Repair of long segmental ulnar nerve defects in rats by several different kinds of nerve transposition
Fei Yu , You-Lai Yu , Su-Ping Niu Pei-Xun Zhang Xiao-Feng Yin Na Han Ya-Jun Zhang Dian-Ying Zhang Yu-Hui Kou ,Bao-Guo Jiang
1 Peking University People’s Hospital, Beijing, China
2 The Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu Province, China
Abstract Multiple regeneration of axonal buds has been shown to exist during the repair of peripheral nerve injury, which confirms a certain repair potential of the injured peripheral nerve. Therefore, a systematic nerve transposition repair technique has been proposed to treat severe peripheral nerve injury. During nerve transposition repair, the regenerated nerve fibers of motor neurons in the anterior horn of the spinal cord can effectively grow into the repaired distal nerve and target muscle tissues, which is conducive to the recovery of motor function.The aim of this study was to explore regeneration and nerve functional recovery after repairing a long-segment peripheral nerve defect by transposition of different donor nerves. A long-segment (2 mm) ulnar nerve defect in Sprague-Dawley rats was repaired by transposition of the musculocutaneous nerve, medial pectoral nerve, muscular branches of the radial nerve and anterior interosseous nerve (pronator quadratus muscle branch). In situ repair of the ulnar nerve was considered as a control. Three months later, wrist flexion function, nerve regeneration and innervation muscle recovery in rats were assessed using neuroelectrophysiological testing, osmic acid staining and hematoxylin-eosin staining, respectively. Our findings indicate that repair of a long-segment ulnar nerve defect with different donor nerve transpositions can reinnervate axonal function of motor neurons in the anterior horn of spinal cord and restore the function of affected limbs to a certain extent.
Key Words: nerve regeneration; nerve transposition repair; conical sleeve; small gap sleeve bridging; ulnar nerve; target organ; muscle; nerve reinnervation; neural regeneration
Introduction
Peripheral nerve injury is a common condition, and can cause motor and sensory dysfunction. Delayed treatment can result in a poor prognosis and lead to disability (Lu et al., 2016; Bergmeister et al., 2018). Early recovery of the continuity of injured nerves is beneficial for the functional recovery of peripheral nerves. The treatment method varies for different peripheral nerve injuries. Currently, nerve sutures (van der Avoort et al., 2013; Zhang et al., 2013; Leuzzi et al., 2014), neural transplantation (Lee and Wolfe, 2000;Lin et al., 2013; Trehan et al., 2016) and artificial nerve bridging (Ahmad et al., 2017; Bozkurt et al., 2017; Liu et al.,2017; Quan et al., 2017) are commonly used to repair damaged peripheral nerves. A damaged peripheral nerve without obvious defects can be repaired by suturing the epineurium or perineurium (Gosk et al., 2005; Bamba et al., 2016; Shi et al., 2018). Middle- and short-segment peripheral nerve defects can be repaired by nerve lengthening or small-seg-ment nerve transplantation (Kou and Jiang, 2016; Sallam et al., 2017; Pollins et al., 2018). However, severe peripheral nerve injury, such as long-distance peripheral nerve defect and proximal donor nerve loss (such as brachial plexus injury and sacral plexus nerve injury), cannot be repaired by traditional methods. Thus, most patients with severe injuries are unable to get effective treatment, highlighting an urgent need to find new repair methods (Houschyaret al., 2016;Emamhadi and Andalib, 2018; Jiang and Lao, 2018).
From the joint efforts of clinicians and researchers, the repair methods of peripheral nerve injury have been improved, and remedial repair methods by nerve or muscle transposition have been proposed to achieve partial repair of limb function (Afshari et al., 2018; Li et al., 2018; Smith et al., 2018). As early as 1966, researchers reported the translocation of the musculocutaneous nerve to the median nerve(Benassy, 1966). Later, thoracic medial nerve transposition of the musculocutaneous nerve and axillary nerve also achieved good results (Samardzic et al., 2002). An anatomical study on the displacement and repair of the anterior interosseous nerve has also been performed (Wang et al.,1997). Thus, the transposition repair mode is gaining more and more recognition.
