The effectiveness and safety of the RESTORE? drug-eluting balloon versus a drug-eluting stent for small coronary vessel disease: study protocol for a multi-center, randomized, controlled trial
Yi–Da TANG, Shu–Bin QIAO, Xi SU, Yun-Dai CHEN, Ze-Ning JIN,Hui CHEN,Biao XU, Xiang-Qing KONG, Wen-Yue PANG, Yong LIU, Zai-Xin YU,Xue LI, Hui LI, Yan-Yan ZHAO, Wei LI, Jian TIAN,Chang-Dong GUAN, Bo XU, Run–Lin GAO, the RESTORE SVD China Investigators
?
The effectiveness and safety of the RESTORE?drug-eluting balloon versus a drug-eluting stent for small coronary vessel disease: study protocol for a multi-center, randomized, controlled trial
Yi–Da TANG1,*, Shu–Bin QIAO1,*, Xi SU2, Yun-Dai CHEN3, Ze-Ning JIN4,Hui CHEN5,Biao XU6, Xiang-Qing KONG7, Wen-Yue PANG8, Yong LIU9, Zai-Xin YU10,Xue LI11, Hui LI12, Yan-Yan ZHAO13, Wei LI13, Jian TIAN1,Chang-Dong GUAN14, Bo XU14, Run–Lin GAO1, the RESTORE SVD China Investigators
1Department of Cardiology, Fu Wai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences, Beijing, China2Department of Cardiology, Wuhan Asia Heart Hospital, Wuhan, Hubei China3Department of Cardiology, Chinese PLA General Hospital, Beijing, China4Department of Cardiology, Beijing Anzhen Hospital, Capital Medical University, Beijing, China5Department of Cardiology, Beijing Friendship Hospital, Capital Medical University, Beijing, China6Department of Cardiology, Affiliated Nanjing Drum Tower Hospital of Nanjing University School of Medicine, Nanjing, Jiangsu, China7Department of Cardiology, Jiangsu Province Hospital, the First Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu, China8Department of Cardiology, Shengjing Hospital of China Medical University, Shenyang, Liaoning, China9Department of Cardiology, The Fourth Central Hospital of Tianjin, Tianjin, China10Department of Cardiology, Xiangya Hospital of Central South University, Changsha, Hunan, China11Department of Cardiology, Tangdu Hospital of the Fourth Military Medical University, Xi’an, Shannxi, China12Department of Cardiology, Daqing Oilfield General Hospital, Daqing, Heilongjiang, China13Division of Biometrics, National Center for Cardiovascular Diseases of China, Beijing, China14Catheterization Laboratories, Fu Wai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences, Beijing, China
Small coronary vessel disease (disease affecting coronary vessels with main branch diameters of ≤ 2.75 mm) is a common and intractable problem in percutaneous coronary intervention (PCI). This study was designed to test the theory that the effectiveness and safety of drug-eluting balloons for the treatment oflesions in small coronary vessels are non-inferior to those of drug-eluting stents.We designed a prospective, multicenter, randomized, controlled clinical trial aiming to assess the effectiveness and safety of the RESTORE?(Cardionovum, Bonn, Germany) drug-eluting balloon (DEB) versus the RESOLUTE?(Medtronic, USA) drug-eluting stent (DES) in the treatment of small coronary vessel disease. This trial started in August 2016. A total of 230 patients with a reference vessel diameter (RVD) ≥ 2.25 mm and ≤ 2.75 mm were randomly assigned to treatment with a DEB or a DES at a 1:1 ratio. The study was also designed to enroll 30 patients with an RVD ≥ 2.00 mm and ≤ 2.25 mm in the tiny vessel cohort.The key baseline data include demographic characteristics, relative medical history, baseline angiographic values and baseline procedural characteristics. The primary endpoint is in-segment diameter stenosis at nine months after the index procedure. Secondary endpoints include acute success, all-cause death, myocardial infarction, target vessel revascularization, target lesion revascularization and stent thrombosis.The study will evaluate the clinical efficacy, angiographic outcomes, and safety of DEBs compared to DESs in the treatment ofcoronary artery lesions in small vessels.
