNF-α)] during the induction of BPH in dogs with testosterone and estrogen.
2. Materials and methods
2.1. Animals
This study was performed under the supervision of the Committee on Animal Ethics, Shiraz University, Shiraz, Iran (IACUC No.4687/63). The dogs were owned and kept by Shiraz University School of Veterinary Medicine, and all dogs were selected with a known history. All the dogs were castrated at the end of the study.They were treated with antiparasitic drugs (one tablet of praziquantel for 10 kg/b.w. and one tablet of mebendazole for 5 kg/b.w.) for two weeks. All dogs were fed 300 g/dog/day of commercial dog food(NUTRI?Dry Dog Food; Behintash Co. Iran) daily. Dogs were given at libitum access to water.
2.2. Study design
This research was designed as a case-control study. Twenty adult intact and mixed-breed dogs (age: 1-3 years old; weight: 15-20 kg) were divided into two groups (the normal group and the BPH group), with 10 dogs in each group. In the BPH group, BPH was induced by the injection of testosterone (75.00 mg/dog, i.m.) and estrogen (0.75 mg/dog, i.m.) at days 0 (day of the first injection), 21,42, and 63. The doses of testosterone were doubled at days 21, 42,and 63[10]. The induced BPH was confirmed in accordance with the volume of prostate and concentration of prostate-specific antigen and canine prostate specific esterase in serum at day 63. The normal group did not receive any injection. Blood sampling was performed from the jugular vein into plain glass tubes at days 0, 21, 42, and 63 before injection of hormones. Blood serum was prepared with the centrifugation of blood samples at 750 × g for 10 min. Serum samples were stored at -20 ℃ by the end of the study.
2.3. Inflammatory cytokine assays
The concentrations of inflammatory cytokines (IL-8, IL-10, and TNF-α) were assayed by enzyme-linked immuno sorbent assay(ELISA) kits: Canine IL-8 ELISA kit (Bioassay Technology Laboratory, China) with sensitivity 2.34 pg/mL and assay range 5-1 500 pg/mL; canine IL-10 ELISA kit (Bioassay Technology Laboratory, China) with sensitivity 1.02 pg/mL and assay range 2-800 pg/mL; and TNF-αELISA kit (Bioassay Technology Laboratory, China) with sensitivity 0.01 ng/L and assay range 0.03-9 ng/L. Intra-assay and inter-assay were coefficients of variation <8% and coefficients of variation <10% for all kits.
2.4. Confirmation of induced BPH in dogs
The induced BPH in dogs was confirmed by the calculation of prostate volume [(width ××length × diameter) ÷÷2.6] +1.8[11] related to normal volume prostate calculated by the following formula in accordance with the body weight of dogs [0.33 × body weight (kg)+3.28][12] and concentration of canine prostate specific esterase and prostate specific antigen in serum of dogs at day 63 of study[13].
2.5. Statistical analysis
The distribution of data was not normal, so data were analyzed by non-parametric Friedman test and SPSS Statistics for windows,version 16 (SPSS Inc, Chicago, USA). The differences between groups (the normal and BPH-induced groups) and between groups in each day of sampling were analyzed. The data were expressed as median (Q1, Q3) and P value less than 0.05 was considered as significant in the statistical analyses.
3. Results
3.1. Prostate volume, prostate-specific antigen and canine prostate specific esterase concentration
The mean prostate volume and concentrations of prostate-specific antigen and canine prostate specific esterase in the normal and BPH groups at day 63 of study were presented in Table 1. At day 63 of induction of BPH, prostate volume and blood serum concentrations of prostate-specific antigen and canine prostate specific esterase in BPH-induced dogs were significantly higher than those in the normal dogs (P<0.05).
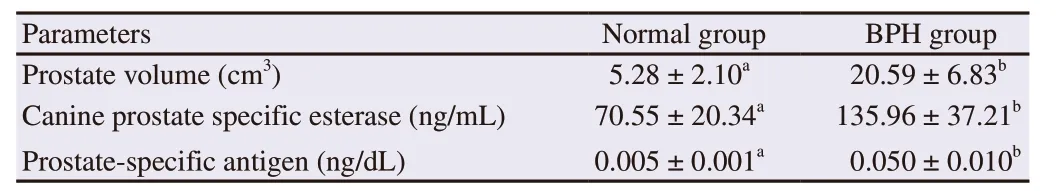
Table 1. Prostate volume and serum canine prostate specific esterase and prostate-specific antigen concentrations in normal group and benign prostate hyperplasia (BPH) group of dogs at day 63 of study.