Our previous studies have shown that a single new axon can produce many new axons; when a distal nerve has enough regeneration space, a proximal single nerve fiber can regenerate to a distal nerve with three or four mature axonal buds (Jiang et al., 2007). This study confirmed multiple regeneration during peripheral nerve regeneration, suggesting that it is possible to repair the gross nerve with a single fine nerve. Follow-up studies based on this phenomenon of multiple regeneration have used a single donor nerve to simultaneously repair two or more donor nerves (Yu et al., 2016),and a partial donor nerve to repair the whole nerve trunk(Zhao et al., 2007). The above studies have demonstrated good nerve regeneration and functional recovery, suggesting that there is a certain regenerative potential in peripheral nerves. The potential of nerve regeneration can be fully utilized during nerve repair to solve the difficult problems that cannot be solved clinically at present. More importantly,in the previous study in which peripheral nerve injury was repaired by cross-innervation, the authors also found nerve remodeling at the spinal cord levels during the repair of the peripheral nerve (Yu et al., 2015, 2016). Therefore, they proposed a new idea that the repair and regeneration after peripheral nerve injury reflects the system remodeling of the interaction between the nerve and terminal effector, and thus considered that nerve transposition repair technology has potential as a clinical repair method for severe peripheral nerve injury.
This study used different donor nerves, the musculocutaneous nerve, medial pectoral nerve, muscular branches of the radial nerve and anterior interosseous nerve (pronator quadratus muscle branch), to repair a long-segment ulnar nerve defect in rats so as to explore the regeneration and nerve functional recovery of peripheral nerve injury repaired by different nerve transposition.
Materials and Methods
Experimental animals
Thirty-six male Sprague-Dawley rats, aged 6–7 weeks and weighing 200–220 g, were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd., China (license No. SCXK (Jing) 2016-0006). All rats were housed in individual cages of a specific-pathogen-free region of the Experimental Animal Center of Peking University People’s Hospital,China, at 24 ± 2°C and relative humidity of 50–55%. They were allowed free access to standard pellet feed and clean water in a 12:12-hour light/dark cycle daily. The rats were intraperitoneally anesthetized with 1% sodium pentobarbital, 30 mg/kg (Merck, Darmstadt, Germany). Efforts were made to ensure that the experimental operation minimized the pain of the animals. All animal care and animal surgeries were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No.85-23, revised 1985). This study was approved by the Animal Ethics Committee of Peking University People’s Hospital on December 9, 2015 (approval No. 2015-50).
Preparation of conical sleeve with different inner diameters at both ends
The sleeve was prepared in accordance with our patented technology (patent number ZL01 136314.2.). Briefly, the mold was composed of a guide roller, contour shaping sleeve and fixed base. Chitosan was dissolved in dilute acetic acid aqueous solution. Stock solution without foam was obtained by vacuuming and defoaming. The mold was evenly coated with foam-free stock solution, and solidified in NaOH aqueous solution. The hollow tube with different inner diameters at both ends was obtained after setting. The hollow round tube combined with the sleeve mold was fixed on the base.The sleeve corresponding to the mold was sleeved from the thin end and fixed to the base. The sample was placed in double-distilled water for molding. The inner diameter of one end of the sleeve was 0.5–1.0 mm, and the inner diameter of the other end was 0.8–1.2 mm. The thickness of the wall was controlled at approximately 0.3 mm.
