Synthesis of Monoglycerides with Cinnamomum Burmannii Seeds Oil and Its Application in Moisturizing Cream
Zhenhao Dong
Shanghai Geotechnical Engineering Detecting Center, China
Xiaoxia Zhao
Shanghai ChemPartner Co., Ltd., Shanghai, China
Glycerin Monostearate (MG) is a kind of nonionic surfactant in polyhydric alcohols type and it has two polyhydric alcohols ofα-MG andβ-MG which were divided depending on the position of hydroxyl. As a polyhydric alcohols type surfactant, which included a long-chain alkyl and two hydrophilic hydroxyl groups,it has good surface activity and is mainly used as a kind of excellent emulsifier and stabilizer in the fields of food,medicine, detergent and cosmetics industry and so on.[1]
Cinnamomum burmanniiis also called Guangdong cinnamon or wild cinnamon. It is a kind ofLauraceae Cinnamomumevergreen broad-leaved trees, which is a common tree species in south subtropical.[2]The sunny, warm and humid climate is suitable for the growth ofCinnamomum burmannii. It usually grows in noncalcareous soil environment covered with thick soil.It has strong resistance. Hence, it is one of the tree species that have strong capability of sterilization[3].Cinnamomum burmanniileaves have strong effect of antibacterial and moth-proofing and as essential oil, the extraction has been widely used in the fields of pharmaceutical, food,cosmetics and other fields.[4]Cinnamomum burmanniihas high yield and its fruit contains rich sugar, protein, fat,pectin and procyanidins.Cinnamomum burmanniiseeds are rich in oil and the mass fraction of the laurel acid reached 84.21% in the oil.[5]Monoglycerides synthesized byCinnamomum burmanniiseeds oil mainly included glycerol monolaurate. It is a kind of high performance surfactant;[6,7]as the carbon chain of MG is shorter, it has good dispersion in water and strong emulsifying properties. When monoglyceride content reached higher than 90%, it demonstrated excellent anti-corrosion and emulsifying performance in the neutral to slightly alkaline condition. Thus, it is a kind of safe antibacterial agent and preservative with efficient broad-spectrum,and can be widely used in daily cosmetic industry.[8]In addition to the synthesis of monoglyceride which is used as a kind of environmental material to replace emulsifier composition in cosmetics,Cinnamomum burmanniiseeds oil can also be regarded as a kind of new cosmetics raw material oil to replace the raw mineral oil in cosmetics.[9]It is in line with the people’s pursuit of returning to nature and the pursuit of green health products in the meantime. In this experiment,the author discussed the extraction conditions ofCinnamomum burmanniiseeds oil and the synthetic conditions of monoglyceride by the extracts in order to establish a theoretical foundation for the green and environmental application of monoglyceride.
Experimental
Materials:Oil extracted fromCinnamomum burmanniiseeds which was picked from Jiangxi Agricultural University.[5]Monoglyceride was Analytical Reagent Grade and purchased from Guangzhou Jia De Le biotech Co., Ltd..
Reagents:Glycerol, chloroform and acetic acid (AR,Tianjin Hengxing Chemical Reagent Co., Ltd.); BHT (two butyl hydroxy toluene), Span 80 and perchloric acid (AR,Tianjin Da Mao chemical reagent factory); Methylparaben,Ethylparaben(AR, Tianjin Kemiou Chemical Reagent Co.,Ltd.); Tween 80 (AR, Wuxi Chemical Reagent Co., Ltd.);Periodic acid(AR, Zhejiang OceanPower Chemical Co.,Ltd.); Sodium thiosulfa (AR, National Pharmaceutical Group Chemical Reagent Co., Ltd.).
Apparatus:PPV-4060 synthesis device, (TOKYO RIKAKIKAI Co., Ltd.); MD-S80 molecular distillation equipment (Guangzhou Hanwei Machinery Co., Ltd.);DHG-9101-2 DHG Series heating and drying oven(Shanghai Sanfa Scientific Instrument Co., Ltd.); DC-3006 low-temperature thermostat bath (Shanghai Precision Scientific Instrument Co., Ltd.); FM200 high shearing dispersion emulsifying machine (Shanghai FLUKO Fluid Machinery Manufacturing Co., Ltd.); DDB-6200 conductivity meter (Shanghai Honglan instrument and Meter Co., Ltd.); UV-2600 double beam ultraviolet visible spectrophotometer (Beijing Rayleigh Analytical Instrument Co., Ltd.).
