Neuroprotective effects of rapamycin on spinal cord injury in rats by increasing autophagy and Akt signaling
Xi-Gong Li, Jun-Hua Du, Yang Lu, Xiang-Jin Lin
Department of Orthopedic Surgery, The First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, Zhejiang Province, China
Abstract Rapamycin treatment has been shown to increase autophagy activity and activate Akt phosphorylation, suppressing apoptosis in several models of ischemia reperfusion injury. However, little has been studied on the neuroprotective effects on spinal cord injury by activating Akt phosphorylation. We hypothesized that both effects of rapamycin, the increased autophagy activity and Akt signaling, would contribute to its neuroprotective properties. In this study, a compressive spinal cord injury model of rat was created by an aneurysm clip with a 30 g closing force.Rat models were intraperitoneally injected with rapamycin 1 mg/kg, followed by autophagy inhibitor 3-methyladenine 2.5 mg/kg and Akt inhibitor IV 1 μg/kg. Western blot assay, immunofluorescence staining and terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling assay were used to observe the expression of neuronal autophagy molecule Beclin 1, apoptosis-related molecules Bcl-2, Bax, cytochrome c, casp ase-3 and Akt signaling. Our results demonstrated that rapamycin inhibited the expression of mTOR in injured spinal cord tissue and up-regulated the expression of Beclin 1 and phosphorylated-Akt. Rapamycin prevented the decrease of bcl-2 expression in injured spinal cord tissue,reduced Bax, cytochrome c and caspase-3 expression levels and reduced the number of apoptotic neurons in injured spinal cord tissue 24 hours after spinal cord injury. 3-Methyladenine and Akt inhibitor IV intervention suppressed the expression of Beclin-1 and phosphorylated-Akt in injured spinal cord tissue and reduced the protective effect of rapamycin on apoptotic neurons. The above results indicate that the neuroprotective effect of rapamycin on spinal cord injury rats can be achieved by activating autophagy and the Akt signaling pathway.
Key Words: nerve regeneration; rapamycin; mammalian target of rapamycin, mTOR; autophagy; Beclin 1; 3-methyladenine; acute spinal cord injury; apoptosis; Bax; Akt; neural regeneration
Introduction
Spinal cord injury (SCI) is a serious traumatic disease which results in neurological deficits and motor dysfunction (Singh et al., 2014; Berlowitz et al., 2016; Bravo-Esteban et al., 2017;Noonan et al., 2017). Primary damage is results from an irreversible mechanical injury to the spinal cord. In contrast,secondary damage presents with a variety of biochemical and pathological events, including hypoxia, edema, inflammation and apoptosis (Cevikbas et al., 2011). Several pharmacological agents have been developed to attenuate secondary damage after SCI.
Autophagy, a normal catabolic mechanism, degrades cytosolic organelles and proteins in cells (Klionsky et al., 2000;Levine et al., 2004, 2008; Hayashi et al., 2009). Rapamycin can trigger autophagy and subsequently provide neuroprotective effects in several models of neurodegenerative disorders (Yamamoto et al., 2006; Michiorri et al., 2010; Wold et al., 2016;Moloudizargari et al., 2017). Increased autophagic activity by rapamycin also has been found to have protective roles in various traumatic and ischemic brain injuries (Diskin et al., 2005;Erlich et al., 2007; Xiangnan et al., 2013; Song et al., 2017).Several recent studies demonstrate that autophagic activity increases at the neural lesion after SCI (Kanno et al., 2009;Akira et al., 2011; Chen et al., 2012, 2013; Hong-Yu et al.,2013; Tang et al., 2014; Lin et al., 2017; Hu et al., 2017; Yan et al., 2017; Zhou et al., 2017). Our previous studies showed that rapamycin activated autophagy, markedly reduced spinal cord damage and improved motor function in rat models of SCI (Li et al., 2016; Du et al., 2017).
Phosphoinositide 3-kinase/serine-threonine kinase (PI3K/Akt) signal pathway is a significant pathway for the survival of cells. Pro-survival kinase Akt can be phosphorylated following PI3K activation, which had benefits in protecting against cell death (Markman et al., 2010). PI3K/Akt signal activation has been observed in damaged neural tissue after SCI(Peixun et al., 2015; Gu et al., 2017; Wang et al., 2017). Rapamycin treatment can activate Akt phosphorylation (O’Reilly et al., 2006; Wullschleger et al., 2006), which has effects on apoptotic suppression in several models of ischemia reperfusion injury (Lee et al., 2004; Carloni et al., 2009).