3.2. IL-8
Minimum and maximum levels of IL-8 were 197.33 (181.44,358.49) pg/mL and 285.14 (190.58, 930.64) pg/mL in the normal group,and 232.67 (173.62, 368.81) and 445.28 (207.71, 622.56) pg/mL in the BPH group. There were not significant differences in IL-8 levels between groups overally, and each day of sampling between groups(Figure 1).
3.3. IL-10
In the normal group, the concentration of IL-10 was between 98.43(82.07, 173.95) pg/mL and 150.60 (139.21, 181.34) pg/mL, and in the BPH group, its concentration was between 121.28 (88.12,211.90) pg/mL and 138.16 (114.79,1 86.54) pg/mL. The differences in IL-10 concentrations between the normal and BPH groups were not significant and each day of sampling between groups (Figure 2).
3.4. TNF-α
The level of TNF-αwas between 1.50 (1.09, 1.90) pg/mL and 1.69 (1.19, 2.21) pg/mL in the normal group. In the BPH group,the level of TNF-αwas between 1.53 (1.16, 2.16) pg/mL and 2.33 (1.64,3.84) pg/mL. There were no significant differences between two groups and between groups in each day of sampling(Figure 3).
4. Discussion
The level of serum inflammatory cytokines (IL-8, IL-10, and TNF-α) fluctuated in the normal and BPH dogs, and the mean concentrations were not significantly different between the groups at different times.
The mechanisms involved in the disturbances of prostate homeostasis were not understood definitely. A group of growing documents has referred to inflammation as a key component in the pathogenesis of BPH[9]. The cell-mediated and humoral immune responses were observed in the biopsy samples and it was found that they were predated by hyperplasia[9]. In the experimentallyinduced BPH in dogs, the imbalance in the hormonal levels, as a primary event, caused pronounced inflammation, and the actions appeared to occur in a harmony. In this model, the inflammation may be a cause, an outcome, or an incisive advancing factor in the prostate hyperplasia and its development[6]. In a study on humans,the cytological and immunohistochemically evaluation of surgicallytreated BPH specimens revealed a significant increase in the inflammatory process, and a greater prostate volume was found to be linked significantly with a high level of inflammation[14].
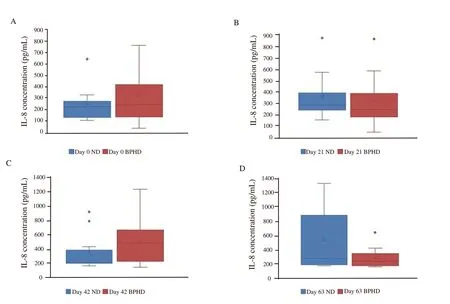
Figure 1. Comparisons of serum interleukin-8 (IL-8) level between benign prostate hyperplasia dogs (BPHD) and normal dogs (ND). Box and whiskers indicate serum IL-8 concentration [median (Q1, Q3)] in each day of sampling in each group.
