Voluntary running delays primary degeneration in rat retinas after partial optic nerve transection
Hong-Ying Li , Xi Hong Mi Huang , Kwok-Fai So ,
1 Department of Anatomy, School of Medicine, Jinan University, Guangzhou, Guangdong Province, China
2 Guangdong-Hongkong-Macau Institute of CNS Regeneration, Ministry of Education CNS Regeneration Collaborative Joint Laboratory, Jinan University, Guangzhou, Guangdong Province, China
3 Guangdong Key Laboratory of Brain Function and Diseases, Jinan University, Guangzhou, Guangdong Province, China
4 Department of Ophthalmology and State Key Laboratory of Brain and Cognitive Sciences, The University of Hong Kong, Hong Kong Special Administrative Region, China
Abstract Running is believed to be beneficial for human health. Many studies have focused on the neuroprotective effects of voluntary running on animal models. There were both primary and secondary degeneration in neurodegenerative diseases, including glaucoma. However, whether running can delay primary or secondary degeneration or both of them was not clear. Partial optic nerve transection model is a valuable glaucoma model for studying both primary and secondary degeneration because it can separate primary(mainly in the superior retina) from secondary (mainly in the inferior retina) degeneration. Therefore,we compared the survival of retinal ganglion cells between Sprague-Dawley rat runners and non-runners both in the superior and inferior retinas. Excitotoxicity, oxidative stress, and apoptosis are involved in the degeneration of retinal ganglion cells in glaucoma. So we also used western immunoblotting to compare the expression of some proteins involved in apoptosis (phospho-c-Jun N-terminal kinases, p-JNKs), oxidative stress (manganese superoxide dismutase, MnSOD) and excitotoxicity (glutamine synthetase) between runners and non-runners after partial optic nerve transection. Results showed that voluntary running delayed the death of retinal ganglion cells vulnerable to primary degeneration but not those to secondary degeneration. In addition, voluntary running decreased the expression of glutamine synthetase, but not the expression of p-JNKs and MnSOD in the superior retina after partial optic nerve transection. These results illustrated that primary degeneration of retinal ganglion cells might be mainly related with excitotoxicity rather than oxidative stress; and the voluntary running could down-regulate excitotoxicity to delay the primary degeneration of retinal ganglion cells after partial optic nerve transection.
Key Words: voluntary running; optic nerve injury; oxidative stress; excitotoxicity; JNKs; primary degeneration; secondary degeneration
Introduction
Glaucoma ranks the second leading cause for irreversible blindness in the world (Weinreb and Khaw, 2004; Zhang et al., 2012a). The most important symptom of glaucoma is the gradual vision loss from peripheral to central field of the eyes. There are different types of glaucoma and the most common type is open-angle glaucoma in which the drainage of aqueous humor is blocked and eye pressure rises to induce injury to retinal ganglion cells (RGCs) (Liebmann and Lee,2017). There is also normal-tension-type glaucoma and the precise mechanisms of RGC death were not clear in this type although several possible factors of pathogenesis have been put forward (Killer and Pircher, 2018). Nevertheless, for most glaucoma patients, the common and only effective treatment is to reduce the intraocular pressure by surgery or drugs, even in normal hypertension glaucoma patients. However, even the intraocular hypertension is reduced, RGCs will continue to die and the vision loss will further deteriorate in some patients. Therefore, except for primary degeneration, secondary degeneration is believed to exist in glaucoma (Calkins, 2012;Li et al., 2014).
To study primary and secondary degeneration in specific locations in the retina, a new partial optic nerve transection(PONT) model in monkeys and rats was established in the first decade of this century. Two main points had been verified using this model: one is the existence of secondary degeneration, and the other is the distribution pattern of primary and secondary degeneration in the retina (Levkovitch-Verbin et al., 2001; Levkovitch-Verbin et al., 2003; O’Hare Doig et al., 2017). Previous studies using the PONT model have demonstrated that the death mechanisms between primary and secondary degeneration of RGCs are different. For example, the polysaccharides from Lycium barbarum (LBP) exert neuroprotective effect only on RGCs which would die from secondary degeneration but not on those would die from primary degeneration (Li et al., 2013, 2015). Recently, Chiha et al. (2018) showed the difference in the retinal gene activation nature after PONT between the region vulnerable to primary degeneration and the one vulnerable to secondary degeneration. All these findings support that the mechanisms of RGC death are different between primary and secondary degeneration after PONT.
Exercise has been shown to be beneficial for central nervous system (CNS) diseases in both human and animal models. There are various styles of exercise, such as swimming,dancing, running and walking. The role of exercise has been detected in the following CNS disease models: Parkinson’s disease (Crowley et al., 2018), amyotrophic lateral sclerosis(Desseille et al., 2017), cerebral ischemia (Gabriel-Salazar et al., 2018), dementia (Kurucz et al., 2018), and depression (Yau et al., 2014a). Voluntary running is a kind of exercise which is common in human daily life, and this method is also used in animal studies to imitate human actual exercise.
Previous studies showed that voluntary running improved cognitive dysfunction, increased the production of neurotrophic factors in a rat model of Parkinson’s disease(Hsueh et al., 2018), increased neurogenesis in the brains of rat models of depression (Yau et al., 2014a, b), increased the production of anti-oxidant enzyme HO-1 in the brains of rat models of dementia (Kurucz et al., 2018), and up-regulated the production of Sirt3 in cortical neurons. Lacking of voluntary running has been reported to increase the vulnerability to oxidative stress and excitotoxicity (Cheng et al., 2016).All these results point to a conclusion that exercise is beneficial for different CNS diseases through various mechanisms.