The aim of this study is to investigate whether administration of rapamycin simultaneously triggers both autophagy and Akt signaling in a rat model of SCI. We also evaluate the possible effects of activation of autophagy and Akt signaling following rapamycin treatment in neural tissue damage after SCI. We hypothesize that both enhanced autophagy activity and Akt signaling played important roles in protective roles of rapamycin in a rat model of SCI.
Materials and Methods
Animals
A total of 72 adult male Sprague-Dawley rats weighing 300 to 330 g, provided by the Laboratory Animal Center of Zhejiang Province of China, were used in this study. The surgical procedures were performed in an aseptic manner and were approved by the Research Animal Resources and Care Committee of Zhejiang University (SCXK (Zhe) 2014-0001).An initial batch of 48 rats were divided into the following three groups: sham, SCI + vehicle, and SCI + rapamycin(RAPA) (n = 16 per group). In the SCI groups, rats received laminectomy with SCI. In the SCI + RAPA group, RAPA(one single dose of 1 mg/kg body weight; Selleck, Houston,TX, USA) was injected intraperitoneally on the onset of SCI.In the SCI + vehicle group, the corresponding volume of saline was injected intraperitoneally on the onset of SCI.
To examine the potential roles of activation of autophagy and Akt signaling, an additional 24 rats were used and allocated into two groups: SCI + RAPA + PI3K inhibitor,3-methyladenine (3-MA), and SCI + RAPA + Akt inhibitor IV (IV) (n = 12 per group). In the SCI + RAPA + 3-MA group, 3-MA (one single dose of 2.5 mg/kg body weight; Selleck) was injected intraperitoneally 20 minutes before SCI.In the SCI + RAPA + IV group, Akt inhibitor IV (one single dose of 1 μg/kg body weight; Santa Cruz Biotechnology,Santa Cruz, CA, USA) was injected intrathecally 20 minutes before SCI. Intrathecal injection was performed as described previously (Kanno et al., 2009).
SCI model establishment
The rats were intraperitoneally anesthetized with chloral hydrate (400 mg/kg). A mid-line skin incision (15 to 20 mm length) was made to expose the laminae of the T6–10 vertebrae. Laminectomy was performed about the level of T8. A compressive SCI model was created with an aneurysm clip (30 g closing force). The clip was removed after flaccid paralysis was observed in the rat’s hind legs. This technique created a model of complete and irreversible SCI with complete paralysis of the lower extremities (Rivlin et al., 1978). Hand compression of the urinary bladder was performed twice a day until spontaneous voiding began. Rats in the sham group received laminectomy only, without spinal cord contusion. All rats were fed singly in a room at 27°C. The rats were killed with an overdose of sodium pentobarbital (100 mg/kg) and intracardially perfused with 0.1 mmol phosphate buffered saline (PBS) (pH 7.4) and 4% paraformaldehyde (pH 7.4)at 24 hours after SCI. A 10 mm length of spinal cord tissue containing the center of the lesion site was then collected.The serial 7 μm transverse sections cut at 250-μm intervals around the epicenter were used for immunohistochemical and terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay as described below.