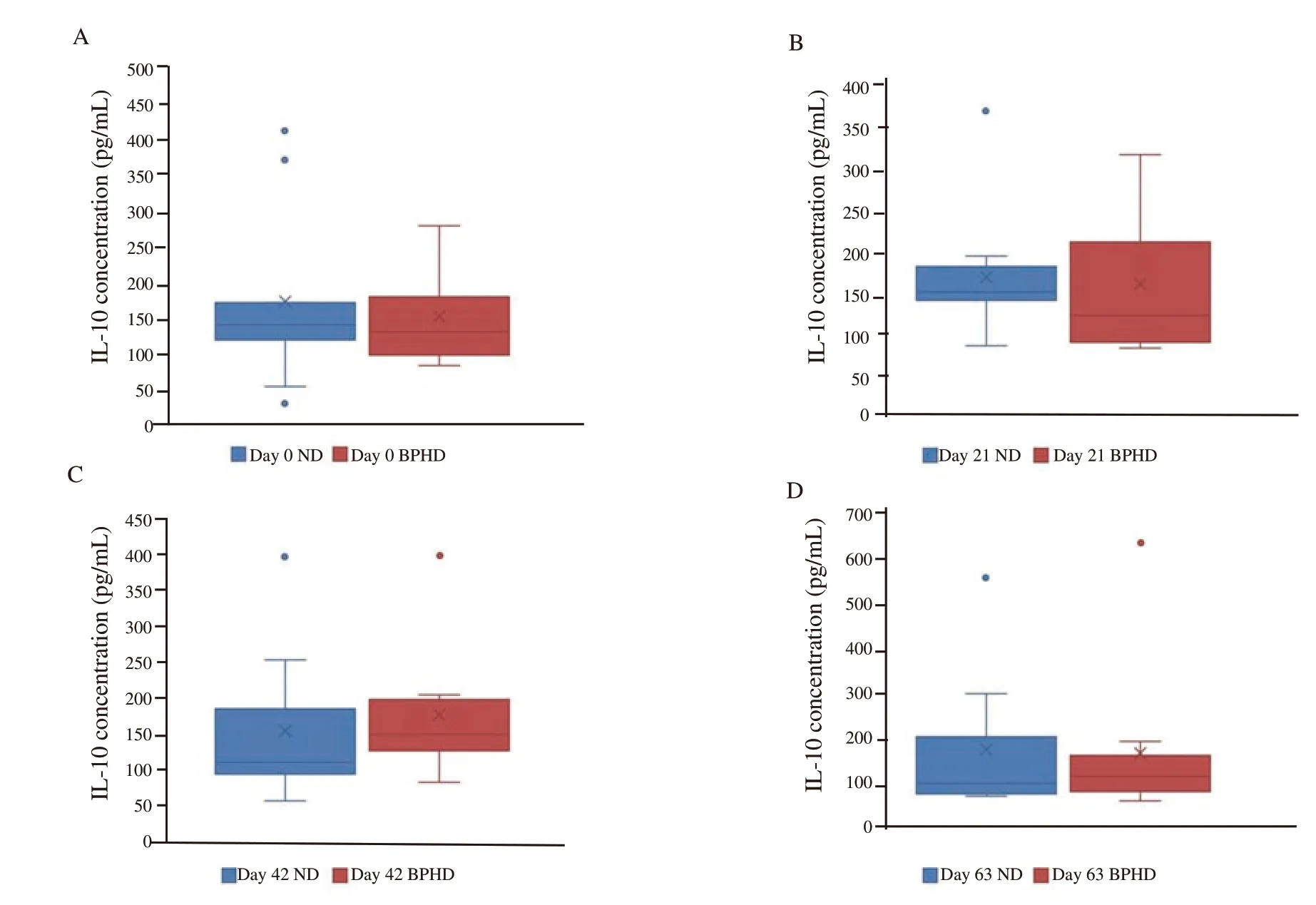
Figure 2. Comparisons of serum interleukin-10 (IL-10) level between prostate hyperplasia dogs (BPHD) and normal dogs (ND). Box and whiskers indicate serum IL-10 concentration [median (Q1, Q3)] in each day of sampling in each group.

Figure 3. Comparisons of serum tumor necrosis factor-α(TNF-α) level in benign prostate hyperplasia dogs (BPHD) and normal dogs (ND). Box and whiskers indicate serum TNF-αconcentration [median (Q1, Q3)] in each day of sampling in each group.
Chronic inflammation at the pathologic evaluation was found in about 55% of men with BPH-related lower urinary tract symptoms[15]. Progressively, chronic prostate inflammatory might lead to BPH, which is likely to increase the size of the prostate,worsening lower urinary tract symptoms[16]. Chronic inflammation begins with inflammasome, which is activated by the canonical and noncanonical pathways[17]. The first step in the canonical pathway,priming, is associated with the ligand binding to non-nucleotidebinding oligomerization domain-like receptors. This will enhance the expression of inflammasome ingredients. One of the components is protein-lipopolysaccharide binding to toll-like receptor 4, which then enhances the expression of the apoptosis-associated speck-like protein, PYD domains-containing protein 3, caspase-1, and pro-IL-1b. IL-18 is typically expressed[18].
