Repair of peripheral nerve defects by nerve transposition using small gap bio-sleeve suture with different inner diameters at both ends
Yu-Hui Kou , You-Lai Yu , Ya-Jun Zhang Na Han Xiao-Feng Yin Yu-Song Yuan Fei Yu Dian-Ying Zhang Pei-Xun Zhang ,Bao-Guo Jiang
1 Peking University People’s Hospital, Beijing, China
2 The Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu Province, China
Abstract During peripheral nerve transposition repair, if the diameter difference between transposed nerves is large or multiple distal nerves must be repaired at the same time, traditional epineurial neurorrhaphy has the problem of high tension at the suture site, which may even lead to the failure of nerve suture. We investigated whether a small gap bio-sleeve suture with different inner diameters at both ends can be used to repair a 2-mm tibial nerve defect by proximal transposition of the common peroneal nerve in rats and compared the results with the repair seen after epineurial neurorrhaphy. Three months after surgery, neurological function, nerve regeneration, and recovery of nerve innervation muscle were assessed using the tibial nerve function index, neuroelectrophysiological testing, muscle biomechanics and wet weight measurement, osmic acid staining, and hematoxylin-eosin staining. There was no obvious inflammatory reaction and neuroma formation in the tibial nerve after repair by the small gap bio-sleeve suture with different inner diameters at both ends. The conduction velocity, muscle strength, wet muscle weight, cross-sectional area of muscle fibers, and the number of new myelinated nerve fibers in the biosleeve suture group were similar to those in the epineurial neurorrhaphy group. Our findings indicate that small gap bio-sleeve suture with different inner diameters at both ends can achieve surgical suture between nerves of different diameters and promote regeneration and functional recovery of injured peripheral nerves.
Key Words: nerve regeneration; bio-sleeve; small gap; sleeve suture; nerve transposition; nerve defect; nerve conduit; nerve reinnervation;peripheral nerve; neural regeneration
Introduction
For patients with severe peripheral nerve injury, especially proximal nerve injury (such as brachial plexus avulsion) and long-distance nerve defects (Koshima et al., 1996; Ramos and Zell, 2000; Arzillo et al., 2014; Houschyar et al., 2016),full recovery of nerve function is rare and patients often experience limb disability or permanent sensory loss (Li et al.,2014; Levi et al., 2016; Liu et al., 2017; Barakat-Walter and Kraftsik, 2018). For this kind of nerve injury, nerve transposition is often used for repair in the clinic, which is effective in some patients (Maldonado et al., 2017; Ma et al., 2018; Vu et al., 2018).
In some cases of peripheral nerve damage, the diameter difference between transposed nerves is large, or many distal nerves need to be repaired at the same time. In these situations, traditional epineurial neurorrhaphy has the problem of high tension at the suture site, or even the failure of nerve suture (Rodkey et al., 1980). In recent years, our research team has developed a small gap bio-sleeve suture technique for repairing peripheral nerve injury. In the case of sleeve suture, the repaired nerve stumps need not be directly sutured, but are separately sutured through the catheter with which they are sleeved. Therefore, only the inner wall of the sleeve catheter is matched with the diameter of the sutured nerve to achieve the effect of tension-free suturing (Kou et al., 2015; Zhang et al., 2015; Deng et al., 2017). Therefore, we investigated repair following the small gap bio-sleeve suture method with different diameters at both ends for tibial nerve transposition. The repair effect was evaluated using the tibial nerve function index, neuroelectrophysiological measurements, muscle biomechanics and wet weight measurement,and nerve and muscle histology.
Materials and Methods
Preparation of chitin bio-sleeve with different inner diameters at both ends
The sleeve-making mold comprises three parts, as follows:a bio-sleeve guiding mold (guide roller), a contour-shaping sleeve, and a fixed base. The bio-sleeve guiding mold is made of stainless steel. The two ends of the guiding mold are 5-cm cylindrical guiding rods with different diameters. The two guiding rods are connected by a conical structure. The diameters of the two ends of the conical structure are the same as those of the guiding rods on both sides. The contour-shaping sleeve is a similar shape to the guiding mold, and a uniform gap of 0.3–2 mm can be formed between the base and the guiding mold after fixing them. The mold is used to fix and correct the catheter in the preparation of the making of the absorbable bio-sleeve and has different inner diameters at both ends.
