New Application of a Series of Preservatives Derived from Amino Acid for Cosmetic Products
Shujuan Huang
Shanghai Zhongshi Science and Technology Development Co., Ltd., China
Huiyu Wang, Hongyu Xue
Nanjing Hua Shi New Materials Co., Ltd., China
Introduction
According to the Safety and Technical Standards for Cosmetics of China, a preservative is the material which is added into the cosmetic products, in order to inhibit the microbial growth and to ensure a long shelf life.Usually, the traditional preservatives used in cosmetic products are DMDM hydantoin, parabens, benzalkonium chloride, methylisothiazolinone, imidazolidinyl urea,phenoxyethanol and so on. But the traditional preservatives are proved to be hazard to human health more or less,[1-5]and as the list of globally-approved preservatives is limited,so cosmetic manufacturers are continually interested in new alternative preservation systems.
Ethyl lauroyl arginate HCl (LAE) is a new surfactant derived from lauric acid and arginine. It is a non-aromatic active ingredient with strong antimicrobial properties, and it can be rapidly metabolized by humans to the naturally occurring dietary components lauric acid and arginine.Besides, LAE was Generally Recognized as Safe (GRAS)for use as antimicrobial in some food categories by the FDA in 2005. So it is considered as a safe product for the consumer.
The low toxicity in conjunction with remarkable antimicrobial features makes LAE a product of wide application in the food preservation field.[6]Some tests in this article have been carried out that prove series products of compounds of LAE can be used as efficient preservatives for cosmetic products. The addition of LAE to the cosmetic products preservation area means a great technological innovation towards the manufacturing of products with better and safer preservation qualities.
Properties of LAE
Molecular structure and anti-microbial mechanism.The molecular formula of LAE is C20H41N4O3Cl, and the molecular structure of LAE is as shown in the following Figure 1.
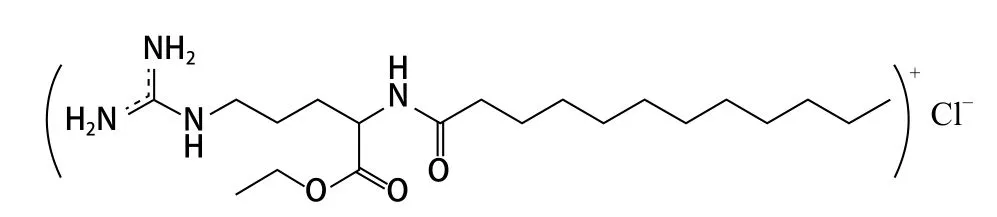
Figure 1. Structural formula of LAE
LAE is a white hygroscopic solid which has a melting point at 50℃ to 58℃. And it has good solubility in water with the number of 24.7 g/100 g. The LAE molecule is mainly distributed in water phase, because its partition coefficient between water and oil (olive, sunflower,soy) is higher than 10. And since water phase is more susceptible to microbial contamination, the property gives LAE an advantage of more efficacy compared with other preservatives with a different chemical structure intended for the same applications.
The antimicrobial properties of LAE are due to the properties of its cationic molecular structure. It can act on the cytoplasmic membranes of the microorganisms in a manner which their metabolic processes are altered and their normal cycle is inhibited but without cellular lysis.
Antimicrobial efficacy of LAE. The following tables (Table 2 to Table 5) show the minimum inhibitory concentrations of LAE against different types of microorganisms, mostly pathogens, which give an insight into the wide range of activity and great effectiveness of LAE.[7]
The results showed that LAE has a slightly higher sensitivity of Gram positive bacteria than Gram negative bacteria. In general, these values show that LAE has a greater sensibility of bacteria, yeasts and moulds.