Locally, the expression of IL-7 and IL-15 genes in the prostate biopsies and serum titers of these cytokines are significantly enhanced in men with early-stage prostate cancer compared with men affected by BPH. In their study, IL serum titers were not significantly associated with their gene expression in the autologous prostate biopsies;therefore, they suggested that cells other than tumor cells were likely at least in part responsible for the production of IL[19].
The inflammatory infiltration related to BPH specifically reveals a nodular pattern with an enhanced superiority of CD4+T-cells and the release of inflammatory cytokines including interferon-γ, IL-2, IL-4,IL-5, and IL-13[20]. Toll-like receptors may somewhat mediate tissue responses in the prostate epithelial cells locally[21]. The release of local inflammatory mediators such as IL-1, IL-6, IL-8, and C-X-C motif chemokine ligand stimulates the activation of the toll-like receptor[21].These intermediaries recruit further inflammatory cells as well as agitate the inflammatory response in the local tissue. Stromal cells may act as antigen presenting cells to excite chronic inflammatory responses through the production of IL-12 and IL-23[21].
The pro-inflammatory cytokines concentrations were affected by the inflammatory conditions and causes. Today, the researchers have suggested that multiplex measurements of pro-inflammatory cytokines are useful for preventing the effect of fluctuation of single measurement of pro-inflammatory cytokine and confirming diagnosis of diseases and monitoring body condition and health[22].In our study, multiplex measurement of pro-inflammatory cytokines was performed. Non-significant differences between the normal and BPH groups indicated that there were not abnormal conditions or causes in the normal and BPH dogs to lead to increasing all proinflammatory cytokines.
IL-8 level was affected by the breed of dogs and method of the assay[22], and serum or plasma quality[23]. It appears that the most reliable and predictive marker of prostatitis is seminal plasma IL-8(sIL-8)[24]. Moreover, there is a growing body of evidence that sIL-8 connection in the inflammation is not only of the prostate but also,and specially, in the epididymis and seminal vesicles. However,sIL-8 connection is not connected with the inflammation in the testis.
Although the early mentioned evidence revealed that inflammation most likely was one of the triggers and inducing factors of BPH in dogs and humans, all of the reports have either referred to the chronic inflammation in the histopathological examination of prostate samples as a local factor and change or investigated the local inflammatory cytokines in the prostate secretions.
Inflammatory cytokines in blood serum did not change during experimentally-induced BPH in dogs. Further investigation of the local inflammatory cytokines in the seminal plasma or the secretions of the prostate is suggested, so that more can be understood about the relationship between the inflammatory changes in BPH-prostate tissue and the cytokine levels.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Acknowledgments
The authors would like to thank the Research Council of Shiraz University and School of Veterinary Medicine, Shiraz University for financial and technical support of this study.
Foundation project
This study was supported financially by the School of Veterinary Medicine, Shiraz University, Iran (Grant No. 96INT1M154630).
 Asian Pacific Journal of Reproduction2019年6期
Asian Pacific Journal of Reproduction2019年6期
- Asian Pacific Journal of Reproduction的其它文章
- Effect of L-carnitine supplementation during in vitro maturation and in vitro culture on oocyte quality and embryonic development rate of bovines
- Effects of Crassocephalum bauchiense (Hutch) leaf aqueous extract on toxicity indicators and reproductive characteristics in Oryctolagus cuniculus exposed to potassium dichromate
- Protective effect of Citrus reticulata peel extract against potassium dichromateinduced reproductive toxicity in rats
- Prolonged post-thaw culture of embryos does not improve outcomes of frozen human embryo transfer cycles: A prospective randomized study
- Effect of cognitive behavioral therapy on anxiety and depression of infertile women:A meta-analysis
- Human blastomere rotation in early cleavage embryos is not associated with reduced implantation: Evidence from time-lapse videography
