Prolonged post-thaw culture of embryos does not improve outcomes of frozen human embryo transfer cycles: A prospective randomized study
Minh Tam Le, Van Trung Nguyen, Thanh Tung Nguyen, M. Blake EVans, Thai Thanh Thi Nguyen, Tam An Thi Nguyen, Dinh Duong Le, Vu Quoc Huy Nguyen, Ngoc Thanh Cao,, Micah J.Hill
1Department of Obstetrics and Gynecology, Hue University of Medicine and Pharmacy, Hue University, 06 Ngo Quyen Street, Hue, Vietnam
2Center for Reproductive Endocrinology and Infertility, Hue University of Medicine and Pharmacy, Hue University, 06 Ngo Quyen Street, Hue, Vietnam
3Department of Histology and Embryology, Hue University of Medicine and Pharmacy, Hue University, 06 Ngo Quyen Street, Hue, Vietnam
4Walter Reed National Military Medical Center, 8901 Rockville Pike, Bethesda, MD20889, USA
5Department of Public Health, Hue University of Medicine and Pharmacy, Hue University, 06 Ngo Quyen Street, Hue, Vietnam
Keywords:Embryo vitrification Post-thaw culture Frozen embryo transfer Biochemical pregnancy rate Clinical pregnancy rate Implantation rate
ABSTRACT Objective: To evaluate the impact of prolonged post-thaw embryos culture on pregnancy outcome during frozen embryo transfer cycles.Methods: This prospective cohort study evaluated 324 thaw transfer cycles with 819 embryos from 269 patients at the Center for Reproductive Endocrinology and Infertility of Hue University Hospital in Vietnam. These frozen embryo transfer cycles were divided into two groups at the time of thawing: the short culture group (2-hour post-thaw culture) and the overnight culture group (overnight culture for 18 h) before the embryo was transferred into the uterus. The rates of embryo intact, grade A embryo at frozen and transfer time and continuing cleavage were recorded. The clinical outcomes including serum beta-human chorionic gonadotropin, clinical pregnancy and implantation rate were evaluated after 14 days, 4 weeks,6 weeks, respectively, after embryo transfer.Results: Human chorionic gonadotropin positive occurred in 39.5% of patients in the short culture group compared to 25.9% in the overnight culture group with risk difference(RD)=13.6%, relative risk (RR)=1.343, 95% confidence interval (CI) 1.085-1.663, P<0.01.Clinical pregnancy rate of the short culture group and overnight culture group was 33.3% and 24.1%, respectively (RD=9.2%, RR=1.242, 95% CI 0.996-1.549, P=0.06) and the implantation rate in the short culture group and overnight culture group was 16.5% and 11.0%, respectively(RD=5.5%, RR=1.244, 95% CI 1.046-1.479, P=0.01). In women of advanced age (≥35 years)and women who received 3 embryos, pregnancy outcomes were found to be significantly(P<0.05) higher in the short culture than in the overnight culture group.Conclusions: The prolonged post-thaw culture period does not increase pregnancy outcome in comparison with the short culture.
1. Introduction
Frozen embryo transfer (FET) is a procedure widely used for the cryopreservation and transfer of embryos obtained in assisted reproductive technology. Due to improved laboratory conditions,more good quality embryos are obtained after in vitro fertilization cycles. Additionally, by reducing the number of embryos transferred into the uterus to limit the multiple pregnancy rate, the number of FET cycles increases progressively[1]. During the FET cycles, the uterus was not affected by side effects of ovarian stimulation,better synchronization of embryos and endometriosis than FET cycles, preventing ovarian hyperstimulation syndrome[2-4].Therefore, FET has drastically increased in annual frequency,contributing to more than 50% of embryo transfers in assisted reproduction centers[5].