J Geriatr Cardiol 2018; 15: 469?475. doi:10.11909/j.issn.1671-5411.2018.07.006
Drug eluting balloon; Percutaneous coronary intervention; Small vessel disease
1 Introduction
Small coronary vessel disease (a disease affecting coronary vessels with main branch diameters of ≤ 2.75 mm) is commonly addressed with percutaneous coronary intervention (PCI); more than 30% of procedures occur in female, diabetic or elder patients.[1,2]The upper limit of small coronary vessel disease is still controversial. Caputo,[3]defined the upper limit of the small coronary vessel as 2.5 mm, while most clinical trials consider 2.75 mm as the upper limit.[4,5]Currently, the PCI procedure is mainly completed using plain balloon angioplasty, a drug-eluting balloon (DEB) or a drug-eluting stent (DES). The incidence of rupture or dissection of the intima and restenosis of target vessels caused by single balloon dilation angioplasty is relatively high.[6,7]DESs exhibit unsatisfactory therapeutic efficacy. Multiple studies have found that the incidences of in-stent restenosis, in-stent thrombosis and long-term adverse events (AE) are high after stent implantation.[8–11]The DEB coated with paclitaxel, a potent cell inhibitor that irreversibly inhibits arterial smooth muscle cell proliferation, has emerged as an alternative therapeutic tool for coronary atherosclerotic disease.[12]This non-stent-based device has the potential to have sustained anti-restenosis efficacy without the limitations of permanent vascular implants. Such a device has shown promising results with high-con-centration, rapid local delivery of paclitaxel without the use of drug reservoirs, thus reducing the inflammation caused by permanent metal implantation.[13]The strategy of DEB therapy with bail-out stenting may have a clinical role, particularly in the setting of small vessels, where the effect of neointimal hyperplasia is greater, and DESs perform poorly.[14]Data from small randomized trials suggest inferior results with a DEB strategy when compared to a DES strategy;[5]however, data from the Balloon Elution and Late Loss Optimization (BELLO) trial suggest low major ad-verse cardiac event (MACE) rates with DEBs used in this setting.[4]Therefore, the role of DEBs in treating small coronary vessels when compared with that of next-gene-ration DESs needs further exploration.
This study aims to test the theory that the effectiveness and safety of the RESTORE?(CARDIONOVUM, Germany) drug-eluting balloons for the treatment oflesions in small coronary vessels are non-inferior compared to those of the RESOLUTE?(MEDTRONIC, USA) zotarolimus drug-eluting stents.
2 Methods
2.1 Study design
This prospective, randomized, open-label, multiple-cen-ter trial is designed to assess the safety and efficacy of the RESTORE?DEB in the treatment ofcoronary lesions in small or tiny vessels. This study will be divided into a small vessel cohort and a tiny vessel cohort. In the small vessel cohort, patients with a visual reference vessel diameter (RVD) ≥ 2.25 mm and ≤ 2.75 mm will be randomly assigned to the RESTORE?DEB or the RESOLUTE?DES group in a 1:1 ratio. The study is powered to detect the non-inferiority of the RESTORE?DEB versus the RESOLUTE?DES for a primary endpoint of diameter stenosis at 9 months. In the tiny vessel cohort, patients with visual RVD ≥ 2.00 mm and ≤ 2.25 mm will be selected and treated with the RESTORE?DEB of an appropriate size. The protocol of the trial has been registered on ClinicalTrials.gov (NCT02946307).
2.2 Study device
The RESTORE?DEB holds a paclitaxel concentration of 3.0 μg/mm2on the balloon surface. Utilizing a stable nanocrystalline paclitaxel drug-coating, the balloon provides maximum protection from downstream effects to minimize the risk potential of myocardial vascular changes. RESTORE?prevents the endangerment of cathlab personnel and guarantees safe and predictable drug delivery to the target lesion site, benefiting from the integrity of a highly detailed protective coating. Miglonico,.[15]have confirmed the effectiveness and safety of the RESTORE?DEB compared to the DES in in-stent restenosis patients.
The control device to be used in the trial is a RESO-LUTE?zotarolimus-eluting coronary stent. The RESO-LUTE?DES is a metal stent combined with a polymer; the polymer is composed of a hydrophilic biocompatible com-ponent that faces the endoluminal surface and a hydropho-bic component attached to the metal stent surface. The polymer serves as a drug reservoir, enabling the sustained release of zotarolimus to control neointimal hyperplasia.
