Application of New Polymeric Additive in Detergent for Automatic Dishwasher
Bai Jianyun, Qu Xianghua
Lubrizol Specialty Chemicals Manufacturing (Shanghai) Co., Ltd., China
Brijmohan Smita, Hsu Gordon
Lubrizol Advanced Materials Inc., Cleverland, USA
Introduction
Household automatic dishwasher cleaning products include automatic dishwashing powder, liquid, tablet and unit dose. For automatic dishwashing formulations,the following conditions must be met: effective cleaning,bright finish, to prevent spots and film formation, ease of use, safe for utensils, safe for dishwashers, shelf life stability, no phase separation during product shelf life.In automatic dishwashing products, surfactants act as the wetting agent and enhance the spreading and assist the stain removal. Low-foaming nonionic surfactants are generally used, and the cloud point is lower than the temperature at the time of cleaning to ensure no foam formation. Inorganic salts such as sodium tripolyphosphate, sodium citrate, sodium carbonate,sodium silicate, etc., are used for combining calcium and magnesium ions in water, to maintain the high alkalinity during the cleaning process. Calcium and magnesium ions in water not only have a negative impact on the removal of stains, but also form crystals accumulated on the tableware surface, resulting in spotting and filming,which will seriously affect the glass or ceramic surface brightness. Some acrylic polymers may be added to formulations to help disperse and suspend dirt, and to prevent calcium carbonate crystal from growth, and reduce the growth of formed crystals, while also helping prevent spots and films on the tableware.[1]

This paper studied the calcium ion chelation ability of NoveriteTMAD810 polymer as well as its application in chlorine-containing automatic dishwashing products,enzyme-containing automatic dishwashing products and unit dose automatic dishwashing powders. This study provides the basis for applications of this new polymer.
Experiments
Materials
Metrohm auto titrator and calcium ion sensitive electrode, pH meter, self-made light box, automatic dish washer were purchased from Siemens IQ100.
Calcium chloride, sodium citrate, sodium carbonate,sodium silicate, sodium tripolyphosphate (STPP) were purchased from Shanghai Lingfeng Chemical Reagent Factory.
Tetrasodium glutamate diacetate (GLDA) was purchased from Akzol Nobel.
NoveriteTMAD810, Carbopol?676 (Carbomer),NoveriteTMK7058 (acrylate polymer,NoveriteTMK775(acrylate copolymer) , tetraacetylethylenediamine (TAED)were purchased from Lubrizol.
Plurafac?SLF180 (nonionic surfactant) and methylglycine diacetate (MGDA) were purchased from BASF.
Savinase?Ultra (protease,Savinase?6.OT (protease),Termamyl?120T (amylase), Termamyl?330L DX(amylase) were purchased from Novozyme.
Dequest?2016D (hydroxyethylidene diphosphonic acid) was purchased from Italmatch Chemicals.
Calcium ion chelating test
All the additives as Figure 1 disclosed were prepared as 1% aqueous solutions, pH was adjusted to 11.5, temperature was at 24℃, and 0.01 mol / L CaCl2was prepared. Titration was carried out with a calcium ion sensitive electrode by titrating with prepared CaCl2solution to determine the calcium ion concentration. The end point of titration is the calcium ion concentration to reach zero.
Preparation and evaluation of automatic dish detergent gel containing chlorine or enzyme
Deionized water was added to proper beaker firstly,under moderate rotation speed (around 800 r/min)Carbopol?676 polymer was slowly added to the water.The solution was mixed until its appearance was homogeneous. pH was adjusted with sodium hydroxide solution to 8~9. Then NoveriteTMAD810N polymer was transferred to the beaker with moderate rotation speed following with adding the rest raw materials until stir to homogeneous solution.