Immunofluorescence staining
Spinal cord tissues from the lesion epicenter were collected at 24 hours after SCI. These tissues were postfixed at 4°C in the same fixative overnight, and then embedded in paraffin. The transverse sections (7 μm thick) were cut and transferred onto slides. The sections were deparaffinized and rehydrated, and then washed in PBS for 10 minutes,followed by a wash with PBS containing 0.3% Tween for 10 minutes, and blocked with 3% milk and 5% fetal bovine serum in 0.01 M PBS for 2 hours. Subsequently, the sections were incubated using rabbit anti-Beclin 1 polyclonal antibodies (1:1000; Proteintech), anti-phosphor-Akt polyclonal antibody (1:1000; Proteintech) diluted in PBS overnight at 4°C. The sections were washed with PBS and then incubated using goat anti-rabbit IgG secondary monoclonal antibody(1:500; Beyotime, Shanghai, China) at room temperature for 1 hour. Finally, the sections were washed and stained using 4′,6-diamidino-2-phenylindole (DAPI) (1 μg/mL;Roche, Basel, Switzerland). To assess autophagic expression in neurons and astrocytes, the sections were incubated with rabbit anti-Beclin 1 polyclonal antibody (1:200; Proteintech), rabbit anti-NeuN polyclonal antibody (1:200; Affinity)and mouse anti-glial fibrillary acidic protein (GFAP) (1:200;Abcam, London, UK) diluted in PBS overnight at 4°C. After rinsing with PBS, the sections were incubated with donkey anti-rabbit IgG Alexa Fluor antibody (1:500; Beyotime,Shanghai, China) and goat anti-mouse IgG Alexa Fluor antibodies (1:500; Beyotime) for 1 hour at room temperature.Immunostaining was detected under a fluorescent microscope (Olympus, Tokyo, Japan).
TUNEL assay
Spinal cord tissues from the lesion epicenter were collected at 24 hours after SCI and cut into sections, as described above, for TUNEL assay. A TUNEL detection kit was used to perform TUNEL assay according to the manufacture instruction (Roche). The numbers of TUNEL-positive cells were observed using a fluorescent microscope (Olympus).
Western blot assay
Spinal cord tissues from the lesion epicenter (5 mm cephalad and caudally) were homogenized in lysis buffer at 24 hours after SCI. The detailed procedure was performed as described previously (Klionsky and Emr, 2000). Briefly, a protein assay kit (Bio-Rad) was used to measure the protein content in the lysates. The proteins were subjected to 10–14%sodium dodecyl sulfate-polyacrylamide gel electrophoresis and were electro-transferred to nitrocellulose membranes.All membranes were blocked for 1 hour in Tris-buffered saline with Tween 20 buffer containing 3% milk. These membranes were incubated using rabbit anti-Beclin 1 polyclonal antibody (1:2000; Proteintech, Chicago, IL, USA), rabbit anti-phospho-mTOR polyclonal antibody (1:2000; Affinity,Cincinnati, OH, USA), rabbit anti-phosphor-Akt polyclonal antibody (1:2000; Proteintech), rabbit anti-NeuN polyclonal antibody (1:1000; Affinity), rabbit anti-Bcl-2 polyclonal antibody (1:2000; Affinity), rabbit anti-cytochrome c polyclonal antibody (1:1000; Proteintech), rabbit anti-Bax polyclonal antibody (1:2000; Proteintech) and rabbit anti-caspase-3 polyclonal antibody (1:1000; Proteintech) diluted in Tris-buffered saline with Tween 20 buffer overnight at 4°C. After incubation, all these membranes were washed three times with Tris-buffered saline and then incubated using the secondary monoclonal goat antibodies β-actin (1:4000; Cell Signaling Technology, Danvers, MA, USA) for 2 hours at room temperature. Band optical density values were determined using Gel-Pro Analyzer Software (version 4.0, Media Cybernetics,Silver Spring, MD, USA). Quantities of band optical densities were normalized using β-actin.
Transmission electron microscopy
Portions of spinal cord tissues (2.5 mm caudal and cephalic to the lesion epicenter) were collected at 24 hours after SCI and postfixed in 2.5% glutaraldehyde for 2 hours at 4°C, dehydrated in a graded alcohols and amyl acetate, and embedded in Epon618. Ultrathin (70 nm) sections were made on an ultramicrotome and then stained using uranyl acetate and lead citrate. Finally, ultrathin sections were examined using an electron microscope (JSM-IT300LV, JEOL, Tokyo, Japan).
Statistical analysis
Data are expressed as the mean ± SD. All data were analyzed using SPSS 13.0 software (SPSS, Chicago, IL, USA). Multiple comparisons of differences in quantitative measurement were made using a one-way analysis of variance followed by Dunnett’s test. A value of P < 0.05 was considered statistically significant.