Chitosan (Beijing Chemical Factory, Beijing, China) with deacetylation greater than 70% and a molecular weight of 15–50 × 104Da was dissolved in 2% dilute acetic acid aqueous solution to obtain a high viscosity solution, which was defoamed using a vacuum pump until a solution without bubbles was obtained. The bio-sleeve was prepared with the mold using a conical sleeve uniformly stuck foamless stock solution, and then solidified in NaOH aqueous solution after coating the wall with the solution we obtained. The hollow tube with different inner diameters at both ends was obtained after setting. The hollow round tube combined with the sleeve mold was fixed on the base. The sleeve corresponding to the mold was sleeved from the thin end and fixed to the base. The sample was placed in double-distilled water for molding. The inner diameter of one end of the sleeve was 1.0 mm, and the inner diameter of the other end was 1.5 mm.The thickness of the wall was approximately 0.3 mm.
Experimental animals
Eighteen healthy female specific-pathogen-free Sprague-Dawley rats aged 6 weeks and weighing 200–220 g were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd., China (license No. SCXK (Jing) 2016-0006). All rats were housed in individual cages of the Experimental Animal Center of Peking University People’s Hospital, China, at (24 ±2)°C and a relative humidity of 50–55%. Rats were allowed free access to standard pellet feed and clean water in a 12:12-hour light/dark cycle. This study was approved by the Animal Ethics Committee of Peking University People’s Hospital on December 9, 2015 (approval No. 2015-50). All animal care and animal surgeries were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1985).
The rats were randomly divided into three groups (n =6 per group), as follows: sham operation group, epineurial neurorrhaphy group (tibial nerve transection + epineurial neurorrhaphy), and bio-sleeve suture group (tibial nerve transection + bio-sleeve suture).
Preparation and suture of tibial nerve injury models
The rats were intraperitoneally anesthetized with 1% sodium pentobarbital (Merck, Darmstadt, Germany), 30 mg/kg. The right tibial nerve and common peroneal nerve were exposed under aseptic conditions, and cut off with microscissors 10 mm below sciatic nerve bifurcation. The proximal end of the common peroneal nerve and distal end of the tibial nerve received a sleeve suture.
In the epineurial neurorrhaphy group, the proximal end of the common peroneal nerve and the distal end of the tibial nerve with different diameters underwent epineurial neurorrhaphy with a 10-0 needle suture (Ningbo Medical Needle Co., Ltd., Ningbo, China) under a four-fold operating microscope (Figure 1A). In the bio-sleeve suture group, the proximal end of the common peroneal nerve and distal end of the tibial nerve received a bio-sleeve suture (Figure 1B).Under a four-fold operating microscope, the selected nerve conduit was placed between the two nerve stumps with different diameters to be sutured. At the junction of the cylindrical sleeve and the conical sleeve, the nerve wall was penetrated outward and inward. The nerve was sutured to the sleeve at 1 mm from the proximal end of the nerve. The nerve stump was embedded in the sleeve by approximately 2 mm, and the nerves at both ends were sutured with two stitches relative to each suture. After the suture, the nerves at both ends were su-tured into the sleeve at 2 mm, and the nerve stumps were leftwith a 2-mm gap. The other two ends were sutured onto the muscles in opposite direction to avoid self-repair.
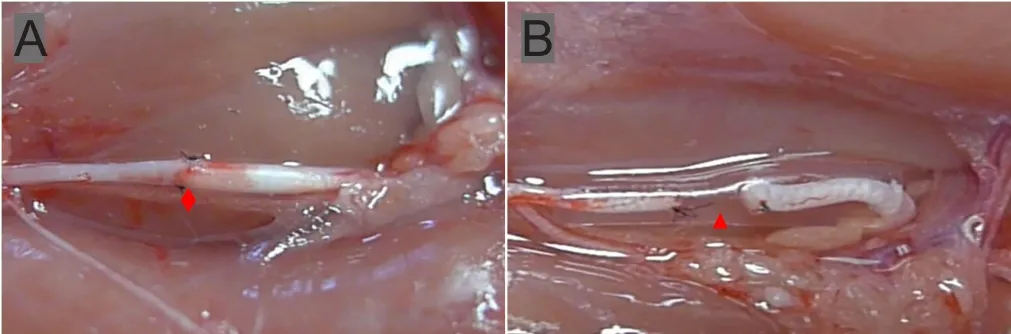
Figure 1 Repair of tibial nerve defects with the small gap sleeve suture and epineurial neurorrhaphy for proximal transposition of the common peroneal nerve with different inner diameters of the bio-sleeve at both ends.
