Surface Performance Testing of Cardanol Polyoxyethylene Ether and Its Application in Detergent
Zhao Xuehua, Wang Limin
Shanghai Key Laboratory of Functional Materials Chemistry, China
Huang Zhongrui, Kang Wenqian
Institute of Fine Chemicals, School of Chemistry and Molecular Engineering, East China University of Science and Technology, China
Che Fei, Zhai Zhaokai
Shanghai Bronkow Chemical Co., Ltd., China
Introduction
Cardanol polyoxyethylene ether (BGF), belonging to the linear alkyl phenol polyoxyethylene ether, synthesizing from natural cardanol as raw material, is a biodegradable and environmental friendly nonionic surfactant. Having excellent acid and alkali resistances,[1]the biodegradable products of BGF do not contain any APEO (alkylphenol polyoxyethylene ether), leaving no effect to the endocrine system and no harm to nature. Its molecular structure is shown in Figure 1. n is the average plus number of EO.
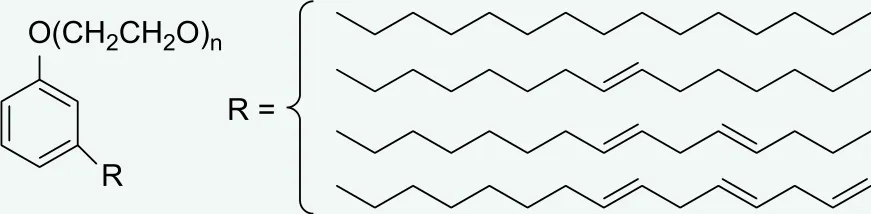
Figure 1. The structure of BGF
Nonylphenol polyoxyethylene ether (TX-10) is one of the main nonionic surfactants widely abandoned in developed countries due to pollution caused by its relevant products. BGF-10, synthesizing from natural cardanol as raw material, perfectly avoided detrimental effects to the environment compared to traditional alkylphenol polyoxyethylene ether products synthesized from petroleum.[2]BGF-10, having a fast and complete biodegradability, obtaining quite satisfying water solubility,is an ideal green environment-friendly surfactant used in various detergents. Starting with three aspects-water hardness, the additives of common electrolyte (NaCl and Na2SO4) and solubilizers (alcohols), this article mainly revolved around the common factors during the process of washing application, discussed and analyzed their influences on the surface activity of BGF-10, and studied the synergistic performance of BGF-10 as a preparation for the study of environment-friendly detergent formulations. At last, the emulsifying performances, the foam performances, thickening performances, the cloud points and detergencies of both BGF-10 and TX-10 were studied in order to find whether BGF-10 is an all-around replacement of TX-10.
Materials and instruments
BGF-10 (99% of the active ingredient is cardanol polyoxyethylene ether, the average plus number of EO is 10, the number average molecular weight Mn is 2179),AEO9(the number average molecular weight Mn is 1353),TX-10 (the number average molecular weight Mn is 715),industrial grade, Shanghai Bronkow Chemical Co., Ltd;calcium chloride, sodium chloride, sodium sulfate,magnesium chloride and hexadecyltrimethylammonium bromide, analytical grade, liquid paraffin, chemical grade,Shanghai Titanchem Co., Ltd; sodium dodecyl benzene sulfonate, methanol, ethanol, n-propanol and n-butanol,analytical grade, Shanghai Lingfeng Chemical Reagent Co. Ltd; JB series dirty cloth (JB-01, carbon black oil dirty cloth; JB-02, protein dirty cloth; JB-03, sebum dirty cloth;JB-04, edible oil dirty cloth, JB-05, starchy dirty cloth)and standard washing powder, China Daily Chemical Industry Research Institute; deionized water; automatic plate tensiometer (JK99C), Shanghai Zhongchen Digital Technic Apparatus Co.,Ltd; digital viscometer(DV-C), Shanghai Qiashen Instrument Co., Ltd;digital thermometer (DM6801A TYPE K), Shanghai Precision Science Instrument Co., Ltd; scrubbing double barrel washing machine (XPB55-17S), Little Swan; digital whiteness meter (WSB-2), Shanghai Xin Rui Instrument Co., Ltd.