Performance evaluation was following ASTM D3556 method.[2]First, new glasses were purchased and washed with 1% citric acid, and followed with a washing with an automatic dishwasher with a setting of normal procedures, and finally hand rinse with deionized water and no heat drying step to ensure no spots on the cups.Test conditions were water hardness of 300 mg/kg, calcium and magnesium ions molar ratio of 2:1; detergent dosage of 45 mL; automatic dishwasher normal procedures for cleaning; the stain used were the mixture of 80%margarine and 20% milk powder, each time used a brush coated with 40 g stains to each of six large dish evenly,smudged large plates were loaded at the lower level of the dishwasher, the upper level was loaded with already precleaned six glasses and 2~4 plastic cups. The test was repeated for 5 cycles. Take photos of final glasses and plastic cups to observe the films and spots. The scores of film-forming property are one for almost no filmforming, and five points for highly filming. The scores of spotting are one for almost spotless, and five for many spots. The addition of the film-forming value and the spotting value is the final performance evaluation.Experienced panelists were asked to give score for filming and spots. The lower the score, the better the performance.
Preparation and evaluation of auto dish detergent powder
Firstly, sodium sulfate and sodium carbonate were added to beaker for 5 minutes mixing, then Plurafac?SLF180 was transferred to the beaker with slow agitation following with the rest raw materials until stir uniformly.
Performance evaluation was conducted by Germany Fresenius Institute with 20 g of unit dose per cycle, washing temperature was 65℃, and water hardness was 376 mg/kg.30 cycles were conducted under IKW test method.[3]Anti-filming scores were: 1 was poor and 8 was very good. Higher score indicated better performance.
Results and discussions
Calcium ion chelating test
As shown in Figure 1, NoveriteTMAD810 polymer was capable of chelating calcium ions at much higher levels than common polyacrylic acids on the market,and similar to MGDA or GLDA, and was weaker than sodium tripolyphosphate. Sodium citrate although it was biodegradable, had a much lower calcium ion chelation than the NoveriteTMAD810 polymer.
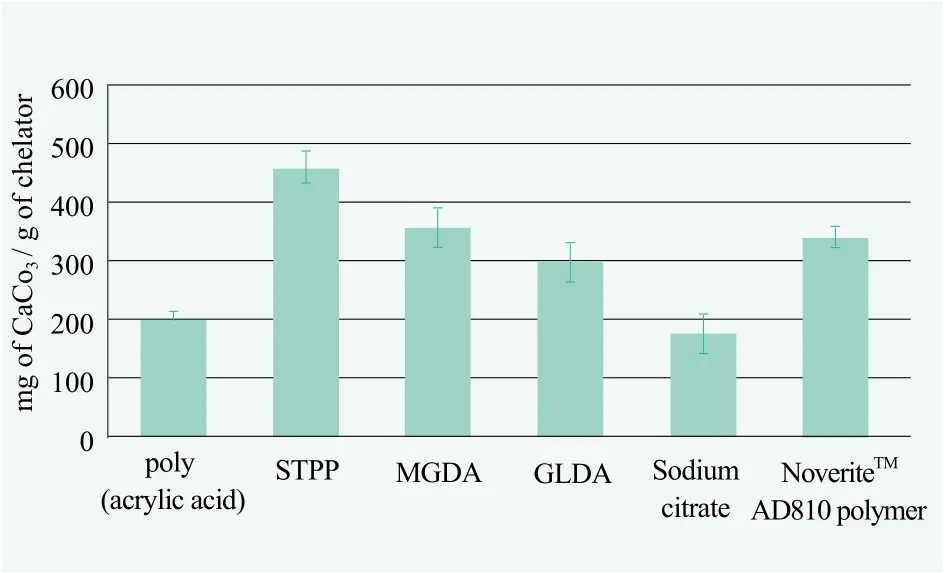
Figure 1. Calcium chelating abilities of various additives
Chlorine containing auto dish detergent gel
Chlorine bleach helps stain removal by bleaching stains. In addition, it also plays a role in disinfection and to avoid spotting on tableware due to the deposition of hydrophilic stains.[4]For formulations containing sodium hypochlorite, formulation technology has a big challenge, mainly due to high alkaline and high chlorine content, therefore the additives have a high restrict.Table 1 formulation was chlorine bleach containing autom-atic dishwashing gel. After mixing, the pH was 12.5~ 13.5, viscosity by Brookfield viscometer at rotation speed 20 r/min was 6,320 mPa·s. After 2months stability under room temperature, viscosity was 6,800 mPa·s.Stability of chlorine content of the formula showed that at room temperature for 60 days after the chlorine content is still maintained at a relatively high level, the original is 1.05%, after 60 days, the chlorine is 0.87%. The stability data had shown that the system exhibited excellent viscosity stability and chlorine stability.