Group assignment
The rats were intraperitoneally anesthetized with 1% sodium pentobarbital (Merck), 30 mg/kg. The right brachial plexus was exposed under aseptic conditions. Corresponding nerves were denervated in the middle of the exposed musculocutaneous nerve, medial pectoral nerve, muscular branches of the radial nerve or anterior interosseous nerve(pronator quadratus muscle branch) and the ulnar nerve.The donor nerve and ulnar nerve received a small-gap conical sleeve suture, leaving a 2-mm gap between the nerves.The donor nerve was placed at the thin end of the conical sleeve and the ulnar nerve was placed in the wide end of the conical sleeve. The other two broken ends of each nerve were reversely sutured to the surrounding muscles to prevent the nerves from repairing themselves.
The rats were randomly assigned to six groups. (1) Musculocutaneous nerve transposition repair group (n = 6): after denervation of the ulnar nerve and musculocutaneous nerve,the proximal end of the musculocutaneous nerve and the distal end of the ulnar nerve received a small gap sleeve suture. (2)Medial pectoral nerve transposition repair group (n = 6): after denervation of the ulnar nerve and medial pectoral nerve,the proximal end of the medial pectoral nerve and the distal end of the ulnar nerve received a small gap sleeve suture. (3)Radial nerve muscular branch transposition repair group (n =6): after denervation of the ulnar nerve and radial nerve, the proximal end of the muscular branches of radial nerve and the distal end of the ulnar nerve received a small gap sleeve suture. (4) Anterior interosseous nerve (pronator quadratus muscle branch) transposition repair group (n = 6): after denervation of the ulnar nerve and anterior interosseous nerve(pronator quadratus muscle branch), the proximal end of the anterior interosseous nerve (pronator quadratus muscle branch) and the distal end of the ulnar nerve received a small gap sleeve suture. (5) Ulnar nerve in situ repair group (n = 6):after ulnar nerve denervation, the epineurium of the two broken ends of the ulnar nerves was sutured in situ. (6) Control group (n = 6): the ulnar nerve did not receive any treatment;the original anatomical relationship was maintained (Figure 1). At 3 months postoperatively, samples were harvested for histological observation.
General observation
After model establishment, rats were fed normally. General conditions of the rats were observed and recorded weekly,including activity, diet, changes in surgical incision and limb movements. The neurological recovery was observed before the rats were sacrificed.
Wrist flexion function in rats determined using neuroelectrophysiological testing
At 3 months postoperatively, the rats were fixed on the operating table after anesthesia. Their forelimbs were fixed with pins. The skin of rat forelimbs was cut under an aseptic state to expose and bluntly dissociate nerves at the site of repair. A stimulating electrode was placed at the nerve trunk after repair. The ulnar nerve was stimulated with a Synergy electrophysiologic apparatus (Medle-cSynergy; Oxford Instrument Inc., United Kingdom) at 50 Hz and 0.9 mA continuous square waves. Wrist flexion of rat forelimbs was photographed and recorded with a digital camera (Canon,Tokyo, Japan) before and after stimulation. Results were synthesized with PowerPoint 2013 software (Microsoft Corporation, Redmond, Washington, USA). The difference in wrist flexion was compared between groups.
Changes of nerve fibers observed by osmic acid staining
At 3 months postoperatively, nerve tissue proximal and distal to the sleeve was anesthetized, trimmed, and fixed in 4%paraformaldehyde (Merck, Darmstadt, Germany). Nerve tissue was washed with running water, stained with 1%osmic acid (Spi, West Chester, USA) for 12 hours, washed with running water, dehydrated through a graded alcohol series, permeabilized, embedded in wax, and serially sliced into 5-μm-thick sections. These sections were permeabilized, mounted, and observed under a light microscope(Olympus, Tokyo, Japan). Five fields at 400× magnification were randomly selected from each rat. ImageJ Image Analysis Software (National Institutes of Health, Bethesda, MD,USA) was used to calculate the number of myelinated nerve fibers proximal and distal to the sleeve, the mean diameter and area of axons of myelinated nerve fibers at 5 mm distal to the sleeve, and mean thickness of the myelin sheath.