Many factors may affect the clinical outcomes in FET cycles,such as the impact of vitrification, the stage of embryo and embryo quality at the time of freezing, the embryo damage after thawing,and the post-thaw culture period[6]. In recent years, embryo vitrification has become a popular method in cryopreservation, and it has better results compared to the slow frozen method. It is found that embryo survival, implantation and pregnancy rate in cases using vitrification were better than them with slow freezing[7,8]. Because cryoprotectants had high viscosity, it is used for replacing water in equilibration and vitrification step in and out blastomere. As a result,cryoprotectants prevented intracellular or extracellular ice formation which was sharp enough to damage cell membrane, cytoskeletal structure, spindle. However, vitrification may cause lethal intracellular ice formation, osmotic injury, and air bubble formation during the cooling/warming process that could effectively reduce the survival rates and quality of the embryo[9]. In the vitrification procedure,embryos change osmotic pressure very quickly when they come into contact with high concentrations of cryoprotectants, and the cells underwent extreme cellular shrinkage, resulting in potential changes to the cytoskeletal structure. Besides these factors, cryopreservation systems may influence frozen and thaw procedure. There are two systems which mostly performed including open and close systems.Although close device could induce less cross contamination risk,it had disadvantage that cooling and warming rate was slower than open device. In contrast, in open device, embryos were directly contacted with liquid nitrogen so that embryo survival rate was higher than it in close device[10,11].
In practice, embryos are evaluated according to a morphological standard to select those with the highest implantation potential and increase the efficiency of frozen embryo transfer[12,13]. In addition to the selection of embryos before vitrification, the prolonged culturing of embryos after thaw can offer more information of embryo development[14-16]. However, the impact of overnight culture on treatment outcomes remains controversial. Currently,there are debates on the benefit from the post-thaw culture in short(2-5 h) or prolonged (18-24 h) duration concerning blastomere survival and proliferation as well as implantation potential of thawed embryos[14,17,18]. Joshi et al performed 415 FET cycles after an overnight post-thaw culture and in 89 FET cycles within 2 h of embryo thawing. The results showed that no significant difference was noticed between FET with overnight culture and 2-hour culture,but the study concerned only the biochemical pregnancy rate[17]. The same results were also observed by Guo et al when they evaluated 2 562 FET cycles in China[14]. However, Rato et al demonstrated that a short post-thaw culture period is associated with higher clinical outcomes compared to long culture when investigating 631 frozenthawed embryos[18]. Therefore, the purpose of this prospective cohort study was to compare the pregnancy outcomes of two different postthaw embryo culture approaches: short culture (2-hour thaw) versus overnight culture (18-hour thaw).
2. Materials and methods
2.1. Study participants
From May 2016 to May 2018 at the Center for Reproductive Endocrinology and Infertility of Hue University Hospital, Vietnam,there were 640 patients who underwent 870 frozen embryo transfer with 2 036 embryos. The inclusion criteria were the patients with the first FET cycle/ovarian pick-up, FET with day 2 embryo vitrification,and the female age not more than 45 years old at the time of oocyte retrieval. The exclusion criteria were the cycles with gamete donor,female with endometriosis, low ovarian reserve, and sperm retrieval by surgery. To test the equivalence of the clinical pregnancy rate in the short and overnight culture methods , an appropriate formula was applied[19]. The sample size was determined by:
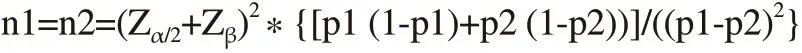
Where Zα/2was the critical value atα= 0.05 and Zβwas the critical value at a desired power of 80% (β=0.20). p1 and p2 were the expected clinical pregnancy rate of the short and overnight culture methods, respectively. Based on the report from Shi et al, we estimated the clinical pregnancy rate was 28.3% and 51.5% in the group with 2-hour culture post-thaw embryos and overnight culture, respectively[20]. The minimum sample was 69 in each group. The present data were collected from 162 samples from each group of the study population.
This prospective randomized study evaluated 269 patients with 324 thaw transfer cycles with 819 embryos, after screening and exclusion (Figure 1). The cycles evaluated were the patient’s first FET and embryos frozen were carried out on day 2 of culture. On day 7 of FET cycles, patients were scanned by ultrasonography for endometrium thickness and then received oral estradiol tablets for artificial endometrial preparation at a dosage of 8 mg/day(Progynova?2 mg × 4 tablets, Bayer, Germany) divided into 2 doses.Secretory transformation with progesterone (Crinone gel?8%,MerckKGaA, Darmstadt, Germany) administered vaginally at a dose of 90 mg twice daily was done when endometrium thickness was at least 7 mm. A total of 324 thawing embryo transfer were divided in half into two groups at the time of thawing by even and odd rule in the order of coming cycles, 2-hour post-thaw culture (short culture group) with 401 embryos, and overnight culture for 18 h (overnight culture group) with 418 embryos before transfer into the uterus. In the short culture group, progesterone was started in the morning of day 0, corresponding to the first day of cultured embryos, and in the overnight culture group, progesterone was started an extra half-day earlier, from the evening of the day before day 0. The information and characteristics of the patients involved in the frozen embryo cycles were collected to facilitate research as well as treatment activities. Body mass index (BMI) was calculated as body weight in kilograms divided by the square of height in meters. Based on the Asian-specific classification for BMI status, BMI values were categorized as underweight (<18.5 kg/m2), normal (18.5–22.9 kg/m2),overweight (23.0–24.9 kg/m2), and obese (≥25 kg/m2).