Toxicological safety. A series of toxicological experiments have been carried out to confirm the safety of LAE. These tests included the metabolism of LAE in animals and in humans, mutagenic, acute, subchronic, chronic, reproductive and developmental toxicity. The results indicate that LAE is non-mutagenic and non-clastogenic, the general toxicological data shows that LAE is a material with high safety.[8]
Additional studies were performed to demonstrate the safety of LAE to humans’ skin and eyes. The parameters studied were: the capacity of LAE to diffuse trough the skin, the potential degree of LAE irritation to the skin and to the eyes, and the potential for sensitization when LAE is repeatedly applied to skin. The results show that the percutaneous absorption value of LAE is 5.24 ± 2.29 μg/cm2, and LAE has non ocular irritation to eyes and non sensitization to skin.
In conclusion, the toxicological profile of LAE looks favourable for its use as a preservative with no risk to the health of consumer.
According to the safety properties, LAE has been used in food and can be used in cosmetic products.
The SCCP (Scientific Committee on Consumer Products)adopted the opinion of LAE using as a preservatives in cosmetics at its 15thplenary on 15 April 2008.
Regulations Commission Regulation (EU) No 344/2013 of 4 April 2013 considers that Ethyl lauroyl arginate HCl is safe for the consumers, the dosage is as followed: 1) up to a maximum authorised concentration of 0.4 % as a preservative in cosmetic products, but excluding products for the lips, oral hygiene products and spray products; 2) up to a maximum authorised concentration of 0.8% in soap,anti-dandruff shampoos, and non-spray deodorants.[9]
The SCCS (Scientific Committee on Consumer Safety)has updated the Opinion on Ethyl lauroyl arginate HCl -submission IV at its 3rdplenary meeting on 19 September 2013, which indicated that Ethyl lauroyl arginate HCl is safe when used as a preservative to a maximum concentration of 0.15% in mouthwashes, though not in oral cosmetic products as a whole.
Chemical Stability of LAE. In addition to these antimicrobial advantages, the antimicrobial properties of LAE remain constant from pH 3-7, suggesting that this substance may be useful as antimicrobial agent for a wide range of cosmetic products. And this capacity offers LAE a significant advantage compared to other preservatives currently available in the market.


LAE keeps its antimicrobial properties when heat is added to. Some technologies of producing cosmetic products involve processes at high temperature, so the antimicrobial agents need to maintain their antimicrobial properties after high temperature treatment. The results showed that MICs and MBCs for E. coli after heating LAE at 90℃ and 120℃ for 15 min were the same as those obtained without treatment.[10]This advantage of LAE makes it can be used in the fields that require high temperature, such as some types of cleansers and creams.
Antimicrobial properties of LAE series products as preservatives
Materials and methods. According to the safety and excellent anti-bacterial abilities of LAE, Sino Lion developed series of preservatives which are compounds of LAE and other safe preservatives or synergy agents.This series of LAE preservatives have good solubility in water, and environmental performance and can be widely used in cosmetic industry. In addition, the series of LAE preservatives have a high anti-microbial efficacy with low toxicity, non-sensitizing and non skin irritating. They can make skin and hair smooth because of the cationic nature.Sino lion’s series of LAE preservatives have been certified by Ecocert, Cosmos, NaTrue. When using Sino lion’s series of LAE preservatives for general cosmetic products,the suggested dosage range is 0.25 % ~ 2.0 %.
EverguardTMLAE-20 (INCI Name: Ethyl lauroyl arginate HCl, Glycerin): is a clear liquid based on a 20%solution of LAE in glycerin. EverguardTMLAE-20 will be referred as Preservative A in the subsequent parts of the article.
EverproTMLCG (INCI Name: Ethyl lauroyl arginate HCl, Caprylyl Glycol, Glycerin): is a liquid based on a 20%solution of LAE in caprylyl glycol and glycerin. EverproTMLCG will be referred as Preservative B in the subsequent parts of the article.
To evaluate the efficacy of antimicrobial preservation ability of LAE series preservatives, the microbiology challenge experiments are carried out to test the effect for bacteria, fungi and yeast. And the method refers to the European pharmacopoeia of EUP 5.1.3.