In the sham operation group, the sciatic nerve and its branches were incised and exposed without injuring the nerve. The incision was closed with a 5-0 suture (Ningbo Medical Needle Co., Ltd.).
Three months after surgery, the tibial nerve function index, neuroelectrophysiology, neuromuscular histology,muscle biomechanics, and wet weight were measured.
General condition and local gross morphology observation of nerve suture sites
After surgery, rats were fed normally. General conditions of the rats were observed and recorded daily, including activity,diet, surgical incision healing, surgical limb activity on the surgical side, and the presence of foot ulcers. At 3 months after surgery, the repaired common peroneal nerve and tibial nerve were exposed to observe local nerve morphology and peripheral soft tissue adhesion at suture sites.
Measurement of tibial function index
We made a 50-cm footprint plastic walking box lined with equal-width white paper. The hind feet to the ankles of rats were immersed in ink and they were allowed to walk freely in the box. Several pairs of clear bilateral footprints were recorded (Zhang et al., 2007) per rat. Three variables were measured, as follows: (1) Print length (PL): the longest distance of the footprint; (2) toe spread (TS): the distance between the first and fifth toes; and (3) intermediate toe spread(IT): the distance between the second and fourth toes. Three factors were calculated using the right foot as test (E) data and the left foot as normal (N) data, as follows: (1) Footprint length factor (PLF) = (EPL - NPL)/NPL; (2) footprint width factor (TSF) = (ETS - NTS)/NTS; and (3) intermediate toe width factor (ITF) = (EIT - NIT)/NIT. These factors were inserted into the following Bain-Mackinnon-Hunter formula to calculate the tibial function index: -37.2 PLF + 104.4 TSF + 45.6 ITF - 8.8. The tibial function index score was between -100 and 0; the lower the absolute value, the better tibial nerve function was.
Neuroelectrophysiological tests
After footprint measurements, the rats were intraperitoneally anesthetized with 1% sodium pentobarbital (Merck)30 mg/kg to expose the right tibial nerve. The stimulating electrodes were placed at the distal and proximal ends of the nerve trunk to be measured. The recording electrode was inserted into the middle of the corresponding innervated muscle. Compound muscle action potentials were measured,and then motor nerve conduction velocity was calculated.The nerve was stimulated with a Synergy electrophysiological apparatus (Medle-cSynergy; Oxford Instrument Inc.,United Kingdom) at 1 Hz and 0.9 mA continuous square waves and 0.1 ms wave width to record compound muscle action potentials. The latencies of compound muscle action potentials obtained by stimulating the proximal and distal nerve trunks were recorded, respectively. The difference (dt)between two latencies was calculated; the length (dl) of the nerve trunk between proximal and distal stimulation sites was measured; and motor nerve conduction velocity was calculated by dl/dt.
Muscle biomechanical measurement
After neuroelectrophysiological testing, the gastrocnemius muscle was dissected and separated. The rat knee joint was fixed on the holder. The distal gastrocnemius tendon is connected to the traction receptor to ensure that the muscle and the receptor are maintained in the same straight line. Tension(0 < F < 0.1 N) was applied to keep the muscles straight. The sample was stimulated with an electrophysiological apparatus(Medle-cSynergy; Oxford Instrument Inc., United Kingdom)at 50 Hz initially, and 0.9 mA and 0.1 ms wave width. The stimulus current was gradually increased until the waveform of the tonic contraction force did not increase. Tonic contraction force was recorded and measured using the Biomedical Signal Acquisition and Processing System (PCLAB-UE;Beijing Microsignal Star Technology Development Co., Ltd.,Beijing, China) to assess gastrocnemius muscle strength.