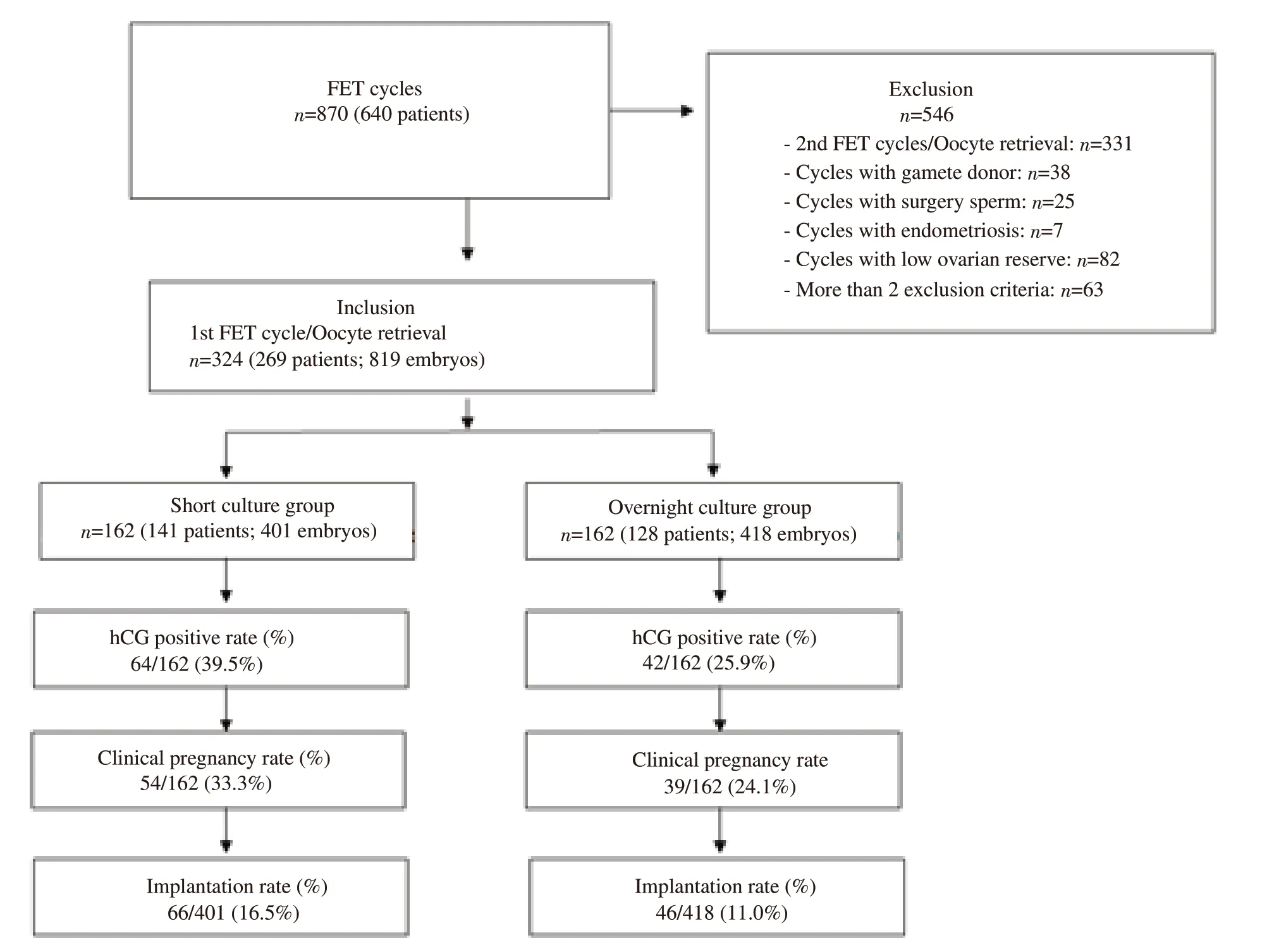
Figure 1. Flow chart summarizing the study. FET: frozen embryo transfer.