The addition of Carbopol?676 Polymer imparted the viscoelasticity and shear-thinning of chlorine-containing gel systems. These properties helped the dishwashing gel remain in the automatic dishwasher detergent compartment, without leaking out and easy to disperse in the water.
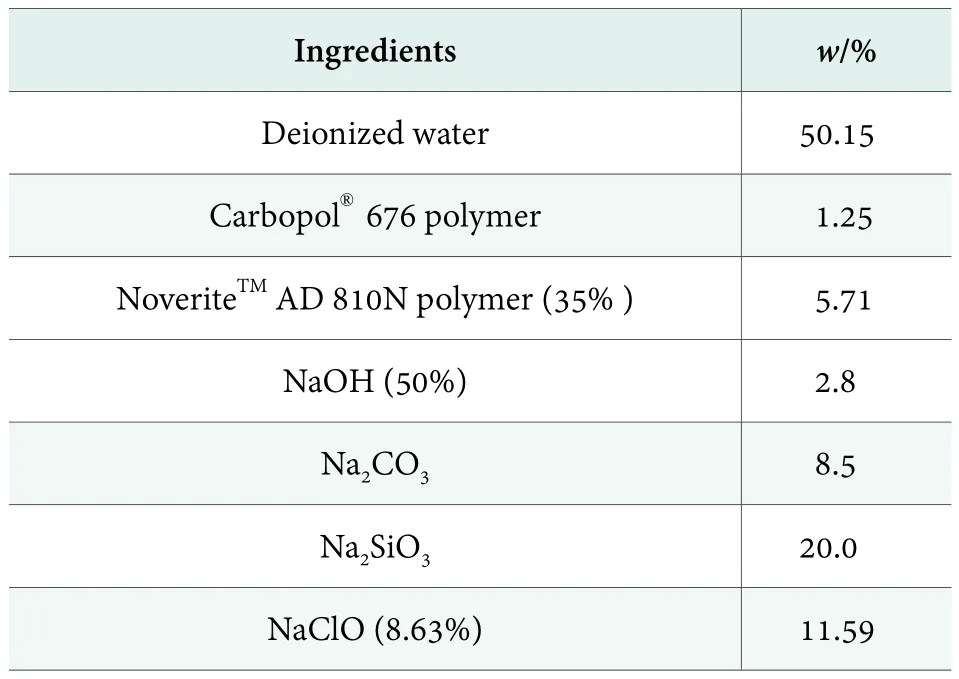
Table 1. Formula of chlorine bleach-based automatic dishwashing gel
Formula similar to Table 1 was prepared with an equal solid content replacement of NoveriteTMAD 810 N polymer by 1:1 ratio of NoveriteTMK7058 acrylic acid homopolymer and NoveriteTMK775 acrylic acid copolymer. Two formulas’ performances were compared with commercial chlorine gel.
After 5 washes, the glasses of commercial chlorinecontaining automatic dishwashing product had shown a lot of spots and misty outlook, the filming and spot score was 6 (Figure 2a). Glasses washed with an equal solid content product of 1:1 ratio of polyacrylic acid homopolymer and copolymer were also hazy, the filming and spot score was 5.6 (Figure 2b). As Figure 2c shows the glass washed with NoveriteTMAD 810N polymer was as bright as new, the filming and spot score was 4.4.