Changes of innervated muscle observed using hematoxylin-eosin staining
At 3 months postoperatively, flexor carpi ulnaris was harvested at the site of repair after anesthesia. Wet weight of muscle was measured and recorded with an analytical balance(Tianjin D&T Precision Instrument Co., Ltd., Tianjin, China). After trimming, samples were fixed in 4% paraformaldehyde, dehydrated through a graded alcohol series, permeabilized, embedded in wax, and serially sliced into 5-μm-thick sections. These sections were stained with hematoxylin (Beijing Yili Fine Chemicals Co., Ltd., Beijing, China) for 5 minutes, washed with running water for 1 minute, and stained with 0.5% eosin (Beijing Yili Fine Chemicals Co., Ltd.) for 1 minute. After washing with running water, the samples were permeabilized and mounted. Histological characteristics of transverse slices of muscle were observed under the light microscope (Olympus). Five fields were randomly selected from each rat at 200× magnification. Muscle cross-sectional area was measured with QWin software package Q550 IW image analysis system (Leica Imaging Systems Ltd., Cambridge, England), and the mean value was calculated.
Statistical analysis
The data were analyzed using SPSS 17.0 software (SPSS,Chicago, IL, USA). Measurement data were expressed as the mean ± SD. The difference among groups was compared with one-way analysis of variance. Paired comparison was conducted using the least significant difference test. A value of P < 0.05 was considered statistically significant.
Results
General status of rats with ulnar nerve injury after repairing with several kinds of nerve transposition
All rats survived and were in good condition. They ate and drank water normally. There was no inflammation around the incision. No infection was detectable at the site of the incision or elsewhere in the body. No other complications were found.The models were successfully established. Compared with the control group, at 3 months postoperatively, various degrees of muscle atrophy at the innervation site were observed in the nerve transposition and in situ repair groups before the rats were sacrificed. In the transposition repair groups, neural adhesions were mild, and the sleeve was partially degraded and absorbed. After careful removal of the unabsorbed sleeve, we found that the nerve tissue was evenly connected at the repair site, and no obvious neuroma had formed. In the in situ repair group, obvious neuroma was seen at the site of repair.
Wrist flexion function in rats with ulnar nerve injury after repairing with several kinds of nerve transposition
After continuous electrical stimulation, muscle contraction and wrist flexion were evident in rat forelimbs of each repair group (Figure 2). There was no significant difference in stimulus response between the affected limbs in each group.
Several kinds of nerve transposition promote ulnar nerve regeneration in rats
The myelinated nerve fibers proximal to the sleeve had a uniform morphology in each group, showing normal nerve fibers. The number of myelinated nerve fibers was less in the transposition repair groups than in the in situ repair and control groups (Table 1). The number of myelinated nerve fibers was more at the distal end than at the proximal end in each repair group (P < 0.01). Compared with the in situ repair group, the transposition repair groups had a decreased number of myelinated nerve fibers at the distal end (P < 0.01).However, axon diameter, myelin area, and myelin thickness of myelinated nerve fibers were not significantly different between the in situ repair group and the transposition repair groups (P > 0.05; Table 1 and Figure 3).
Several kinds of nerve transposition promote muscle recovery in rats with ulnar nerve injury
Muscles atrophy to varying degrees was detected in each transposition and in situ repair group compared with the control group (Figure 4). Muscle wet weight was significantly lower in each transposition repair group than in the control group (P < 0.05), and the cross-sectional area of muscle fibers was significantly smaller in each transposition repair group compared with the control group (P < 0.01).However, no significant differences in gross shape, muscle wet weight or cross-sectional area of muscle fibers of the ulnar wrist flexor were found between the transposition repair groups and in situ repair group (P > 0.05; Table 2).