Glaucoma is a disease characterized by the death of RGCs which is caused by multiple factors, including deprivation of neurotrophic factors (Sleeman et al., 2000; Ghaffariyeh et al., 2011; Levkovitch-Verbin, 2015), oxidative stress(Pinazo-Duran et al., 2015; Giacci et al., 2018), excitotoxicity(Ju et al., 2009; Lee et al., 2014); inflammation (Takada et al.,2011; Husain et al., 2012; Vohra et al., 2013), and apoptosis(Levkovitch-Verbin, 2015). Among these factors, several could be modulated by voluntary running as mentioned above, therefore, we hypothesized that voluntary running would be beneficial in glaucoma model by modulating these pathways although whether it would act on primary or secondary degeneration or both remained unknown.
Materials and Methods
Animals and housing conditions
The experimental plan was approved by the Committee on the Use of Live Animals in Teaching and Research at the University of Hong Kong (Animal license #14-465 approved on May 5, 2014) and given a license by the Jinan University Institutional Animal Care and Use Committee.
Adult Sprague-Dawley (SD) rats, aged 10–12 weeks, weighing 250–280 g, were housed individually in cages with a locked wheel (non-runners) or unlocked wheel (runners). All animals were maintained on a 12-hour light/dark cycle with ad libitum access to water and food. In this study, animals (n= 4 to 6/group, total 30 rats) were used for the following three experiments: 1) To compare the RGC survival 4 weeks after PONT in runners and non-runners, the schedule of treatments and animal allocation are demonstrated in Figure 1A.The PONT surgery (detailed procedure described below) was administrated on the day after 4 weeks of voluntary running.After the surgery, the runners would continue voluntary running for 4 more weeks. RGC retrograde labeling from superior colliculi (SC) was administrated 1 week before euthanasia.Then all rats would be euthanatized after the cease of voluntary running. 2) To detect the changes in the protein levels of MnSOD and p-JNKs in the superior retina since the changes in the inferior retina had been detected and reported in a previous study (Li et al., 2013); and to detect the protein levels of glutamine synthetase (GS) both in the superior and inferior retinas 1 day after PONT (The timeline of treatments and animal grouping are illustrated in Figure 1B). 3) To compare the changes in the expression levels of these proteins between the runners and non-runners and the schedule of treatments and animal grouping are shown in Figure 1C. The PONT surgery was administrated after voluntary running ceased and the rats were euthanized 1 day after PONT.
Exercise training
The running system for rats in this study was used in several reports (Yau et al., 2012, 2014a; Lee et al., 2016). Runners were housed in cages individually with unlocked wheels (diameter, 31.8 cm; width, 10 cm; Nalgene Nunc International,Rochester, NY, USA), while non-runners were housed in cages with locked wheels as well as other similar conditions.Turns of wheel were recorded using the VitalViewer software per hour (Mini Mitter Company, Inc, Bend, OR, USA).The perimeter of the wheel was used as the length of running per turn. Then the average daily running distance (km/day) was calculated for each animal both in the group used for western blot analysis and in the group used for RGC survival estimation. The time points for locking and unlocking management are demonstrated in Figure 1A & B.
PONT surgery
The PONT surgery was described previously (Chu et al.,2013; Li et al., 2013, 2014, 2015). Before surgery, SD rats were anesthetized with ketamine (80 mg/kg) and xylazine(8 mg/kg) via intraperitoneal injection. Sterile eye lubricant ointment was applied to prevent drying of the corneas during surgery. The partial incision in the optic nerve was cut 1.0 mm from the optic disc and was completed using a diamond knife (G-31480, Geuder AG, Hertzstrasse, Heldelberg, Germany) with the blade fixed to a length of 200 μm. After surgery, ophthalmic ointment (0.3% tobramycin)was applied to the cornea to reduce inflammation, and the fundus of the eyes was inspected to ensure that the retinal blood supply was not compromised by the surgery (Chu et al., 2013). The buprenorphine (100 mg/kg) was added to the drinking water for 7 days after the surgery to reduce the pain of animals. The exclusion criterion was the compromise of the blood supply after surgery.
Superior colliculi labeling
Superior colliculi labeling was conducted 1 week before euthanasia according to a previously described method (Chiu et al., 2008; Li et al., 2013). In brief, before surgery, SD rats were anesthetized with ketamine (80 mg/kg) and xylazine (8 mg/kg) via intraperitoneal injection. Then the rat scalp was cut open in the mid-line and the sagittal, coronal and transverse sutures of the skull were exposed. Two skull holes with the area of 4 × 4 mm2and 0.5 mm from both sagittal and transverse sutures were drilled on both sides of the sagittal suture. The brain tissue covering the superior colliculi was sucked away using a vacuum pump until four borders of superior colliculi could be seen under microscope. Then a thin layer of gel-foam soaked with 6% Fluro-Gold was spread on the surface of superior colliculi for labeling of RGCs. The space of the hole was filled with gel foam and the skin was closed with suture clips. After surgery, buprenorphine (100 mg/kg) was added to the drinking water for 7 days or until euthanasia to alleviate the pain of animals.