Experimental Method
Synthesis of monoglycerides with Cinnamomum burmannii seeds oil.There are many preparation methods for monoglycerides and the method we used in this experiment was ester exchange method.[10]The specific procedures were as follows:Cinnamomum burmanniiseeds oil as raw material, glycerol in certain molar ratio to oil and a certain quantity of NaOH as catalyst were added to the solution. The experiment was conducted in the PPV-4060 organic synthesis reaction device after a certain period of time in the corresponding temperature and under the protection of nitrogen, and then the reaction products was obtained.
The solvent crystallization method[11]was used to remove residual impurities such as glycerol and catalyst in reaction products. Specific methods were as follows:reaction products were centrifuged and isolated from the upper clear solution,n-hexane with 1:5 of the volume ratio of reaction product to solvent amount was added to the solution. If it was slightly turbid solution, it can be heated in water bath to the temperature of 60 ℃ and be shocked until the product was clear. Then put it into the refrigerator under 5 ℃ for crystallization. After precipitation, it was filtered by vacuum filtering until dry.Finally, white powder was obtained, which was the coarse monoglycerides products.
Monoglycerides Purification.Molecular distillation device was used to purify the monoglycerides.[11-12]Specific procedures were as follows:
① Completely melt the coarse monoglycerides in water bath at 80 ℃, and then pour it into the distillation equipment storage tank where the temperature was at 75 ℃,in order to prevent condensation of material (charging valve was closed at this time).
② Open the water pump so that the material in the storage tank could be degassed. After degassing complete, water pump was closed and oil pump was open for vacuum operation. When air residual was down to 30 Pa, the booster pump was open to reduce the residual pressure to 2 Pa, and to maintain a constant.
③ Open the feed valve, making sure the velocity of material flow less than 1, 000 mL/h, and distillation was started. First, the lauric acid and glycerin were steamed out when the distillation temperature was 90 ℃, the cooling surface temperature was 60 ℃, liquid flow rate of 500 mL/h,and the residual air pressure under the condition of 4~5 Pa.
④ Finally, the steamed section was distillated when distillation temperature was 100 ℃, the cooling surface temperature was 75 ℃, material liquid flow rate of 500 mL/h, the residual air pressure under the condition of 4~5 Pa.Finally, the obtained fraction was monoglycerides.
Determine the content of monoglycerides by periodatemethod.Refer to the national standard GB/T 22328-2008 and the method in literature:[13]adequate amount of sample was dissolved in 100 mL of chloroform, and 100 mL of 5% (volume fraction) acetic acid solution was added into the tube containing the sample. Then the solution was transferred to the separatory funnel and was shocked until it was homogeneous. After 2~3 hours, used pipette to remove 25 mL of solution containing samples from chloroform solution, and 0.07 mL of 56% (mass ratio)perchloric acid was added to the solution. Then 25 mL of periodate-ice acetic acid solution (2.7 g periodate in 1,000 mL ice acetic acid) was added to the above solution.Shook it until it was homogeneous and let it stand for 30 min in the dark. Then added 10 mL of 20% (mass ratio)potassium iodide solution to the solution. After standing in the dark for 5 min, added 50 mL distilled water to the solution. Conducted the titration with the 0.1 mol/L sodium thiosulfate solution, with 10 g/L starch solution as an indicator. At the same time, we used chloroform solution without samples for check and recorded the consumption volume of sodium thiosulfate solution. The content of monoglycerides was calculated as the following formula:
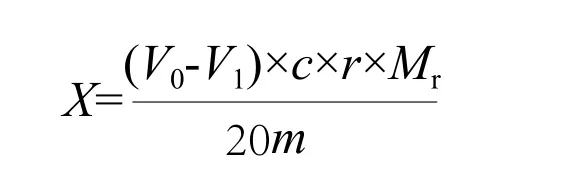
In the formula,Xstands for the content of monoglycerides;V0stands for the volume of sodium thiosulfate standard solution consumed by titration of blank solution mL;V1stands for the volume of sodium thiosulfate standard solution consumed by titration of sample solution, mL;cis the concentration of sodium thiosulfate standard solution, mol/L;Mr is the relative molecular mass of monoglycerides;mstands for the mass of the sample, g;rstands for the ratio of volume capacity of the chloroform to volume of the chloroform fetched by titration.
Among them,the linear velocity viof the centroid of each link Liinvolved in link Lprelative to the coordinate systemis
The preparation of the emulsion.Added the right amount of water to dissolve one gram of self-made MG by molecular distillation. Mixed with, at 45 ℃, 99 g of mixture of oil phase and aqueous phase of which molar ratio of oil to water were 2 : 8, 3 : 7, 4 : 6, 5 : 5, 6 : 4, 7 : 3, 8 : 2,respectively. First, heated and stirred. Then, prepared the emulsion using Micro Jet Homogenization Machine. The stability of emulsion was observed at room temperature.Prepared control emulsion using commodities monoglycerides to replace self-made monoglycerides.
