Cosmetic Safety Evaluation Based on In Vitro Three-dimensional Reconstructed Human Epidermis(3D-RHE) Models
Xue Kong, Wendan He, Pengju Nie, Ying Tang
Beijing Key Laboratory of Plant Resource Research and Development, School of Science, Beijing Technology and Business University, China
Department of Cosmetics, School of Science, Beijing Technology and Business University, China
Ding Cao
State Key Laboratory of Chemical Resource Engineering, Beijing University of Chemical Technology, China
Recently, frequent occurrence of cosmetic-induced adverse skin reactions has led to an increasing concern of cosmetic safety from both cosmetic consumers and enterprises. Cosmetic safety evaluation, as a hazard identification at both qualitative and quantitative levels,is aimed to predict potential risks caused by daily use of cosmetics, as well as to determine the safety exposure dosage.[1]In history, safety evaluation of cosmetics is mainly reguired the use of animal testing which has met with opposition for its cruelty, inferior reproducibility and lack of scientific correlation with human data. According to the 7th Amendment to the European Cosmetics Directive (2003/15/EC, 2003),[2]the European Union has set a deadline for eliminating animal testing of both cosmetic ingredients and products by 11 March 2013, which was reinforced by a market ban by all the member states. Therefore,in vitroalternative methods to animal experimentation employing cultured human cells or other non-animal systems are booming in the current cosmetic industry, bringing advantages of shorter testing periods, more controllable experimentation, and better scientific quality.
3D RHE models
3D RHE model has been a focus in the field ofin vitrosafety evaluation of cosmetics. As a mimic to the real human epidermis, 3D-RHE model is a 3D skin tissue reconstructed from cultured human skin cells on an inert filter substrate at the air-liquid interface.[3]Compared with monolayer cultured cells, 3D-RHE models have intact stratum corneum derived from human keratinocytes,which advantages the direct testing of “insoluble” solid or extremely acidic/basic ingredients and formulated products. 3D-RHE models can also be an ideal model for phototoxicity evaluation by adjusting intensity, time and wavelength of the exposure radiation. Besides, since 3D-RHE models are derived from primary cultured human cells, inter-species extrapolation can be avoided.[4]Validated 3D-RHE models possess high biological consistency with clinical human data in gene expression,microstructure, cytokines, tissue viability.[5]3D-RHE models were first developed for clinical treatment of large-area skin damage. In 1970s, American scientists reconstructed the first RHE model from cultured epithelial cells, which is regarded as a milestone in the development of skin models.