Different formulas of rinse-off and leave-on cosmetic products that contained Preservative A or Preservative B were prepared. The change of microbe quantity in the solution at hour 48, day 7, day 14, day 21 and day 28 respectively after inoculation was studied. In day 21, carry out the secondary inoculation, which will increase the difficulty to pass the antimicrobial efficacy test, to study the inhibitory effect of secondary contamination.
The microbial strains selected for use in the test are shown in Table 1.
Antimicrobial efficacy test of a moisturizing toner.The antimicrobial efficacy of Preservative A in a toner formula was studied while adding 0.30% Preservative A and 0.45% 1, 2-hexylene glycol (HG) as preservative system. The formula of the moisturizing toner for testing is as shown in Table 2.
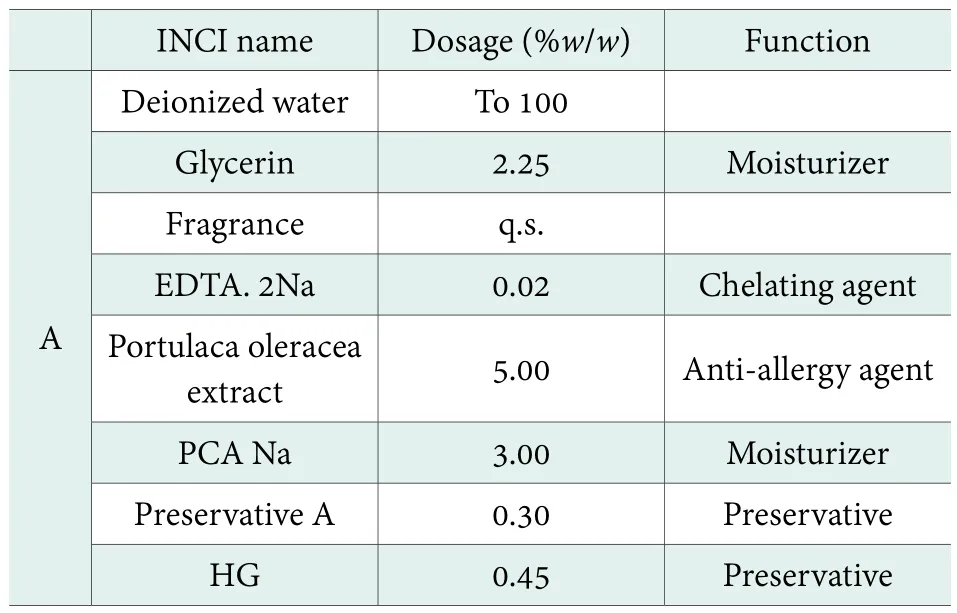
Table 2. Formula of moisturizing toner: 0.30% Preservative A + 0.45% HG
Antimicrobial efficacy test of an amino acid hair shampoo. The antimicrobial efficacy of Preservative B in a formula of transparent amino acid hair shampoo with 0.60% Preservative B was studied. The formula of the transparent amino acid hair shampoo for testing is as shown in Table 3.