Determination of emulsion type.Because most of the oil in the emulsion is poor in conductivity, and the conductivity of the water is better (the water often contains electrolytes). So the conductivity measurement of the emulsion can be used to determine the type of the emulsion.[14]According to the standard of HG/T 3506-1999 for the determination of the conductivity of emulsion, when the emulsion conductivity and water conductivity is relatively low, the emulsion is W/O type;when the emulsion conductivity is higher and similar to the water conductivity, emulsion for O/W type. With distilled water as the water phase, the conductivity of the water was measured by DDB-6200 type electrical conductivity meter.
Determination of emulsifying ability.The emulsifying ability of the sample was determined by spectrophotometry.[15]One milliliter of prepared emulsion was transferred to a 100 mL volumetric flask, diluted to the mark with distilled water and mixed well. Removed one milliliter of mixed liquor, and then 0.1% (volume fraction)sodium dodecyl sulfate (SDS) solution was added to the mixture to 10mL, determined the absorbance in 500 nm wavelength. 0.1% (mass ratio) SDS solution was used as blank control, absorbance of size represented the emulsifying capacity of the emulsifier.
Formula design and preparation of moisturizing cream.According to the cosmetics formula design principle and preparation technology,[14,16]we obtained the optimum formula of moisturizer (Table 1) by adjusting the ratio of raw materials. Oil phase and water phase were added into the beaker respectively, according to designed proportion, heating to 90~95 ℃, under the continuous stirring to dissolve evenly. Added the homogeneously dissolved oil phase to the water phase at a constant speed and stirred constantly with a glass rod about 1 min. Then dispersed with high shear homogeneous emulsifying machine about 1 min. After emulsification, continued to stir and cooled down to about 50 ℃ and added a moderate amount of essence. Cooled it to near room temperature (21 ℃or so), stop stirring and let it stand for a while.

Result and discussion
Optimized conditions for the preparation of monoglycerides by Cinnamomum burmannii seeds oil
Effect of molar ratio of alcohol to oil on the yeild of monoglycerides.According to synthesis method of monoglycerides, under the aegis of N2, reaction temperature at 180℃, reaction time for 3 hours and 0.6% of the oil mass as catalyst dosage, effect of the molar ratio of glycerol to oil on the yield of monoglycerides was studied and the results were shown in Figure 1.
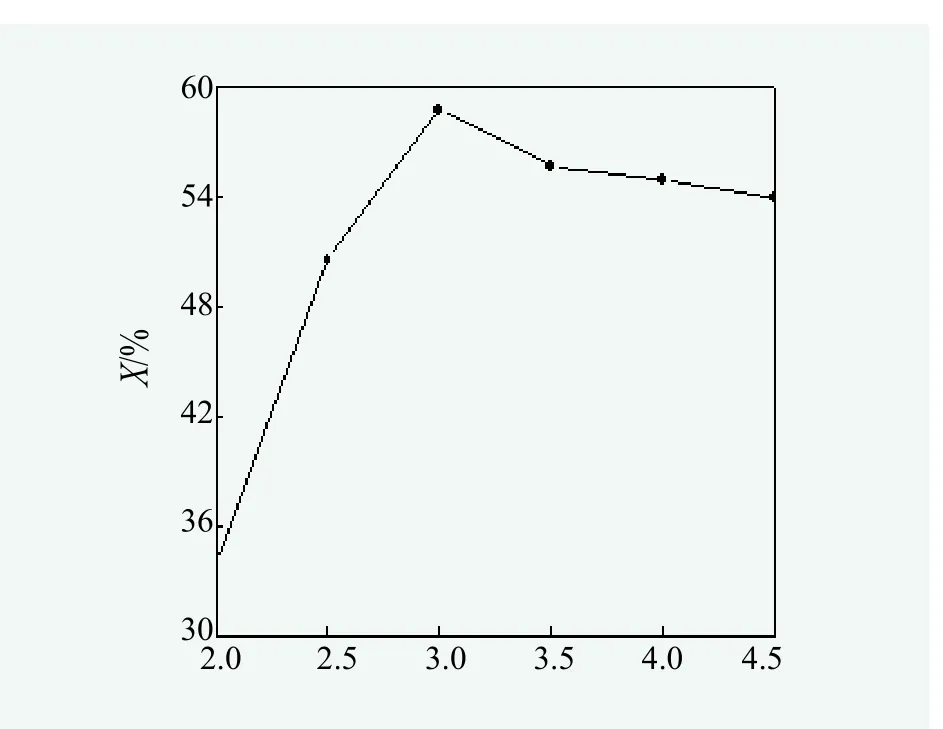
Figure 1. Effect of glycerol/oil molar ratio on monoglyceride yield
Figure 1 showed that when the molar ratio of glycerol to oil between 2 : 1 to 3 : 1, monoglycerides content continued to rise with the increase of molar ratio. When the glycerol to oil molar ratio reached 3 : 1, monoglycerides content went up to 58.73%; When the mole ratio of was higher than 3 : 1, monoglycerides content didn’t improve, instead, it went down to lower. As esterification is a kind of reversible reaction, an increase of glycerol can prompt reaction to the positive direction, but too much of glycerol can reduce the catalyst concentration and lower efficiency. Therefore, the glycerol to oil molar ratio should be 3 : 1.