Measurement of wet muscle weight
After biomechanical measurement, the right gastrocnemius muscle, including the starting and ending sites of tendons,was harvested, and its dominant nerve was cut off. The deep fascia coated on the surface of muscles was peeled off, and the blood was wiped away. The wet weight of gastrocnemius muscle was recorded.
Measurement of a cross-sectional area of the gastrocnemius muscle
Gastrocnemius muscle belly was fixed in 4% paraformaldehyde, washed with tap water, dehydrated through a graded alcohol series, permeabilized, embedded in wax, and serially sliced into 5 μm-thick sections. These sections were stained with hematoxylin (Beijing Yili Fine Chemicals Co., Ltd.,Beijing, China) for 5 minutes, washed with tap water for 1 minute, stained with 0.5% eosin (Beijing Yili Fine Chemicals Co., Ltd.) for 1 minute, washed with tap water, permeabilized, and mounted. Histological features of muscle cross sections were observed under a light microscope (Olympus,Tokyo, Japan). Muscle fiber area of muscle cross sectiona was measured using Image-Pro Plus 4.5 software (Media Cybernetics, Silver Spring, USA).
Osmic acid staining of myelinated nerve fibers
Nerve tissue at distal and proximal ends at the nerve repair site was harvested in the epineurial neurorrhaphy group and bio-sleeve suture group. Tibial nerve tissue at both the distal and proximal ends at the same level was harvested in the sham operation group. Tissue samples were fixed in 4%paraformaldehyde, washed with tap water, stained with 1%osmic acid (Spi, West Chester, USA) for 12 hours, washed with tap water, dehydrated through a graded alcohol series,permeabilized, embedded in wax, and serially sliced into 5 μm-thick sections. These sections were permeabilized,mounted, and observed under a microscope. The number of myelinated nerve fibers, the myelin sheath thickness, axon diameter, and cross-sectional area of axons in the repaired distal regenerated nerves were measured.
Statistical analysis
All data are expressed as the mean ± SD. The data were analyzed using SPSS 17.0 software (SPSS, Chicago, IL, USA).Between-group differences were tested using a one-way analysis of variance. Further paired comparisons were conducted using the least significant difference test. A value of P< 0.05 was considered statistically significant.
Results
General conditions of rats
All rats were in good condition after surgery, and no rats had systemic or local wound infection or any other complications.In the epineurial neurorrhaphy group and bio-sleeve suture group, limb function on the operative side was restored to varying degrees after three months, and the muscles of the posterior leg on the operative side were atrophied to varying degrees. In the epineurial neurorrhaphy group, the nerve suture site adhered heavily to the surrounding tissues, and local fusiform swelling was apparent at the suture site with obvious neuroma formation. In the bio-sleeve suture group, there was no obvious inflammatory reaction around the sleeve at the nerve suture site. Some of the absorbed sleeve tissues slightly adhered to the surrounding tissues, and nerve growth in the sleeve was smooth. Careful dissection of the incompletely absorbed sleeve and local soft tissue showed that the proximal nerve was evenly connected with the distal nerve, and there was no obvious neuroma in the local area.
Bio-sleeve suture improves neurological function in rats with tibial nerve injury
In both the epineurial neurorrhaphy group and bio-sleeve suture group, tibial nerve function was restored, with no significant between-group difference (P > 0.05). Recovery of nerve function was significantly worse in the epineurial neurorrhaphy and bio-sleeve suture groups compared to the sham operation group (P < 0.05; Figure 2).
Bio-sleeve suture improves nerve conduction velocity and muscle strength in rats with tibial nerve injury
Nerve conduction velocity and gastrocnemius muscle strength were restored three months after surgery compared to the sham operation group, and there were no significant differences between the epineurial neurorrhaphy and bio-sleeve suture groups (P > 0.05). Nerve conduction velocity was significantly lower in the epineurial neurorrhaphy and bio-sleeve suture groups than in the sham operation group (P < 0.05; Table 1).