Methods
The surface tensions of aqueous solutions were measured with an automatic plate tensiometer at 25 ℃; the emulsifying performances of BGF-10 and TX-10 for paraffin oil were determined by water separation method;[3,4]the foam performances of BGF-10 and TX-10 were measured by shaking method;[3,4]the thickening performances were tested by the viscosity method; the decontamination abilities of BGF-10 and TX-10 were tested by the reference GB/T 13174-2008.[5]
Surface tension measurements
Sixteen aqueous solutions with different concentrations of BGF-10 were prepared and the surface tensions were measured at 25 ℃. The curve of γ-lgc was plotted and the CMC value was determined from the break point of the curve exhibited in a tension (log) concentration plot.
The effects of water hardness
The aqueous solutions with concentrations of 0,3.75×10-3mol/L, 7.50×10-3mol/L respectively (calculated as Ca2+) were prepared and the surface tensions of BGF-10 under different concentrations of hard water were tested.
The effects of inorganic salts
The inorganic salts solutions of NaCl and Na2SO4with concentrations of 0.1 mol/L, 0.5 mol/L and 0.9 mol/L were prepared respectively and the surface tensions of BGF-10 under different concentrations of inorganic salts solutions were tested.
The effects of alcohols
The aqueous solutions of methanol, ethanol, n-propanol and n-butanol with concentrations of 0.1 mol/L, 0.3 mol/L,0.5 mol/L and 0.7 mol/L were prepared respectively and the surface tensions of BGF-10 under different concentrations of alcohols solutions were tested.
The synergistic performances of BGF-10 with other surfactants
Determination of CMC. A series of SDBS, AEO9and CTAB solutions with different concentrations were prepared. The surface tension of each solution was measured at 25 ℃.
Theoretical CMC value of composite systems.The theoretical synergistic performances were calculated by the weighted average of the single component critical micelle concentrations CMC1, CMC2and the minimum surface tension γCMC1, γCMC2, used by Wang Zhengwu et al.,[6]as shown in equations (1) and (2).
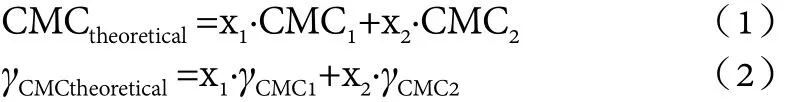
In the formula, x1and x2were the mass fraction of single component 1 and 2 respectively. CMCtheoreticalwas the theoretical CMC value of the composite systems,and γCMCtheoreticalwas the theoretical γCMCof the composite systems. In the actual composite system, when the actual CMC (CMCactual) or actual γCMC(γCMCactual) is smaller than the CMCtheoreticalor γCMCtheoretical, it indicates that the composite system is more effective, conversely, the efficiency of the composite system is reduced.
Actual CMC value of composite systems.BGF-10 was mixed with SDBS, AEO9and CTAB at a certain proportion to test the CMC value of each composite solutions at 25 ℃.
The emulsifying performances of BGF-10 and TX-10
The BGF-10 and TX-10 solutions with concentrations of 2.28×10-5mol/L were prepared and the emulsifying performances of BGF-10 and TX-10 for paraffin oil were determined by water separation method respectively.
The foam performances of BGF-10 and TX-10
The BGF-10 and TX-10 solutions with concentrations of 2.28×10-5mol/L were prepared and the foam performances of BGF-10 and TX-10 were measured by shaking method respectively.
The thickening performances of BGF-10 and TX-10
Two kinds of detergents that BGF-10 and TX-10 had the same proportion respectively in the detergent formulations were prepared and the viscosities of the two detergents were tested by a digital viscometer (DV-C).
The cloud points of BGF-10 and TX-10
The 1 wt% aqueous solutions of BGF-10 and TX-10 were prepared and the cloud points of BGF-10 and TX-10 were tested by a digital thermometer.
The detergencies of BGF-10 and TX-10
A mild detergent formulation (laboratory homemade was chosen, the main ingredients were SDBS, K12, BGF-10 or TX-10, AEO9, AES, Na2SO4, isopropyl alcohol, triethanolamine, ethylenediaminetetraacetic acid disodium salt,NaOH and water), two kinds of detergents that BGF-10 and TX-10 had the same proportion respectively in the detergent formulations were prepared. Referring to GB/T 13174-2008, the detergencies of BGF-10 and TX-10 were measured. The detergent solution was hardened with 250 mg/kg of hard water (expressed as calcium carbonate,where the molar ratio of calcium ions to magnesium ions was 6∶4). The specific calculation method was found in reference 5. The size of dirty cloth was 6 cm × 6 cm, and each group selected 6 pieces of dirty clothes. Then each batch of dirty cloth was washed in a scrubbing double barrel washing machine for 20 minutes at room temperature(25 ℃),dehydrated for 3 minutes, and dried at room temperature(25 ℃) for 24 hours to test the detergencies of BGF-10 detergent, TX-10 detergent and standard washing powder to each of the dirty cloth. The decontamination value R and decontamination ratio P of two kinds of detergents were caluated according to the different kinds of dirty cloth method.