Discussion
It is important to restore the continuity of injured nerves as soon as possible after peripheral nerve injury (Wang et al.,2017; Costales et al., 2018; Hoang et al., 2018). Nevertheless, when the nerve is severely damaged or the nerve has a large defect, often nerve continuity cannot be effectively restored due to the loss or defect of the donor nerve, which poses a difficult problem for clinicians (Post et al., 2012;Limthongthang et al., 2013; Grinsell and Keating, 2014). In view of this kind of serious peripheral nerve injury, clinical experts have been trying many ways to repair the nerve.In 1986, Gu Yudong applied a C7 nerve transfer on the healthy side to treat a brachial plexus root avulsion injury, and achieved effective functional recovery (Gu, 2007).Muhetidier et al. (2011) confirmed the effect of this operation. Wu et al. (2017) repaired the posterior interosseous nerve and reconstructed the abduction function of the hand by transposition of the radial nerve branch innervating the supinator muscle, and achieved good results. The method of nerve transposition repair has gradually been recognized by the medical community (Chepla and Bafus, 2018; Leland et al., 2018). However, except for radial avulsion injury, nerve transposition repair has not been recognized as an active repair method, and lacks systematic examination, and theoretical and experimental support. In our previous studies, we have found multiple regeneration and potential for nerve repair during the repair of peripheral nerve injury. Therefore,in this study, the ulnar nerve was selected for the peripheral nerve injury. The musculocutaneous nerve, medial pectoral nerve, radial nerve muscle branch and anterior interosseous nerve (pronator quadratus muscle branch) originating from brachial plexus were transposed to repair the injured ulnar nerve through a small gap suture of a conical sleeve. The results demonstrate effective regeneration and functional recovery of the ulnar nerve repaired by different kinds of nerve transposition, which provides theoretical support for the clinical application of transposition repair in the treatment of severe peripheral nerve injury.
Chitin cannula with a small gap is effective for repairing peripheral nerve injury. The degradation of the material will not affect the repair of the injured nerve. This material is beneficial for nerve regeneration, which has been confirmed in our previous study (Zhang et al., 2005). This study performed nerve transposition with a conical sleeve with different inner diameters at both ends by small-gap-sleeve bridging. Based on the cylindrical chitin sleeve developed earlier by our research group, the sleeve used in this study with different inner diameters at both ends was made by improving the manufacturing process. The advantages of this sleeve suture are mainly reflected in: (1) simplified operation of the nerve suture. Only two to three needles are needed tosuture the nerves at both ends of the transposition to achieve a stable suture effect. (2) Nerve tension-free suture is achieved.During nerve transposition repair, the two nerves sutured are usually two different nerves, creating a problem when the diameters of the two nerve ends do not match. The suture method in this study can achieve the effect of tension-free suture only if the inner wall of the sleeve matches the diameter of the sutured nerve. (3) During nerve repair, the blocked axoplasmic transport near the broken end results in increased pressure, disordered protein and collagen synthesis, and proximal regenerated axonal buds that are entangled with collagen,which can lead to the formation of neuromas (Willard and Skene, 1982; Seckel et al., 1984). The use of a conical sleeve can effectively reduce the generation of neuromas; the sleeve surrounds the nerve, and after nerve injury, local tissue swells and fills the sleeve gap. This can reduce the escape of regenerated nerve fibers to the outside of the nerve and reduce the occurrence of neuromas. (4) Providing a good regeneration microenvironment and forming a closed nerve regeneration chamber by sleeve suture can reduce the interference of the local inflammatory response of the repaired nerve, and provide a good microenvironment for nerve regeneration, which is conducive to the repair after injury. In addition, the application of the small gap suture of the biological sleeve with different inner diameters at both ends in the transposition repair of rat peripheral nerve is not only limited to the model of one donor nerve repairing one receptor nerve, but also to the simultaneous repair of two or more nerves by one nerve.