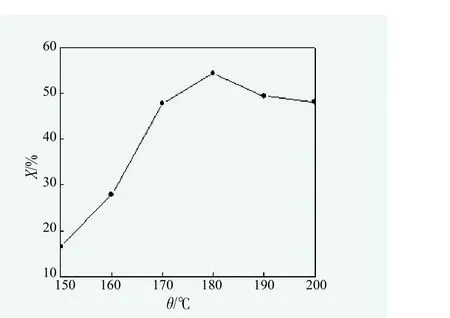
Figure 2. Effect of reaction temperature on monoglyceride yield
Figure 2 showed that along with the rise of reaction temperature, the content of monoglycerides in the reaction product gradually increased; when the temperature reached 180 ℃, the content of monoglycerides rose no longer, but decreased instead. As the low reaction temperature could influence the reaction velocity of generating monoglycerides, and reduce the content of monoglycerides. However, more carbon chain adverse reactions such as dehydrogenation coking, glycerin dehydration condensation would happen when the reaction temperature was higher than 180 ℃, and led to monoglycerides content decreased consequently.Therefore, reaction temperature should be 180 ℃.
Effect of reaction time on the yeildof monoglycerides.According to monoglycerides synthesis method, under the aegis of N2, when the glycerol to oil molar ratio of 3 : 1, reaction temperature at 180 ℃ and 0.6% of the oil mass as catalyst dosage, effect of reaction time on the monoglycerides yield was studied and the results were shown in Figure 3.
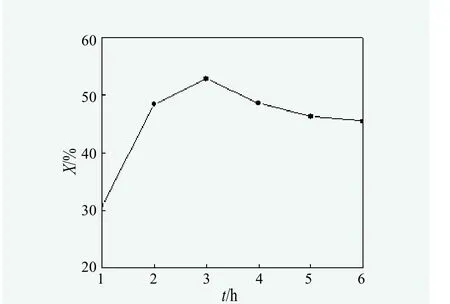
Figure 3. Effect of reaction time on monoglyceride yield
Figure 3 showed that during the first 1~3 hours, the longer reaction time, the higher content of monoglycerides in the reaction product. Monoglycerides content reached 52.86% when the reaction time got to 3 hours. However,monoglycerides content gradually decreased after 3 hours. The main reason is that the extended reaction time made monoglycerides further react withCinnamomum burmanniiseeds oil and large amounts of diglyceride was generated. Thus, in the production process, in order to get higher content of monoglycerides and effectively control the reaction time to save energy, reaction time should be 3 hours.
Effect of catalyst dosage on the yeild of monoglycerides.According to synthesis methods of monoglycerides, under the aegis of N2, when the glycerol to oil molar ratio of 3 : 1, reaction temperature at 180 ℃ and reaction time for 3 hours, effect of the dosage of NaOH catalyst was studied and the results were shown in Figure 4.
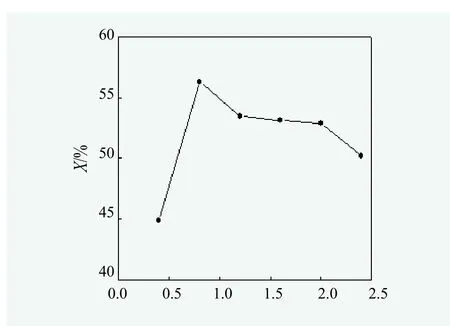
Figure 4. Effect of catalyst dosage on monoglyceride yield
From Figure 4, the content of monoglycerides in the reaction product gradually increased when the mass of catalyst to oil was between 0.5% and 0.8%. Instead, the content of monoglycerides decreased when it was more than 0.8%. As larger amount of catalyst catalytically generated diglyceride more quickly and it lowered the content of monoglycerides obviously. Hence, the mass of NaOH catalyst was 0.8% of the oil mass should be better.