2.2. Ovarian stimulation, oocyte retrieval, and intracytoplasmic sperm injection procedures
All of the patients were treated with the controlled ovarian stimulation by a gonadotropin-releasing hormone antagonist protocol, and recombinant follicle-stimulating hormone(follitropinalfa) was administered with a starting dose of 225 IU(Gonal F?, Merck KGaA, Darmstadt, Germany). Oocyte retrieval was performed 35-36 h after human chorionic gonadotropin (hCG)10 000 IU intramuscularly (Pregnyl?, Merck Sharp & Dohme Limited, UK) administration by ultrasound-guided follicle aspiration with a single-lumen needle (Kitazato, Japan). The collected mature oocytes were inseminated via intracytoplasmic sperm injection 3 h after retrieval. On the following day, the oocytes were examined for fertilization and cultured in 20 μL of G-TL (Vitrolife, Sweden)covered in 3 mL of Ovoi (Vitrolife, Sweden) under conditions of 5.5%CO2and 5.0% O2.
The embryos were evaluated according to the number of blastomeres, their symmetry, and cytoplasmic fragmentation.Embryos with less than 25% fragmentation, and embryos with more than 2 blastomeres on day 2 were selected for vitrification. Grade A embryos contained less than 10% fragmentation with 4 or more blastomeres on day 2.
2.3. Embryo vitrification procedure
Since oocytes were retrieved around 8:00-9:00 am, cryopreservation was performed in the afternoon of day 2 post-fertilization,corresponding to an embryo age of 50-52 h, using the Cryotop method with commercially available medium (Kitazato, Japan). The vitrification solutions were warmed at room temperature for at least 1 h. Embryos were placed in 150 μL of equilibration solution for 5 min and then transferred to 2 drops of 150 μL vitrification solution for a maximum of 60 s in the vitrification solution before loading on cryotop and plunging into liquid nitrogen follow open method for vitrification.
2.4. Thawing of embryos
Thawing solution was warmed to 37 ℃ in an incubator. Dilution solution and washing solution were warmed up to room temperature.The embryos with the cryotop were plunged directly into the warming solution for 1 min and were transferred to the dilution solution for 3 min. Then, the embryos were placed in the washing solution for 5 min and washed in washing solution for 1 min.After warming, single embryo was cultured in 20 μL of G-TL(Vitrolife, Sweden) covered by 3 mL of Ovoil (Vitrolife, Sweden)under conditions of 5.5% CO2and 5.0% O2to ensure the pH value of the media was 7.28. The environment was balanced in CO2before loading the embryos. The embryo survival was evaluated immediately after warming. Embryos were considered to have survived when more than 50% of the embryo blastomeres were still alive. Embryos without any damaged blastomere were evaluated as intact embryos after warming. The embryos were cultured for 2 h or overnight for 18 h before transferred. For the overnight culture group,embryos were thawed in the afternoon before the embryo transfer day[20]. Total post-thaw-culture time was approximately 18 h, so that the embryo-thawed age was 68 h homogeneously with the time of embryo quality evaluation[21]. The embryo classification was based on the Istanbul consensus workshop on embryo assessment criteria,and the top embryo (grade A) was consistent with good embryo quality following day 2 (42-44 hours after injection) and day 3 (66-68 hours afer sperm injection)[22]. Grade A embryos after thawing were considered the same day 2 criteria and without blastomere damage. After culturing, we determined the continuing cleavage embryos rate which consisted of embryos having increased number of blastomeres. Grade A embryos after overnight culture were like embryonic day 3, they were those with 8 or more blastomeres, a fragment rate less than 10%, equal and symmetrically distributed blastomere size, and no multinucleation present in blastomeres.
2.5. Frozen-thawed embryo transfer
Embryos were placed 1 mL of embryoglue (Vitrolife, Sweden) and incubated for 15 min. Then, embryos were loaded into the transfer catheter (Kitazato, Shizuoka, Japan) and transferred to the uterus under transvaginal ultrasound guidance.