Quantification of RGCs
Preparation of retinas for quantification of RGCs was the same as described previously (Li et al., 2013). After euthanasia, retinas were post-fixed in 4% paraformaldehyde for 1 hour and divided into the superior and inferior halves. Either half was cut into three sectors (roughly equal) before being flat-mounted. Eight photographs with the size of 200 × 200 μm2in each sector were obtained along the median line, from the optic disc to the borders at 500 μm intervals under microscope (DM6000 B, Leica, Wetzlar, Germany) at 400× magnification. Therefore, a total 24 photographs were counted in either half to obtain the average number of RGCs in each photograph (0.04 mm2). Then the densities of surviving RGCs per mm2were calculated for either half. All photographs were quantified by two persons using a double-blind manner.
Western blot analysis
At 1 day after PONT, we detected protein expression and investigated the effects of voluntary running on protein expression because protein changes had been observed as early as this time point after PONT in the previous studies (Fitzgerald et al., 2010; Li et al., 2013). After euthanasia, the superior and inferior retinas were collected individually in 0.01 M sterilized PBS on ice. All agents used were as described previously (Li et al., 2013). After transferring the proteins onto the polyvinylidenedifluoride (PVDF) membranes, the membranes were blocked with 5% non-fat dry milk or 3%bovine serum albumin in Tris-buffered saline with 0.05%Tween 20 for 1 hour. Mouse monoclonal antibody manganese superoxide dismutase (MnSOD or SOD2, 1:20 000) was supplied by Abcam (Cambridge, MA, USA). Rabbit polyclonal phospho-c-jun N-terminal kinases (p-JNKs, 1:500),JNK (1:500) and glyeraldehayde-3-phosphate-dehydrogenase (GAPDH, 1:10,000) antibodies were bought from Cell Signaling Technology (Beverly, MA, USA). Mouse monoclonal antibody glutamine synthetase (GS, 1:10,000) was purchased from Millipore (Billerica, MA, USA). The primary antibodies were incubated in Tris-buffered saline with 0.05%Tween 20 overnight at 4°C. The secondary antibodies (dilution 1:10,000 for MnSOD and GS and 1:2000 for others) were incubated for 1 hour at room temperature. Protein loading was controlled using the monoclonal mouse antibody against alpha-tubulin (1:20 000, Sigma-Aldrich, St. Louis, MO, USA)or GAPDH (1:10000). Densitometric analysis was conducted using ImageJ software (National Institutes of Health, Bethesda, MD, USA) on the autoradiographic photographs acquired from the ChemiDocTMTouch Imaging System (Bio-Rad, Hercules, California, USA).
Statistical analysis
Data were compared between two groups. Student’s t-test was utilized. Data were statistically analyzed using the Sigmastat software (Sigmastat 3.5; Systat Software Inc., Chicago, IL, USA). The level of P < 0.05 was decided to be statistically significant. All data are expressed as the mean ± SEM.
Results
Running distance of runners
The average daily running distance of runners was 4.9 ± 0.8 km/day in rats used for RGC survival estimation and it was 5.6 ± 0.6 km/day in rats used for western blot analysis. There was no significant difference in running distance between these two groups (Figure 1D).
The RGC survival rates in the superior and inferior retinas of both non-runners and runners 4 weeks after PONT
The densities of RGCs in the superior retina of non-runner(n = 5, one rat died after anesthesia for PONT) and runners(n = 6) were: 828 ± 182 RGCs/mm2and 1345 ± 106 RGCs/mm2, respectively. The number of surviving RGCs in the superior retina in runners significantly increased compared with that in non-runners (P < 0.05, Figure 2A–C). The densities of existing RGCs in the inferior retina of non-runner and runners were: 855 ± 120 RGCs/mm2and 1024 ± 178 RGCs/mm2, respectively. There were no significant differences in the densities of RGCs in the superior and inferior retina between non-runners and runners (P > 0.05; Figure 2A, D & E).
The expression pattern of MnSOD, GS, and p-JNKs in the retina of non-injured rats and the rats subjected to PONT 1 day after surgery
At 1 day after PONT, the protein levels of MnSOD and p-JNK2/3 in the inferior retinas increased (Li et al., 2013). Results from this study showed that the protein level of MnSOD in the superior retinas also increased 1 day after PONT (Figure 3A). The expression of p-JNK2/3 increased but p-JNK1 did not change in the superior retinas (Figure 3B). The expression of GS increased in the superior retinas, but not in the inferior retinas 1 day after PONT (Figure 3C).
The expression pattern of MnSOD, GS, and p-JNKs in the retina of non-runners and runners 1 day after PONT
After 4 weeks of voluntary running, there were no signifi-cant differences in MnSOD and p-JNKs expression in the superior retina between runners and non-runners 1 day after PONT (Figure 4A and C). The density of GS bands decreased in the superior retina but not in the inferior retina in runners (Figure 4B).
Discussion
The average daily running distance in the group used for estimation of RGC survival and the group used for western blot analysis was estimated. Results showed that there was no significant difference between these two groups. The running exercise in these two groups was conducted at different time periods. The data shows that the average daily running distances are comparable between different runners who ran at different time periods.
Results from this study showed that voluntary running could delay RGC degeneration in the superior retina but not in the inferior retina. This suggests that voluntary running mainly delayed primary degeneration of RGCs but not secondary degeneration since we found that most primary degeneration occurred in the superior retina and secondary degeneration mainly occurred in the inferior retina (Li et al.,2013).