[6]The application of 3D-RHE models in cosmetic safety evaluation took off in the 1990s. With 25 years’ development, various 3D-RHE models have been developed (Table1) and are now widely used in hazard identification of skin irritation,[7]eye irritation,[8]phototoxicity,[9]genotoxicity,[10]skin sensitization,[5]etc.
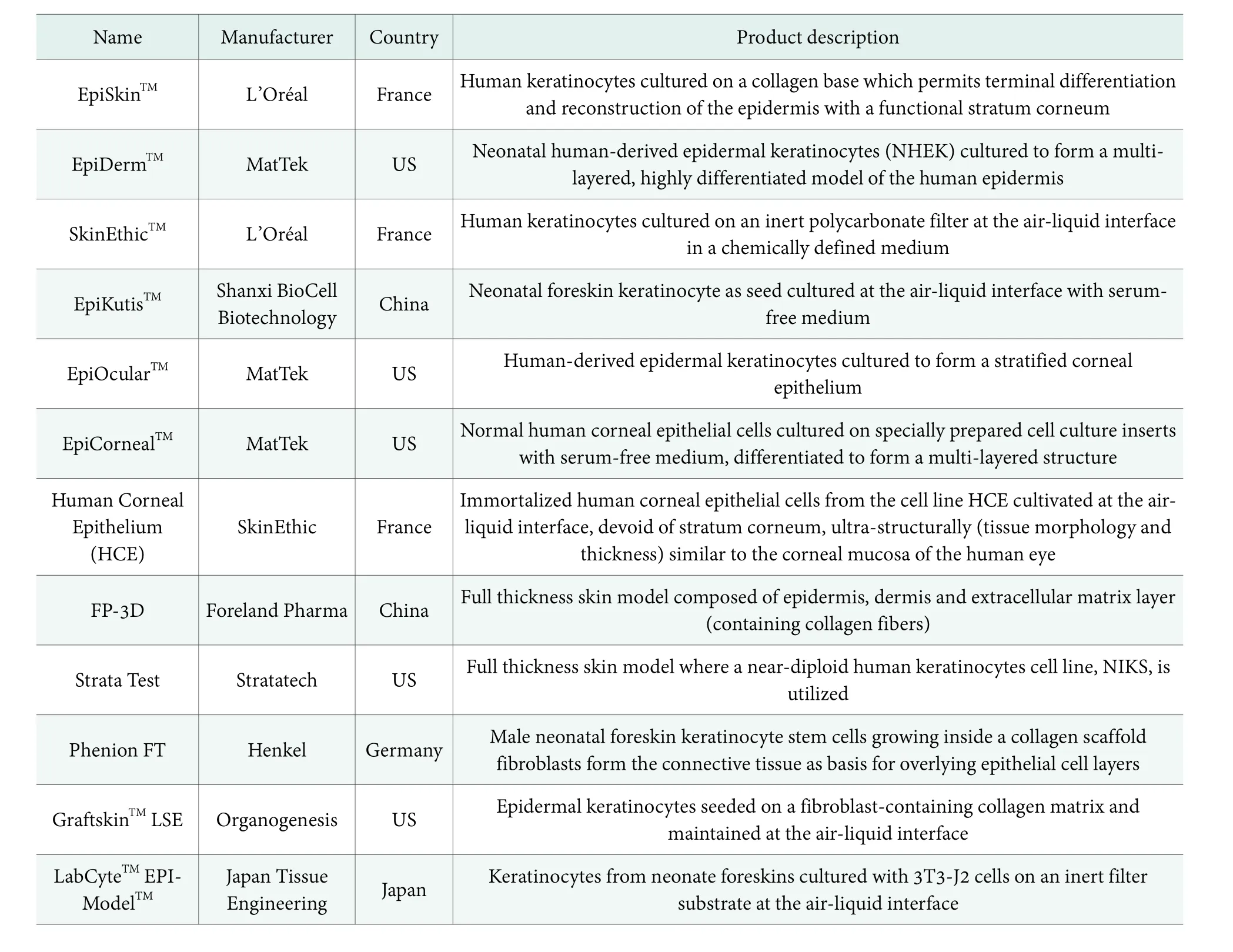
Table 1. Some commercially available 3D-RHE models
Application of RHE models in Safety Evaluation of Cosmetics
Skin/eye irritation, phototoxicity and genotoxicity are necessities for the safety evaluation of most cosmetic ingredients. In China, skin/eye irritation and phototoxcity tests use animal models (rabbits and guinea pigs),[12]which are banned in EU and US for their cruelty and lack of scientific accuracy. Taking eye irritation for example,the rabbit Draize test in most cases may over-predict human responses because of the lack of lacrimal glands in the rabbit eye. Genotoxicity studies can employ cultured cells and microorganisms and have good sensitivity but suffer from a high occurrence of false positives and a limitation for sample solubility. By contrast, 3D-RHE models have the advantages of good similarity with human skin, high experimental accuracy, sensitivity and reproducibility, and thus are increasingly applied in these cosmetic safety areas. Today, various 3D-RHE models are available for different toxicity studies with different endpoints (Table 2).
Skin irritation refers to reversible damage to the skin following the application of a test article for up to four hours.[13]Testing chemicals for their potential to cause skin irritation is required for all ingredients of products that come into contact with skin. Today,in vitroassessment of skin irritation usually employs cultured cells (e.g.human epidermal keratinocytes), isolated human/animal skins and 3D-RHE models. Cellular tests have a high degree of standardization and are less expensive, but suffer from the lack of a skin barrier function which may result in overestimation of toxic effects. Methods using isolated skin suffer from low predictivity and high cost as human skins are mainly obtained from corpses or beauty surgery.Therefore, 3D-RHE models have been an idealin vitromodel to evaluate skin irritation since early 1990s. Three models (EpiSkinTM, EpiDermTM, and SkinEthicTM) were validated by the European Centre for the Validation of Alternative Methods (ECVAM) as stand-alone test replacements for the rabbit Draize test and received regulatory approval from the Organization for Economic Co-operation and Development (OECD).