In summary, the optimum conditions for the preparation of monoglycerides withCinnamomum burmanniiseeds oil as material were as follows: under the aegis of N2, the glycerol to oil molar ratio of 3 : 1, NaOH catalyst dosage of 0.8% of the oil mass, reaction temperature at 180 ℃,reaction time for 3 hours. Under these conditions, the results showed that the average content of the ester was 62.6% and the rest products were diacylglycerol and a small quantity of triacylglycerol and so on. The effect of anticorrosion and emulsifying of diacylglycerol is far less than that of monoglyceride. Therefore, product with more than 70% of monoglycerides content had good emulsification effect and that with more than 90% monoglycerides content had good anticorrosion effect. Crude monoglyceride was purified by molecular distillation and the monoglyceride content reached to 92.8%. The monoglycerid have better emulsifying properties, corrosion resistance and good application value after the molecular distillation purification
Emulsifying capacity
According to the determination method of emulsion type, the conductivity of the water was 28.7 S/cm and the conductivity of the emulsion with strongest emulsifying ability is 1.68 S/cm. Therefore, the emulsion type is W/O.
In accordance with the determination of the emulsifying capacity, the emulsifying properties of self-made and commercial monoglyceride were determined by spectrophotometry and compared in Figure 5.
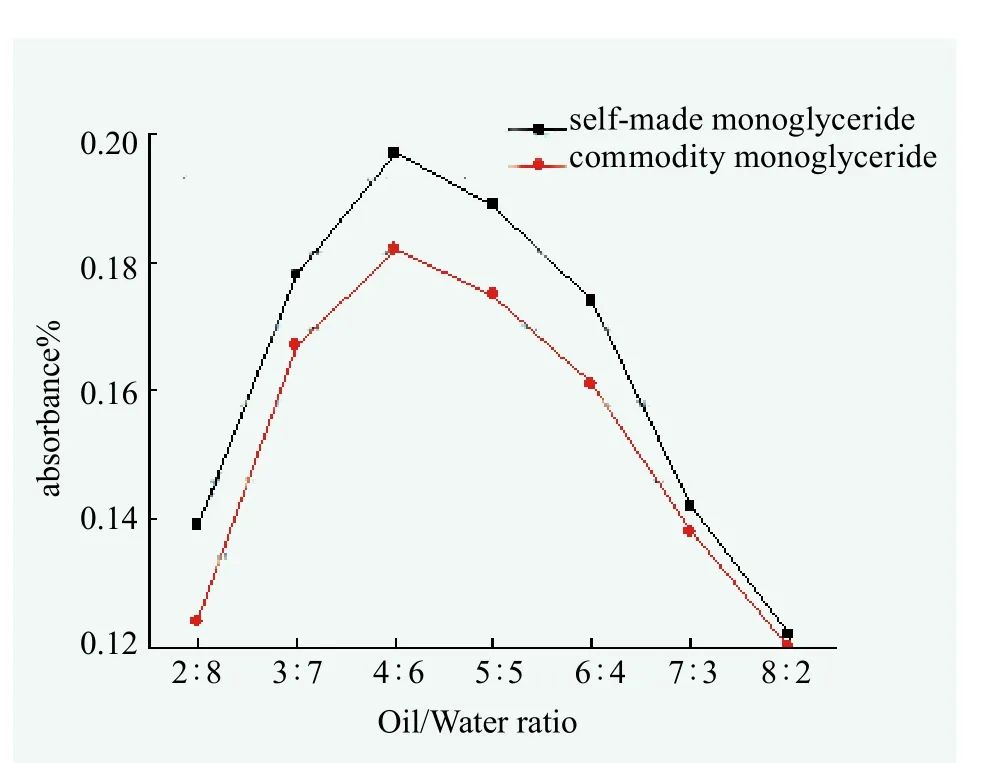
Figure 5. Emulsifying power of monoglyceride
From Figure 5, it can be seen that the curve tendency of self-made monoglyceride lipid and commercial monoglyceride were similar, but emulsifying capacity of commercial monoglyceride was slightly lower than that of selfmade monoglyceride, indicating that the self-made monoglyceride had better emulsifying ability than that of purchased monoglyceride. So the self-made monoglyceride was an excellent natural emulsifier of raw materials.
Emulsifying properties of self-made monoglyceride increased with the increasing of the amount of water when the mass ratio of oil to water was less than 4 : 6. And it became weak gradually when the mass ratio was larger than 4 : 6 (Figure 5). Glycerol Monolaurate, the main component in self-made monoglyceride, was hydrophilic emulsifier. Increasing the amount of water can promote the emulsion process. But continuous increase of water quantity could reduce the emulsion viscosity, which was not propitious to the formation of emulsion.