2.6. Assessment of hCG positive, clinical pregnancy and implantation rates
Serum beta-hCG was measured 14 days post embryo transfer, and if the test was more than 50 mIU/mL (positive test), pregnant women were followed with serial ultrasound evaluations, and the numbers of gestational sacs with fetal cardiac activity were determined 4 weeks after embryo transfer (clinical pregnancy), otherwise it is biochemical pregnancy if positive hCG without gestational sac found by ultrasound. Implantation rate was defined as the number of gestational sacs observed at ultrasound screening at 6 weeks of pregnancy divided by the number of embryos transferred into the uterine cavity. Progesterone (Crinone gel 8%, Merck KGaA twice daily) vaginal supplementation was continued until the 10th week of gestation.
2.7. Statistical analysis
Variables were analyzed by using the Statistical Package for Social Sciences version 20.0 (SPSS, Chicago, USA) and MedCal version 12(MedCal Software, Ostend, Belgium). All continuous variables, such as maternal age and number of embryos transferred, were presented as mean ± standard deviation (mean ± SD) and were compared between treatment groups by means of a 2-tailed, unpaired t-test.All categorical variables, such as biochemical pregnancy, clinical pregnancy, and implantation rates were compared between treatment groups by Chi-squared test. Results were reported as percentages,risk difference (RD), relative risk (RR), or odds ratios (OR) with 95%confidence intervals (CI). All analyses were considered significant at P<0.05.
2.8. Ethics approval
The research protocol was approved by the Ethics Committee of Biomedicine Research of Hue University of Medicine and Pharmacy,with registration number H2019/383. Informed and written consents were obtained from all participants.
3. Results
3.1. Demographic, embryological, and clinical characteristics of short and overnight culture groups
As expected in a prospective cohort study, there was no significant difference between the two groups with respect to the mean maternal age, BMI, endometrial thickness, number of transferred embryos,embryo intact rate, and grade A embryo before frozen rate (P>0.05).It was excited that the continuing cleavage and grade A embryo before transfer rates was significantly different between the two groups: 43.1%, 72.8% in the short culture group and 74.9%, 43.8%,respectively in the overnight culture group (P both <0.01) (Table 1).Besides, we found that the rate of grade A embryos decreased from before frozen to transfer time in overnight culture group.
3.2. Pregnancy outcomes of FETs in short and overnight culture groups
Comparison of clinical outcomes of FETs with short or overnight culture was shown in Table 2. Compared to the overnight culture group, the use of short culture was associated with a significantly higher hCG positive and implantation rates (39.5% vs 25.9%,16.5% vs 11.0%, respectively). The clinical pregnancy rate in short culture was also better than it in long culture group but there was no significant statistically with P=0.06.
3.3. Results of binary logistic regression analysis of related factors on clinical pregnancy rate
The results of binary logistic regression analyses of related factors on the clinical pregnancy rate were presented in Table 3. It was shown that BMI had no impact on the clinical pregnancy rate(P=0.40) and endometrial thickness (P=0.89) variables. However,maternal age and the number of transferred embryos were found to impact clinical pregnancy rate (P<0.01, P<0.05, respectively).
3.4. Pregnancy outcomes related to maternal age in short and overnight culture groups
To assess the effect of age on the pregnancy outcomes of FETs following distinct post-thaw culture periods, the patients were divided into 2 subgroups according to the maternal age (< 35 and≥ 35 years). In women of advanced age (≥ 35 years), pregnancy outcomes were found to be significantly decreased compared to those in young women (< 35 years). The hCG positive (42.6% vs 30.8% with P=0.06) and clinical pregnancy rates (38.0% vs 30.0%with P=0.19) in the < 35 years subgroup did not reach statistical significance between the short and overnight culture groups.Meanwhile, the hCG positive (33.3% vs 9.5% with P<0.01) and clinical pregnancy rates (24.1% vs 7.1% with P<0.01) in the ≥ 35 years subgroup was found to be significantly higher in the short culture group than in the overnight culture group. Additionally, in both age subgroups, implantation rates in the short culture group were significantly higher than that in the overnight culture group(with P<0.05 and P<0.01 in group under and above 35 years old,respectively) (Table 4).