Currently, skin irritation tests based on 3D-RHE models can be grouped as skin irritation tests (SIT)[14]and time-to-toxicity tests.[15]According to OECD Test Guideline 439,[15]skin irritation test distinguish a test article as irritant (I) or non-irritant (NI) following a single exposure for a fixed period of time. A sample is classified as an irritant if the tissue viability, assessed by an MTT reduction assay, relative to the negative control treated tissue is reduced below 50%. For some mild personal care and cosmetic products, it is necessary to extend the exposure time in order to further distinguish mild to moderate irritation using the time-to-toxicity test which measures the exposure time at which 50% of cultured skin tissues (ET50) remain viable.[15]Time-totoxicity test is particularly useful for industrial safety screening of cosmetic ingredient or formula: the smaller the ET50, the stronger the potential to cause skin irritation.Considering that keratinocytes, the major cell type in the epidermis, express a group of inflammatory cytokines (e.g.IL-1α, IL-6, IL-8, TNF-α) in the irritation process, the release of inflammatory cytokines (especially IL-1α) can be measured as important supplementary endpoints to the MTT viability assay.[11,15]
Kandárová[7]has developed SIT using the EpiDermTMmodel for the irritation evaluation of cosmetic and pharmaceutical ingredients which allows discrimination between irritants of GHS category 2 and non-irritants.Spiekstra[16]addresses the variation between different skin models and proposes a method which may in the future be used to determine irritant potency according to the EC50values. But the author also indicated that the results of the assay may be influenced by both the properties of the chemical and the testing system, so irritant thresholds should be taken into account for the further development ofin vitroassays based on 3D-RHE models.[16]In conclusion,3D-RHE models can reliably distinguish between irritants and non-irritants, and evaluate skin irritancy of new ingredients or formulations.
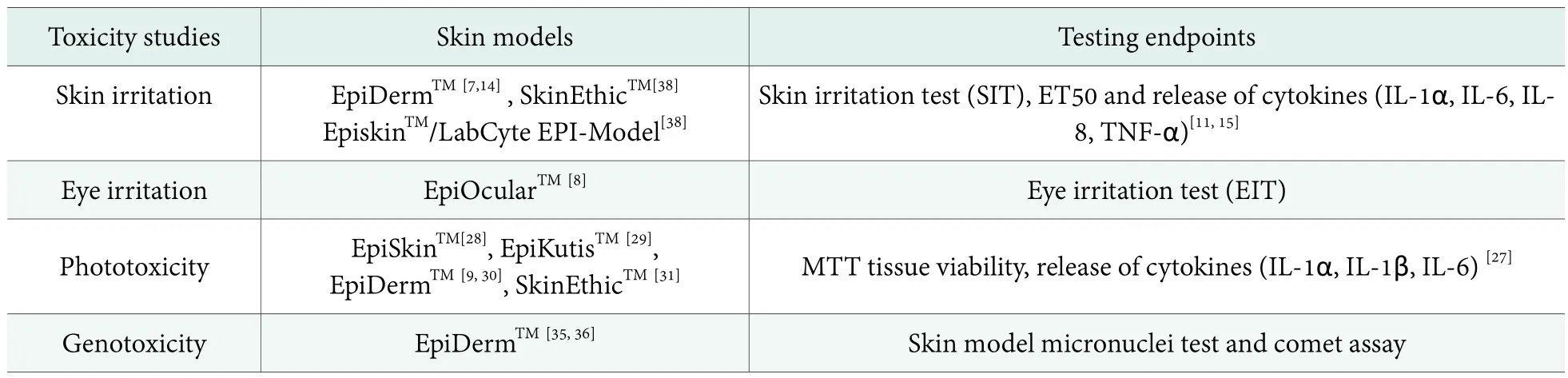
Table 2. Skin models used in various safety evaluation & testing endpoints
Eye Irritation refers to testing articles that produce significant changes in the eye that are fully reversible within 21 days of application.[17]Currentin vitromethods for eye irritation evaluation approved by ECVAM include : (i) tests using isolated animal organs: Bovine Corneal Opacity and Permeability tests[18]and Isolated Chicken Eye (ICE)tests;[19](ii) tests based on cytotoxicity and cell function:Cytosensor Microphysiometer (CM)[20]and Fluorescein Leakage (FL) Test;[21]and (iii) the EpiOcularTMEye Irritation Test (EIT).[17]However, no single test of the above is considered appropriate to replace the rabbit eye test, and a grading strategy incorporating two or more test systems is suggested for full replacement.
The 3D reconstructed human cornea-like epithelium(3D-RhCE) tissue construct used in the eye irritation study has no stratum corneum, leading to higher sensitivity compared with the other 3D-RHE models.[22]According to OECD Test Guideline 492, the EpiOcularTMEIT used a single exposure period for each chemical and a prediction model based on a cut-off of 60% relative tissue viability, assessed by MTT assay, to distinguish between non-classified chemicals (NC) and eye irritants(I) (GHS categories 2 and 1).[17]Kaluzhny[8]verified that the overall accuracy of this method for prediction of eye irritation was 88% with a sensitivity of 100% and a specificity of 75%. In 2013, Cosmetic Europe (formerly COLIPA) reported an inter-laboratory validation study of two test methods based on reconstructed cornea-like skin models–EpiOcularTM[23]and SkinEthicTMHuman Corneal Epithelium (HCE).[24]The results demonstrated that all testing articles analyzed were categorized similarly for eye irritancy and that the two tests based on 3D-RhCE models are quite reproducible.[24,25]In China, a corneal epithelial model reconstructed from Chinese human cells has been developed by Shanxi BioCell Biotechnology and the test results on standard reference chemicals were consistent with the rabbit eye test.