In this study, we used rat models of PONT. In a previous study, oxidative stress was involved in both the primary and secondary degeneration after PONT and increased MnSOD could be detected as fast as 5 minutes after PONT and maintained at least 3 days after PONT (MnSOD at later time points was not detected in this study) (Fitzgerald et al., 2010). Therefore, we chose 1 day after PONT as the time point for analyzing the effects of voluntary running on oxidative stress. Since excitotoxicity was often thought to be related with oxidative stress (Bondy and LeBel, 1993; Lee et al., 2014), so we also wanted to detect the excitotoxicity simultaneously. The excitatory neurotransmitter glutamate in the retina is mainly taken up and degraded to nontoxic glutamine by Müller cells in the retinas. If the metabolism of glutamate is abnormal, then excitotoxicity will occur. The increase in extracellular level of glutamate could up-regulate GS. Therefore, the increase in GS after PONT might suggest excess glutamate after PONT (Ishikawa, 2013).
Glutamate is secreted by photoreceptors and bipolar cells in the retinas. Increased glutamate level indicates the malfunction or degeneration of photoreceptors or bipolar cells after PONT. This has been verified in a previous study involving the same model (Chu et al., 2013). Therefore, GS up-regulation indirectly indicates the existence of excitotoxicity in primary degeneration region after PONT. In this study, up-regulation of GS is only detected in the primary degeneration region but not in the secondary degeneration region. This is consistent with a recent study by Chiha et al. (2018). Chiha et al. (2018) found the gene related with excitotoxicity changed obviously in the primary degeneration region but not in the secondary degeneration region.Both results show that excitotoxicity is involved in primary degeneration of RGCs and that the mechanism underlying primary degeneration of RGCs should be different from that underlying secondary degeneration of RGCs.
In this study, we also identified the increase of MnSOD in primary degeneration area. But what surprises us is that voluntary running has effect only on GS but not on Mn-SOD in the superior retinas after PONT, suggesting that excitotoxicity can occur independently of oxidative stress.Although this explanation is contrary to the opinion in most related publications, Boldyrev et al. (1999) pointed out that excitotoxicity death could be independent of oxidative stress in their study. In addition, although the involvement of JNK pathway in RGC death was proved in a previous study (Tezel et al., 2004; Wakabayashi et al., 2005; Kim et al., 2013) and in this study, the neuroprotective effect of voluntary running was not dependent on this pathway. This is consistent with a recent study by Fahrenthold et al. (2018) who reported that excitotoxicity to RGCs induced by N-methyl-D-aspartate(NMDA) could not be attenuated when JNK component was absent. This result indicates that excitotoxicity is not mediated by JNK pathway and the initial driver of it remains unknown. Therefore, it is possible that voluntary running can only influence excitotoxicity, but not oxidative stress, in RGC protection and this pathway is independent of the JNK pathway. The possible explanation for voluntary running influencing GS is that the degradation of glutamate needs ATP to supply the energy (Ishikawa, 2013). More ATP is produced when there is enough oxygen. One important role of exercise is to increase the blood supply of organs and to provide more oxygen to the retina (Zhang et al., 2012b), therefore supply of enough energy promotes the degradation of glutamate in this case and protects RGCs from degeneration. There is also another possibility that GS down-regulation after voluntary running is an indirect result of less RGC death. This study may provide a potential direction when considering the mechanisms of RGC death and the neuroprotective measures.
In summary, these data demonstrate that voluntary running can delay primary degeneration, but not secondary degeneration, of RGCs. This finding is significant since there is currently a lack of intervention which clearly demonstrates the delay of primary degeneration of RGCs following optic nerve damage. However, neuroprotection for RGCs which will die from secondary degeneration is important because degeneration of RGCs will continue for a long time period after the primary damage stops. In previous research, the polysaccharides extracted from Lycium barbarum (LBP) is found to delay secondary, but not primary, degeneration after PONT, therefore in future we will investigate the combined effects of voluntary running and LBP on the retinas after PONT.
Acknowledgments:The authors thank Jada Chia-Di Lee, The University of Hong Kong, for her assistance in the set-up of the voluntary running system.
Author contributions:Study concept and design, data analysis, providing reagents/materials/analysis tools, and manuscript preparation: KFS,HYL; experiment conduction: HYL, XH, MH; study guarantor: HYL. All authors approved the final version of this manuscript for publication.
Conflicts of interest:None declared.
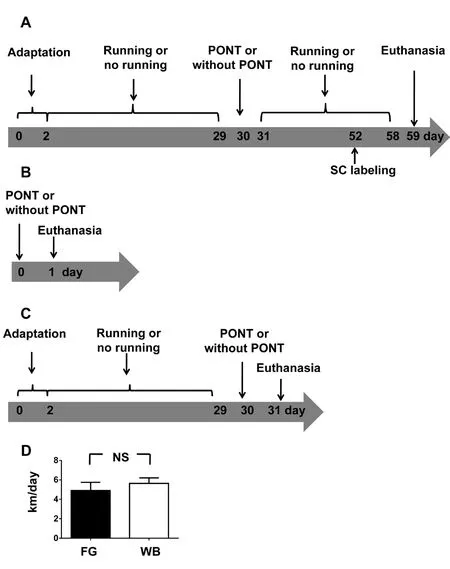
Figure 1 Schematic diagrams demonstrating the experimental procedure.