Table 1 Quantitative analysis of regenerated nerves of each group
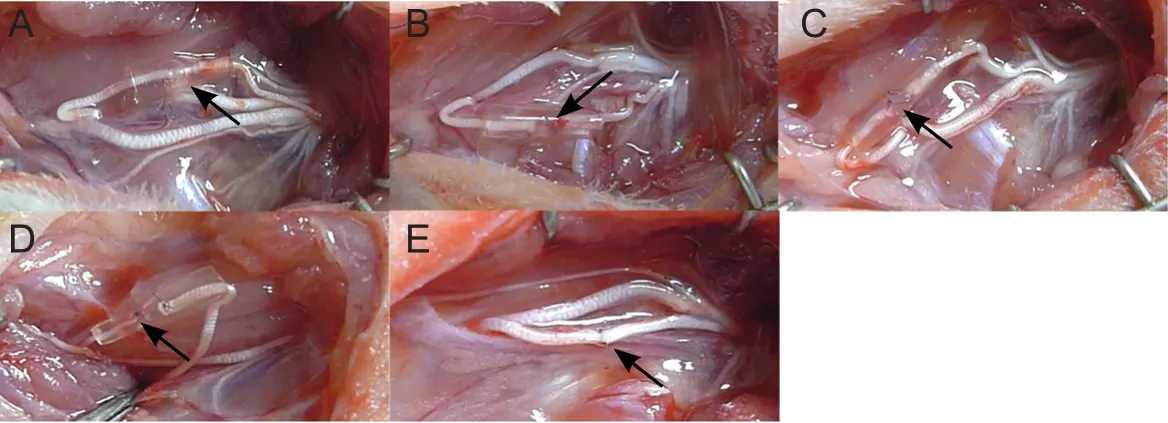
Figure 1 Repair of ulnar nerve defects in rats with several kinds of nerve transposition.
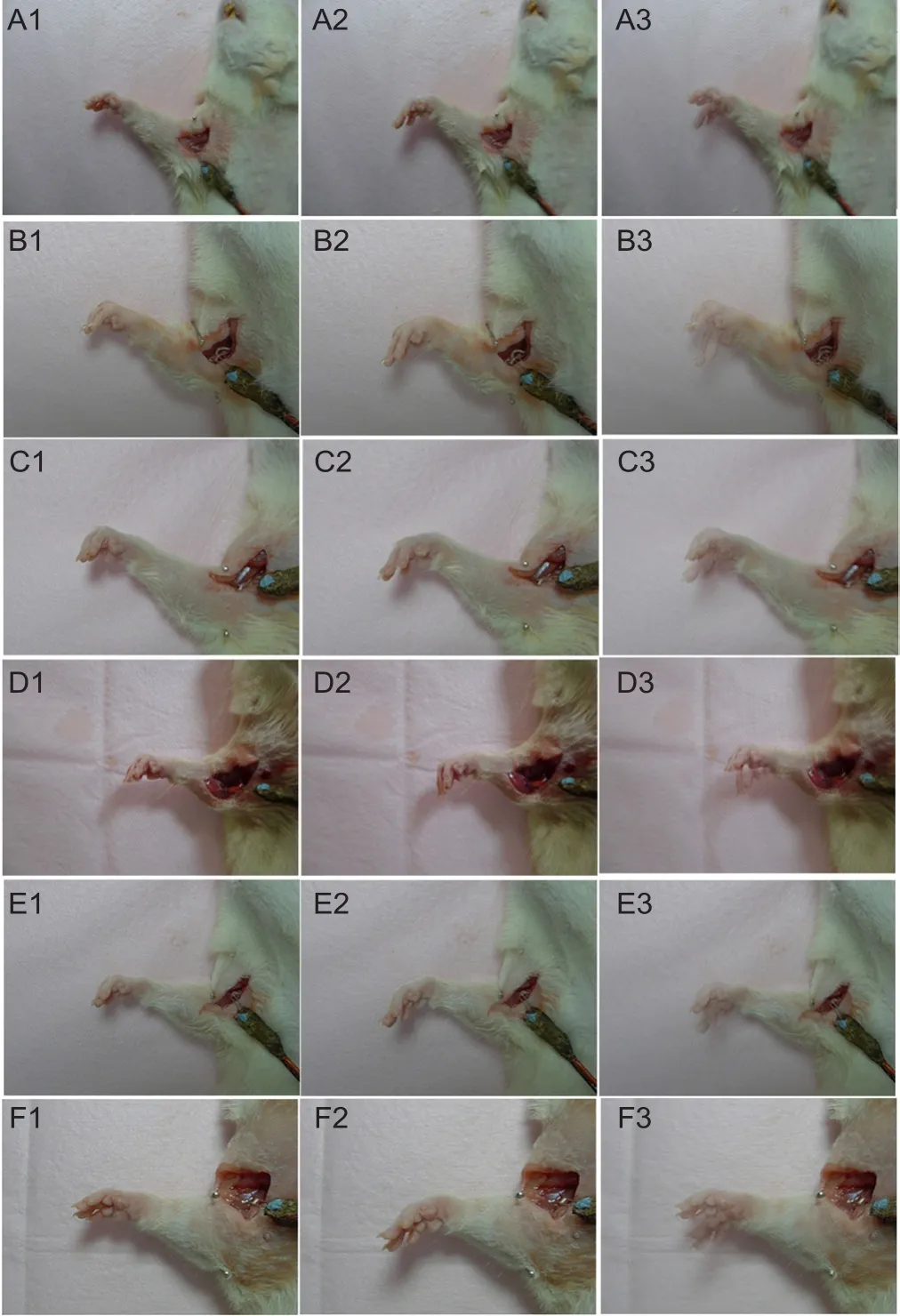