3.5. Pregnancy outcomes related to number of transferred embryos in short and overnight culture groups
To assess the effect of the number of transferred embryos on the pregnancy outcomes of FETs following distinct post-thaw culture periods, the patients were divided into 2 and 3 embryos transfer subgroups. The results showed that hCG positive (49.4% vs 28.7%with P<0.01) and clinical pregnancy rates (44.2% vs 26.6% with P<0.05) and implantation rate (19.5% vs 10.6% with P<0.01)significantly increased in short versus overnight culture groups in the 3 embryo transfer subgroup. Meanwhile, pregnancy outcomes did not reach statistical significance between short versus overnight culture groups when transfer 2 embryo (Table 5).
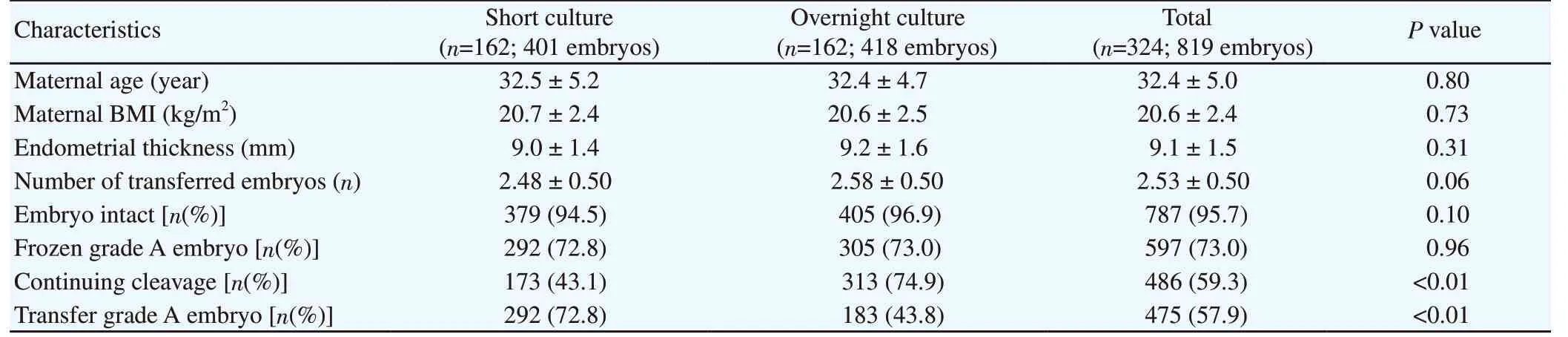
Table 1. Demographic, embryological, and clinical characteristics of short culture and overnight culture groups.

Table 2. Influence of 2 different post-thaw culture periods on outcomes of frozen embryo transfer cycles.

Table 3. Binary logistic regression of factors related to clinical pregnancy rate.
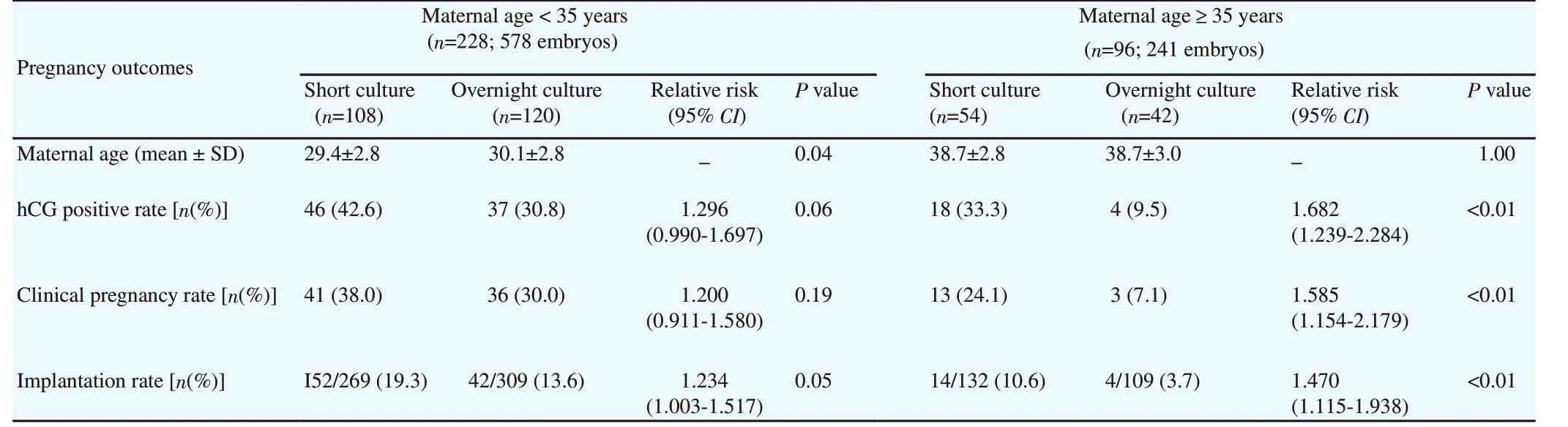
Table 4. Pregnancy outcomes related to maternal age at oocyte pick-up in two different post-thaw culture periods.