Financial support:The work was supported by the National Natural Science Foundation of China, No. 81501091 (to HYL), the Natural Science Foundation of Guangdong Province of China, No. 2015A030310201(to HYL); Medical Scientific Research Foundation of Guangdong Province of China, No. A2015393 (to HYL); the funds of Leading Talents of Guangdong Province of China, No. 2013 (to KFS); Programme of Introducing Talents of Discipline to Universities, No. B14036 (to KFS);the National Basic Research Program of China (973 Program), No.2015CB351800 (to KFS); the Fundamental Research Funds for the Central Universities, No. 21609101 (to KFS). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
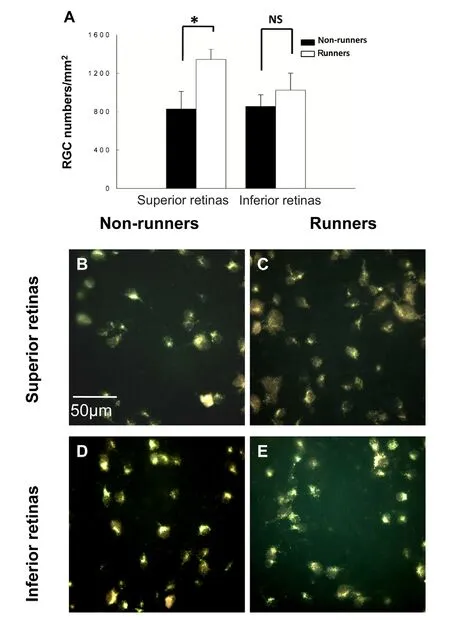
Figure 2 Comparisons of surviving RGCs both in the superior and inferior retinas between non-runners and runners 4 weeks after PONT.
Open peer reviewer:Fei Gao, West Virginia University School of Medicine, USA.
Additional file:Open peer review report 1.
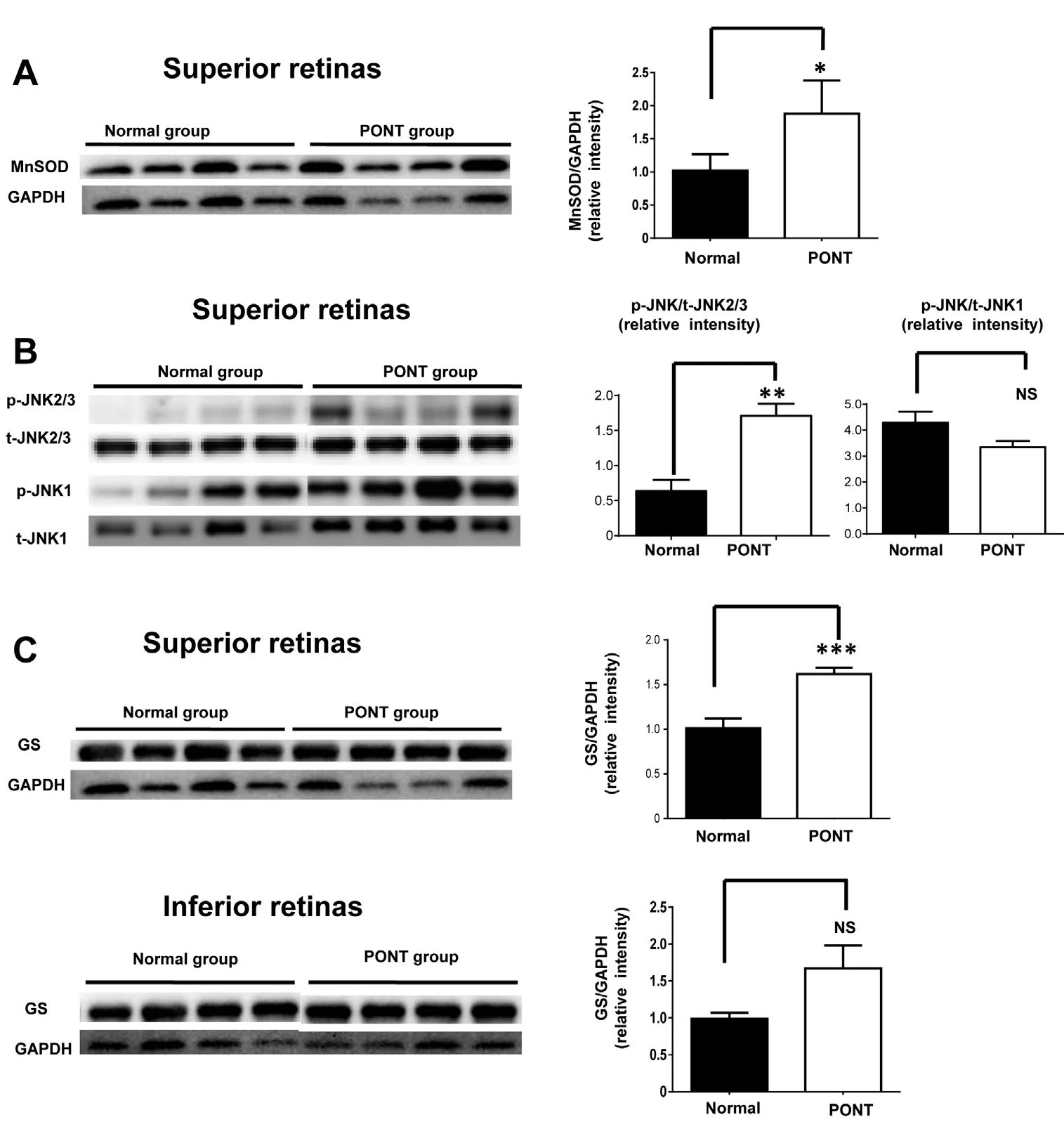
Figure 3 Comparisons of MnSOD,p-JNKs and GS expression between normal retinas and the retinas from rats subjected to PONT 1 day after surgery.