2.3 Patient selection
Adult patients (age ≥ 18 years) who present with stable or unstable angina or with recently stabilized myocardial infarction will be recruited from participating hospitals. Pa-tients will be eligible if they have (1) only one lesion in the target small vessel with a visual stenosis of ≥ 70% or ≥ 50% complicated by evidence of ischemia according to visual in-spection before PCI; (2) lesion length limited to < 26 mm; and (3) visual diameters of the target lesions limited to ≥ 2.25 mm and ≤ 2.75 mm in the small vessel cohort and ≥ 2.00 mm and < 2.25 mm in the tiny vessel cohort. Major exclu-sion criteria include acute myocardial infarction within 1 week of the study, a left ventricular ejection fraction of < 35%, total occlusion, bifurcation and left main lesions, or patients with more than two non-target lesions requiring treatment. Full inclusion and exclusion criteria are shown in the appendix.
Subjects are expected to participate in this study volun-tarily and to sign informed consent forms. No study-asso-ciated operation is allowed before the written informed consent forms are obtained by the investigators.
After informed consent forms are obtained, information from the subjects such as medical history, physical examina-tions and adjuvant examinations will be collected by the in-vestigators to confirm whether the subjects satisfy the inclusion/exclusion criteria. Subjects who conform to all inclusion criteria and do not meet any of the exclusion criteria will be enrolled in this study. PCI and combined therapy will be performed in accordance with the regulations of this protocol.
2.4 Randomization and registration
The randomization and registration process will be based on the Interactive Web Respond System (IWRS). Once informed consent is obtained, subjects enrolled in the small vessel cohort (visible RVD ≥ 2.25 mm and ≤ 2.75 mm) will be randomly divided into a study group and a control group at a 1:1 ratio. The study group will be treated with the RESTORE?DEB while the control group will be treated with the RESOLUTE?DES. Stratification factors for randomization include whether the subject is complicated by (1) non-target lesions and (2) diabetes. Subjects enrolled in the tiny vessel cohort (visible RVD ≥ 2.00 mm and ≤ 2.25 mm) will be registered in the IWRS. The complete study flow chart is represented in Figure 1.
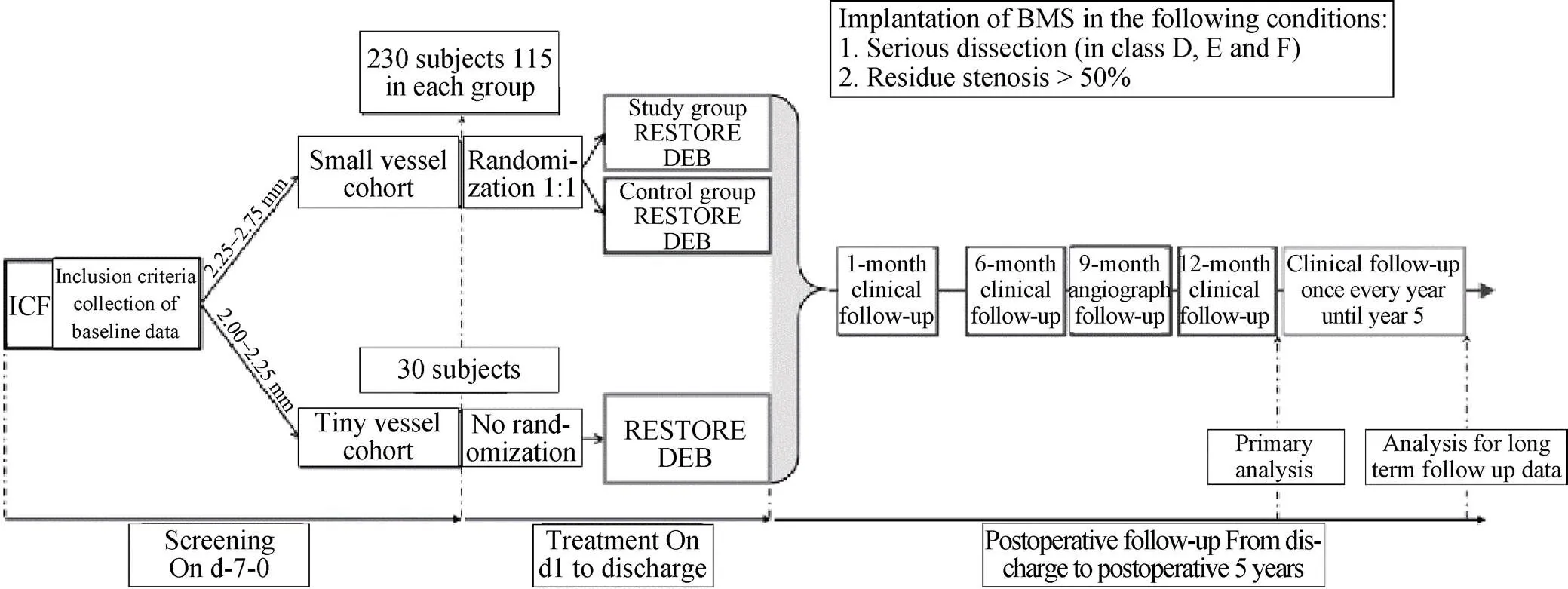
Figure 1. Study Flowchart. DEB: drug-eluting balloon; DES: drug-eluting stent; ICF: informed consent form.