Results and discussions
Surface tension of BGF-10
The observed change law of surface tension and the concentration of BGF-10 at 25 ℃ are shown in Figure 2. The CMC of BGF-10 in deionized water was 6.09×10-6mol/L and the minimum surface tension γCMCwas 38.08 mN/m.According to γCMC, the surface tension of water (about 72.00 mN/m at 25 ℃) was remarkably reduced, which indicated the great surfactivity of BGF-10.
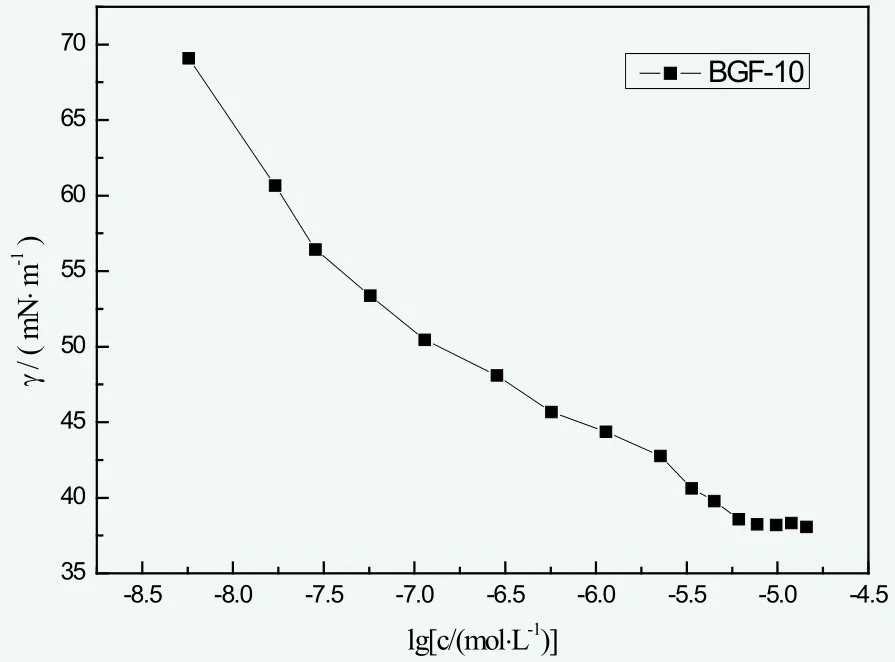
Figure 2. The surface tension curve of BGF-10
Effect of water hardness on the surface performance of BGF-10
The effect of water hardness on the surface tension of BGF-10 is shown in Figure 3. It was obvious in Figure 3 that with the growth of the hardness of water, BGF-10 became less efficient reducing the surface tension of water, while γCMCwas unchanged. This indicated the ability to reduce water surface tension stayed the same. The slight change on CMC value of the solution was possibly, because Ca2+ion did minor influence on hydrophilic part of BGF-10, which enabled micelle to form up normally. This indicated fine hard water resistance of BGF-10.