(A) There was significant difference in MnSOD expression in the superior retina between normal rats and the rats subjected to PONT. (B) There were no significant differences in p-JNK1 and p-JNK2/3 expression in the superior retina between normal rats and the rats subjected to PONT. (C) Significant difference in GS expression both in the superior and inferior retinas was observed between normal rats and the rats subjected to PONT. There were four rats in each group; *P < 0.05, **P< 0.01, ***P < 0.001; mean ± SEM. NS:Not significant; PONT: partial optic nerve transection; p-JNKs: phosphoc-Jun N-terminal kinases; MnSOD:manganese superoxide dismutase; GS:glutamine synthetase.
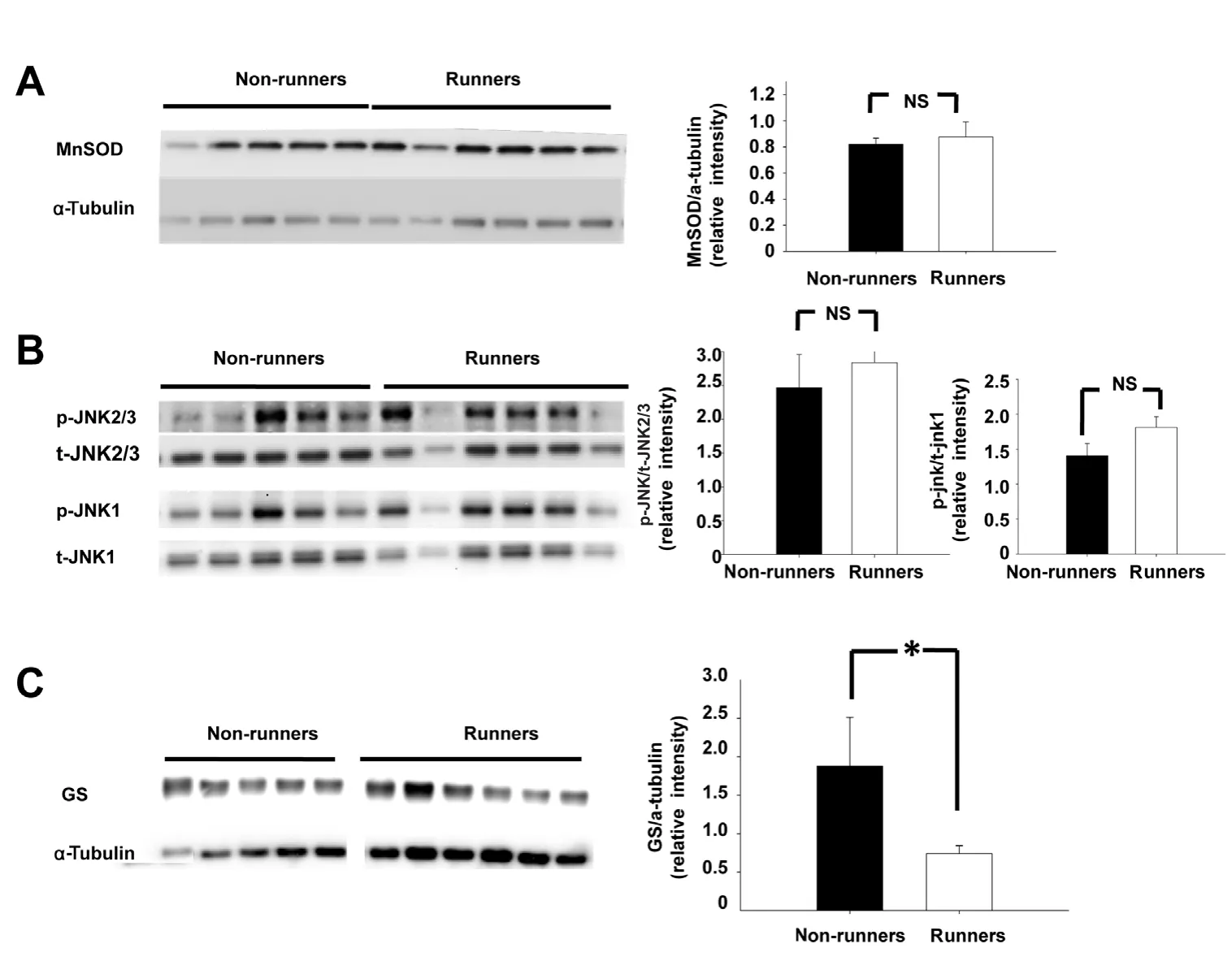
Figure 4 Comparisons of MnSOD,p-JNKs and GS expression in the superior retinas between nonrunners and runners.