The presence of calcium and magnesium ions during the cleaning process can lead to the spotting and thin film formation on the dishes, due to the interactions between calcium and magnesium ions, some protein stains, fatty acids, anionic surfactants, and carbonate to form insoluble complexes. Spotting and filming are caused due to the hard water ion scale build-up and due to inadequate sheeting of water from the surface of the glass. During the last rinse, the water droplets flow from the vessel,when the drainage is uneven, the water droplets will stay on glass cups. If there are stains in water droplets, spots would be formed after drying. There are two main routes to prevent spotting and film formation, primarily by the use of chelating agents to chelate ions of water hardness.Furthermore, the introduction of dispersants in products could slow down the crystallization of calcium carbonate or reduce the growth of existing crystals. NoveriteTMAD 810 N was very superior in chelating calcium, magnesium ions and dispersing calcium carbonate crystal as well as preventing its deposition.
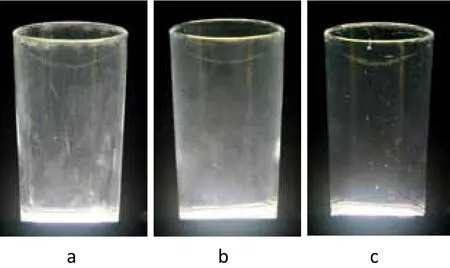
Figure 2. Photographs of glasses after 5 washes
Enzyme containing automatic dish detergent gel