Bio-sleeve suture promotes the recovery of innervated gastrocnemius in rats with tibial nerve injury
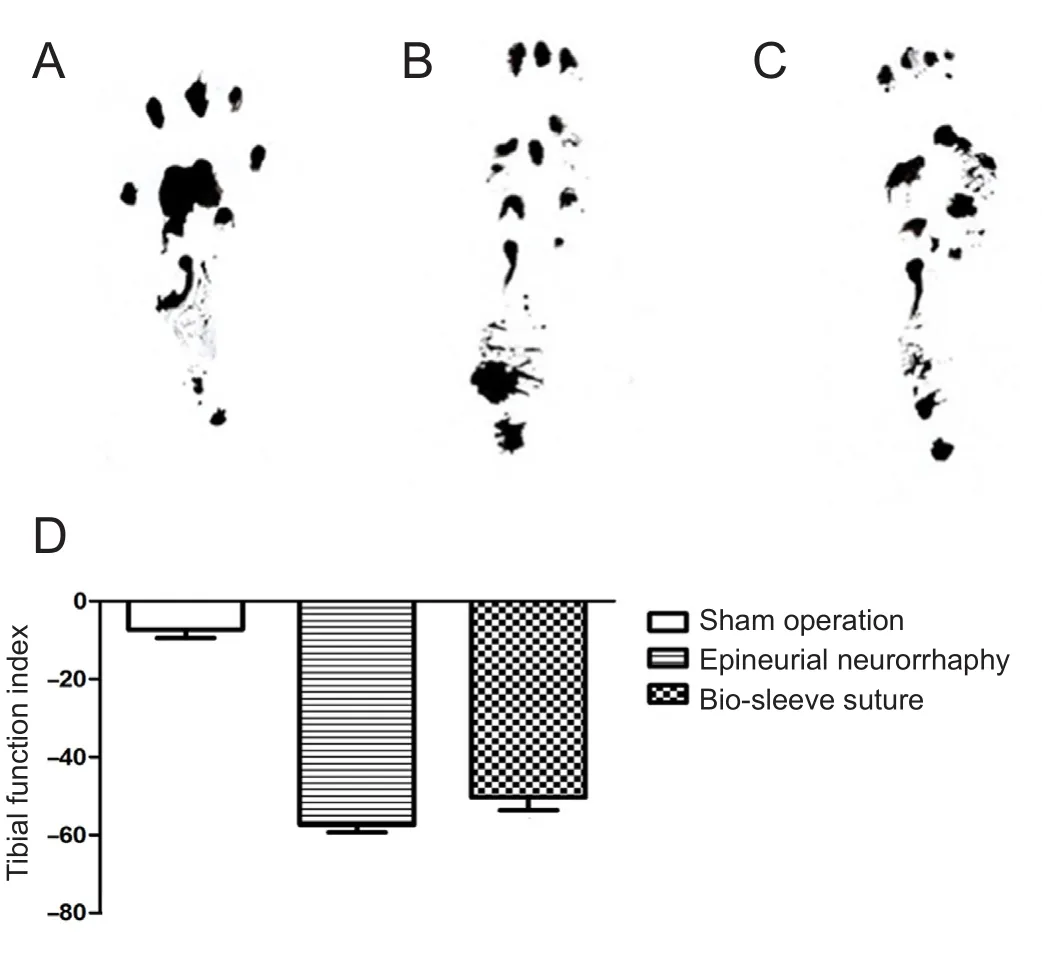
Figure 2 Tibial nerve function in rats.