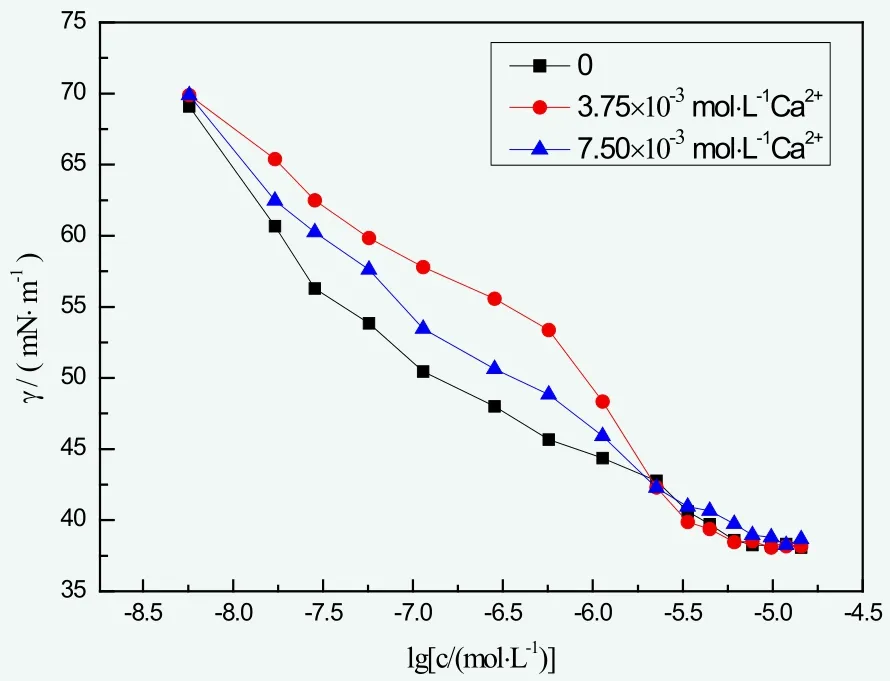
Figure 3. The surface tension of BGF-10 in different water hardness
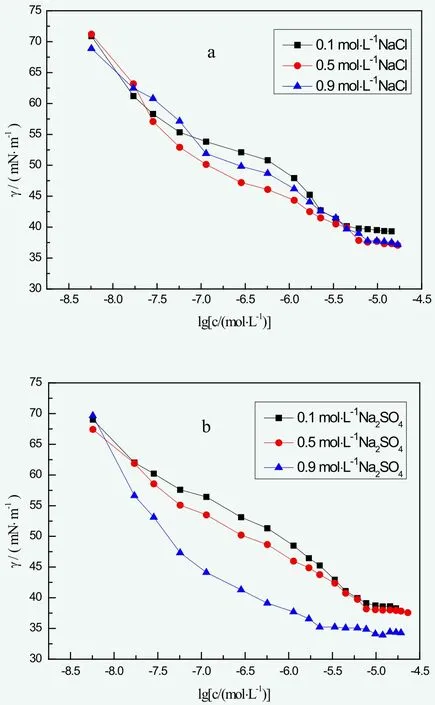
Figure 4. The surface tension of BGF-10 under different concentrations of NaCl and Na2SO4
Effect of inorganic salts on surface performance of BGF-10
It is usually considered that inorganic salt electrolytes exert an influence to the surface performance of non-ionic surfactants by influencing the property of water structure—main body of the entire hybrid system.[7]Since NaCl and Na2SO4shares the same cation, the key factor affecting the surface performance of BGF-10 should be Cl- and SO42-. In this case, the influence to BGF-10 surface tension of NaCl and Na2SO4solution under different concentration can be found in Figure 4 as followed. According to Figure 4,CMC value of BGF-10 barely changed under low NaCl concentration and it slightly increased under rather high NaCl concentration. On another hand, γCMCof BGF-10 slightly increased under low NaCl concentration, gently decreased under high concentration. In short, NaCl concentration hardly did any influence on BGF-10 surfactivity, which indicated great saltresistance.
For Na2SO4, CMC value stayed the same while γCMCslightly decreased as the concentration increased from 0.1 mol/L to 0.5 mol/L. At the concentration point of 0.9 mol/L, CMC was 2.13×10-6mol/L, nearly 1/3 of the original value, while γCMCwas 33.92 mN/m, nearly 7/8 of the original. In short, SO42-obtained a larger influence on BGF-10 surfactivity than Cl- under high salinity.
