Single-territory incomplete surgical revascularization improves regional wall motion of remote ventricular areas: results from a propensity-matched study
Cristiano Spadaccio, Antonio Nenna, Francesco Nappi, Raffaele Barbato, Salvatore Matteo Greco, Annunziata Nusca, Luigi Sommariva, Massimo Chello
?
Single-territory incomplete surgical revascularization improves regional wall motion of remote ventricular areas: results from a propensity-matched study
Cristiano Spadaccio1,2,*, Antonio Nenna1,*, Francesco Nappi3, Raffaele Barbato1, Salvatore Matteo Greco1, Annunziata Nusca4, Luigi Sommariva5, Massimo Chello1
1Department of Cardiovascular Surgery, Università Campus Bio-Medico di Roma, Rome, Italy2Department of Cardiac Surgery, Golden Jubilee National Hospital, Glasgow, United Kingdom3Department of Cardiac Surgery, Centre Cardiologique du Nord, Saint Denis, Paris, France4Department of Cardiology, Università Campus Bio-Medico di Roma, Rome, Italy5Department of Cardiology, Belcolle Hospital, Viterbo, Italy
Basic science studies demonstrated a general intramyocardial angiogenetic response potentially responsible for the creation of a microvascular neocapillaries network assisting myocardial function. We hypothesized that the benefit provided by the reperfusion of left anterior descending (LAD) territories and the biological angiogenetic drive triggered by the revascularization could translate in a global improvement in ventricular contractility, not restricted to the grafted area.High-risk patients with multivessel coronary artery disease and preoperative wall motion abnormalities were retrospectively analyzed to compare outcomes and regional ventricular function of those who received optimal medical therapy (OMT) versus those who underwent off-pump coronary artery bypass grafting (OPCABG) and received an incomplete myocardial revascularization using left internal mammary artery (LIMA) on LAD (OPCABG group). From January 2007 to December 2014, 206 patients (OMT,= 136, OPCABG,= 70) were propensity-score matched to have 70 matched pairs. Variables included in propensity score analyses were ejection fraction (EF), left ventricular end diastolic volume (LVEDVi), EuroSCORE II. Primary endpoint was the variation in the global wall motion score index (ΔWMSI) as evaluated by transthoracic echocardiography. Follow up was completed at 3 years from surgery or hospital discharge.Regional analysis of ventricular function revealed a regional WMSI improvement in the OPCABG group not only for LAD territories but also for non-LAD regions, associated with a reduction in the negative left ventricular ischemic remodeling, compared to patients discharged in optimal medical therapy. Global ΔWMSI was negative in OPCABG group (-3.4 ± 2.8%) and positive in the OMT group (5.9 ± 3.1%), indicating a better wall motion score for OPCAB patients. Surprisingly, regional WMSI improved also in non-grafted territories in the off-pump CABG group with a delta value of -3.7 ± 5.3% for left circumflex artery (LCX) area and -3.5 ± 5.4% for right coronary artery (RCA) area.In patients with multivessel coronary artery disease, LIMA-to-LAD grafting is associated with an improvement in the WMSI involving also the surrounding non-LAD ungrafted segments and with the attenuation of negative global and regional ischemic ventricular remodeling.
J Geriatr Cardiol 2018; 15: 479?485. doi:10.11909/j.issn.1671-5411.2018.07.003
Cardiac surgery; Coronary artery bypass graft; Incomplete revascularization; Wall motion score index
1 Introduction
After its initial introduction by Dauerman in 2011,[1]the concept of “reasonable incomplete surgical revascularization” continued to be considered an acceptable option in certain circumstances, as testified by a comprehensive metanalysis by Zimarino and colleagues summarizing recent percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG) trials and demonstrating the long-term satisfactory outcomes of this “incomplete” approach.[2]Results from large surgical trials, as the BARI trial, suggested the idea that grafting more than one target other than left anterior descending artery (LAD) did not confer any long-term advantage and was associated with an increased mortality risk.[3]Interestingly, large real life study demonstrated that an incomplete revascularization (ICR) of the circumflex or right coronary artery territories when the LAD was grafted with the left internal mammary artery (LIMA) to LAD graft, did not adversely affect early or long-term survival in patients with multivessel coronary artery disease (CAD).[4]Also, in our group we recently demonstrated that in a subset of high-risk patients with multivessel coronary artery disease, not eligible to on-pump surgery or percutaneous procedures, an off-pump CABG (OPCABG) approach with LIMA to LAD territory provided a survival benefit when compared to optimal medical therapy.[5]Possible explanations of these findings are thought to be found in the reperfusion extent and the biological effects on the myocardium provided by LAD revascularization. Indeed, on a side the reperfusion of the LAD is thought to guarantee blood supply to the vast majority of the anterior wall and protect from fatal arrhythmias. On the other side, basic science studies demonstrated a general intramyocardial angiogenetic response initiated by the ischemia/reper-fusion phenomenon potentially responsible for the creation of a microvascular neocapillaries network assisting myocar-dial function.[6–9]However, the mechanisms underlying the survival benefit in incomplete revascularization remain still unrevealed. We hypothesized that the benefit provided by the reperfusion of LAD territories and the mentioned biological angiogenetic drive triggered by the revascularization could translate in a global improvement in ventricular function and contractility, i.e. not restricted to the grafted area, ultimately justifying the survival benefit described in the literature.
2 Methods
2.1 Study design and patients’ characteristics
From January 2007 to December 2014, 206 high-risk patients with multivessel CAD and multiple comorbidities were retrospectively analysed to compare outcomes and regional ventricular function of those who received optimal medical therapy (OMT,= 136) versus those who underwent OPCABG (= 70) and received an incomplete myocardial revascularization using LIMA on LAD, in our Centre (Università Campus Bio-Medico di Roma, Rome, Italy). The study was designed to target “high-risk” patients with coronary anatomy unfavourable to percutaneous coronary intervention and not eligible for any other technique of revascularization because of the hostile comorbidity profile. Patients received a complete and multidisciplinary clinical evaluation, and underwent blood tests, electrocardiography, chest X-rays, echocardiogram, epiaortic Doppler-ultrasound and coronary angiography. Surgical option was proposed to all patients. Patients who refused the operation due to personal decision, were discharged in good clinical conditions with indication to medical therapy to control ischemia-related symptoms according to the guidelines for ischemic heart disease[10]and were assigned to the OMT group in the study. Alternatively, patients who gave consent for surgery, underwent OPCAB with LIMA to LAD and were discharged in OMT (OPCABG group).
Definition of “high-risk” and enrolment criteria included both EuroSCORE > 6% and presence of at least one risk factors discussed in Table 1, as previously described.[11–14]Patients with multivessel CAD who received OPCAB for procedural or anatomical reasons were not considered in our study. Inclusion and exclusion criteria are listed in Table 1. To flatten potential biases related to patient selection and the retrospective nature of the study, a propensity score matching analysis was performed. Variables included in the propensity score analysis were ejection fraction (EF), left ventricular end diastolic volume (LVEDVi), EuroSCORE II. This led to the generation of 70 paired couples (OMT = 70, OPCABG = 70) with perioperative similar characteristics (see methods).
Patients were all discharged in antiplatelet therapy with aspirin, associated with a maximum tolerated nitrate and anti-hypertensive therapy with angiotensin converting enzyme inhibitor ramipril or, if not tolerated, angiotensin receptor antagonist valsartan. Beta-blocker therapy with bisoprolol was provided to all patient, and in case of contra-indication it was replaced with cardio-selective calcium channel antagonists diltiazem. Underlying domiciliary therapy was continued. Patients were followed at our Centre every 6 months after discharge with interview and clinical examination. Follow up was concluded in December 2017 and was updated at the third year after surgery or discharge, with a telephonic interview with the patients or one of his relatives and an echocardiographic evaluation performed at out Centre by two blinded experienced cardiologists.