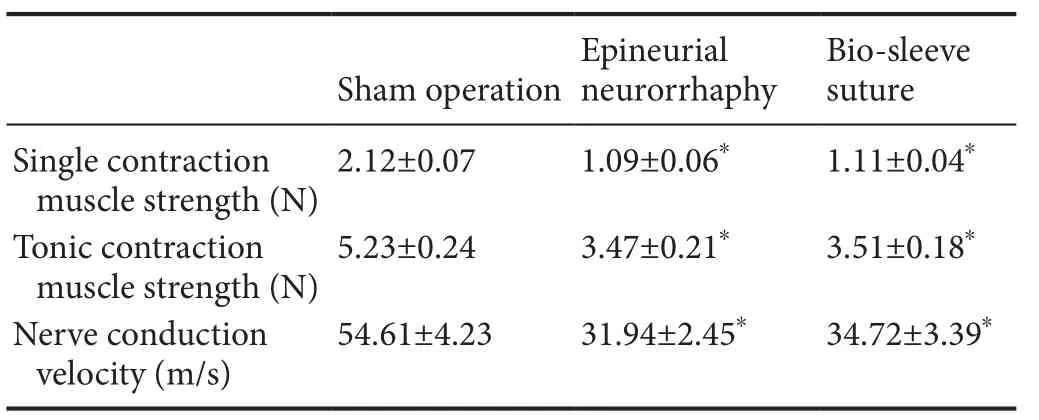
Table 1 Nerve conduction velocity and gastrocnemius muscle strength after bio-sleeve suture or epineurial neurorrhaphy in rats with tibial nerve injury
There was no significant difference in wet muscle weight between the epineurial neurorrhaphy group and bio-sleeve suture group (P > 0.05). However, wet muscle weight was significantly lower in the epineurial neurorrhaphy and biosleeve suture groups than in the sham operation group (P< 0.05; Table 2). Varying degrees of gastrocnemius muscle atrophy were observed on the surgical side in the epineurial neurorrhaphy and bio-sleeve suture groups compared with the sham operation group (Figure 3). In the sham operation group, hematoxylin-eosin staining showed that the muscle fibers had clear boundaries, uniform dyeing, and uniform diameter in muscle tissue cross-sections. The uniformity of diameter of muscle fibers on the surgical side was worse than that of normal muscle. The cross-sectional area of muscles was significantly lower in the epineurial neurorrhaphy and bio-sleeve suture groups than in the sham operation group (P< 0.05). There was no significant difference in cross-section area of muscle tissue between the epineurial neurorrhaphy and bio-sleeve suture groups (P > 0.05).
Bio-sleeve suture promotes regeneration after tibial nerve injury
Regenerated myelinated nerve fibers were observed in the epineurial neurorrhaphy and bio-sleeve suture groups at 3 months. The homogeneity of regenerated myelinated nerve fibers was poor in the epineurial neurorrhaphy and biosleeve suture groups. The density of myelinated nerve fibers was higher in the epineurial neurorrhaphy and bio-sleeve suture groups than in the sham operation group. Axon diameter and myelin sheath thickness were significantly smaller in the epineurial neurorrhaphy and bio-sleeve suture groups than in the sham operation group. The number of regenerated myelinated nerve fibers on the distal end was significantly higher in the epineurial neurorrhaphy and biosleeve suture groups than in the sham operation group (P <0.05). The number of regenerated myelinated nerve fibers on the distal end was not significantly different between the epineurial neurorrhaphy and bio-sleeve suture groups (P > 0.05).The number of regenerated myelinated nerve fibers on the distal end was 2.66 and 2.74 times that of the proximal end for the epineurial neurorrhaphy and bio-sleeve suture groups,respectively. In addition, axon diameter, axon area, and myelin sheath thickness were not significantly different between the epineurial neurorrhaphy and bio-sleeve suture groups (P> 0.05), but were significantly lower in the two groups than in the sham operation group (P < 0.05; Figure 4).
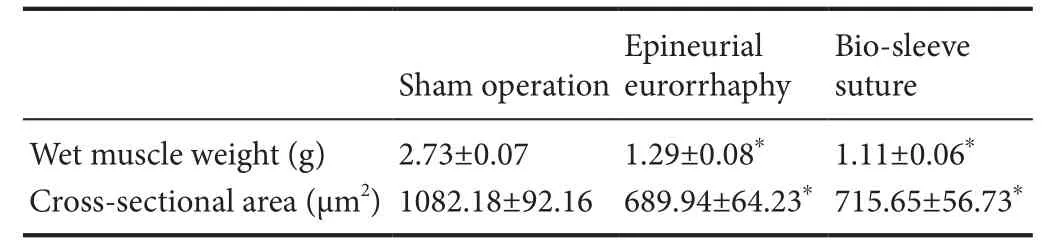
Table 2 Wet muscle weight and cross-sectional area after bio-sleeve suture or epineurial neurorrhaphy in rats with tibial nerve injury
Discussion
Peripheral nerve transposition repair has been widely used in the clinic (Hu and Gu, 2014; Huan et al., 2017; Mathews et al., 2017). An increasing number of transposition repair techniques have been developed by medical workers, such as ipsilateral/contralateral cervical spinal nerve 7, accessory nerve, and phrenic nerve transposition in the repair of brachial plexus injury (Tsai et at., 2015; Dahlin et al., 2017;Prasad, 2018), musculocutaneous nerve transposition in the repair of median nerve injury, muscular branches of the pronator quadratus in the repair of ulnar nerve injury, and facial nerve transposition repair (Bianchi et al., 2012; Cage et al., 2013; Rui et al., 2016). During nerve transposition repair,effective surgical suture of nerves after transposition is a key factor affecting transposition repair (Yang et al., 2016; Menu et al., 2017; Bijon et al., 2018).