Table 2 shows automatic dish detergent gel with amylase and protein. After mixing, pH was 7.8 ~ 8.2, viscosity by Brookfield viscometer at rotation speed 20 r/min was 1,500~ 2,500 mPa·s. Proteases can hydrolyze protein matrix which can be broken down into small fragments,such as amino acids or oligopeptides. Amylase can break the ester bonds of starch sugar by catalytic hydrolysis to render water-insoluble large starch molecules into small water soluble molecules.[5]
The following two formulations were evaluated according to the ASTM D3556 method described previously,spotting and filming were evaluated after 5 wash cycles with experienced panelists’ visual rating. Figure 3 reveals formulation a, formulation b and market sample were comparable on glass performance. Figure 4 showes formulation a and b, which were similar performance on plastic cups, were better than market sample containing GLDA. However, formulation a need extra 2.5% of polymer dispersant to boost 40% GLDA’s performance which increased the cost in use. Formulation b need 8.6%NoveriteTMAD 810N together with 15% sodium citrate to reduce the cost and improve the performance.
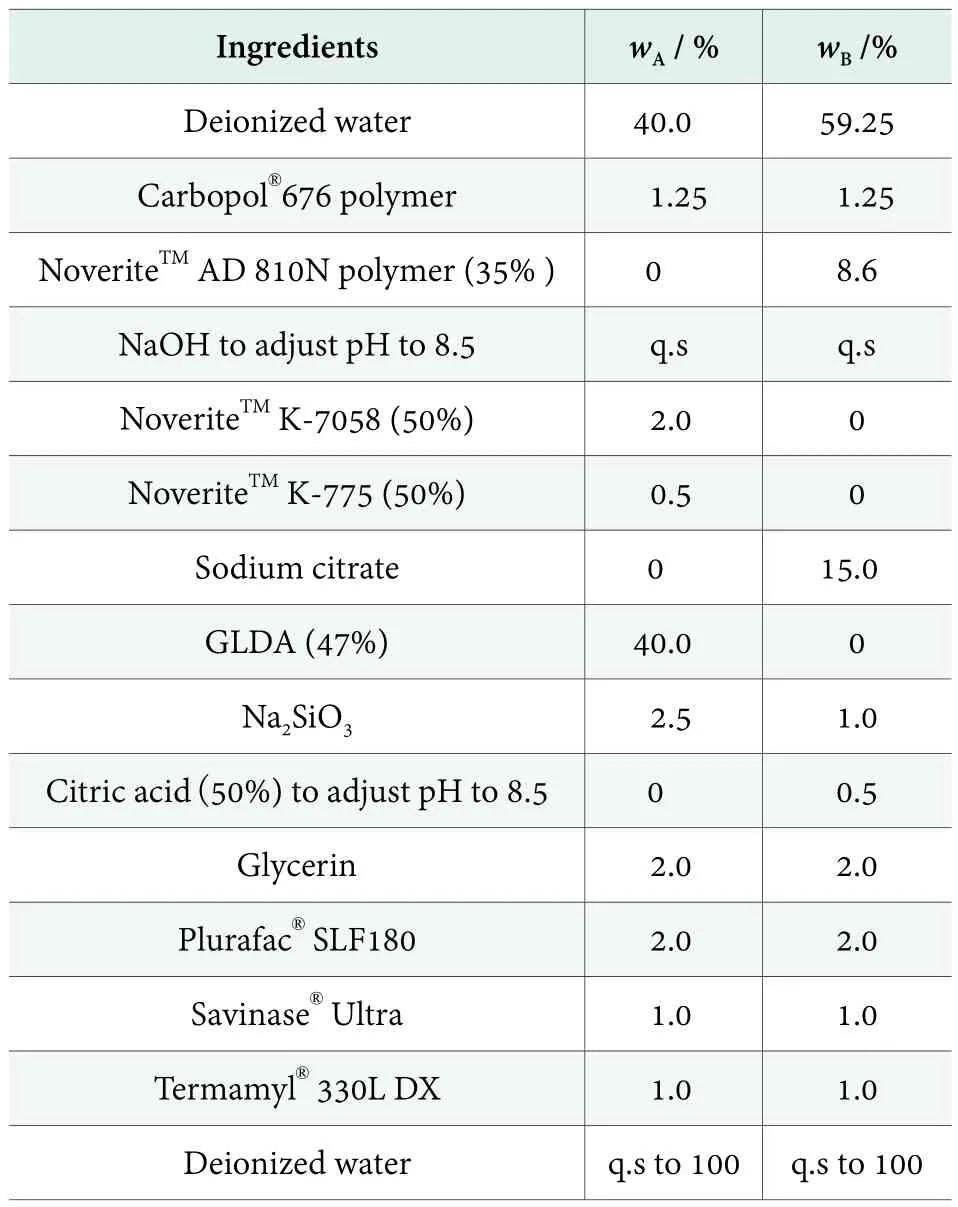
Table 2. Enzyme containing automatic dishwashing gel formula
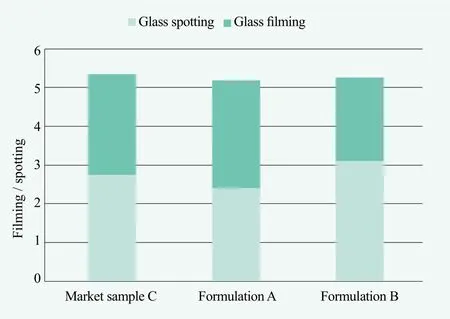