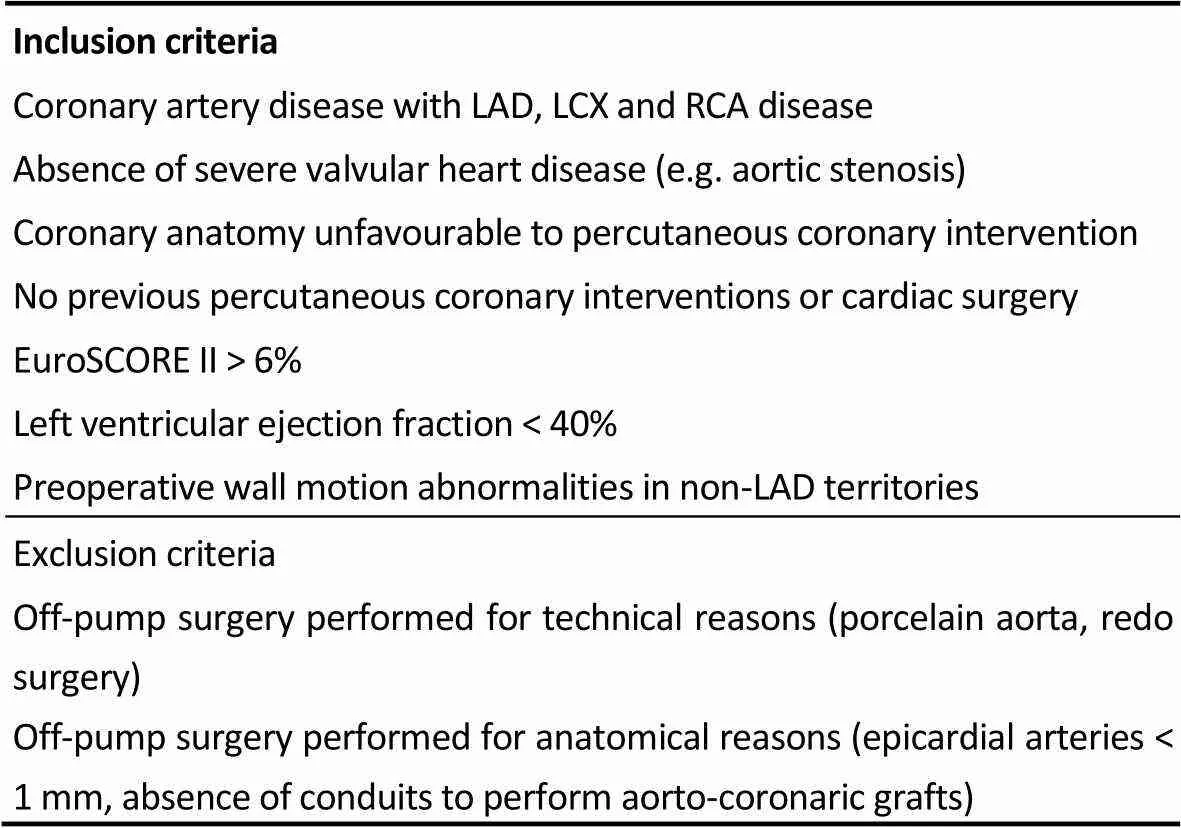
Table 1. Inclusion and exclusion criteria.
LAD: left anterior descending artery; LCX: left circumflex artery; RCA: right coronary artery.
2.2 Endpoints
Primary endpoint was the variation in the global wall motion score index (ΔWMSI) (17 segments) as evaluated by transthoracic echocardiography. Secondary endpoints included regional ΔWMSI [LAD, left circumflex artery (LCX), right coronary artery (RCA) territories], variation in ejection fraction (ΔEF), changes in indexed left ventricular end diastolic volume (ΔLVEDVi), indexed left ventricular end systolic volume (ΔLVESVi), left ventricular end diastolic diameter (ΔLVEDD), left ventricular end systolic diameter (ΔLVESD), and clinical outcomes. Delta-variables are expressed as the % difference from baseline [(follow up – preop) / pre op * 100], with “follow up” values evaluated at 3 years from surgery. Clinical outcomes included survival from all-cause mortality, cardiac mortality, and major adverse cardiac events (MACEs: cardiac mortality, myocardial infarction, rehospitalization for heart failure).
Regional WMSI re-evaluated after angiography to assess variable segments according to angiography’s results. Segments for LAD were 1, 2, 7, 8, 13, 14, 17; segments for LCX were 5, 6, 11, 12, 16; segments for RCA were 3, 4, 9, 10, 15. Segment 9 could be attributed to RCA or LAD, segments 5 and 11 could be attributed to RCA or LCX, segments 6, 12 and 16 could be attributed to LAD or LCX.
2.3 Operative technique
OPCAB was performed under general anaesthesia, median sternotomy, with an epicardic stabilizer (Octopus Tissue Stabilizers, Medtronic), using the common surgical procedure. Bypass graft was performed using the skeletonized LIMA on LAD. Patients were extubated after a mean time of four hours after surgery, and 12 patients (17.1%) required blood products. Intensive Care Unit stay was 1.45 ± 1.14 days. Patients were discharged in good clinical conditions 5.24 ± 1.77 days after surgery.
2.4 Statistical analysis
The comparison between mean values was performed with Student t-test or Mann-Whitney test for independent samples, according to D’Agostino-Pearson test for Normal distribution. Comparisons in tables were performed using Chi-square test. Matched pairs data were analysed with paired tests or McNemar’s test. Causes of death were classified as cardiac or non-cardiac depending on the medical records documentation, and deaths of unknown causes were considered as cardiac. For the matched population, survival and freedom from MACEs were evaluated using Kaplan-Meier plots, and significance was studied with stratified Log Rank for matched pairs data. Cox regression was performed using “enter” method and stratified on the matched pairs, and proportionally-hazard assumption was tested and verified with Schoenfeld residuals. Unmatched population was analyses with non-stratified tests. Propensity score analysis was performed with a 1-to-1 nearest neighbour matching algorithm using a logistic regression. Variables included in propensity score analyses were EF, LVEDVi, EuroSCORE II. Propensity score was estimated and evaluated with STATA packages “psmatch2” and “pstest” (Supplementary Table 1, Supplementary Figure 1). Statistical analysis was performed using STATA Statistics/Data Analysis (StataCorp 13.0, College Station, Texas; 2013). Results were considered statistically significant in case of avalue less than 0.05.
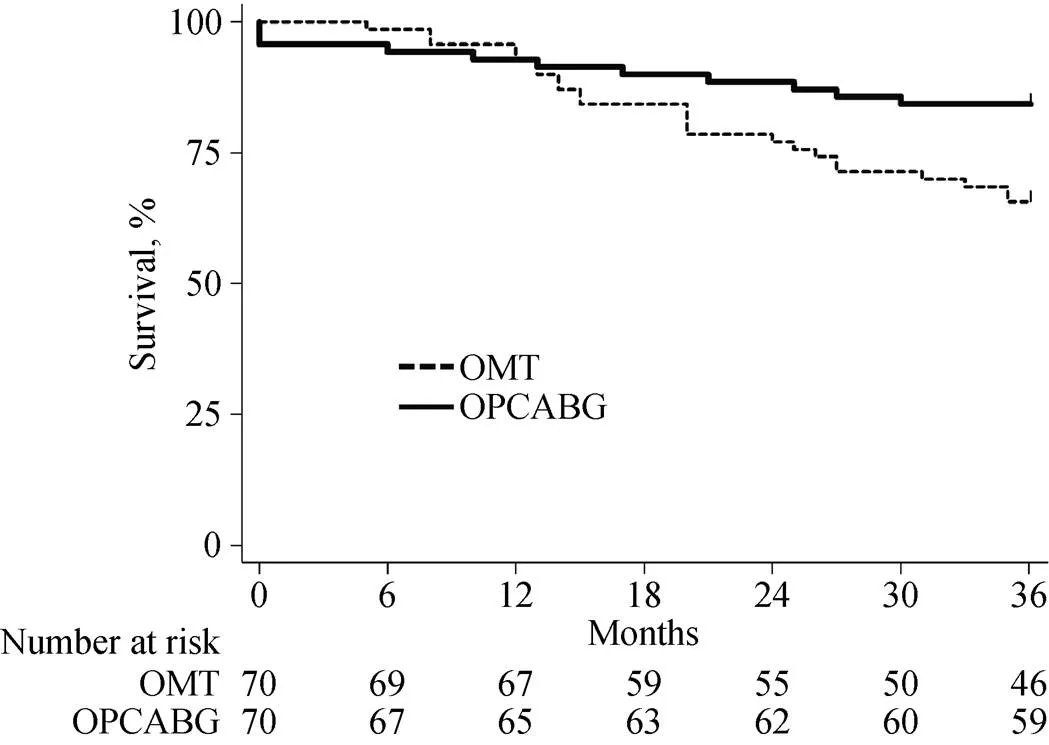
Figure 1. Overall survival in the matched population. OMT: optimal medical therapy; OPCABG: off-pump coronary artery bypass grafting.