2.5 Medication
Aspirin 300 mg should be taken at least 24 hours before the intervention treatment; clopidogrel 300 mg will be administered at least 6 hours before the intervention treatment and then maintained at 75 mg once per day. Otherwise, ticagrelor 180 mg will be orally administered and then maintained at 90 mg twice per day. Unfractionated heparin (100 U/kg iv) will be administered before PCI, or activated clotting time (ACT) will be maintained for 250–350 seconds (HemoTec method). According to PCI guidelines, the recommended medications should be given to the subjects by investigators based on clinical practice. After discharge from the hospital, dual antiplatelet drugs should be administered for at least six months.
2.6 Coronary angiography
In this study, the coronary angiographic results will be objectively evaluated by the central laboratory of angiography. All angiograms will be carefully recorded in all critical surgical periods. At least two orthographic views (reference views) will be required in preoperative nidus angiograms, accurate DEB balloon location angiograms obtained before dilation and two postoperative angiograms with a similar projection angle as the preoperative angiograms. Follow-up angiograms should be recorded with a similar projection angle as the postoperative angiograms. Information to be recorded includes the types of angiographic catheters and guiding catheters for intervention treatment; the brand, length and labeled diameter of the balloons; and the maximum pressure and dilation duration of each balloon. All angiography should be performed under the same standard conditions, and coronary artery angiograms should provide proper images for quantitative coronary artery analysis (QCA), which will be conducted in an angiographic core laboratory by professional technicians. QCA will be performed using the QAngio XA system, Version 7.3 (Medis Medical Imaging System BV, Leiden, the Netherlands). During analysis, angiographic body positions with the most severe coronary artery stenosis should be selected.
2.7 PCI description
2.7.1 Small vessel cohort
The enrolled subjects will be randomly divided into the study group and the control group. The study group will receive the RESTORE?DEB with lengths of 15, 20, 25 or 30 mm and diameters of 2.25, 2.50 or 2.75 mm. The control group will receive the RESOLUTE Zotarolimus DES with lengths of 8, 12, 14, 18, 24 or 30 mm and diameters of 2.25, 2.50 or 2.75 mm.
2.7.2 Tiny vessel cohort
The tiny vessel cohort will be treated with the RESTORE?DEB with lengths of 15, 20, 25 or 30 mm and a diameter of 2.00 mm.
In the DEB group, the intervention will be performed according to international guidelines and the recent China Expert Consensus on DEBs. Common balloons with smaller diameters will be used for the pre-dilation of lesions; for these cases, selection and pre-dilation procedures will be determined by the individual study sites. Stenosis ≤ 50% after pre-dilation is regarded as successful pre-dilation. Each DEB should be used once (detachment of balloon from nidus cannot be considered as one single use). Post-dilation balloons should not be used for re-dilation after the application of a DEB.
In the control group treated with the DES, pre-dilation or post-dilation methods and procedures should be determined by investigators according to clinical judgment. The balloons will be selected based on the conditions set by the individual study sites. Stenosis ≤ 50% after pre-dilation is regarded as successful pre-dilation.
The intervention treatment will be considered success-ful if the visual postoperative residual stenosis is ≤ 50% in the DEB group and ≤ 30% in the DES group after PCI. If there is severe intraoperative dissection (in classes D, E, and F), or the visual residual stenosis is > 50% immedi-ately after PCI, bare-metal stents (BMS) should be implanted for rescue treatment by investigators based on clinical judgment. Remedial stents are not recommended, as the arterial dissec-tion in classes A, B, and C does not influence blood flow.