Figure 3. Performance evaluation on glasses after 5 washes
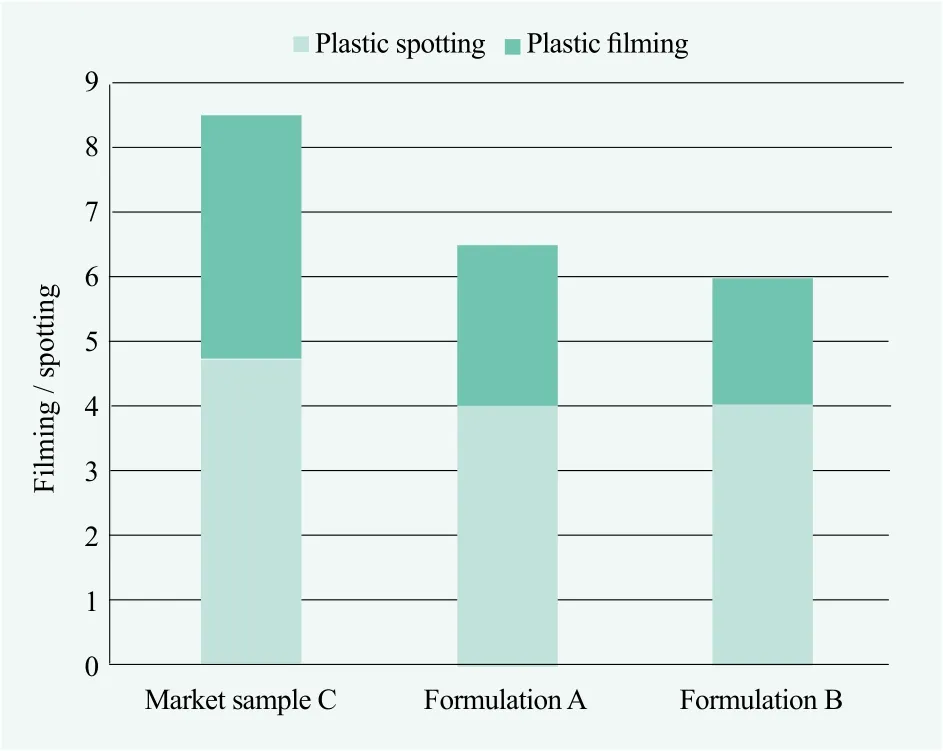
Figure 4. Performance evaluation on the plastic cups after 5 washes
Unit dose of automatic dishwashing powder
Unit dose package of automatic dishwashing powder is preferred by some consumers due to its convenience. The combination of sodium percarbonate with bleach activator TAED is often used in helping stain removal and prevents the deposition of hydrophobic stains on the vessel to form water spots. After 30 wash cycles, Table 3 formula provides better cleaning performance of the tableware for different materials and anti-filming was very good.
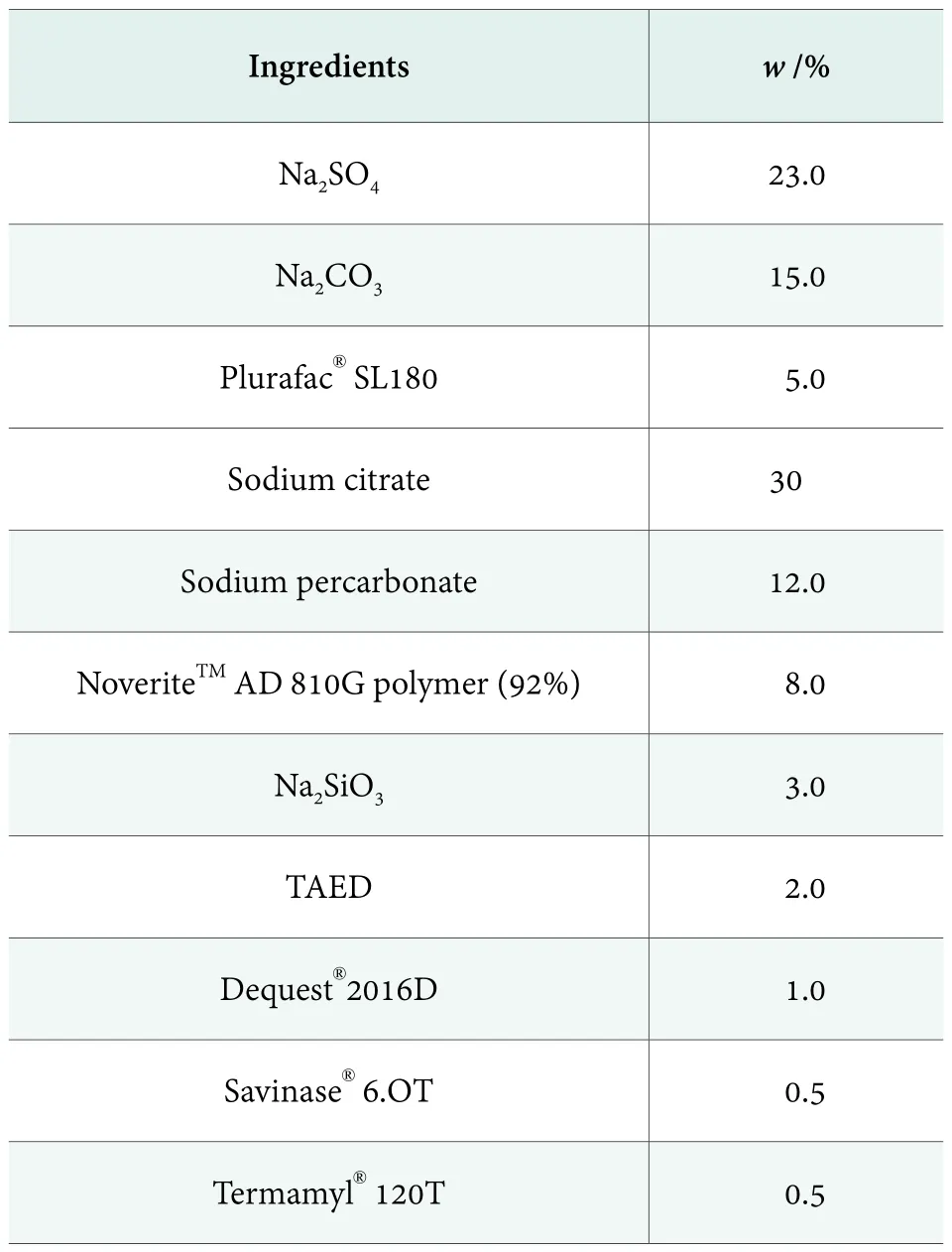
Table 3. Formula of automatic dishwashing powder in unit dose

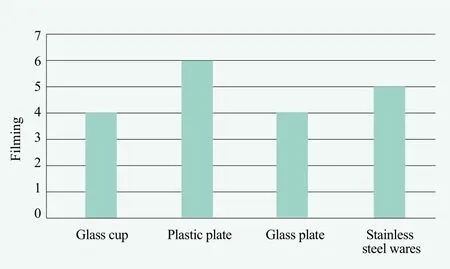
Figure 5. Film-forming performance of tableware made of different materials after 30 washes
Summary
NoveriteTMAD810 polymer which is novel acryilc terpolymer is on US TSCA Inventory, and is lited in WERCS. It was approved by the EPA program (US government—Environrnental Protection Agency) as a“safer ingredient” for use in formulations. NoveriteTMAD810 polymer shows higher calcium chelation performance than acrylate polymer and sodium citrate, it can be