Figure 2. Survival from cardiac events in the matched population. OMT: optimal medical therapy; OPCABG: off-pump coronary artery bypass grafting.
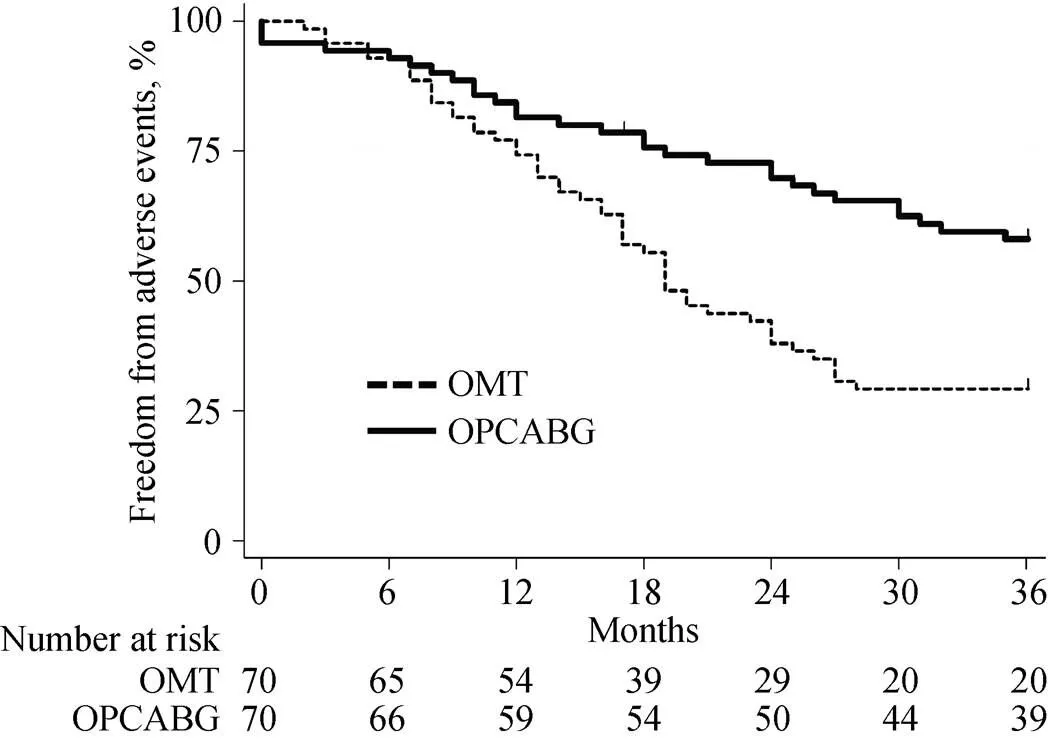
Figure 3. Freedom from major adverse cardiac events in the matched population. OMT: optimal medical therapy; OPCABG: off-pump coronary artery bypass grafting.
3 Results
Results are presented and discussed for the matched population. Results of the unmatched population are shown in Supplementary tables and figures. Preoperative charac-teristics are given in Table 2. Mean age was 79.1 ± 4.6 for OPCABG and 78.4 ± 4.0 for OMT group with a mean Eu-roSCORE II of 8.79 ± 1.66 and 8.87 ± 1.83, respectively. Demographic variables, echocardiographic parameters and WMSI evaluation was similar in the two groups.
3.1 Clinical outcomes
In-hospital mortality was 4.2% (3 patients) with no other significant postoperative complication in the OPCAB group. At the end of the follow up, overall mortality and car-diac-related mortality were higher in the OMT group (= 0.023 and= 0.050, respectively) (Figure 1 & 2). Major cardiac events occurred in 41.4% of OPCABG group and in 70.0% of OMT group (= 0.001) (Figure 3). Hazard ratio for clinical complica--tions for OMT group was more than two-fold than OP-CABG group (Table 3).
3.2 Echocardiographic outcomes (Table 4, Table 5)
Regional analysis of ventricular function revealed a regional WMSI improvement in the OPCABG group not only for LAD territories but also for non-LAD regions, associated with a reduction in the negative left ventricular is-chemic remodeling, compared to patients discharged in optimal medical therapy.
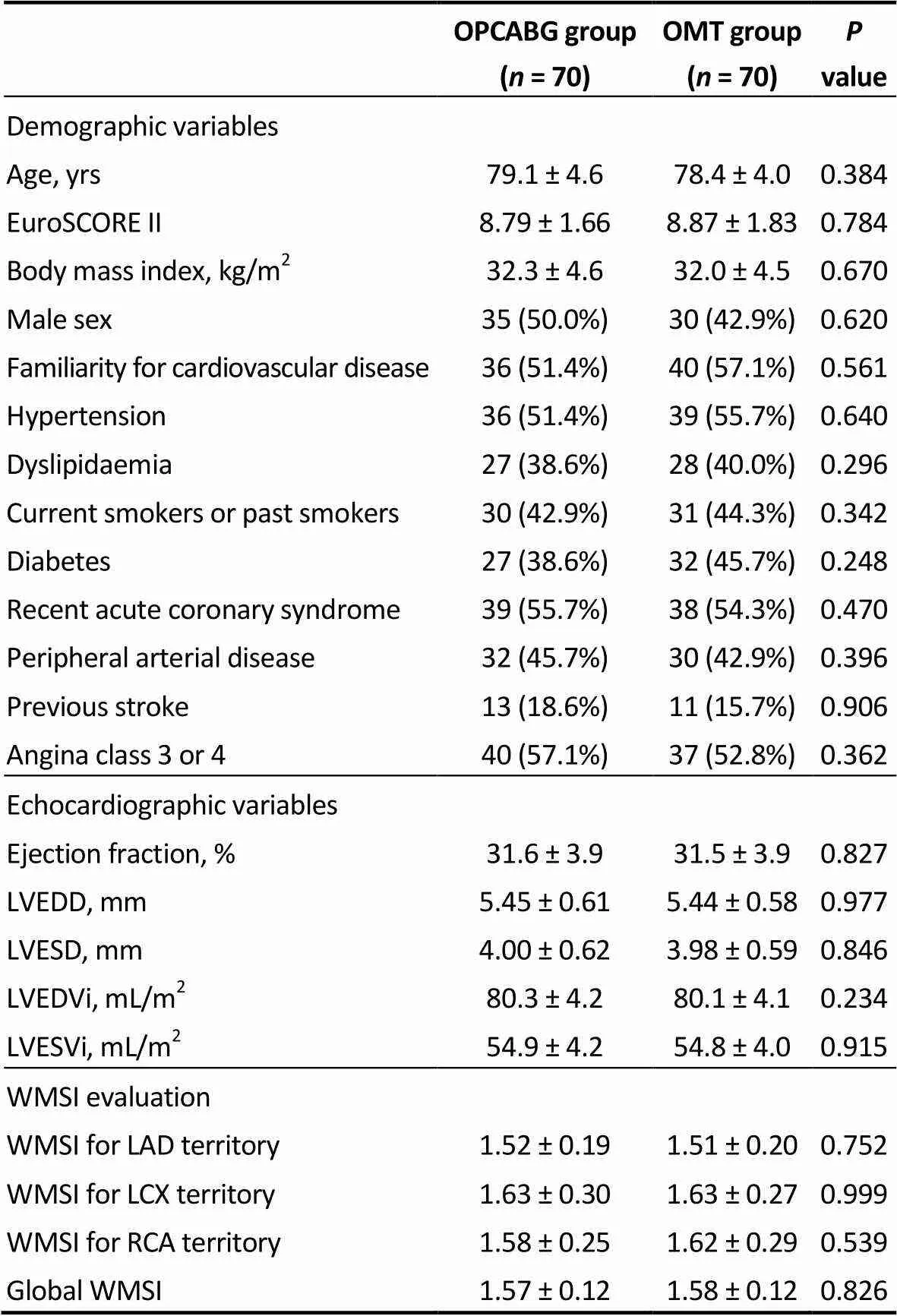
Table 2. Preoperative variables of the matched population.