Results
RAPA enhances autophagic activity in the dorsal column by suppressing mTOR signal pathway
Beclin 1 is one biomarker of autophagy in mammalian cells(Carloni et al., 2009, Cevikbas et al., 2011). The immunofluorescence analysis showed that the staining density of Beclin 1 in the dorsal column was weak in the sham group,but greater in the SCI + vehicle and SCI + RAPA groups at 24 hours (Figure 1). Beclin 1 expression was observed in NeuN-labeled neurons and GFAP-labeled astrocytes (Figure 2). Additionally, the development of autophagic vacuoles was detected after SCI under transmission electron microscope (Figure 3). In western blot assay, the level of phosphorylated mTOR protein was significantly less in the SCI +RAPA group than in the SCI + vehicle and sham groups at 24 hours after SCI (P < 0.05; Figure 4). This indicated that RAPA enhanced autophagic activity after SCI by suppressing mTOR signal pathway. Autophagic activity might affect the process of neuronal injury and astrogliosis after SCI.
Rapamycin simultaneously increases both autophagy and Akt signal expression
Immunofluorescence analysis showed that the staining density of p-Akt was weak in the sham group, but greater in the SCI + vehicle and SCI + RAPA groups at 24 hours (Figure 1).The western blot assays (Figures 5 & 6) showed that levels of p-Akt and Beclin-1 proteins following RAPA treatment appreciably increased in the SCI + RAPA group compared with the sham and SCI + vehicle groups at 24 hours (P <0.05). This suggests that RAPA treatment simultaneously activated both autophagy and Akt signal expression after SCI.
RAPA treatment reduces neural apoptosis after SCI
To test RAPA effects on neural apoptosis, the expression levels of Bcl-2, Bax, cytochrome c and caspase-3 in spinal cord homogenates were evaluated by western blot assay at 24 hours after SCI. Densitometric quantification of the protein band densities demonstrated that Bcl-2 protein level was significantly reduced in the SCI + vehicle group compared with the sham group at 24 hours after SCI. However, RAPA treatment significantly blunted the SCI-induced inhibition of Bcl-2 expression (P < 0.05; Figure 7). The levels of Bax,cytochrome c and caspase-3 had appreciably increased in the SCI + vehicle group over those in the sham group at the same time point. However, RAPA treatment reduced the levels of Bax, cytochrome c and caspase-3 expression induced by SCI (P< 0.05; Figure 7). The immunofluorescence analysis further confirmed that the staining density of TUNEL in the SCI +vehicle group was also greater than in the sham group (Figure 8). Administration of RAPA reduced the staining density of TUNEL compared with vehicle treatment at 24 hours after SCI (Figure 8). All these results indicated that RAPA treatment reduced neural apoptosis after SCI by preventing the attenuation of the anti-apoptotic pathway and blocking activation of the pro-apoptotic pathway.
Protective effects of RAPA on neural apoptosis are attenuated by both 3-MA and Akt inhibitor IV
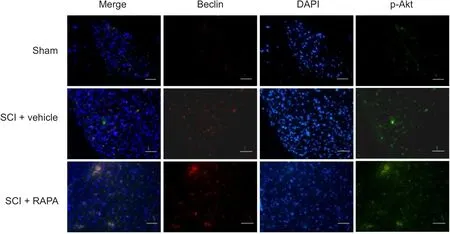
Figure 1 Immunofluorescence staining of Beclin-1 and p-Akt expression in the dorsal column at 24 hours after SCI.
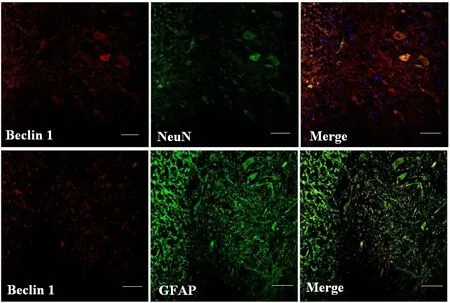
Figure 2 Immunofluorescence staining of Beclin 1, NeuN and GFAP in the dorsal column along the epicenter at 24 hours after SCI(fluorescence microscope).

Figure 4 Phosphorylated mTOR protein expression in the dorsal column 24 hours after SCI.