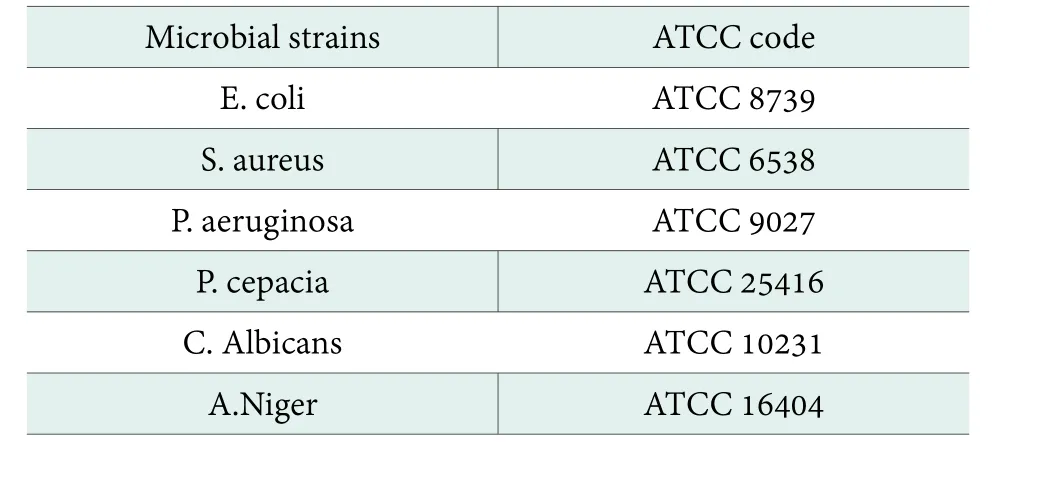
Table 1. List of measurement microbial strains
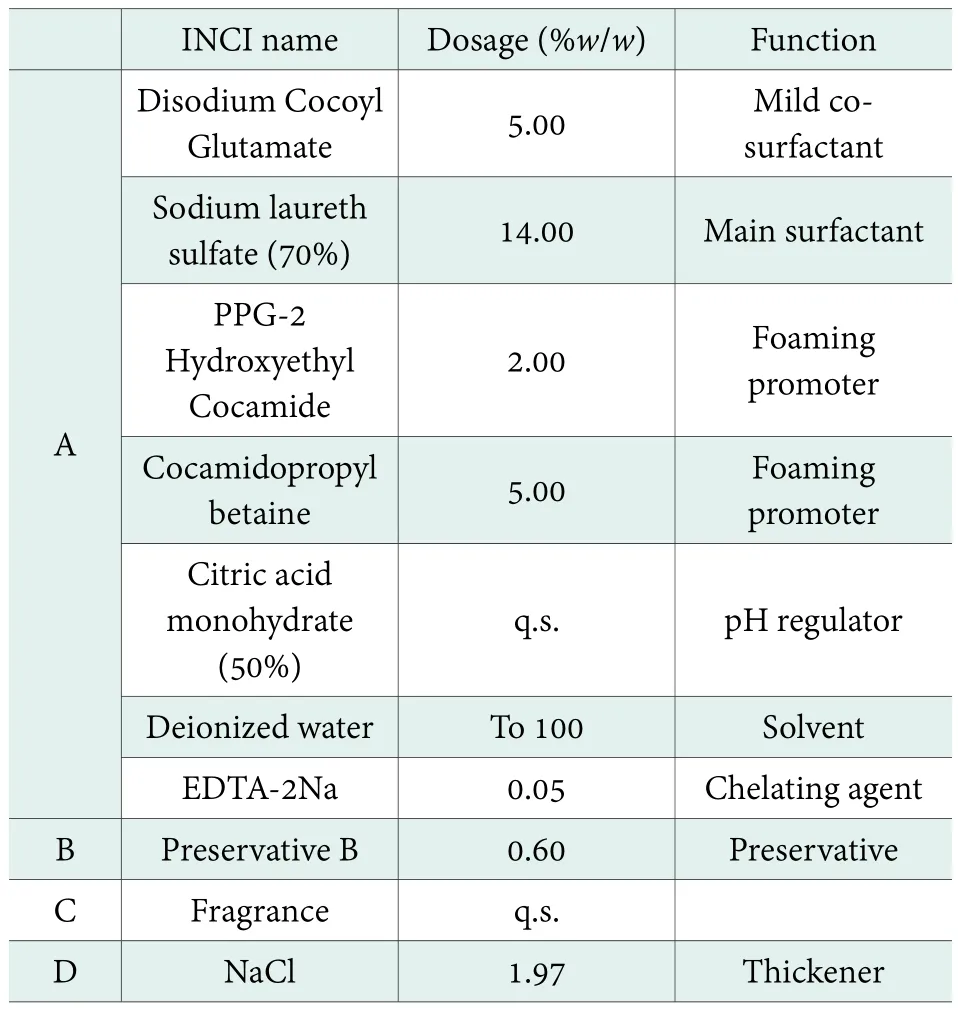
Table 3. Formula of transparent amino acid hair shampoo:0.60 % Preservative B
Results and discussion
Antimicrobial efficacy test of a moisturizing toner.Table 4 shows the antimicrobial efficacy test result of the toner with the preservative system of 0.30% Preservative A compounding with 0.45% HG. The values in the table shows that when adding 0.30% Preservative A compounding with 0.45% HG as the antimicrobial system into the toner formula for testing, the bacteria, moulds and yeasts count tested at 48 hours were all less than 10 CFU/ml. And in day 7, day 14 and day 28, the bacteria, moulds and yeasts count tested were all less than 10 CFU/ml.