LAD: left anterior descending artery; LCX: left circumflex artery; LVEDD: left ventricular end diastolic diameter; LVESD: left ventricular end systolic diameter; LVEDVi: indexed left ventricular end diastolic volume; LVESVi: indexed left ventricular end systolic volume; OMT: optimal medical therapy; OPCABG: off-pump coronary artery bypass grafting; RCA: right coronary artery; WMSI: wall motion score index.
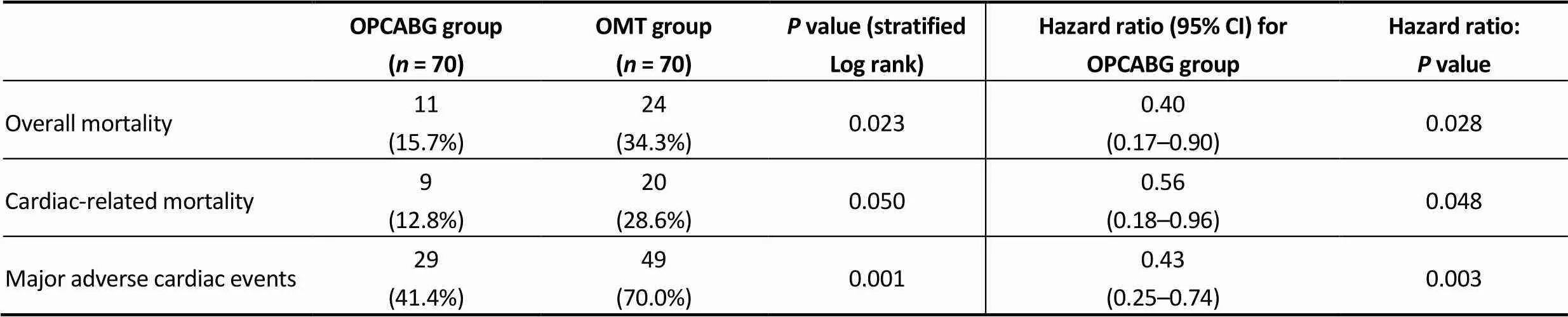
Table 3. Clinical endpoints in the matched population.
Results are expressed as events and percentages. CI; confidence intervals; OMT: optimal medical therapy; OPCABG: off-pump coronary artery bypass grafting.
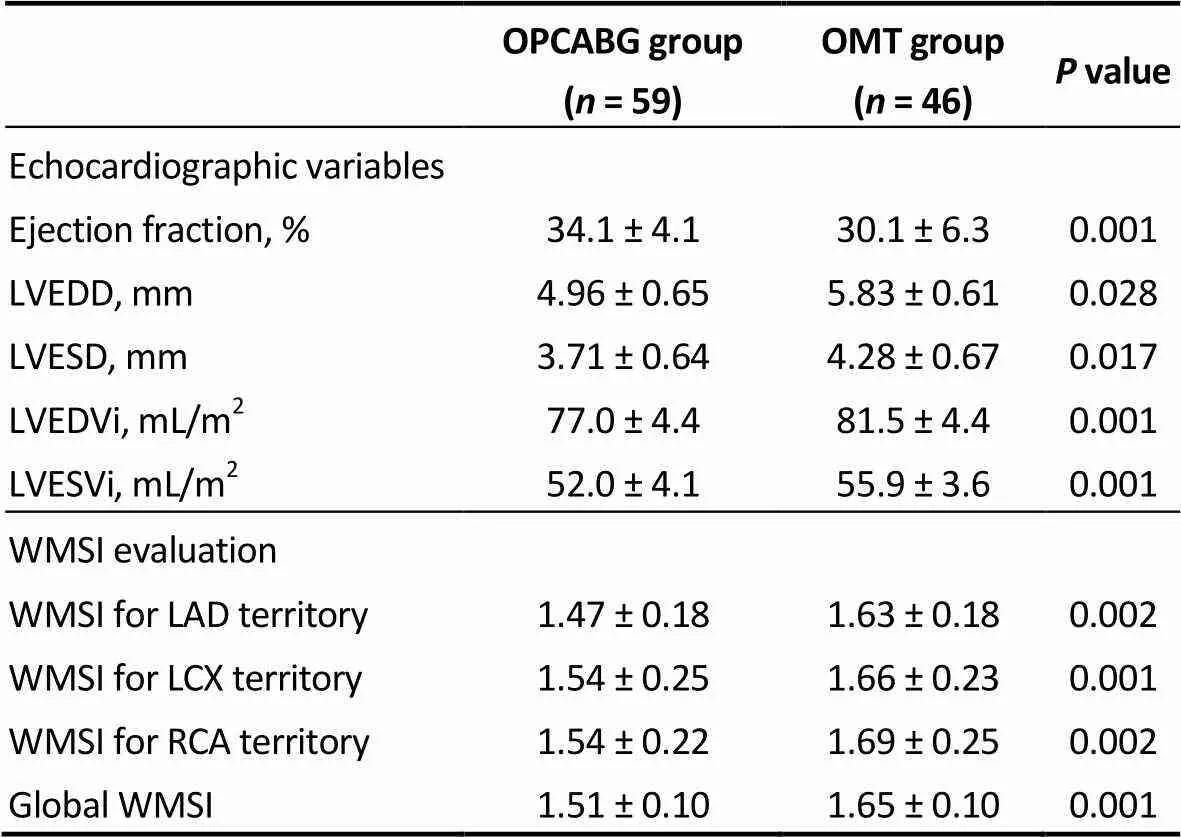
Table 4. Echocardiographic variables and WMSI evaluation of the matched population at 3-year follow up.
LAD: left anterior descending artery; LCX: left circumflex artery; LVEDD: left ventricular end diastolic diameter; LVESD: left ventricular end systolic diameter; LVEDVi: indexed left ventricular end diastolic volume; LVESVi: indexed left ventricular end systolic volume; OMT: optimal medical therapy; OPCABG: off-pump coronary artery bypass grafting; RCA: right coronary artery; WMSI: wall motion score index.