Monoglycerides in the application of the cream
Formula design of moisturizing cream and preparation procedure.In accordance with the design and preparation methods of cosmetics, commonly used emulsifier and self-made monoglycerides were compared in Table 1.
Table 1 showed that formulation 2 was obtained in which 2% of monoglycerides in formulation 1 was replaced by 2% of self-made monoglycerides; meanwhile,formulation 3 was archived in which the emulsifier Span 80 and Tween 80 in formulation 1 was replaced by 3%of self-made monoglycerides. The designed formulation needs to be tested in terms of physical and chemical properties and the effect on trial.
Moisturizing cream sensory and physical and chemical indicators of evaluation.According to the national standard GB 11431-89, the items such as color,aroma, pH value, cold resistant, heat resistant, centrifugal experiment and paste structure and so on[14]have to be tested. The test results of three formulations in the mentioned chapter, were in Table 2 and the color of formulation 1,formulation 2 and formulation 3 were all white.
Table 2 showed that the sensory, physical and chemical indicators of moisturizer cream adding monoglyceride transesterificated byCinnamomum burmanniiseeds oil conformed to the requirements of the cream cosmetics.
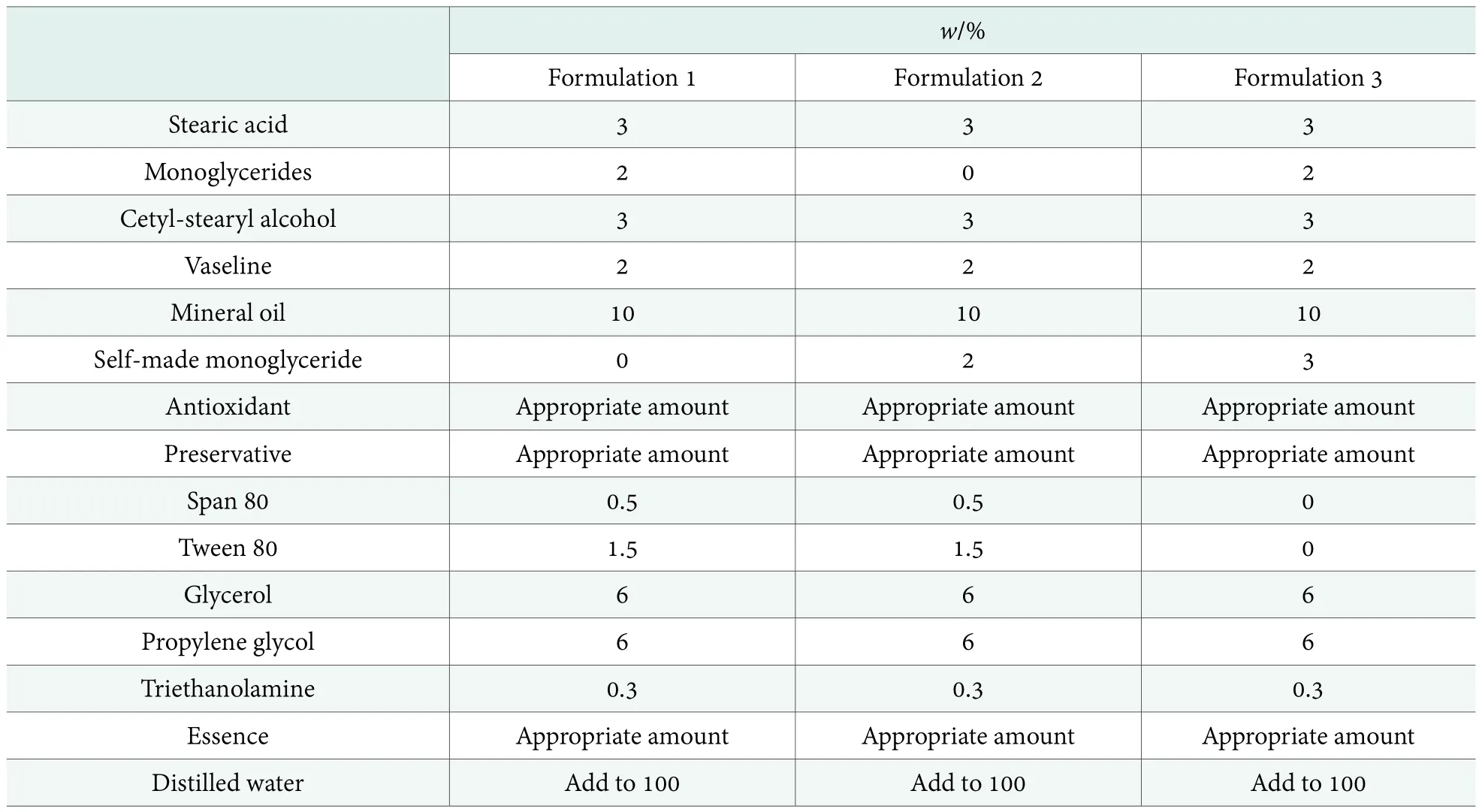
Table 1. Formulation of moisturizing cream
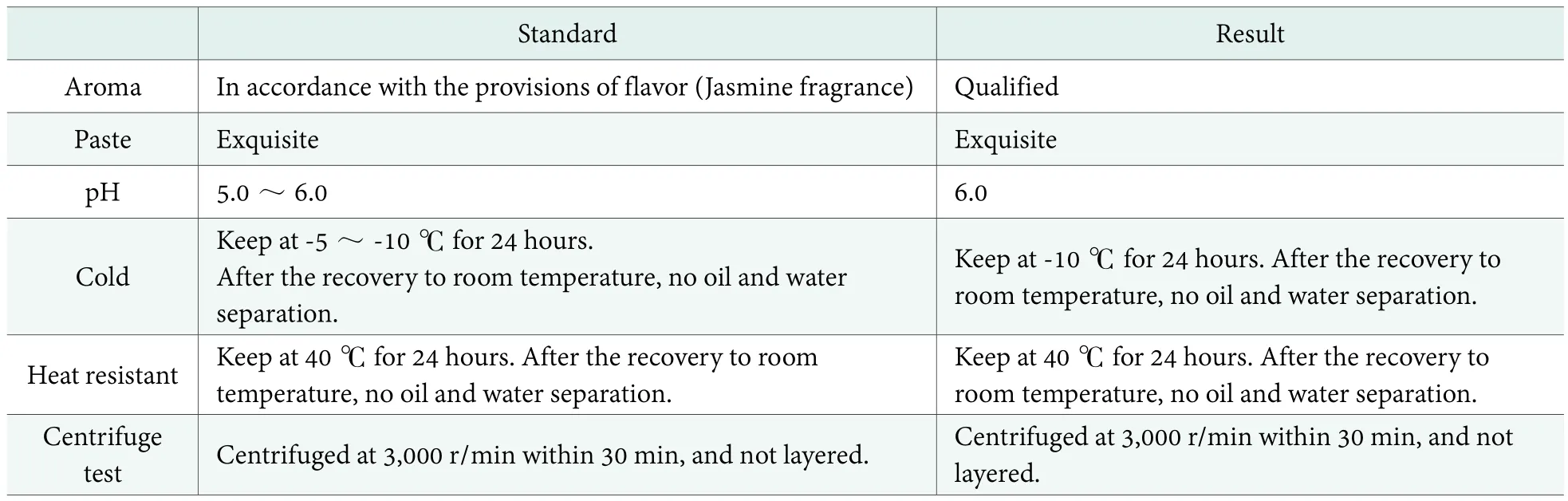
Table 2. Moisturizing cream’s sensory, physical and chemical indices
Efficacy of the prepared moisturizing cream.20 skin properties of different clinical trials in human subjects were selected and compared, the results are shown in Table 3.
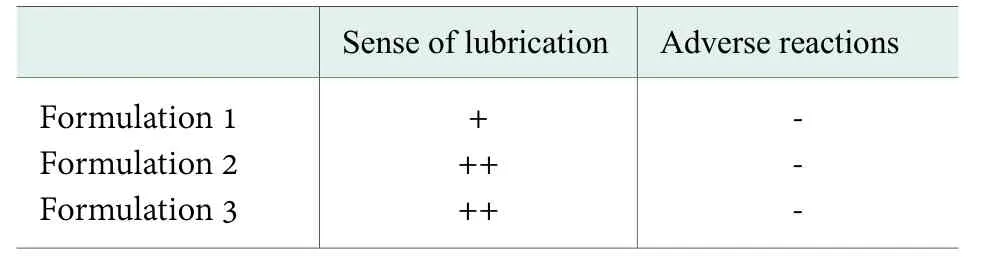
Table 3. Efficacy of the prepared moisturizing cream
Table 3 showed that after the application of moisturizing cream with self-made monoglyceride, all skins were comfortable without red, pain, itching and other adverse reactions and the sense of lubrication is good. Therefore,monoglyceride transesterificated byCinnamomum burmanniiseed oil, as a kind of green, natural cosmetics raw materials to replace the application effect of monoglyceride and traditional emulsifier in cosmetics,satisfying people’s pursuit of consumption concept of returning to nature.