Effects of monohydric alcohols on the surface performance of BGF-10
The influences on BGF-10 surfactivity of different monohydric alcohols are shown as Figure 5. Monohydric alcohols can reduce the surface tension of BGF-10, and the reduced rate advanced with the growth of carbon chain number. However, the influence on CMC value of BGF-10 was rather complicated.[8]It reduced CMC value of BGF-10 with the decrease of alcohol concentration, however, as the concentration increased the CMC value also rose.Overall, the CMC value decreased first, and then increased.This was probably because the surfactant micelle forming process with alcohol molecule participation was a free energy decreasing, spontaneous process, the appearance of alcohol molecules led to easier micelle forming, and CMC value decreased. Water changed to alcohol molecule since the solution property changed as the concentration increases, causing higher surfactant solubility, led to higher CMC value.[9]
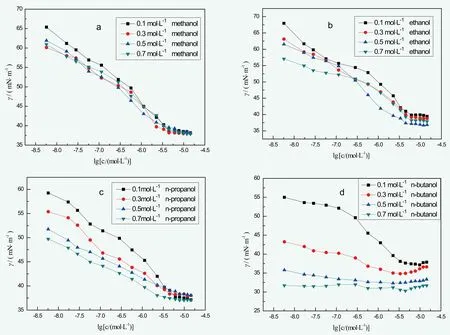
Figure 5. The surface tensions of BGF-10 under different concentrations of methanol, ethanol, n-propanol, and n-butanol
The surface tension hit lowest point while using n-butanol as solubilizer under low concentration, BGF-10 concentration around 3.36×10-6~ 4.46×10-6mol/L. Remarkably, surface tension increased when BGF-10 concentration was higher than 4.46×10-6mol/L, this was because BGF-10 would drastically form micelles when the concentration hits a certain high level, and n-butanol participated in the forming process of micelle. Surface tension barely changed as n-butanol concentration stayed at a high level as the curve was basically horizontal, this could be solution property changed due to high n-butanol concentration,which made BGF-10 to dissolve in n-butanol, losing its surfactivity.
The synergistic performance of BGF-10
The common anionic surfactant SDBS, non-ionic surfactant AEO9 and cationic surfactant CTAB were selected to match the BGF-10. The optimal concentration ratios for the composite systems were derived from the phase separation model of Rosen et al.,[10]as shown in equations (3).
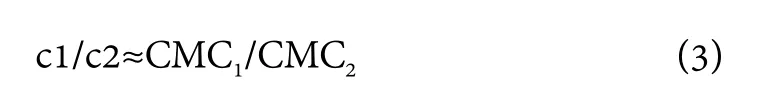
The surface tensions of SDBS, AEO9 and CTAB were tested at 25 °C respectively. The results are shown in Table 1.It was obvious that CMC value of BGF-10 was much less than SDBS, AEO9and CTAB. Although the γCMCof BGF-10 was higher than the others, it still effectively reduced the surface tension of water. In accordance with the CMC values in Table 1 and equations (3), the ratios of the composite systems were calculated. The results of composite systems are shown in Table 2.