(A) There was no significant difference in MnSOD expression in the superior retina between non-runners and runners 1 day after PONT. (B)There were no significant differences in p-JNK1 and p-JNK2/3 expression levels in the superior retina between non-runners and runners. (C) A significant difference in GS expression was detected in the superior retina but not in the inferior retina at 1 day after PONT between non-runners and runners. There were five rats in the non-runner group and six rats in the runner group. *P < 0.05; mean± SEM. NS: Not significant; PONT:partial optic nerve transection;p-JNKs: phospho-c-Jun N-terminal kinases; MnSOD: manganese superoxide dismutase; GS: glutamine synthetase.
Chiha W, LeVaillant CJ, Bartlett CA, Hewitt AW, Melton PE, Fitzgerald M, Harvey AR (2018) Retinal genes are differentially expressed in areas of primary versus secondary degeneration following partial optic nerve injury. PLoS One 13:e0192348.
Chiu K, Lau WM, Yeung SC, Chang RC, So KF (2008) Retrograde labeling of retinal ganglion cells by application of fluoro-gold on the surface of superior colliculus. J Vis Exp pii: 819.
Chu PH, Li HY, Chin MP, So KF, Chan HH (2013) Effect of lycium barbarum (wolヅerry) polysaccharides on preserving retinal function after partial optic nerve transection. PLoS One 8:e81339.
Crowley EK, Nolan YM, Sullivan AM (2018) Neuroprotective effects of voluntary running on cognitive dysfunction in an alpha-synuclein rat model of Parkinson’s disease. Neurobiol Aging 65:60-68.
Desseille C, Deforges S, Biondi O, Houdebine L, D’Amico D, Lamaziere A,Caradeuc C, Bertho G, Bruneteau G, Weill L, Bastin J, Djouadi F, Salachas F, Lopes P, Chanoine C, Massaad C, Charbonnier F (2017) Specific physical exercise improves energetic metabolism in the skeletal muscle of amyotrophic-lateral- sclerosis mice. Front Mol Neurosci 10:332.
Fahrenthold BK, Fernandes KA, Libby RT (2018) Assessment of intrinsic and extrinsic signaling pathway in excitotoxic retinal ganglion cell death. Sci Rep 8:4641.
Fitzgerald M, Bartlett CA, Harvey AR, Dunlop SA (2010) Early events of secondary degeneration after partial optic nerve transection: an immunohistochemical study. J Neurotrauma 27:439-452.
Gabriel-Salazar M, Morancho A, Rodriguez S, Buxo X, Garcia-Rodriguez N, Colell G, Fernandez A, Giralt D, Bustamante A, Montaner J, Rosell A (2018) Importance of Angiogenin and endothelial progenitor cells after rehabilitation both in ischemic stroke patients and in a mouse model of cerebral ischemia. Front Neurol 9:508.
Ghaffariyeh A, Honarpisheh N, Heidari MH, Puyan S, Abasov F (2011)Brain-derived neurotrophic factor as a biomarker in primary open-angle glaucoma. Optom Vis Sci 88:80-85.
Giacci MK, Bartlett CA, Smith NM, Iyer KS, Toomey LM, Jiang H,Guagliardo P, Kilburn MR, Fitzgerald M (2018) Oligodendroglia are particularly vulnerable to oxidative damage after neurotrauma in vivo.J Neurosci 38:6491-6504.
Hsueh SC, Chen KY, Lai JH, Wu CC, Yu YW, Luo Y, Hsieh TH, Chiang YH (2018) Voluntary physical exercise improves subsequent motor and cognitive impairments in a rat model of Parkinson’s disease. Int J Mol Sci 19. pii: E508.
Husain S, Abdul Y, Crosson CE (2012) Preservation of retina ganglion cell function by morphine in a chronic ocular-hypertensive rat model.Invest Ophthalmol Vis Sci 53:4289-4298.
Ishikawa M (2013) Abnormalities in glutamate metabolism and excitotoxicity in the retinal diseases. Scientifica (Cairo) 2013:528940.
Ju WK, Kim KY, Angert M, Duong-Polk KX, Lindsey JD, Ellisman MH,Weinreb RN (2009) Memantine blocks mitochondrial OPA1 and cytochrome c release and subsequent apoptotic cell death in glaucomatous retina. Invest Ophthalmol Vis Sci 50:707-716.
Killer HE, Pircher A (2018) Normal tension glaucoma: review of current understanding and mechanisms of the pathogenesis. Eye (London, England) 32:924-930.
Kim BJ, Liu Y, Silverman S, Wordinger R, Libby R, Pang IH, Clark A(2013) Protective effects of JNK inhibition in retinal ganglion cells and in retinal ischemia/reperfusion injury. Invest Ophthalmol Vis Sci 54:2624-2624.
Kurucz A, Bombicz M, Kiss R, Priksz D, Varga B, Hortobagyi T,Trencsenyi G, Szabo R, Posa A, Gesztelyi R, Szilvassy Z, Juhasz B (2018)Heme oxygenase-1 activity as a correlate to exercise-mediated amelioration of cognitive decline and neuropathological alterations in an aging rat model of dementia. Biomed Res Int 2018:7212861.
Lee D, Shim MS, Kim KY, Noh YH, Kim H, Kim SY, Weinreb RN, Ju WK (2014) Coenzyme Q10 inhibits glutamate excitotoxicity and oxidative stress-mediated mitochondrial alteration in a mouse model of glaucoma. Invest Ophthalmol Vis Sci 55:993-1005.
Lee JC, Yau SY, Lee TMC, Lau BW, So KF (2016) Voluntary wheel running reverses the decrease in subventricular zone neurogenesis caused by corticosterone. Cell Transplant 25:1979-1986.