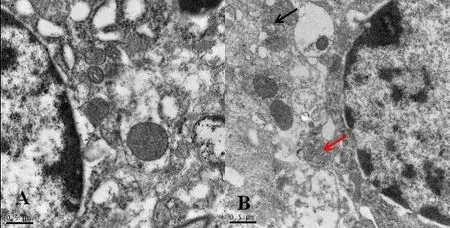
Figure 3 Ultrastructure of neurons in the dorsal horn area at 24hours after spinal cord injury (transmission electron microscope).
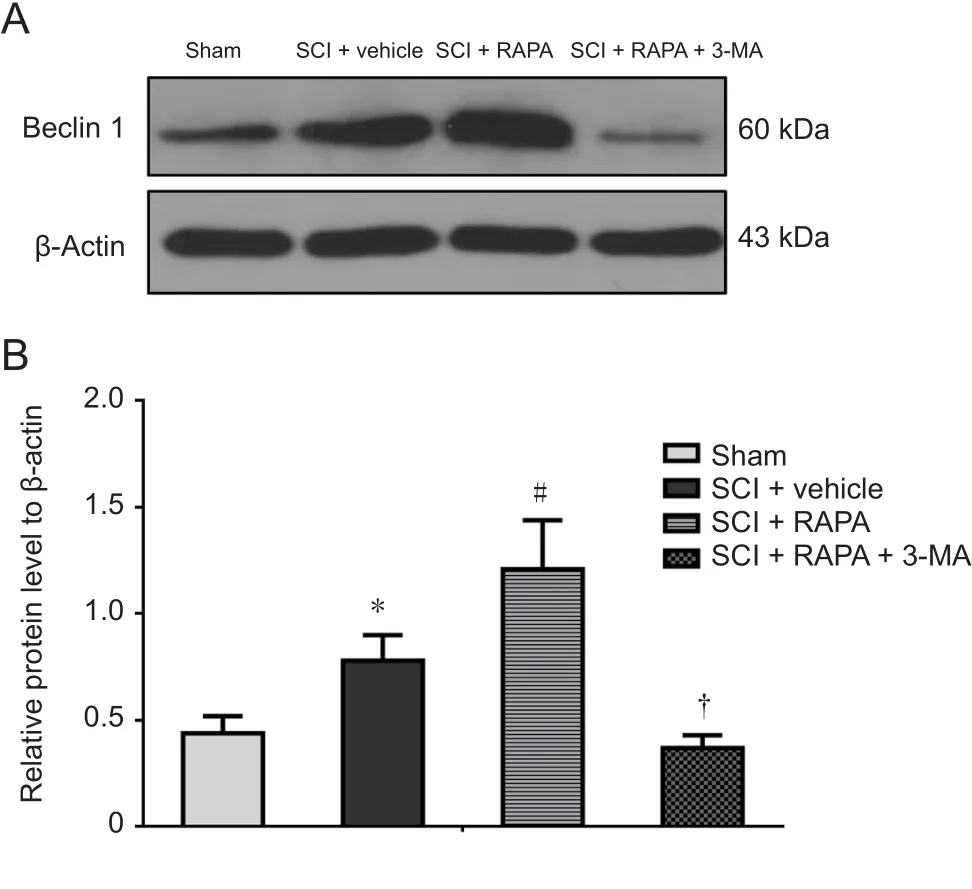
Figure 5 Beclin-1 protein expression in the dorsal column at 24 hours after SCI.
To investigate the roles of autophagic activation and Akt signaling following RAPA treatment in neural apoptosis, RAPA-treated rats were first injected with either 3-MA or Akt inhibitor IV. 3-MA, as a PI3K inhibitor, can block the later stages of autophagy and inhibit the formation of autophagosomes (Lee et al., 2004). In the SCI + RAPA + 3-MA group,3-MA pre-administration inhibited the RAPA-enhanced expression of Beclin 1 observed in the SCI + RAPA group (P< 0.05; Figure 5). After inhibiting the autophagic activity by 3-MA, the anti-apoptotic effect of RAPA was counteracted in the SCI + RAPA + 3-MA group, resulting in relatively lower levels of anti-apoptotic protein Bcl-2 and relatively higher levels of pro-apoptotic proteins Bax, cytochrome c and caspase-3 (P < 0.05; Figure 7). Similar effects were also observed in the SCI + RAPA + IV group. Treatment with Akt inhibitor IV reduced the expression of p-Akt compared with the SCI + RAPA group (P < 0.05; Figure 6). Blocking p-Akt expression by Akt inhibitor IV resulted in lower Bcl-2 expression and higher Bax, cytochrome c and caspase-3 expression in the SCI + RAPA + IV group than in the SCI +RAPA group (P < 0.05; Figure 7). All these results demonstrated that both 3-MA and Akt inhibitor IV attenuated the effects of RAPA on neural apoptosis.