2.8 Follow-up
The clinical follow-up (by telephone when necessary) will be performed at postoperative months 1, 6, and 12 and performed once every year thereafter until year 5 to analyze the incidences of clinical events at each time point. The schedule for visits and evaluation of the subjects is shown in the Appendix.
2.9 Endpoint measurement
The primary endpoint is in-segment diameter stenosis at 9 months after the index procedure. Secondary endpoints include acute success, all-cause death, myocardial infarction, target vessel revascularization, target lesion revascularization and device thrombosis, as defined by the Academic Research Consortium. Details of the endpoints are provided in Table 1.
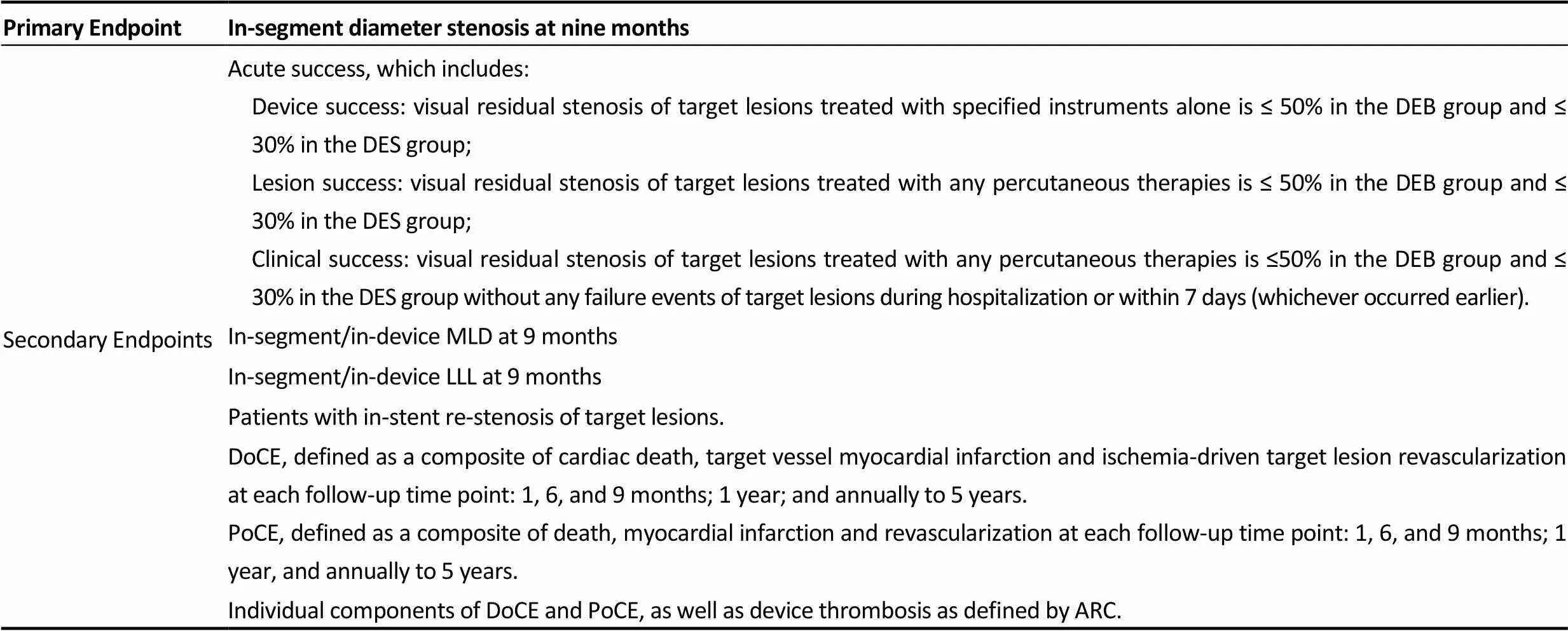
Table 1. Clinical endpoints.
ARC: Device oriented composite endpoints; DEB: drug-eluting balloon; DES: drug-eluting stent; LLL: late lumen loss; MLD: minimal luminal diameter; PoCE: Patient oriented composite endpoints.