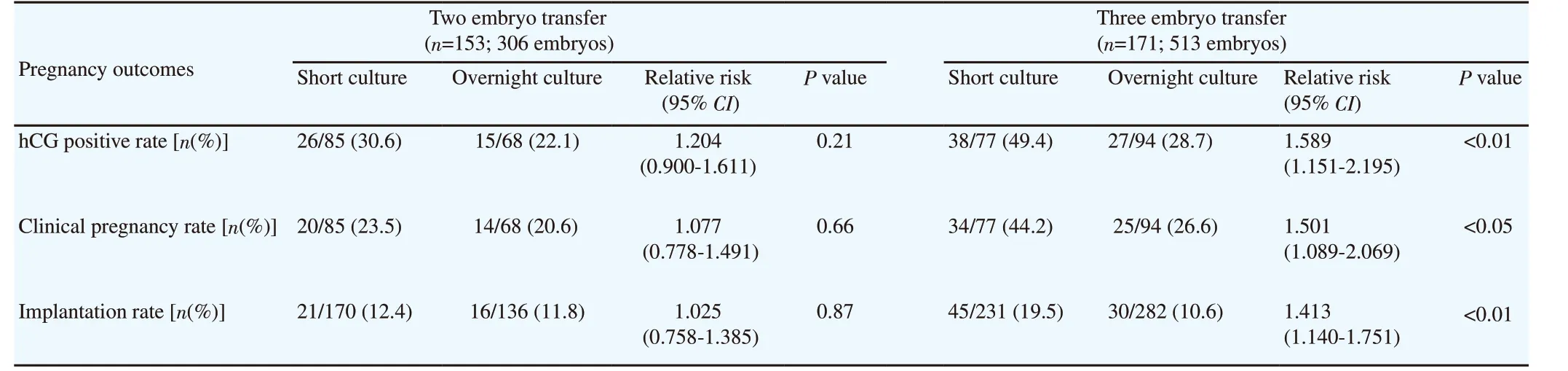
Table 5. Pregnancy outcomes related to number of transferred embryos in two different post-thaw culture periods.
4. Discussion
FET is an indispensable procedure in assisted reproductive cycles.Embryo overnight post-thaw culture may provide more information for predicting the possibility of clinical pregnancy after being vitrified-thawed and subsequently undergoing embryo transfer.Landuyt et al reported that human embryo survival after vitrified/warming was higher than slow frozen/thawing. Additionally,they found that resumption of mitosis after overnight culture has an increased potential for implantation. When embryos without further cleavage were transferred, they only found 2 patients who successfully achieved pregnancy. However, one pregnancy was biochemical and another was early miscarriage[6]. Selection of embryos with further cleavage after thawing can significantly improve the implantation rate perfrozen embryo transfer cycle.
In this prospective randomized study, there was no systematic baseline difference between short and overnight post-thaw culture groups with respect to maternal age, BMI, endometrial thickness,number of transferred embryos, embryo intact rate, and grade A embryo rate. Especially, the open device using in vitrification embryos seemed to be have advantage because the result of survival embryos rate after thawing attended 100%. However, there was a significant difference between the study groups regarding continuing cleavage rate. Embryos continuing cleavage occurred in 173 of 401 embryos (43.1%) in the short culture group and in 313 of 418 embryos (74.9%) in the long culture group (P<0.01), but this was an expected finding. The short culture was revealed to be an insufficient time period for observation of the continuing cleavage of surviving embryos compared to the prolonged culture method. The continuing cleavage rate (74.9%) of our study in the overnight culture group was higher than that of Joshi’s study (47.0%)[17].