The results indicated that 0.30% Preservative A compounding with 0.45% HG behave as a fast and efficient bactericidal agent against bacteria, moulds and yeasts.
For the tested toner formula, 0.30% Preservative A compounding with 0.45% HG was a suitable preservativesystem that can be an alternative for traditional preservatives in leave-on products.
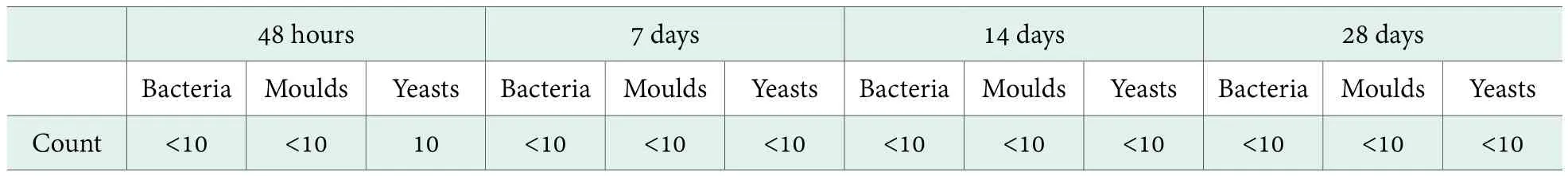
Table 4. Antimicrobial efficacy results of a toner formula (0.30% Preservative A+ 0.45% HG)
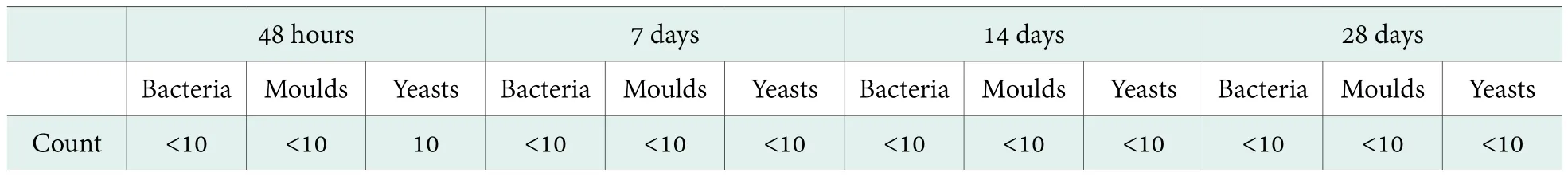
Table 5. Antimicrobial efficacy results of a transparent amino acid hair shampoo formula (0.60% Preservative B)


Antimicrobial efficacy test in transparent hair shampoo products
Table 5 shows the antimicrobial efficacy test result of the amino acid transparent hair shampoo formula with 0.60% Preservative B. As can be seen, when the dosage of Preservative B is as low as 0.60%, the bacteria, moulds and yeasts count tested at 48 hours were all less than 10 CFU/ml,which indicated that Preservative B behaves as fast bactericidal agents. And in day 7, day 14 and day 28, the bacteria, moulds and yeasts count tested were all less than 10 CFU/ml,which indicated that Preservative B behaves as long lasting bactericidal agent for bacteria, moulds and yeasts.