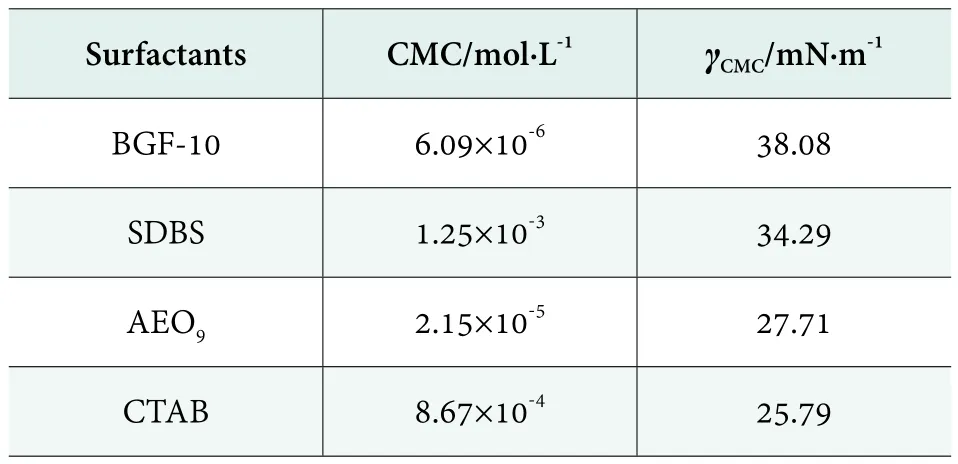
Table 1. The surface properties of BGF-10, SDBS, AEO9,CTAB
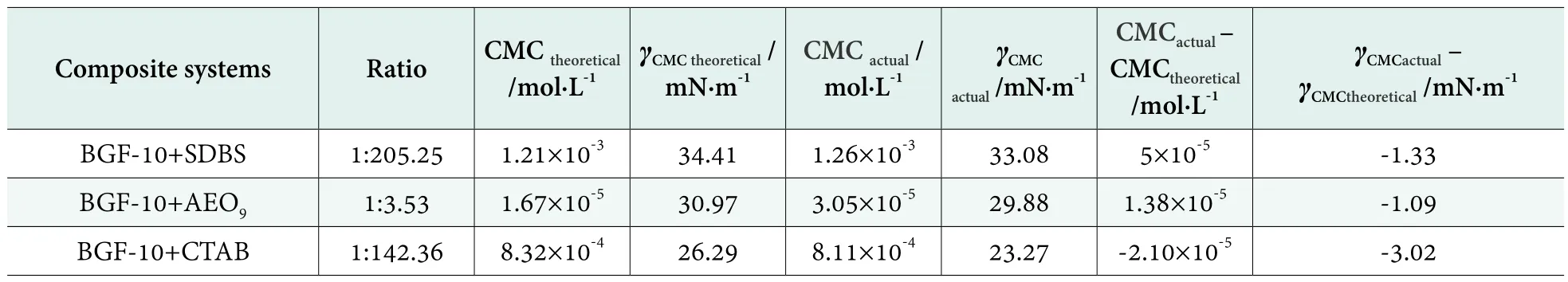
Table 2.The results of composite systems
From Table 2, it can be seen that the synergistic effect of BGF-10 with CTAB was the most obvious. The CMC value of the composite system was 8.11×10-4mol/L decreased by 2.52%, and the γCMCwas 23.27 mN/m decreased by 11.49%. This was mainly due to the addition of the nonionic surfactant BGF-10 to the cationic surfactant CTAB, which would form mixed micelles in the solution system. The formation of mixed micelles of this composite system was the result of the interaction of the ionic groups of the cationic surfactant CTAB with the polar polyoxyethylene groups of the nonionic surfactant BGF-10.[11]
After the synergism of BGF-10 with SDBS and AEO9,the CMC values were 1.26×10-3mol/L and 3.05×10-5mol/L respectively, which were greater than the theoretical CMC values, but the γCMCwere 33.08 mN/m and 29.88 mN/m respectively, with a decrease of 3.87% and 3.52% respectively.
In general, the orders of synergistic effect of BGF-10 with these three surfactants as followed:
The ability to form micelles was: CTAB > SDBS > AEO9.
The ability to reduce the surface tension of the solution was: CTAB > SDBS > AEO9.
The emulsifying performances of BGF-10 and TX-10
The emulsifying performances of BGF-10 and TX-10 for liquid paraffin were shown in Table 3. It can be seen that the emulsifying performance of BGF-10 for paraffin oil was better than that of TX-10.
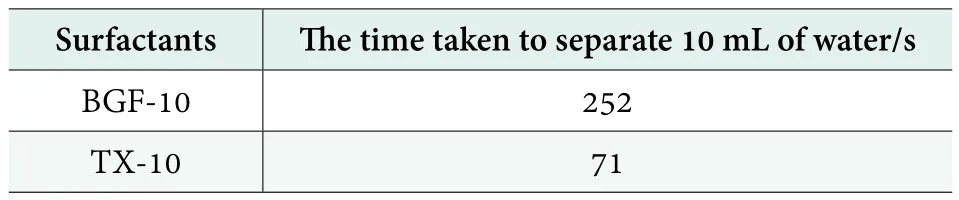
Table 3. The emulsifying properties of BGF-10 and TX-10 for liquid paraffin
The foam performances of BGF-10 and TX-10
The foam performances of BGF-10 and TX-10 are shown in Table 4. As can be seen in Table 4, there is little difference between BGF-10 and TX-10 in their foaming ability and foam stability. BGF-10 has the properties of low foaming.
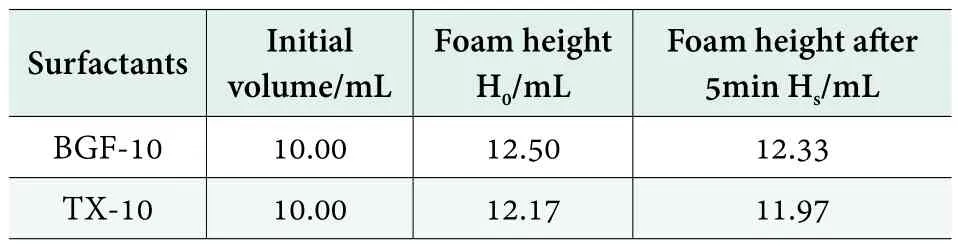
Table 4. The foam properties of BGF-10 and TX-10
The thickening performances of BGF-10 and TX-10
The viscosities of the detergents containing BGF-10 and TX-10 are shown in Table 5. As can be seen in Table 5,the viscosity of the detergent containing BGF-10 is higher than that of the detergent containing TX-10 under the same conditions. The thickening performance of BGF-10 is better than TX-10 in this detergent.