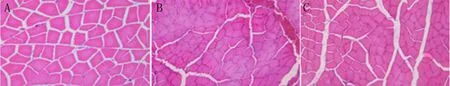
Figure 3 Hematoxylin-eosin staining in each group.
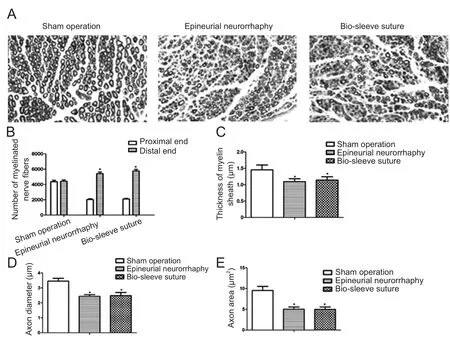
Figure 4 Regeneration of the injured tibial nerve in rats after bio-sleeve suture.
Unlike the usual suture method for treating nerve injury,during nerve transposition repair, the proximal donor nerve and the recipient nerve to be repaired are two different nerves. Moreover, the donor nerve is usually smaller and less important than the repaired recipient nerve, so there is a mismatch between the diameters of two nerve stumps during suture (Li et al., 2018). In this case, traditional epineurial neurorrhaphy often results in excessive local tension at the suture site. This means that neuroma can occur at the suture site, which can result in neuralgia after repair. More seriously, local high tension can affect the effective growth of the proximal nerve into the distal nerve, which affects nerve regeneration after transposition repair (Zhang et al., 2013).Therefore, we developed a nerve transposition method that uses a small gap bio-sleeve suture with different diameters at both ends. This method can effectively reduce the local high tension at the suture site during transposition repair. The formation of local neuromas and secondary neuralgia can be reduced by effective restraint of the sleeve gap. Compared with traditional epineurium suture, this has the following advantages: (1) Both ends can be sutured in an almost tension-free condition. (2) The gap between nerve stumps is very small. This kind of gap cannot affect nerve regeneration and improves the accuracy of nerve regeneration selection.(3) The sleeve surrounds the nerve outside. After nerve injury, local tissue swells and fills the gap, which can reduce the escape of regenerated nerve fibers outside the nerve and reduce the regeneration of neuromas. This has also been validated in our research results. This suture method is expected to replace the traditional epineurium suture in the repair of nerve transposition, especially when the diameters of donor and recipient nerves are different.
This study used chitin as the main raw material to make the bio-sleeve. In our previous experiments, we found that chitin has good biocompatibility. Using this kind of biosleeve in the repair of peripheral nerve injury, the regenerated nerve can smoothly pass through the gap and grow into the distal nerve, achieving good repair (Zhang et al.,2013; Yu et al., 2015, 2016). In models of nerve transposition repair, for sleeve selection, we suggest that the inner diameter of both ends of the sleeve is approximately 0.2 mm larger than that of the nerve; this is convenient for suture,and nerves can be easily inserted into the sleeve while avoiding tension at the suture point. On the other hand, nerve tissue swelling is caused by degeneration in a small part of the proximal and distal nerves after nerve injury. Thus, one day after suture, the swollen nerve completely fills the wall.Using this sleeve size also avoids the compression of nerve tissue that can occur when using a sleeve with an equal inner diameter to the nerve during neural degeneration,which limits blood supply to the stump. Simultaneously,the degenerated swollen nerve fills the gap of the sleeve, so the local neuroma can be effectively avoided during nerve regeneration (Seckel et al., 1984). The application of a small gap bio-sleeve suture with different inner diameters at both ends in transposition repair of the rat peripheral nerve is not only limited to the model of one donor nerve for repairing one receptor nerve, but can also be used in the simultaneous repair of two or more nerves by one nerve. For example,it has a potential clinical value for brachial plexus injury.However, this kind of repair would require improvements in the bio-sleeve shape, such as making the distal nerve sleeve into an ellipse.