For the testing formula, 0.60% Preservative B was a suitable alternative antimicrobial system that can avoid using the traditional preservatives (paraben e.g.) in the rinse-off products.
Conclusion
The series of LAE preservatives show high safety and effective antimicrobial properties. The series of LAE preservatives can be added into the shower gel products and toner products conveniently. The results indicated that the series of LAE preservatives can be used as a new type of preservatives for cosmetic products.
As can be seen from the above data, the series of LAE preservatives are derived from the source of natural.The series of LAE preservatives are safe and mild and have broad spectrum antibacterial, high antimicrobial efficiency, high stability to heat and pH range. The series of LAE preservatives can be applied to various kinds of cosmetic products, including leave-on and rinse-off products. The series of LAE preservatives can be used alone, or together with other preservatives to form the anti-microbial systems for the cosmetic products.
From the above advantages, the series of LAE preservatives may be possible alternatives to traditional preservatives.
[1] Wolfgang U.; Peter J.F.;et al. Contact Allergy from DMDM Hydantoin, 1994-2000. Contact Dermatitis 2002, 47, 57-58.
[2] Sayaka S.; Yayoi S.; Jun Y.;et al. Urinary Excretion of Parabens in Pregnant Japanese Women. Reproductive Toxicology 2013,35, 96–101.
[3] Margherita L.; Chiara R.; Brigida D.; et al. Toxicity and Genotoxicity of the Quaternary Ammonium Compound Benzalkonium Chloride (BAC) Using Daphnia Magna and Ceriodaphnia Dubia as Model Systems. Environmental Pollution 2016, 210, 34-39.
[4] Thyssen J.P.; Sederberg-Olsen N.; Thomsen J.F;et al. Contact Dermatitis from Methylisothiazolinone in a Paint Factory.Contact Dermatitis 2006, 54(6), 322-324.
[5] J. Garc′ ?a-Gav′ ?n; D. Gonz′ alez-Vilas; V. Fern′ andez-Redondo;et al. Allergic Contact Dermatitis in A Girl Due to Several Cosmetics Containing Diazolidinyl-Urea or Imidazolidinyl-Urea.Contact Dermatitis 2010, 63, 49–54.
[6] Laura H.; Gracia L.C.; Pilar H.M.; et al. Development of a Novel Antimicrobial Film Based on Chitosan with LAE.International Journal of Food Microbiology 2013, 165, 339–345.
[7] Dr. Manresa. Antimicrobial Susceptibility in Terms of the Minimal Inhibitory Concentration (MIC) of LAE and Mirenat-N. University of Barcelona Spain: Dr. Manresa 2004.
[8] Stephen A. Ruckmana; Xavier Rocabayera; Joseph F. Borzellecac;et al. Toxicological and Metabolic Investigations of the Safety of N-α-Lauroyl-L-arginine Ethyl Ester Monohydrochloride (LAE).Food and Chemical Toxicology 2004, 42, 245–259.
[9] EU Cosmetic Regulation (EC) No 344/2013.
[10] R. Becerril; S. Manso; C. Nerin; et al. Antimicrobial Activity of Lauroyl Arginate Ethyl (LAE), against Selected Food-Borne Bacteria. Food Control 2013, 32, 404-408.
 China Detergent & Cosmetics2016年2期
China Detergent & Cosmetics2016年2期
- China Detergent & Cosmetics的其它文章
- Synthesis of Monoglycerides with Cinnamomum Burmannii Seeds Oil and Its Application in Moisturizing Cream
- Cosmetic Safety Evaluation Based on In Vitro Three-dimensional Reconstructed Human Epidermis(3D-RHE) Models
- Problems and Suggestions for Domestic Non-special Use Cosmetics Record Filing in China
- Patent Protection of Daily Chemical Industry in China
- New Trends of Comsmetic Regulations in China
- Development of Household Care Chemical Industry in Pakistan