Rapamycin treatment reduces neuronal loss after SCI
NeuN expression in the SCI + RAPA group was increased compared with that in the SCI + vehicle group (P < 0.05;Figure 9). However, administration of 3-MA or Akt inhibitor IV led to decreased NeuN expression in the SCI + RAPA+ 3-MA and SCI + RAPA + IV groups compared with the SCI + RAPA group. This confirms that the neuroprotective effects of RAPA treatment are associated with increased autophagic activity and stimulated p-Akt signaling pathway.
Discussion
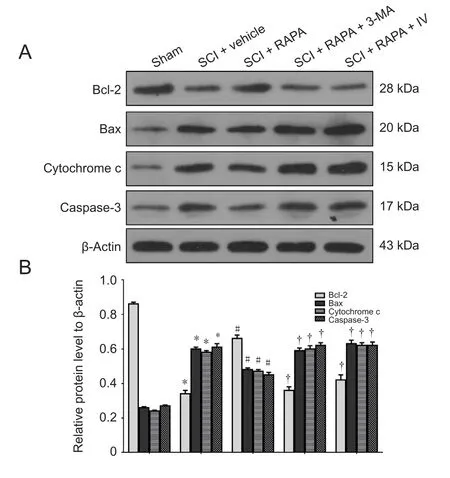
Figure 7 Expression of Bcl-2, Bax, cytochrome c and caspase-3 protein in the dorsal column at 24 hours after SCI.
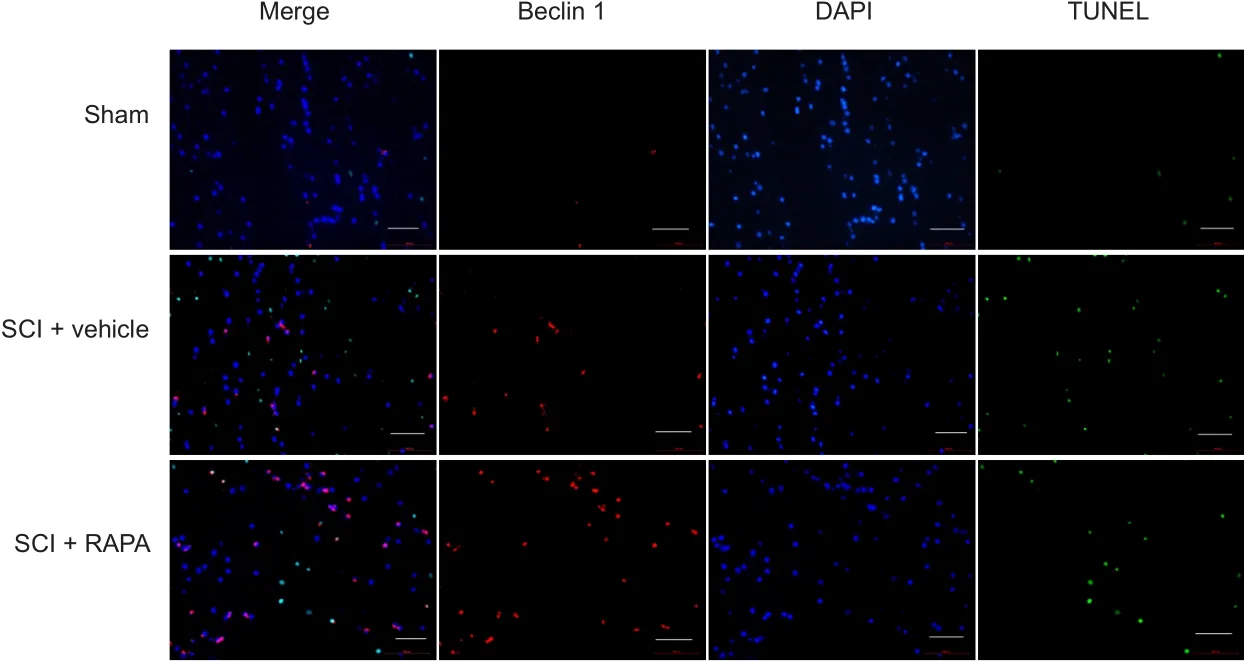
Figure 8 Immunofluorescence staining of TUNEL and Beclin 1 expression in the dorsal column at 24 hours after SCI (fluorescence microscope).