Conclusion
The optimal transesterification conditions for synthesis of monoglycerides byCinnamomum burmanniiseed oil were as follows: the glycerol to oil molar ratio of 3 : 1; NaOH catalyst dosage of 0.8% of the oil mass; reaction temperature at 180 ℃ for 3 hours. The yield reached 62.6%. The crude product was purified by molecular distillation and the purity of the final product achieved 92.8%. As a green, natural raw materials, the monoglyceride synthesized withCinnamomum burmanniiseeds oil achieved batter performance than commercial monoglyceride and emulsifier replaced in moisturizing cream. It is in line with people’s pursuit of the consumption concept of returning to nature.

[1] Y. J. Gu; S. G. Ma; J. L. Preparation of A Series of Fatty Acid Monoglycerides of High Purity. China Surfactant Detergent &Cosmetics 2006, 36(1), 12-14.
[2] Institute of Botany, the Chinese Academy of Sciences. Higher Plants of China. Beijing: Science Press Ltd., 1983.
[3] Zh. H. Zhang; S. C. Lian; G. H. Interspecific Relationships among Main Species of Cinnamomum Burmannii Community on Karst Hills of Guilin. Mountain Research 2007, 25(4), 475-482.
[4] W. L. Zhang; R. X. Qiao; F. Yi; etc. Antifungal Activity of Extracts from 13 Species of Plants Against Five Pathogenic Fungi of Fruits and Vegetables. Journal of South China Agricultural University 2009, 30(2), 40-43.
[5] Zh. H. Dong; G. B. Liu; X. X. Zhao; etc. Optimization of Ultrasound-assisted Extraction of the Seeds Oil from Cinnamomum Burmannii by Response Surface Methodology.China Oils and Fats 2014, 39(10), 10-13.
[6] D. H. Gan; Ch. Y. Wang; Y. M. Pan; etc. The Extraction and Application of Cinnamomum Burmannii Seed Oil. Journal of Guangxi Normal University (Natural Science Edition) 2009(3), 50-53.
[7] C. P.; Zh. L. Zeng; Zh. K. Dai; etc. Synthesis of Monoglycerides from Camphor Tree Seed Oil and Glycerol Catalyzed by Acid Ionic Liquid. China Oils and Fats 2010, 35(08), 61-65.
[8] Y. G. Lv; X. X. Dong; J. Ch; etc. Study on Antimicrobial Effect of Glycerol Monolaurate Microemulsion. Cereals&Oils 2011(2), 46-49.
[9] Tom Krawczyk; Y. J. Zhou; Y. Zh. Xin; etc. Lipids in Cosmetics.Detergent&Cosmetics 1998(1), 12-14.
[10] Sh. L. Liu; Y. L. Bi; T. K. Yang. Synthesis of Monoglycerides and Their Application. Cereals&Oils 2001(11), 30-31.
[11] H. J. Yang. Study on Synthesis and Purification of Glycerol Monolaurate. Zhejiang University 2004.
[12] H. J. Yang; F. Q. Feng. Development of High-purity Glycerol Monolaurate by Molecular Distillation. Science and Technology of Food Industry 2004(5), 110-112.
[13] F. H. Wang; F. Ch.; X. X. Pan; etc. Process for Producing Stearic Acid and Fatty Acid Derivative. China Light Industry Press 1991, 34.
[14] D. Y. Tang; B. C. Liu. Cosmetic Formulation Design and Preparation Process. Chemical Industry Press 2003.
[15] J. Y; Y. F. Hua; J. J. Lu; etc. Comparison of Emulsifying Properties and Mechanisms of Soy Proteins. China Oils and Fats 2005, 30(5), 35-38.
[16] X. X. Zhao; Zh. H. Dong; G. B. Liu; etc. Extraction of Camphor Seed Oil and Its Application in Cosmetics. Applied Chemical Industry 2013, 42(9), 1620-1623.
 China Detergent & Cosmetics2016年2期
China Detergent & Cosmetics2016年2期
- China Detergent & Cosmetics的其它文章
- New Application of a Series of Preservatives Derived from Amino Acid for Cosmetic Products
- Cosmetic Safety Evaluation Based on In Vitro Three-dimensional Reconstructed Human Epidermis(3D-RHE) Models
- Problems and Suggestions for Domestic Non-special Use Cosmetics Record Filing in China
- Patent Protection of Daily Chemical Industry in China
- New Trends of Comsmetic Regulations in China
- Development of Household Care Chemical Industry in Pakistan