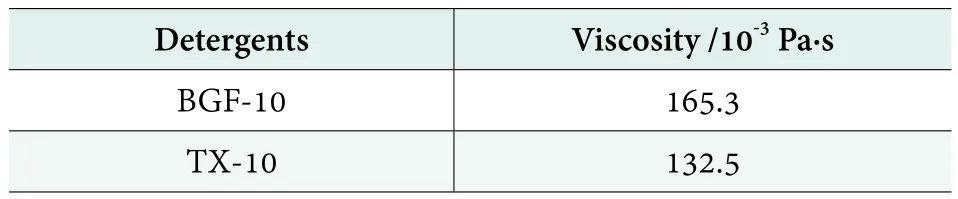
Table 5.The thickening properties of BGF-10 and TX-10
The cloud points of BGF-10 and TX-10
In the washing process, it is necessary to mention the cloud point of the surfactant. The cloud points of BGF-10 is 79.87°C, while the cloud point of TX-10 is 60.33°C.BGF-10 has a higher cloud point than TX-10.
The detergencies of BGF-10 and TX-10
Referring to the GB-T/13174-2008, the decontamination value of a dirty cloth (Ri) was calculated according to formula (5); the decontamination ratio (Pi) was calculated in accordance with the formula (6). The results are shown in Table 6. As can be seen in Table 6, for JB-01 and JB-05 dirty clothes, detergents containing BGF-10 and TX-10 showed equivalent R and P; for JB-02 and JB-03 dirty clothes, the detergent containing BGF-10 showed higher R and P than the detergent containing TX-10; however, for JB-04 dirty clothes, R and P of the detergent containing BGF-10 were slightly lower than the detergent containing TX-10, but the difference was not great. In most cases, BGF-10 can be used instead of TX-10 in detergents.
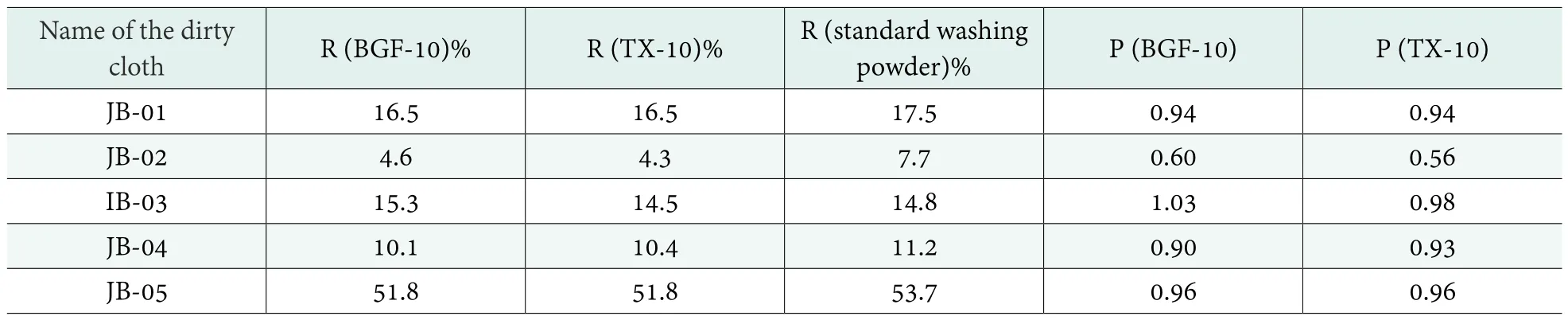
Table 6. The detergencies of BGF-10 and TX-10

i —the ithtype of dirty cloth;
F1i—the spectral reflectance of the ithtype of dirty cloth before washing, %;
F2i—the spectral reflectance of the ithtype of dirty cloth after washing, %;
n —the effective number of each group of dirty clothes after Q test;
—the decontamination value of the sample, %;
—the decontamination value of the standard washing powder, %.
Conclusions
1) The cardanol polyoxyethylene ether (BGF-10) has great surface activity and strong ability to resist hard water. At 25°C,the CMC value was 6.09×10-6mol/L and γCMCwas 38.08 mN/m.