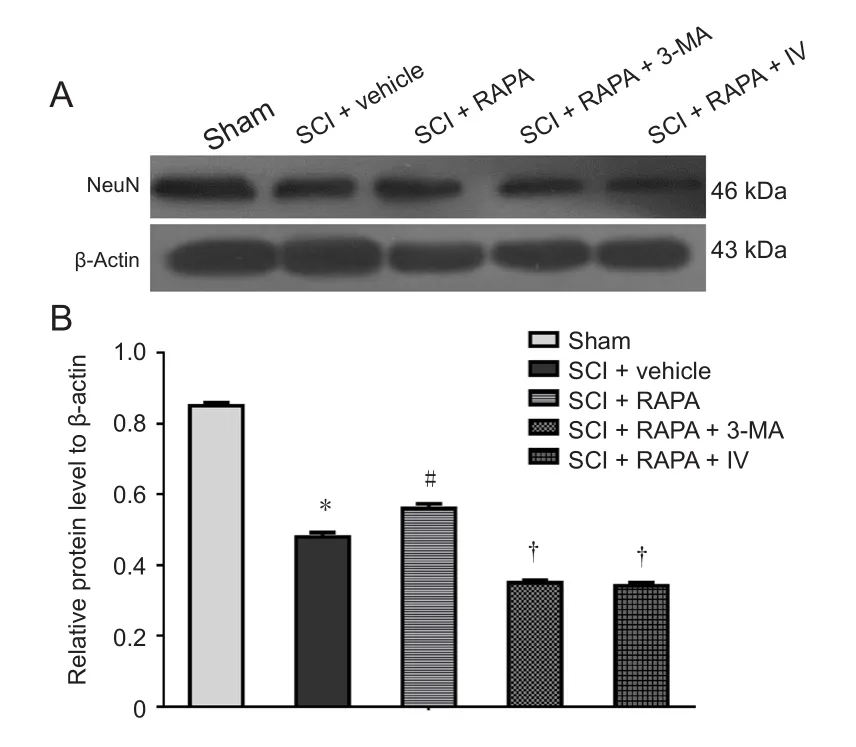
Figure 9 NeuN expression in the dorsal column at 24 hours after SCI.
Traumatic SCI is a severe central nerve system damage that commonly involved a series of pathological process after the initial mechanical injury (Zhang et al., 2018). Previous studies have demonstrated that increased autophagy occurs after the neural lesions and contributes to the secondary damage after SCI (Kanno et al., 2009; Sekiguchi et al., 2011; Chen et al., 2012; Zhang et al., 2013a, b; Tang et al., 2014; Zhou et al., 2017). It is well-known that autophagy is a normal physiological process for maintaining cellular homeostasis. In some circumstances, however, autophagy is also involved in the process of cell death and can affect the development and progression of several diseases (Levine et al., 2008; Hayashi et al., 2009; Hale et al., 2013; Chagin, 2016; Romana et al.,2016). However, the specific effects of autophagy in acute SCI are still debated. Our previous results have shown that activated autophagy following RAPA treatment played an important role in neuronal protection and locomotor recovery in rat models of SCI (Li et al., 2016; Du et al., 2017). In this current study, RAPA resulted in high expressions of Beclin 1 within the injured spinal cord. In addition, apoptotic cell death and neuronal loss in the injured spinal cord were significantly less in the rapamycin-treated rats than in the vehicle-treated rats. This indicates that RAPA had a neuroprotective effect following SCI.