used in high-performance, non-phosphorus formulations in automatic dishwashing formulations to chelate hard water ions, prevent surface spotting and filming. The synergies with sodium citrate can improve the cost of formula.
[1] KUO-YANN L. Liquid detergents (second edition). New York:Taylor & Francis Group, 2006.
[2] ASTM D. Standard Guide for Deposition on Glassware during Mechanical Dishwashing. United States, 2014.
[3] IKW Part B. Methods for Ascertaining the Cleaning Performance of Dishwasher Detergents. German, 2005.
[4] URI Z. Handbook of Detergent, Part E, Application. New York:Taylor & Francis Group, 2009.
[5] Michael S S. Handbook of Detergent, Part D, Formulation.New York: Taylor & Francis Group, 2006.
 China Detergent & Cosmetics2017年3期
China Detergent & Cosmetics2017年3期
- China Detergent & Cosmetics的其它文章
- Surface Performance Testing of Cardanol Polyoxyethylene Ether and Its Application in Detergent
- Study of a Whitening and Anti-wrinkle Bio-functional Ingredient Based on Epigenetics
- Review of Cosmetic Regulations and the Spot Check in China 2016
- China National Standard
——Alycosides (GBl/kT 1y9l4p64o-20l1y4g) - Technology Development and Market Trends of Laundry Detergent Sheets
- New Development and Technology of Sun Protection