Figure 2 Wrist flexion function in rat forelimbs.
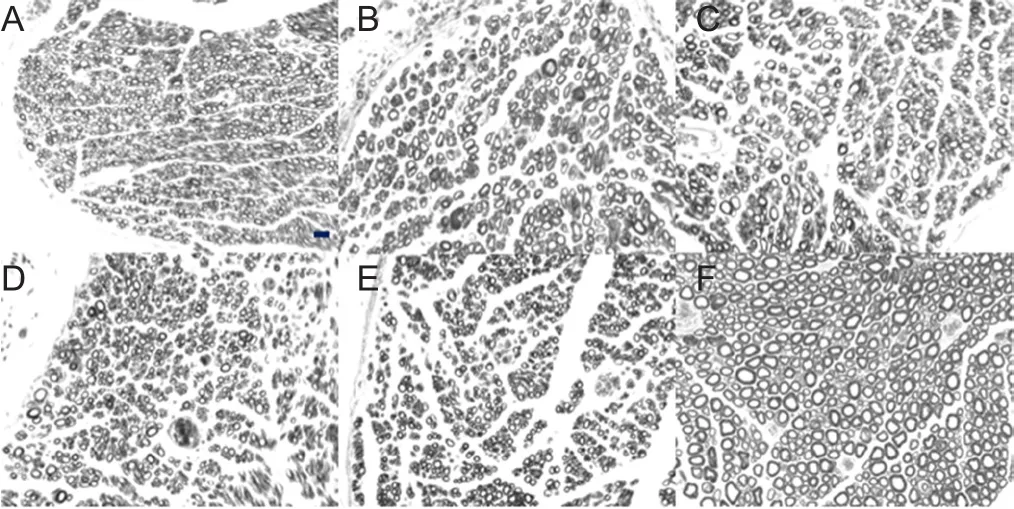
Figure 3 Osmic acid staining of transverse sections of new myelinated nerve fibers at the distal end in rats with ulnar nerve injury.