QCA will be analyzed by the core laboratory while related events will be analyzed by the Clinical Event Committee. The endpoint indexes at postoperative months 1, 6, 9 and 12 will be included in the primary analysis and documented in the clinical study report for product registration. The analysis of long-term follow-up data will be recorded in the follow-up clinical study report.
2.10 Sample size calculation
According to the results reported in published literature,[4,16–18]the severity of stenosis diameter by month 9, which is the primary endpoint, will be assumed to be 25% in the control group treated with the RESOLUTE?DES and 32.5% in the study group treated with the RESTORE?DEB; the combined standard deviation of both groups is conservatively estimated to be ± 18%, and the threshold of non-in-feriority will be set at 15%. In statistical data analysis, the significance level is set as 5% of both sides, while the power of the test will be set as 80%. The subjects will be randomly divided at a ratio of 1:1. According to calculation, the minimum sample size is 91 patients in each group. Plans call for enrolling 230 subjects, considering a dropout rate of < 20% for the angiographic follow-up.
A total of 230 subjects will be enrolled in the randomized controlled small vessel cohort. Another 30 subjects in the study group, whom will receive balloons with diameters of 2.0 mm, will be enrolled in the tiny vessel cohort to evaluate the clinical efficacy of DEBs with diameters of 2.0 mm.
2.11 Statistical analysis
The continuous correction χ2test will be used for the intergroup comparison of enumeration data, while the Fisher exact test will be applied if the theoretical frequency is less than 5 in more than 25% of the cells. The group-test will be used for the intergroup comparison of measurement data with normal distribution, while the Wilcoxon rank sum test will be used for the comparison of data with non-normal distribution. Analysis of covariance that can control for central and baseline effects will be applied for the intergroup comparison. The minimum mean square of the dependent variable, the minimum intergroup mean-variance and 95% confidence interval (CI), as well as the difference of intergroup efficacy and the estimated 95% CI, will be provided after the homogeneity of variance is tested. Whether the hypothesis of non-inferiority is available will be judged by comparing the 95% CI of the difference in intergroup efficacy with the threshold of prespecified non-inferiority, which is of great clinical significance in this protocol.
All statistical data analysis will be conducted with significance levels of 0.05 for both sides. SAS?9.4 will be used for all data analyses.
3 Results
Our study started in August 2016. The estimated primary completion date is set for December 2018. The key baseline characteristics include demographic characteristics, relative medical history, baseline angiographic characteristics and baseline procedural characteristics. The statistical analysis was described in the corresponding part of the manuscript. The primary endpoint is in-segment diameter stenosis at nine months after the index procedure. Secondary endpoints include acute success, all-cause death, myocardial infarction, target vessel revascularization, target lesion revascularization and stent thrombosis.
4 Discussion
Percutaneous revascularization of small coronary vessels represents a real challenge for the interventional cardiologist because it may be associated with an increased risk of adverse clinical events.[19–22]Small vessel size has, in fact, been reported as a powerful independent predictor of res-tenosis and repeat revascularization.[23,24]This is mainly due to the limited ability of the vessel to accommodate for the even limited neointimal proliferation that might develop after stent implantation.
Great expectations have been placed on DESs in the treatment of small vessel lesions due to their strong effect in inhibiting neointima proliferation. Many studies have proved that although the DES is effective for small vessel lesions, its efficacy in normal vessel lesions is more pronounced. Elezi,.[25]divided 2058 subjects with sirolimus-eluting stents (SES) or paclitaxel-eluting stents (PES) into three groups by vascular diameter (< 2.41 mm group, 2.41–2.84 mm group, and > 2.84 mm group). Although the incidences of composite endpoint events (death and myocardial infarction) were similar among the three groups, the rate of target lesion revascularization (TLR) was significantly higher in the < 2.41 mm group. Multivariate analysis indicated that vascular diameter was an independent factor for the prediction of TLR after DES implantation.