2) BGF-10 has a strong resistance to NaCl. When the concentration of inorganic salt Na2SO4is low, the surface activity of BGF-10 changes little; when the concentration of Na2SO4is higher, the surface activity of BGF-10 is significantly increased; the divalent SO42-has a greater impact on the surface properties of BGF-10 than monovalent Cl-.
3) The short carbon alcohol could decrease the surface tension of BGF-10, and with the increase of carbon chain, the decrease is greater. CMC value decreases first and then increases with the increase of alcohol concentration. For the longer chain monohydric alcohols, when the BGF-10 concentration exceedes a certain value, the surface tension would rise.
4) BGF-10 has certain synergistic effects with SDBS, AEO9and CTAB, and among these three, CTAB is the most obvious one. The critical micelle concentration of the composite system is decreased by 2.52%, and the minimum surface tension is decreased by 11.49%. The order of synergistic effect was CTAB > SDBS > AEO9.
5) BGF-10 has better emulsifying, thickening performances and a higher cloud point than TX-10, and low foaming performance; in most case, the detergency of BGF-10 is superior to or equivalent to TX-10, and BGF-10 could substitute for TX-10 in detergents.
Cardanol polyoxyethylene ether is a new generation of mild, safe, biodegradable and green natural nonionic surfactant. It has the characteristics of low surface tension, low foam, easy bleaching, decontamination and excellent washing power, etc.[12]When BGF-10 is applied in the detergents, the dosage is little, the compatibility is better, and the utility model has good economic and social benefits.
Acknowledgment
The authors would like to thank Shanghai Bronkow Chemical Co., Ltd.
[1] Zhou Yawen; Zhang Lei; Su Pengquan;et al. Research progress in biomass cardanol-based surfactants. New Chemical Materials 2013, (11), 10-12.
[2] Yang Xiaohui; Xiao Guomin; Wang Zhimin;et al. Research Advance of cardanol-based surfactants. Chemistry and Industry of Forest Products 2013, (04), 144-148.
[3] Liu Jiuzhu. Study and synthesis of novel cardanol based quaternary ammonium surfactant. Zhengzhou University, 2013.
[4] Liu Jianbang. Synthesis and properties of a new cardanol cationic surfactant. Northeast Petroleum University, 2013.
[5] GB/T 13174-2008 Determination of detergency and cycle washing ability of detergents.
[6] Wang Zhengwu; Li Ganzuo; Li Ying;et al. Study on surface activity of gleditsin and its mixed system.China Surfactant Detergent & Cosmetics 2000 (06), 1-4.
[7] Zhang Zhiguo; Yin Hong. Effect of Concentrations of Additives on the Cloud Point of Nonionic Surfactant AEO9. Journal of Chemical Engineering of Chinese Universities 2007, (01), 155-158.
[8] Nishikido N; Moroi Y; Uehara H; et al. Effect of alcohols on the micelle formation of nonionic surfactants in aqueous solutions.Bulletin of the Chemical Society of Japan 1974, 47 (11), 2634-2638.
[9] Zhao Guoxi. Surfactant Physical Chemistry. Beijing: Peking University Press, 1991, 255-256.
[10] Rosen M J; Xi Y H. Surface concentration and molecular interactions in binary mixtures of surfactants. Journal of Colloid and Interface Science 1982, 86 (1), 164-172.
[11] Wang Shirong; Li Xianggao; Liu Dongzhi. Surfactant Chemistry.Beijing: Chemical Industry Press, 2010: 214-215.
[12] Zhu Huisu; Zeng Yongxiong; Yang Min. Properties of carshew phenol polyoxyethylene ether and its application in laundry liquid detergent. Detergent & Cosmetics 2013, (04), 27-31.
 China Detergent & Cosmetics2017年3期
China Detergent & Cosmetics2017年3期
- China Detergent & Cosmetics的其它文章
- Application of New Polymeric Additive in Detergent for Automatic Dishwasher
- Study of a Whitening and Anti-wrinkle Bio-functional Ingredient Based on Epigenetics
- Review of Cosmetic Regulations and the Spot Check in China 2016
- China National Standard
——Alycosides (GBl/kT 1y9l4p64o-20l1y4g) - Technology Development and Market Trends of Laundry Detergent Sheets
- New Development and Technology of Sun Protection