A number of key molecules that regulate mammalian autophagy have been identified. Rapamycin is a specific inhibitor of mTOR in mammalian cells. RAPA, a lipophilic, macrolide antibiotic, induces autophagy by inactivating mTOR (Harris et al., 2003; Kamada et al., 2004; Sarbassov et al., 2005). The inhibition of mTOR induces the expansion step of the pre-autophagosomal membrane, which is necessary for the formation of autophagic vacuoles. In our study, RAPA treatment increased autophagy activity by inhibiting mTOR signaling after SCI.Currently, two mTOR complex forms are known, mTORC1 and mTORC2 (Sarbassov et al., 2005). mTORC1 controls several cellular processes including autophagy (Sarbassov et al.,2005); mTORC2 was shown to participate in the regulation of Akt activity (Guertin et al., 2006). In the present study, the autophagic marker Beclin 1 and p-Akt signals were simultaneously activated after RAPA treatment in injured spinal cord tissue by inhibiting mTOR. This indicates that both mTOR complexes can be affected by RAPA treatment. However, our study does not determine whether the activated process is mainly mediated by mTORC1 or mTORC2. Our future investigations will examine the mechanism in more detail.
Apoptosis is one important mechanism of secondary spinal cord injury (Liu et al., 2017; Xu et al., 2017; Zhou et al.,2017). Apoptotic cell death following SCI is highly mitochondrial dependent. We found that there were markedly fewer TUNEL-positive cells in the injured spinal cord in the rapamycin-treated rats than in the vehicle-treated rats. Moreover, RAPA treatment blunted the SCI-induced decrease of antiapoptotic signal Bcl-2 expression and reduced the expression of proapoptotic signals, including Bax, cytochrome c and caspase-3. Our results demonstrated that RAPA treatment reduced neural apoptosis by inhibiting the mitochondrial apoptotic pathway. Previous studies also found that RAPA treatment reduced neural tissue damage after neonatal hypoxia-ischemic injury and traumatic brain injury by blocking the apoptotic signaling pathway. The antiapoptotic effect of RAPA may be attributed solely to the activation of autophagy. p-Akt as a pro-survival signal is beneficial for preventing cell death and has been found to prevent cell apoptosis in several models of ischemia reperfusion injury (Lee et al., 2004; Carloni et al., 2009).In the current study, both 3-MA and Akt inhibitor IV administration blocked autophagic activation and Akt phosphorylation and counteracted the antiapoptotic effects of RAPA and exacerbated neuronal loss. Our results indicated that both the activation of autophagy and Akt signals played important roles in the antiapoptotic mechanism of RAPA and that simultaneous activation of these pathways is beneficial for neuroprotection.
The present study confirmed that rapamycin treatment had a neuroprotective effect in a rat model of SCI by blocking apoptotic signals. Both the enhanced autophagy activity and Akt signaling played important roles in the neuroprotection by RAPA.
Acknowledgments:We thank Wenming Pan from the Second People’s Hospital of Changshu, China, for technical assistance and paper revision.
Author contributions:Study design: XGL and XJL; experiment implement:XGL, JHD and YL; data analysis: YL; paper writing: XGL and YL. All authors approved the final version of the paper.
Conflicts of interest:The authors declare that there are no conflicts of interest associated with this manuscript.
Financial support:This study was supported by the National Natural Science Foundation of China, No. 81401004 (to XGL); the Medical and Health Technology Development Program of Zhejiang Province of China, No. 2015-KY1001-036 (to XGL). The funding sources had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Institutional review board statement:All experimental procedures and protocols were approved by Research Animal Resources and Care Committee of Zhejiang University, China. All experimental procedures described here were in accordance with the National Institutes of Health (NIH) guidelines for the Care and Use of Laboratory Animals.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Daniel Cooper, University of Miami School of Medicine,USA; Megan Ryan Detloff, Drexel University College of Medicine, USA.
Additional file:Open peer review reports 1 and 2.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Human survival and immune mediated mitophagy in neuroplasticity disorders
- Voluntary running delays primary degeneration in rat retinas after partial optic nerve transection
- Establishment and verification of a surgical prognostic model for cervical spinal cord injury without radiological abnormality
- Repair of peripheral nerve defects by nerve transposition using small gap bio-sleeve suture with different inner diameters at both ends
- Reinnervation of spinal cord anterior horn cells after median nerve repair using transposition with other nerves
- Repair of long segmental ulnar nerve defects in rats by several different kinds of nerve transposition