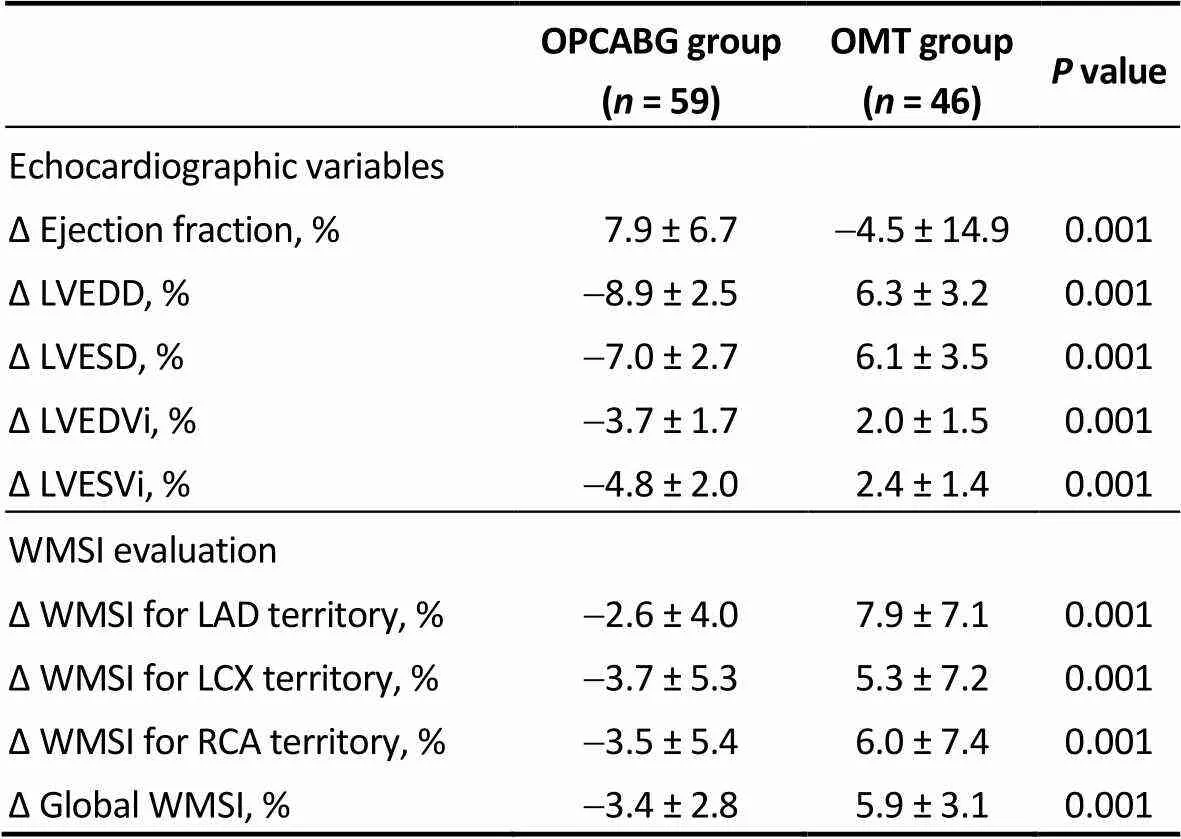
Table 5. Echocardiographic parameters and WMSI evaluation in the matched population: percentage difference from baseline.
“Delta variables” are expressed as the percentage difference from baseline, and were calculated using the following formula: [(follow up – preop) / pre op * 100]. Follow up was concluded three years after surgery. LAD: left anterior descending artery; LCX: left circumflex artery; LVEDD: left ventricular end diastolic diameter; LVESD: left ventricular end systolic diameter; LVEDVi: indexed left ventricular end diastolic volume; LVESVi: indexed left ventricular end systolic volume; OMT: optimal medical therapy; OPCABG: off-pump coronary artery bypass grafting; RCA: right coronary artery; WMSI: wall motion score index.
Global ΔWMSI was negative in OPCABG group (-3.4 ± 2.8%) and positive in the OMT group (5.9 ± 3.1%), indicating a better wall motion score for OPCABG patients. Surprisingly, regional WMSI improved also in non-grafted territories in the OPCABG group with a delta value of-3.7 ± 5.3% for LCX area and-3.5 ± 5.4% for RCA area.
In the OPCAB group, ejection fraction increased from baseline to follow up and this was paired with a reduction in the left ventricular volumes and diameters (ΔEF 7.9 ± 6.7%, ΔLVEDVi-3.7 ± 1.7%, ΔLVESVi-4.8 ± 2.0%). On the other hand, patients in the OMT group tended to have a progressive increase of the left ventricular chambers with impaired EF over time (ΔEF-4.5 ± 14.9%, ΔLVEDVi 2.0 ± 1.5%, ΔLVESVi 2.4 ± 1.4%).
4 Discussion
In this study we found that LIMA-to-LAD revascularization in high-risk patients with preoperative wall motion abnormality resulted in improved global WMSI and cardiac function, with reduction in the negative left ventricular ischemic remodeling, when compared to patients discharged in optimal medical therapy. Surprisingly, regional WMSI was improved in a significant degree also in ungrafted non-LAD territories. These findings were ultimately associated with improved survival and freedom from major adverse cardiac events in the surgical group.
While the survival advantage in the surgical cohort was expected and actually reflects the findings described in the literature,[5]the functional improvement of myocardial segments of the left ventricle not directly involved in the revascularization is intriguing. Beside the caveat of the inter and intra-observer variability with echocardiography, the finding of a statistically significant improvement in global WMSI in the context of a single-territory revascularization allows to reliably speculate the induction of a phenomenon of positive remodeling after LAD reperfusion.
Interestingly, we were able to detect such a positive effect also remotely from the LAD territory, in regions not topographically involved with the primary revascularization act. Basic science literature demonstrated the existence of a general angiogenetic response initiated by reperfusion and sustained by the local release of cytokines,[6–9]conveying towards the idea of a “pro-angiogenetic wave” progressively expanding the neovascularization process territorially from the region directly subjected to revascularization to the adjacent territories with a paracrine mechanism.[6–9]The paracrine diffusion of angiogenetic mediators would account for the progressive spreading of a microvascular network of neocapillaries within the affected myocardium, justifying the restored function of segments of myocardium not directly involved by the original revascularization procedure.[15]Several factors and molecular converging pathways have been claimed to be at the root of this phenomenon such as nitric oxide mediated angiogenesis, positive left ventricular remodeling and inhibition of apoptosis[7,9,16]and many of these molecular patterns are still to be unrevealed. Obviously, this theory is purely speculative in character and an actual demonstration of such a mechanism would be very hard in the context of this and other human trials, however, it is interesting to note that the general consensus about the benefit of arterial graft LIMA-to-LAD (the only graft used in this study) and its superiority over vein graft relies on the belief that the LIMA graft is able to release higher concentration of nitric oxide[17,18]and pro-angiogenetic growth factors.[19]In this context, recent experimental effort aimed at defining differences between arterial and venous tissues using proteomic approaches have further deepened our understanding in this field elucidating molecular pathways related not only to nitric oxide and angiogenesis but also to cytoskeleton activity regulation,[20]migrative capacity of vascular smooth muscle cells, extracellular matrix composition, coagulation, apoptosis, and heat shock response.[21]Interestingly, the secretome of LIMA, revealed the presence of important mediators involved in crucial steps of atherosclerosis pathogenesis.[22,23]All these evidences borrowed by the basic science literature could actually have a potential translation in the clinical side helping to provide explanations of the mechanisms underlying the clinical findings faced in clinical trials or retrospective analyses. In the current study, beside the limitations of its retrospective nature, of the intrinsic bias of echocardiography analysis and of the impossibility to prove the occurrence of molecular patterns in the clinical setting, the idea of a “pro-angiogenetic wave” nourished by the local release of cytokines and progressively migrating within the myocardium, could potentially explain the remote improvement of WMSI in territories far from LAD and in absence of other blood-supplying grafts. The potential translation of this global and regional motion improvements into improved survival and freedom from MACEs demonstrated in this study would surely deserve larger confirmatory studies, but the idea of a global attenuation of the negative ischemic ventricular remodeling and a survival benefit triggered by an incomplete surgical revascularization (LIMA-to-LAD) is intriguing and might also explain the difference in outcomes described in studies comparing percutaneous versus surgical incomplete revascularization.[24,25]
4.1 Limitations
Authors acknowledge the following limitations in this study. The retrospective nature of the study surely has affected the power and reliability of the results. However, propensity matched analysis has been used to reduce this bias. A potential third group constituted by complete revascularized CABG patients could have been added as “positive control”. However, ethical reasons would prevent to perform such a study in a prospective manner and the population studied was at very high risk for conventional surgery. Indeed, in order to investigate the primary hypothesis, we selected a specific subset of patients and we compared outcomes and regional wall motion in a group receiving salvage incomplete revascularization versus a group only receiving medical therapy. Wall motion abnormalities would have been better identified by magnetic resonance imaging (MRI) perfusion scan, an ad hoc study in this context is on the way in our Centre at the moment. Tissue doppler was not available for all patients considering that was not routinely performed in our patients as a preoperative evaluation. We therefore decided not to include tissue doppler analysis in our data because of the presence of many missing data and the subsequent lack of reliability of results. Indeed, we used WMSI which is considered a relatively simple and reproducible way of evaluating regional motion with echocardiography.
4.2 Conclusions
In patients with multivessel coronary artery disease, LIMA-to-LAD incomplete revascularization improves WMSI also in non-LAD ungrafted segments, with an attenuation of negative global and regional ischemic ventricular remodeling. This might translate in improved survival, freedom from MACCEs and cardiac function compared to optimal medical therapy.