The effectiveness of the evaluation of prolonged culture embryos after thawing remains controversial. The previous study by Shi et al found that the pregnancy rate was significantly higher in the overnight culture group (51.1%) than 2- to 4-h transfer after thawing(28.2%)[20]. Veleva et al reported that there was no significant difference between overnight culture or short culture among the frozen and thawed embryos which were good quality[23]. The research made by Joshi et al showed that pregnancy results were equivalent between the two groups, 24.3% in overnight culture and 20.3% in 2-hour culture groups[17]. In the present study, the results demonstrate that a short post-thaw culture period is associated with significantly higher biochemical pregnancy and implantation rate per FET cycle than prolonged post-thaw culture, 39.5% and 16.5%vs. 25.9% and 11.0%. This result indicated that prolonged post-thaw culture may have negative influences on embryo development. The prolonged culture required at least 1 embryo quality assessment process outside incubator before transfer that maybe change temperature and pH level in the culture medium. The other stress on embryos was that they had to be cultured longer time in condition with volatile organic compounds (VOCs) which were harmful to embryo development in vitro. Nevertheless, despite efforts to improve the in vitro culture condition, it always implies the induction of these stress, explaining the poorer pregnancy outcome after assisted reproductive technology[24,25]. Short post-thaw culture may have less information about embryo development than overnight culture, but it provides more safety for the embryo[18].
The success of FET cycles depends on many factors, such as maternal age, embryo factors, vitrified-thawed embryo protocol, and synchronicity between the embryos and endometrium[15]. However,the maternal age is one of the most crucial factors in determining clinical pregnancy rates following FET[26,27]. In agreement with these studies, our binary logistic regression analyses show a negative association between the maternal age and clinical pregnancy outcomes in FET technique. In the younger (<35 years) subgroup,there were no significant differences in hCG positive and clinical pregnancy rates between short and overnight post-thaw culture.The impact of prolonged post-thaw culture was evident in the case of advanced age. Pregnancy outcomes in the older (≥35 years)subgroup were found to be significantly higher in the short culture group than in the overnight culture group. It is found that the mean age in this subgroup was high (38.7 years old) that meant our study had several patients over 40 years old. The reason that we did not exclude these patients was not only our old study population but also our desire to determine the effect of age on thawed embryos transfer cycles. In a previous study, the embryos obtained from women with advanced age were associated with low embryo quality because of the alterations in the regulation of gene expression in oocytes, thus resulting in higher aneuploidy embryos and lower pregnancy rates[28].
Several comments in Klitzman’s survey have suggested a trend toward higher pregnancy outcomes when transferring more embryos.Meanwhile, the implantation rate, which reflects the number of embryos that implant per total transferred, was not increased with more embryos transferred[29]. Our results showed that biochemical pregnancy and clinical pregnancy did not reach statistical significance between short and overnight culture in the 2 embryo transfer subgroup. When transferring 3 embryos, the biochemical and clinical pregnancy rates significantly increased and reached significantly differences between short and overnight culture groups.
In conclusion, our prospective cohort analysis has demonstrated that the overnight post-thaw culture period causes a decrease in the implantation and developmental potential of embryo maybe because of some stress from culturing in longer time on thawed embryos make it worse than the condition of uterine cavity, although blastomere proliferation was observed more often in the prolonged culture group. By reducing culture duration, the embryos should be thawed and transferred after 2 h of culture to be able to recover and avoid adverse conditions affecting the developing embryo from the artificial culture and provide better treatment outcomes. Prolonged post-thaw culture did not demonstrate an overall benefit and is associated with unnecessary increased time and effort.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Acknowledgments
We thank the staff of the Hue Center for Reproductive Endocrinology and Infertility of Hue University Hospital for their excellent help.
 Asian Pacific Journal of Reproduction2019年6期
Asian Pacific Journal of Reproduction2019年6期
- Asian Pacific Journal of Reproduction的其它文章
- Effect of L-carnitine supplementation during in vitro maturation and in vitro culture on oocyte quality and embryonic development rate of bovines
- Stable state of serum inflammatory cytokines during induction of benign prostate hyperplasia in dogs
- Effects of Crassocephalum bauchiense (Hutch) leaf aqueous extract on toxicity indicators and reproductive characteristics in Oryctolagus cuniculus exposed to potassium dichromate
- Protective effect of Citrus reticulata peel extract against potassium dichromateinduced reproductive toxicity in rats
- Effect of cognitive behavioral therapy on anxiety and depression of infertile women:A meta-analysis
- Human blastomere rotation in early cleavage embryos is not associated with reduced implantation: Evidence from time-lapse videography