At present, the PTX-eluting balloon is the most popular DEB in clinical practice because PTX has advantages of a high lipotropy and adsorption rate and can be quickly absorbed by the intima to persistently and effectively inhibit the proliferation of intimal smooth muscle. In recent years, contradictory conclusions have been drawn when comparing DEBs to DESs in the field of interventional treatment for small coronary vessel lesions. The PICCOLETTO study,[5]the first randomized trial in this field, compared the first-generation Dior-I DEB with the Taxus Liberte DES in vessels < 2.75 mm in diameter. This study was halted prior to complete enrollment because of the clear superiority of the DES, which was associated with a lower rate of angiographic restenosis (10.3%. 32.1%;= 0.04) and MACE (13.8%. 35.7%;= 0.054). In the BELLO study,[4]182 subjects with small vessel lesions (< 2.8 mm) were enrolled in total, with the primary endpoint being late lumen loss. Follow-up results at 6 months demonstrated that the PTX DEB (IN.PACT Falcon) notably reduced the in-stent or in-balloon LLL[(0.08 ± 0.38) mm. (0.29 ± 0.44) mm,= 0.001]. The conflicting results may be related to the different types of DEBs used in the studies above. Technologies in different DEBs are not comparable and differ significantly, such as in the balloon technology, drug coating process, and the excipient that acts as a drug carrier and transport facilitator to the vessel wall. To compare the effectiveness of DEBs to second-generation DESs in small coronary vessels, a subgroup study of the BELLO study compared the IN.PACT Falcon DEB (90 pts) to the Xience V DES (91 pts), demonstrating similar MACE (12.2%. 15.4%;= 0.538) and revascularization rates (4.4%. 5.6%;= 0.72) at the 1-year follow-up.[26]
We, therefore, designed a randomized controlled clinical study to evaluate the efficacy and safety of the RESTORE?DEB by comparing it with the RESOLUTE?Zotarolimus DES in the treatment of Chinese coronary artery disease (CAD) patients with small vessel lesions. If our hypotheses are supported, the study findings will have significant implications for clinical practice. Evidence on whether the efficacy and safety of DEB angioplasty are non-inferior to that of DES implantation in patients withcoronary lesions in small vessels will be highly attractive to clinicians and patients.
Acknowledgments
This work was supported by the CAMS Innovation Fund for Medical Sciences [CIFMS 2016-I2M-1-009] and the Beijing Municipal Commission of Science and Technology [Z181100006318005]. The study design, data collection, statistical analysis, or publications are not influenced by the sponsors and are the exclusive responsibility of the investigators. We also thank Joyce Kong for the critical revisions and language editing (New York Institute of Technology-College of Osteopathic Medicine).
This study was approved by the institutional review board central committee at Fuwai Hospital, NCCD of China, and complies with the Declaration of Helsinki. Informed consent will be obtained from each participating patient.
The authors declare that there are no competing interests.
1 Ma CS, FANG WY, Huo Y,.;People's Medical Publishing House: Beijing, China, 2012.
2 Biondi-Zoccai G, Moretti C, Abbate A,. Percutaneous coronary intervention for small vessel coronary artery disease.2010;11: 189–198.
3 Caputo R, Leon M, Serruys P,. Performance of the resolute zotarolimus-eluting stent in small vessels.2014;84: 17–23.
4 Latib A, Colombo A, Castriota F,. A randomized multi-center study comparing a paclitaxel drug-eluting balloon with a paclitaxel-eluting stent in small coronary vessels: the BELLO (Balloon Elution and Late Loss Optimization) study.2012;60: 2473–2480.
5 Cortese B, Micheli A, Picchi A,. Paclitaxel-coated balloon versus drug-eluting stent during PCI of small coronary vessels, a prospective randomized clinical trial. The PICCOLETO study.2010;96: 1291–1296.
6 Aronson D, Edelman ER. Revascularization for coronary artery disease in diabetes mellitus: angioplasty, stents and coronary artery bypass grafting.2010;11: 75–86.
7 Sugihara M, Miura S, Nishikawa H,. Characteristics of patients and types of lesions in patients with drug-eluting or bare-metal stent implantation in small coronary arteries: from the FU-Registry.2013; 61: 117–121.
8 Mehilli J, Dibra A, Kastrati A,. Randomized trial of paclitaxel- and sirolimus-eluting stents in small coronary vessels.2006;27: 260–266.
9 Godino C, Furuichi S, Latib A,. Clinical and angio-gra-phic follow-up of small vessel lesions treated with paclitaxel- eluting stents (from the TRUE Registry).2008;102: 1002–1008.
10 Aboyans V, Lacroix P, Criqui MH,. Large and small vessels atherosclerosis: similarities and differences.2007;50: 112–125.
11 De Luca G, Suryapranata H, de Boer MJ,. Impact of vessel size on distal embolization, myocardial perfusion and clinical outcome in patients undergoing primary angioplasty for ST-segment elevation myocardial infarction.2009;27: 198–203.