1 Dauerman HL. Reasonable incomplete revascularization.2011; 123: 2337–2340.
2 Zimarino M, Curzen N, Cicchitti V. The adequacy of myocardial revascularization in patients with multivessel coronary artery disease.2013; 168: 1748–1757.
3 Vander Salm TJ, Kip KE, Jones RH,. What constitutes optimal surgical revascularization? Answers from the Bypass Angioplasty Revascularization Investigation (BARI).2002; 39: 565–572.
4 Rastan AJ, Walther T, Falk V. Does reasonable incomplete surgical revascularization affect early or long-term survival in patients with multivessel coronary artery disease receiving left internal mammary artery bypass to left anterior descending artery?2009; 120: S70–77.
5 Prestipino F, Spadaccio C, Nenna A,. Off-pump coronary artery bypass grafting versus optimal medical therapy alone: effectiveness of incomplete revascularization in high risk patients.2016; 13: 23–30.
6 Fujita M, Ikemoto M, Tanaka T,. Marked elevation of vascular endothelial growth factor and basic fibroblast growth factor in pericardial fluid of patients with angina pectoris.1998; 2: 105–108.
7 Wu Z, Zhu D. The important role of catestatin in cardiac remodeling.2014; 19: 625–630.
8 Penna C, Pasqua T, Amelio D,. Catestatin increases the expression of anti-apoptotic and pro-angiogenetic factors in the post-ischemic hypertrophied heart of SHR.2014; 9: e102536.
9 Penna C, Alloatti G, Gallo MP,. Catestatin improves post-ischemic left ventricular function and decreases ischemia/reperfusion injury in heart.2010; 30: 1171–1179.
10 Task Force M, Montalescot G, Sechtem U,. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology.2013; 34: 2949–3003.
11 Puskas JD, Williams WH, Duke PG,. Off-pump coronary artery bypass grafting provides complete revascularization with reduced myocardial injury, transfusion requirements, and length of stay: a prospective randomized comparison of two hundred unselected patients undergoing off-pump versus conventional coronary artery bypass grafting.2003; 125: 797–808.
12 Plomondon ME, Cleveland JC Jr, Ludwig ST,. Off-pump coronary artery bypass is associated with improved risk-adjusted outcomes.2001; 72: 114–119.
13 Oo AY, Grayson AD, Patel NC,. Is off-pump coronary surgery justified in EuroSCORE high-risk cases? A propensity score analysis.2003; 2: 660–664.
14 Al-Ruzzeh S, Ambler G, Asimakopoulos G,. Off-Pump Coronary Artery Bypass (OPCAB) surgery reduces risk-strati-fied morbidity and mortality: a United Kingdom Multi-Center Comparative Analysis of Early Clinical Outcome.2003; 108: Ii1–8.
15 Spadaccio C, Nappi F, Nenna A,. Is it time to change how we think about incomplete coronary revascularization?2016; 224: 295–298.
16 Fornero S, Bassino E, Gallo MP,. Endothelium depen-dent cardiovascular effects of the Chromogranin A-derived peptides Vasostatin-1 and Catestatin.2012; 19: 4059–4067.
17 Harrison DG. Endothelial dysfunction in the coronary micro-circulation: a new clinical entity or an experimental finding?1993; 91: 1–2.
18 Luscher TF, Diederich D, Siebenmann R,. Difference between endothelium-dependent relaxation in arterial and in venous coronary bypass grafts.1988; 319: 462–467.
19 Tarr FI, Sasvari M, Tarr M,. Evidence of nitric oxide produced by the internal mammary artery graft in venous drainage of the recipient coronary artery.2005; 80: 1728–1731.
20 de la Cuesta F, Zubiri I, Maroto AS,. Deregulation of smooth muscle cell cytoskeleton within the human athero-sclerotic coronary media layer.2013; 82: 155–165.
21 de la Cuesta F, Alvarez-Llamas G, Maroto AS,. A proteomic focus on the alterations occurring at the human atherosclerotic coronary intima.2011; 10: M110003517.
22 de la Cuesta F, Barderas MG, Calvo E,. Secretome analysis of atherosclerotic and non-atherosclerotic arteries reveals dynamic extracellular remodeling during pathogenesis.2012; 75: 2960–2971.
23 Spadaccio C, Nappi F, Al-Attar N,. CURRENT DEVELOPMENTS IN DRUG ELUTING DEVICES: Introductory Editorial: drug-eluting stents or drug-eluting grafts? Insights from proteomic analysis.2016; 10: 15–19.
24 Milojevic M, Head SJ, Parasca CA,. Causes of death following PCI versus CABG in complex CAD: 5-year follow- up of SYNTAX.2016; 67: 42–55.
25 Nappi F, Sutherland FW, Al-Attar N,. Incomplete reva-scularization in PCI and CABG: when two plus two does not make four.2016; 68: 877–878.
Supplementary Table 1. Diagnostic test of the propensity score algorithm.
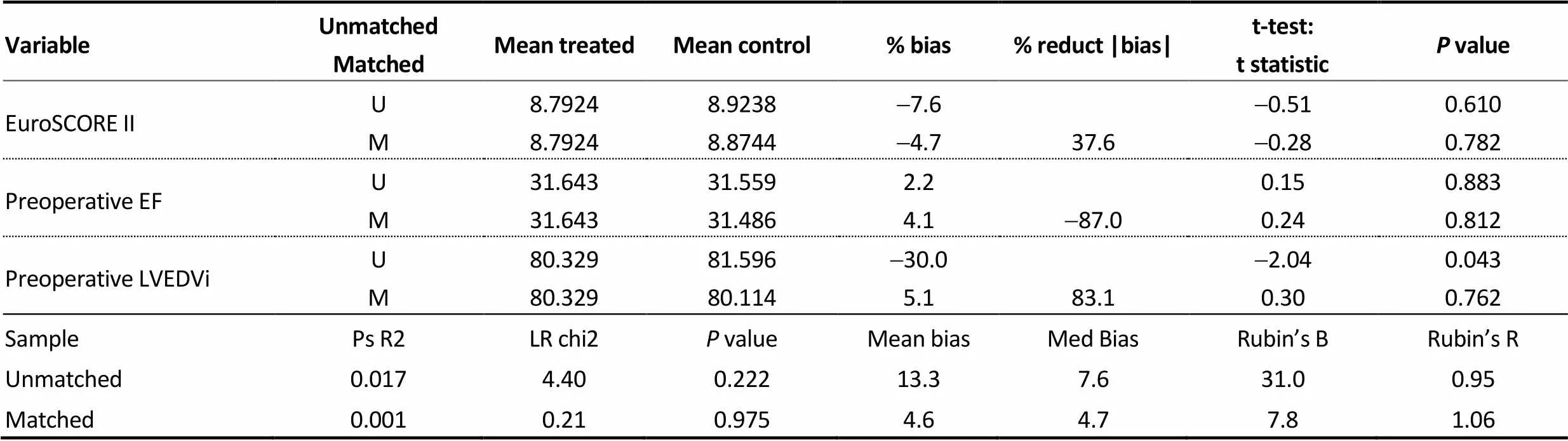
VariableUnmatchedMatchedMean treatedMean control% bias% reduct |bias|t-test:t statisticP value EuroSCORE IIUM8.79248.79248.92388.8744-7.6-4.737.6-0.51-0.280.6100.782 Preoperative EFUM31.64331.64331.55931.4862.24.1-87.00.150.240.8830.812 Preoperative LVEDViUM80.32980.32981.59680.114-30.05.183.1-2.040.300.0430.762 SamplePs R2LR chi2P valueMean biasMed BiasRubin’s BRubin’s R Unmatched0.0174.400.22213.37.631.00.95 Matched0.0010.210.9754.64.77.81.06
EF: ejection fraction; LVEDVi: indexed left ventricular end diastolic volume.
Supplementary Table 2. Preoperative variables of the unmatched population.