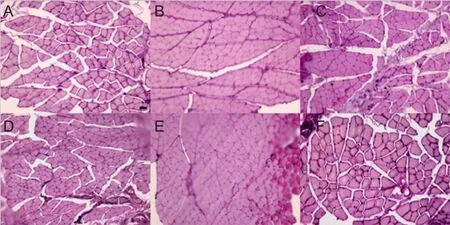
Figure 4 Muscle fiber morphology of the ulnar wrist flexor on the affected side in rats (hematoxylin-eosin staining).

Table 2 Comparison of muscle wet weight and muscle fiber cross-sectional area of ulnar wrist flexor in each group
In this study, the musculocutaneous nerve, medial pectoral nerve, muscular branches of the radial nerve and anterior interosseous nerve (pronator quadratus muscle branch) for proximal transposition were thinner than that of the repaired ulnar nerve and had a fewer number of nerve fibers than the ulnar nerve. However, after transposition, the number of myelinated nerve fibers regenerated at the distal end was obviously increased. Compared with in situ suture, the diameter and area of axons and the thickness of the myelin sheath of myelinated nerve fibers had no significant difference. A similar effect as the in situ suture was achieved in the recovery of nerve function. The results are consistent with the multiple regeneration rule and nerve repair potential of peripheral nerves. The specific transposition repair scheme should be personalized according to the condition of each patient in clinical.
In previous studies, when the proximal common peroneal nerve was used to repair the distal tibial and common peroneal nerves, the motor neurons in the anterior horn of the spinal cord showed corresponding structural and functional remodeling as the nerve function recovered (Yu et al., 2015,2016). Because of the limitations of the experimental methods and design in this study, we did not study the spinal cord and brain function after nerve transposition repair. In subsequent research, experiments will be designed to further study functional remodeling. Additionally, we were unable to measure the electrophysiological nerve conduction velocity and conduction wave amplitude. This is mainly due to the short forelimb nerve of rats. The effective measurement distance after repair cannot meet the needs of detecting electrophysiological conduction velocity and conduction wave amplitude, and cannot be better measured.
In summary, effective regeneration of the ulnar nerve and functional recovery of the nerve and effector can be achieved after nerve transposition repair by different nerves for ulnar nerve defect. The transposition repair method is conducive to the repair of peripheral nerve defect, with a wide range of sources for the donor nerve. These findings provide experimental evidence for clinical repair of long-segment peripheral nerve defect.
Author contributions:Implementation of major experimental operations and paper writing: FY and YLY; implementation of some experimental operations and data analysis: SPN, YJZ, and DYZ; experimental evaluation and revision: PXZ, XFY, and NH; experimental design, evaluation and paper revision: YHK and BGJ. All authors approved the final version of the paper.
Conflicts of interest:The authors declare no conflicts of interest.
Financial support:This research was continuously funded by the National Natural Science Foundation of China, No. 31571236, 31571235(to YHK, PXZ); the National Key Research and Development Program of China, No. 2016YFC1101604 (to DYZ); the National Key Basic Research Program of China (973 Program), No. 2014CB542200 (to BGJ); the Ministry of Education Innovation Program of China, No. IRT_16R01 (to BGJ);the Beijing Science and Technology New Star Cross Program of China, No.2018019 (to PXZ). The funding bodies played no role in the study design,in the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the article for publication.
Institutional review board statement:All animal experimental procedures were approved by the Animal Ethics Committee of Peking University People’s Hospital on December 9, 2015 (approval No. 2015-50) and performed in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23,revised 1985).
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Human survival and immune mediated mitophagy in neuroplasticity disorders
- Voluntary running delays primary degeneration in rat retinas after partial optic nerve transection
- Neuroprotective effects of rapamycin on spinal cord injury in rats by increasing autophagy and Akt signaling
- Establishment and verification of a surgical prognostic model for cervical spinal cord injury without radiological abnormality
- Repair of peripheral nerve defects by nerve transposition using small gap bio-sleeve suture with different inner diameters at both ends
- Reinnervation of spinal cord anterior horn cells after median nerve repair using transposition with other nerves