In this study, the tibial function index was used to evaluate the repaired nerve function (Hou et al., 2018; Lee et al.,2018; Mirzakhani et al., 2018). During transposition repair,we found that the tibial function index was worse than that of the traditional tibial nerve denervation. The number and maturity of regenerated nerve fibers were relatively good in the count of myelinated nerve fibers. By analyzing the reasons, we believe that there is a discrepancy between neurogenesis and functional assessment. One of the important factors is that after this kind of cross-innervation, tissue regeneration and innervation can be quickly completed after effective nerve regeneration, and the real nerve function innervation requires functional remodeling in this kind of repair, which is also an important process in the clinical nerve transposition repair. We found no significant difference in wet muscle weight, forced contraction force, muscle fiber thickness, nerve conduction velocity, myelinated nerve fiber thickness, or nerve fiber count between the epineurial neurorrhaphy and bio-sleeve suture groups. However, some of the mean values of these variables indicated an advantage in either the epineurial neurorrhaphy or bio-sleeve suture groups, which may warrant future studies with larger sample sizes. In future investigations, we will carry out a detailed experimental study on the mechanism of nerve regeneration, the effect of nerve regeneration and repair at different time points, and the effect of local microenvironments by comparing epineurial neurorrhaphy and bio-sleeve suture methods. As mentioned previously, the main purpose of this study was to establish a new technique (a small gap biosleeve suture with different inner diameters) for the transposition and suture of peripheral nerves. Compared with epineurial neurorrhaphy, this method is simpler in terms of surgery, and the experimental results are more accurate.
In summary, during regeneration of transposition repair of peripheral nerve injury, a small gap bio-sleeve suture with different diameters at both ends facilitates the repair and functional recovery of the effector, simplifies the operation process,and adapts to the changes of pathophysiological structures during repair. This provides support for the clinical treatment of severe peripheral nerve injury by transposition repair.
Author contributions:Implementation of main experiments and paper writing: YHK and YLY; implementation of partial experiments and data analysis:YJZ, YSY, and DYZ; experimental evaluation and paper modification: PXZ,XFY, and NH; study design, experimental evaluation, and paper modification:BGJ. All authors approved the final version of the paper.
Conflicts of interest:None declared.
Financial support:This research was continuously funded by the National Natural Science Foundation of China, No. 31571236, 31571235 (to YHK and PXZ); the National Key Research and Development Program of China, No.2016YFC1101604 (to DYZ); the National Key Basic Research Program of China (973 Program), No. 2014CB542200 (to BGJ); the Ministry of Education Innovation Program of China, No. IRT_16R01 (to BGJ); the Beijing Science and Technology New Star Cross Program of China, No. 2018019 (to PXZ). The funding bodies played no role in the study design, in the collection, analysis and interpretation of data, in the writing of the paper, and in the decision to submit the paper for publication.
Institutional review board statement:All animal experimental procedures were approved by the Animal Ethics Committee of Peking University People’s Hospital (approval No. 2015-50) on December 9, 2015 and performed in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1985).
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Human survival and immune mediated mitophagy in neuroplasticity disorders
- Voluntary running delays primary degeneration in rat retinas after partial optic nerve transection
- Neuroprotective effects of rapamycin on spinal cord injury in rats by increasing autophagy and Akt signaling
- Establishment and verification of a surgical prognostic model for cervical spinal cord injury without radiological abnormality
- Reinnervation of spinal cord anterior horn cells after median nerve repair using transposition with other nerves
- Repair of long segmental ulnar nerve defects in rats by several different kinds of nerve transposition