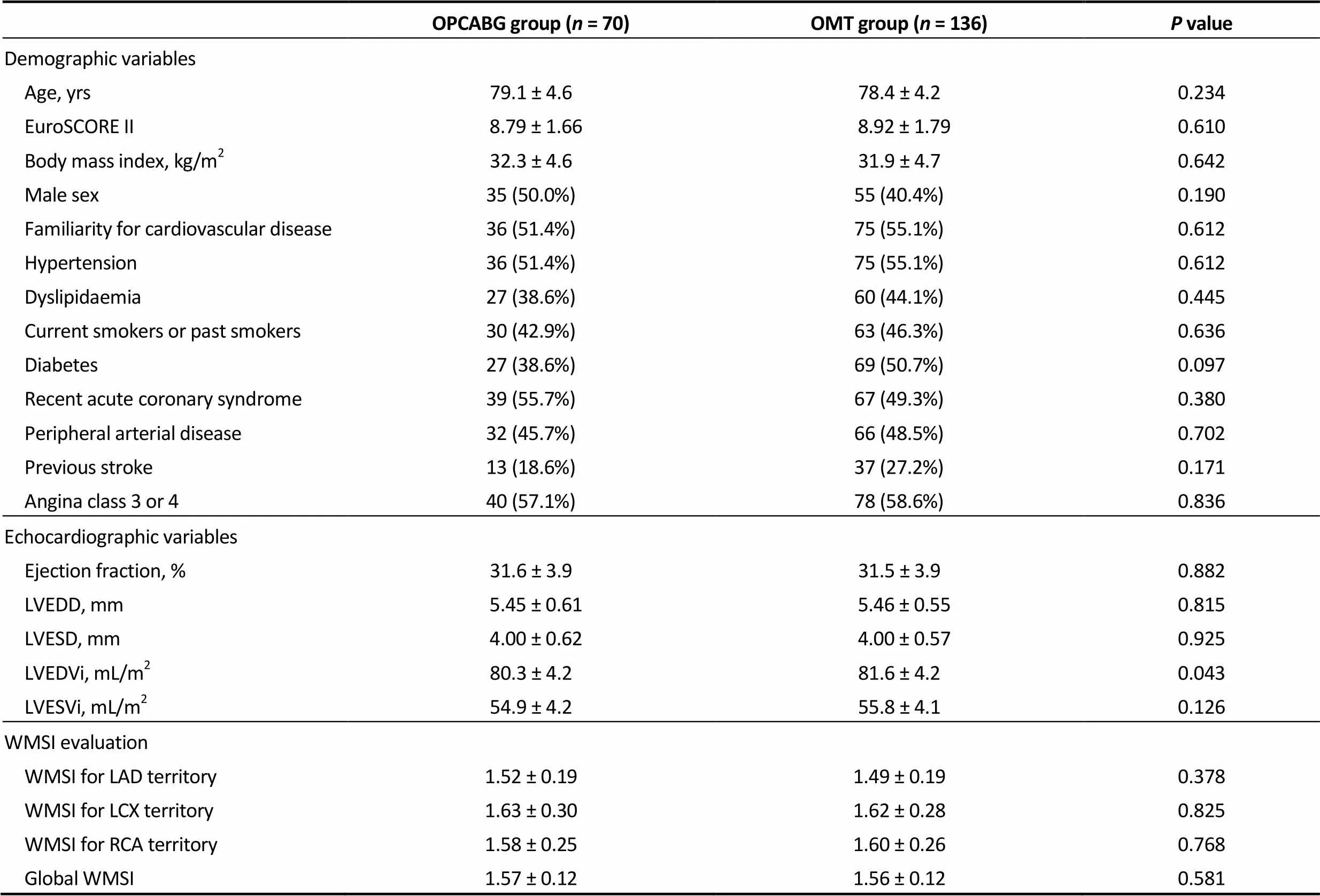
OPCABG group (n = 70)OMT group (n = 136)P value Demographic variables Age, yrs79.1 ± 4.678.4 ± 4.20.234 EuroSCORE II 8.79 ± 1.66 8.92 ± 1.790.610 Body mass index, kg/m232.3 ± 4.631.9 ± 4.70.642 Male sex35 (50.0%)55 (40.4%)0.190 Familiarity for cardiovascular disease36 (51.4%)75 (55.1%)0.612 Hypertension36 (51.4%)75 (55.1%)0.612 Dyslipidaemia27 (38.6%)60 (44.1%)0.445 Current smokers or past smokers30 (42.9%)63 (46.3%)0.636 Diabetes27 (38.6%)69 (50.7%)0.097 Recent acute coronary syndrome39 (55.7%)67 (49.3%)0.380 Peripheral arterial disease32 (45.7%)66 (48.5%)0.702 Previous stroke13 (18.6%)37 (27.2%)0.171 Angina class 3 or 440 (57.1%)78 (58.6%)0.836 Echocardiographic variables Ejection fraction, %31.6 ± 3.931.5 ± 3.90.882 LVEDD, mm 5.45 ± 0.61 5.46 ± 0.550.815 LVESD, mm 4.00 ± 0.62 4.00 ± 0.570.925 LVEDVi, mL/m280.3 ± 4.281.6 ± 4.20.043 LVESVi, mL/m254.9 ± 4.255.8 ± 4.10.126 WMSI evaluation WMSI for LAD territory1.52 ± 0.191.49 ± 0.190.378 WMSI for LCX territory1.63 ± 0.301.62 ± 0.280.825 WMSI for RCA territory1.58 ± 0.251.60 ± 0.260.768 Global WMSI1.57 ± 0.121.56 ± 0.120.581
LAD: left anterior descending artery; LCX: left circumflex artery; LVEDD: left ventricular end diastolic diameter; LVESD: left ventricular end systolic diameter; LVEDVi: indexed left ventricular end diastolic volume; LVESVi: indexed left ventricular end systolic volume; OMT: optimal medical therapy; OPCABG: off-pump coronary artery bypass grafting; RCA: right coronary artery; WMSI: wall motion score index.
Supplementary Table 3. Clinical endpoints in the unmatched population.
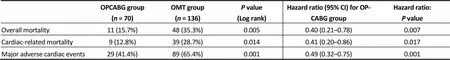
OPCABG group(n = 70)OMT group(n = 136)P value (Log rank)Hazard ratio (95% CI) for OPCABG groupHazard ratio: P value Overall mortality11 (15.7%)48 (35.3%)0.0050.40 (0.21–0.78)0.007 Cardiac-related mortality9 (12.8%)39 (28.7%)0.0140.41 (0.20–0.86)0.017 Major adverse cardiac events29 (41.4%)89 (65.4%)0.0010.49 (0.32–0.75)0.001
Results are expressed as events and percentages. CI: confidence intervals; OMT: optimal medical therapy; OPCABG: off-pump coronary artery bypass grafting.
Supplementary Table 4. Echocardiographic variables and WMSI evaluation of the unmatched population at 3-year follow up.

OPCABG group (n = 59)OMT group (n = 88)P value Echocardiographic variables Ejection fraction, %34.1 ± 4.129.7 ± 5.10.001 LVEDD, mm4.96 ± 0.655.89 ± 0.570.001 LVESD, mm3.71 ± 0.644.30 ± 0.610.001 LVEDVi, mL/m277.0 ± 4.482.9 ± 4.40.001 LVESVi, mL/m252.0 ± 4.157.1 ± 3.90.001 WMSI evaluation WMSI for LAD territory1.47 ± 0.180.61 ± 0.180.001 WMSI for LCX territory1.54 ± 0.251.68 ± 0.230.001 WMSI for RCA territory1.54 ± 0.221.66 ± 0.230.002 Global WMSI1.51 ± 0.101.64 ± 0.100.001
LAD: left anterior descending artery; LCX: left circumflex artery; LVEDD: left ventricular end diastolic diameter; LVESD: left ventricular end systolic diameter; LVEDVi: indexed left ventricular end diastolic volume; LVESVi: indexed left ventricular end systolic volume; OMT: optimal medical therapy; OPCABG: off-pump coronary artery bypass grafting; RCA: right coronary artery; WMSI: wall motion score index.
Supplementary Table 5. Echocardiographic parameters and WMSI evaluation in the unmatched population: percentage difference from baseline.