Levkovitch-Verbin H (2015) Retinal ganglion cell apoptotic pathway in glaucoma: initiating and downstream mechanisms. Prog Brain Res 220:37-57.
Levkovitch-Verbin H, Quigley HA, Kerrigan-Baumrind LA, D’Anna SA,Kerrigan D, Pease ME (2001) Optic nerve transection in monkeys may result in secondary degeneration of retinal ganglion cells. Invest Ophthalmol Vis Sci 42:975-982.
Levkovitch-Verbin H, Quigley HA, Martin KR, Zack DJ, Pease ME,Valenta DF (2003) A model to study differences between primary and secondary degeneration of retinal ganglion cells in rats by partial optic nerve transection. Invest Ophthalmol Vis Sci 44:3388-3393.
Li H, Liang Y, Chiu K, Yuan Q, Lin B, Chang RC, So KF (2013) Lycium barbarum (wolヅerry) reduces secondary degeneration and oxidative stress, and inhibits JNK pathway in retina after partial optic nerve transection. PLoS One 8:e68881.
Li HY, Ruan YW, Ren CR, Cui Q, So KF (2014) Mechanisms of secondary degeneration after partial optic nerve transection. Neural Regen Res 9:565-574.
Li HY, Ruan YW, Kau PW, Chiu K, Chang RC, Chan HH, So KF (2015)Effect of Lycium Barbarum (wolヅerry) on alleviating axonal degeneration after partial optic nerve transection. Cell Transplant 24:403-417.
Liebmann JM, Lee JK (2017) Current therapeutic options and treatments in development for the management of primary open-angle glaucoma.Am J Manag Care 23:S279-292.
O’Hare Doig RL, Chiha W, Giacci MK, Yates NJ, Bartlett CA, Smith NM,Hodgetts SI, Harvey AR, Fitzgerald M (2017) Specific ion channels contribute to key elements of pathology during secondary degeneration following neurotrauma. BMC Neurosci 18:62.
Pinazo-Duran MD, Zanon-Moreno V, Gallego-Pinazo R, Garcia-Medina JJ (2015) Oxidative stress and mitochondrial failure in the pathogenesis of glaucoma neurodegeneration. Prog Brain Res 220:127-153.
Sleeman MW, Anderson KD, Lambert PD, Yancopoulos GD, Wiegand SJ(2000) The ciliary neurotrophic factor and its receptor, CNTFR alpha.Pharm Acta Helv 74:265-272.
Takada K, Munemasa Y, Kuribayashi J, Fujino H, Kitaoka Y (2011) Protective effect of thalidomide against N-methyl-D-aspartate-induced retinal neurotoxicity. J Neurosci Res 89:1596-1604.
Tezel G, Yang XJ, Yang JJ, Wax MB (2004) Role of tumor necrosis factor receptor-1 in the death of retinal ganglion cells following optic nerve crush injury in mice. Brain Res 996:202-212.
Vohra R, Tsai JC, Kolko M (2013) The role of inflammation in the pathogenesis of glaucoma. Surv Ophthalmol 58:311-320.
Wakabayashi T, Kosaka J, Oshika T (2005) JNK inhibitory kinase is up-regulated in retinal ganglion cells after axotomy and enhances BimEL expression level in neuronal cells. J Neurochem 95:526-536.
Weinreb RN, Khaw PT (2004) Primary open-angle glaucoma. Lancet 363:1711-1720.
Yau SY, Li A, Zhang ED, Christie BR, Xu A, Lee TM, So KF (2014a)Sustained running in rats administered corticosterone prevents the development of depressive behaviors and enhances hippocampal neurogenesis and synaptic plasticity without increasing neurotrophic factor levels. Cell Transplant 23:481-492.
Yau SY, Li A, Hoo RL, Ching YP, Christie BR, Lee TM, Xu A, So KF(2014b) Physical exercise-induced hippocampal neurogenesis and antidepressant effects are mediated by the adipocyte hormone adiponectin.Proc Natl Acad Sci U S A 111:15810-15815.
Yau SY, Lau BW, Zhang ED, Lee JC, Li A, Lee TM, Ching YP, Xu AM,So KF (2012) Effects of voluntary running on plasma levels of neurotrophins, hippocampal cell proliferation and learning and memory in stressed rats. Neuroscience 222:289-301.
Zhang K, Zhang L, Weinreb RN (2012a) Ophthalmic drug discovery:novel targets and mechanisms for retinal diseases and glaucoma. Nat Rev Drug Discov 11:541-559.
Zhang Y, Nateras OS, Peng Q, Rosende CA, Duong TQ (2012b) Blood flow MRI of the human retina/choroid during rest and isometric exercise. Invest Ophthalmol Vis Sci 53:4299-4305.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Human survival and immune mediated mitophagy in neuroplasticity disorders
- Neuroprotective effects of rapamycin on spinal cord injury in rats by increasing autophagy and Akt signaling
- Establishment and verification of a surgical prognostic model for cervical spinal cord injury without radiological abnormality
- Repair of peripheral nerve defects by nerve transposition using small gap bio-sleeve suture with different inner diameters at both ends
- Reinnervation of spinal cord anterior horn cells after median nerve repair using transposition with other nerves
- Repair of long segmental ulnar nerve defects in rats by several different kinds of nerve transposition