12 Scheller B, Speck U, Abramjuk C,. Paclitaxel balloon coating, a novel method for prevention and therapy of res-tenosis.2004;110: 810–814.
13 Gray WA, Granada JF. Drug-coated balloons for the preven-tion of vascular restenosis.2010;121: 2672–2680.
14 Zeymer U, Scheller B. PCI in small vessels: the case for a drug-coated balloon based intervention.2011;7: K57–K60.
15 Miglionico M, Mangiacapra F, Nusca A,. Efficacy and safety of Paclitaxel-Coated Balloon for the treatment of in- stent restenosis in high-risk patients.2015;116: 1690–1694.
16 Vaquerizo B, Miranda-Guardiola F, Fernandez E,. Treatment of small vessel disease with the paclitaxel drug- eluting balloon: 6-month angiographic and 1-year clinical outcomes of the spanish multicenter registry.2015;28: 430–438.
17 Saito S, Maehara A, Vlachojannis GJ,. Clinical and angiographic evaluation of the resolute zotarolimus-eluting coronary stent in Japanese patients––long-term outcome in RESOLUTE Japan and RESOLUTE Japan small vessel study.2015;79: 96–103.
18 Mehilli J, Dibra A, Kastrati A,. Randomized trial of paclitaxel- and sirolimus-eluting stents in small coronary vessels.2006;27: 260–266.
19 Koning R, Eltchaninoff H, Commeau P,. Stent placement compared with balloon angioplasty for small coronary arteries: in-hospital and 6-month clinical and angiographic results.2001;104: 1604–1608.
20 Schunkert H, Harrell L, Palacios IF,. Implications of small reference vessel diameter in patients undergoing percutaneous coronary revascularization.1999;34: 40–48.
21 Hirshfeld JJ, Schwartz JS, Jugo R,. Restenosis after coronary angioplasty: a multivariate statistical model to relate lesion and procedure variables to restenosis. The M-HEART Investigators.1991;18: 647–656.
22 Bourassa MG, Lesperance J, Eastwood C,. Clinical, physiologic, anatomic and procedural factors predictive of restenosis after percutaneous transluminal coronary angioplasty.1991;18: 368–376.
23 Rathore S, Terashima M, Katoh O,. Predictors of angiographic restenosis after drug-eluting stents in the coronary arteries: contemporary practice in real-world patients.2009;5: 349–354.
24 Kasaoka S, Tobis JM, Akiyama T,. Angiographic and intravascular ultrasound predictors of in-stent restenosis.1998;32: 1630–1635.
25 Elezi S, Dibra A, Mehilli J,Vessel size and outcome after coronary drug-eluting stent placement: results from a large cohort of patients treated with sirolimus- or paclitaxel- eluting stents.2006;48: 1304–1309.
26 Giannini F, Latib A, Ancona MB,. A propensity score matched comparative study between paclitaxel-coated balloon and everolimus-eluting stents for the treatment of small coronary vessels.2017; 90: 380–386.
*The first two authors contributed equally to this manuscript.
Bo XU, MD & Run–Lin GAO, MBBS. Catheterization Laboratories, Fu Wai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, 167 Beilishi Road, Xicheng District, Beijing 100037, China (XU B); Department of Cardiology, Fu Wai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, 167 Beilishi Road, Xicheng District, 100037 Beijing, China (GAO RL). E-mails: bxu@citmd.com (XU B) & gaorunlin@citmd. com (GAO RL)
May 17, 2018
July 17, 2018
July 17, 2018
July 27, 2018
 Journal of Geriatric Cardiology2018年7期
Journal of Geriatric Cardiology2018年7期
- Journal of Geriatric Cardiology的其它文章
- Single-territory incomplete surgical revascularization improves regional wall motion of remote ventricular areas: results from a propensity-matched study
- A three-year longitudinal study of the relation between left atrial diameter remodeling and atrial fibrillation ablation outcome
- Particular evolution in a 72-year-old diabetic patient with acute coronary syndrome
- Management of hypertensive crises in the elderly
- C-reactive protein aggravates myocardial ischemia/reperfusion injury through activation of extracellular-signal-regulated kinase 1/2
- Prognostic utility of NT-proBNP greater than 70,000 pg/mL in patients with end stage renal disease