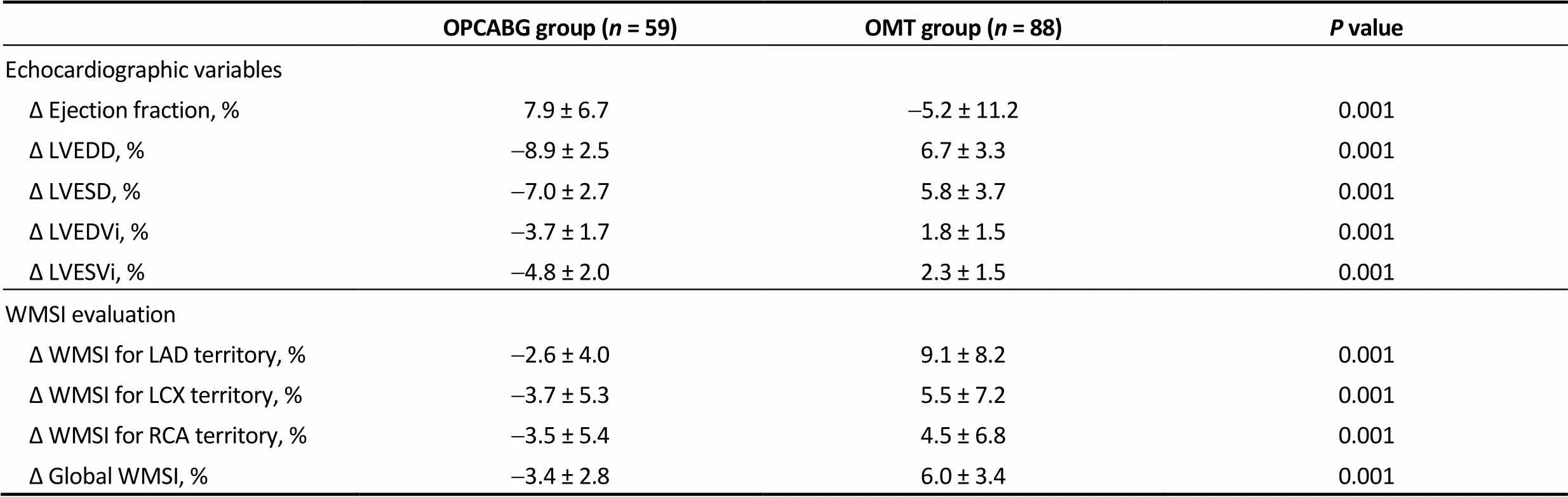
OPCABG group (n = 59)OMT group (n = 88)P value Echocardiographic variables Δ Ejection fraction, % 7.9 ± 6.7-5.2 ± 11.20.001 Δ LVEDD, %-8.9 ± 2.56.7 ± 3.30.001 Δ LVESD, %-7.0 ± 2.75.8 ± 3.70.001 Δ LVEDVi, %-3.7 ± 1.71.8 ± 1.50.001 Δ LVESVi, %-4.8 ± 2.02.3 ± 1.50.001 WMSI evaluation Δ WMSI for LAD territory, %-2.6 ± 4.09.1 ± 8.20.001 Δ WMSI for LCX territory, %-3.7 ± 5.35.5 ± 7.20.001 Δ WMSI for RCA territory, %-3.5 ± 5.44.5 ± 6.80.001 Δ Global WMSI, %-3.4 ± 2.86.0 ± 3.40.001
LAD: left anterior descending artery; LCX: left circumflex artery; LVEDD: left ventricular end diastolic diameter; LVESD: left ventricular end systolic diameter; LVEDVi: indexed left ventricular end diastolic volume; LVESVi: indexed left ventricular end systolic volume; OMT: optimal medical therapy; OPCABG: off-pump coronary artery bypass grafting; RCA: right coronary artery; WMSI: wall motion score index.
Supplementary Figure 1. Standardized differences (expressed in percentage) of bias across covariates. EF: ejection fraction; LVEDVi: indexed left ventricular end diastolic volume.
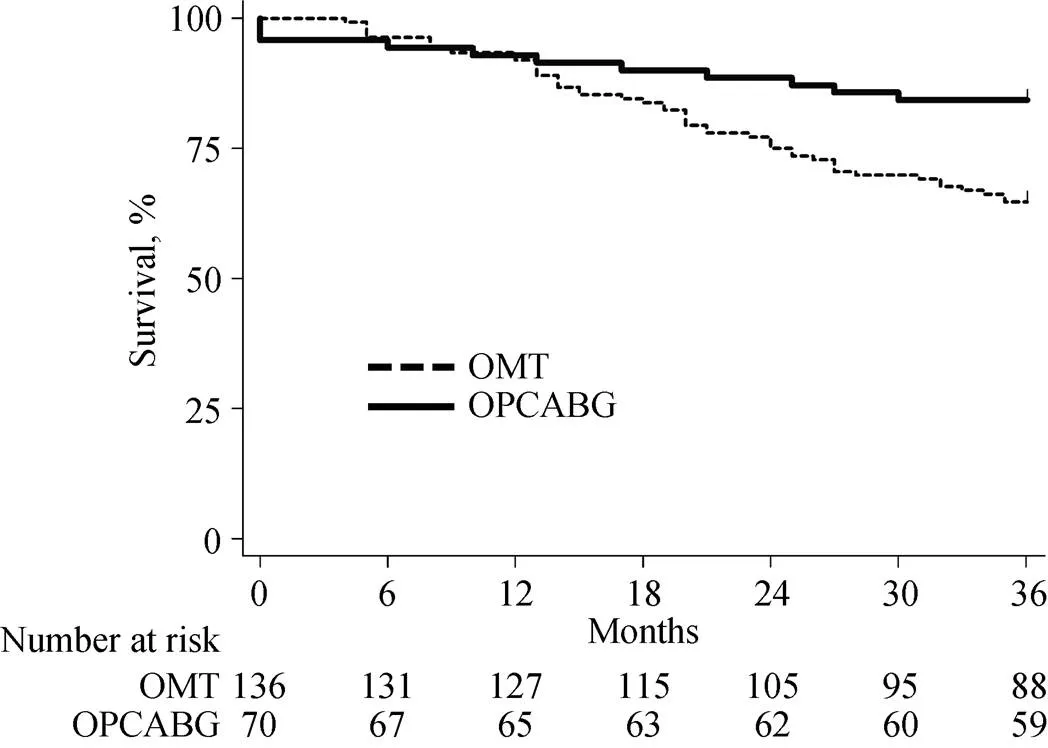
Supplementary Figure 2. Overall survival in the unmatched population. OMT: optimal medical therapy; OPCABG: off-pump coronary artery bypass grafting.
Supplementary Figure 3. Survival from cardiac events in the unmatched population. OMT: optimal medical therapy; OPCABG: off-pump coronary artery bypass grafting.

Supplementary Figure 4. Freedom from major adverse cardiac events in the unmatched population. OMT: optimal medical therapy; OPCABG: off-pump coronary artery bypass grafting.
*The first two authors contributed equally to this manuscript.
Massimo Chello, MD. Department of Cardiovascular Surgery, Università Campus Bio-Medico di Roma, Rome, Italy. E-mail: m.chello@unicampus.it
Telephone: + 39-0622541-1000
Fax: + 39 0622541-1960
February 26, 2018
May 6, 2018
June 12, 2018
July 28, 2018
 Journal of Geriatric Cardiology2018年7期
Journal of Geriatric Cardiology2018年7期
- Journal of Geriatric Cardiology的其它文章
- A three-year longitudinal study of the relation between left atrial diameter remodeling and atrial fibrillation ablation outcome
- Particular evolution in a 72-year-old diabetic patient with acute coronary syndrome
- Management of hypertensive crises in the elderly
- C-reactive protein aggravates myocardial ischemia/reperfusion injury through activation of extracellular-signal-regulated kinase 1/2
- Prognostic utility of NT-proBNP greater than 70,000 pg/mL in patients with end stage renal disease
- The effectiveness and safety of the RESTORE? drug-eluting balloon versus a drug-eluting stent for small coronary vessel disease: study protocol for a multi-center, randomized, controlled trial