Phototoxicity is defined as an acute non-immune toxic reaction which is elicited by topical contact with photosensitive chemicals after exposure to UV light.[25]The clinical features include sunburn, blisters, etc.[26]Phototoxicity test is compulsory for sunscreen cosmetic ingredients that absorb in the UV range.[12]The 3T3 neutral red uptake phototoxicity test (3T3 NRU-PT) is the onlyin vitromethod of formal verification (OECD 432, 25). However, 3T3 NRU PT has some disadvantages:(i) the test uses only cell viability as endpoint, hence only acute phototoxicity can be detected; (ii) the underlying mechanism is not analyzed; (iii) the test shows a high frequency of positive results.
Skin phototoxicity evaluation tests based on 3D-RHE models date back to 1994, using full thickness skin models or epidermis models. In the tests employing epidermis models, articles are applied onto the surface of the model dropwise followed by a UV exposure (typically 1.7 mW/cm2) for one hour, and the toxicity is assessed by the MTT tissue viability assay.[26]For tests using full thickness skin models composed of keratinocytes and melanocytes, phototoxicity can be evaluated by both tissue viability and the release of inflammatory cytokines IL-1ɑ, IL-1β and IL-6.[27]
In recent years, 3D-RHE models are widely used in phototoxicity evaluation of cosmetic ingredients.Using the EpiDermTMmodel, J?′rova′[9]assessed the phototoxicity of bituminous tars (ichthammol and ichthyol pale), a type of anti-pruritic cosmetic ingredients,the results of which showed good correlation between 3D skin model and data of human experiment. A study based on EpiSkinTMshowed very good sensitivity (92.3%) and excellent specificity (100%) with an overall accuracy of 94.1% for identifying phototoxic potency of topically or systemically applied chemicals.[28]In China, EpiKutisTMis a 3D-RHE model reconstructed from Chinese Han human skin cells which has been developed for the phototoxicity assessment.[29]Park used EpiDermTMto test the phototoxicity of polystyrene and TiO2nanoparticles,which is the first report of using skin models to evaluate skin phototoxicity of nanoparticles.[30]The SkinEthicTMmodels were reported as demonstrating high resistance to UVA irradiation, so the irradiation dosage can be increased by (at least) 3-fold without a decrease in tissue viability, making the model an ideal tool for the detection not only of strong but also of weak phototoxic compounds.[31]
Genotoxicity describes the property of chemical agents that damages the genetic information within a cell, thus causing mutations.In vitroassessment of genotoxicity provides information on three major endpoints: (i)mutagenicity at a gene level; (ii) chromosome breakage and/or rearrangements; and (iii) numerical chromosome aberrations.[32]Currently available and regulatorily acceptedin vitromethods include: (i) bacterial reverse mutation test or Ames test; (ii)in vitromicronucleus test; (iii)in vitromammalian cell gene mutation test,mouse lymphoma assay and hypoxanthine-guanine phosphoribosyl transferase test; and (iv)in vitromammalian chromosomal aberration assay.[33]These methods have good sensitivity but low specificity when dealing with rodent carcinogens, which may result in false-positive results.
Cosmetics Europe’s Genotoxicity Task Force has recently driven and funded a “3D skin model” project which is now validating the use of human reconstructed skin (RS) models in combination with the Micronucleus(MN) and Comet assays.[34]An intralaboratory and interlaboratory evaluation of the EpiDermTM3D human reconstructed skin micronucleus (RSMN)assay indicates the 3D human skin model is a promising newin vitrogenotoxicity assay that allows evaluation of chromosome damage following “in vivo-like” dermal exposure.[35,36]Reus[10]reported the genotoxic evaluation of five coded compounds (MMS, N-ethyl-N-nitrosourea,2,4-diaminotoluene, cyclohexanone, p-nitrophenol) by Comet assay using EpiDermTMand EPI-200-MNA skin models. These models were shown to have metabolic capacity comparable to native human skin, confirming their usefulness for genotoxic testing of compounds with a dermal route of exposure.

Summary
Tests based on 3D-RHE models are a trend in today’s cosmetics safety evaluation and offer several advantages: i)better similarity with native human skin; ii) more controllable experimentation, giving good accuracy, sensitivity and reproducibility; iii) shorter testing periods compared with animal and clinical studies; iv) few requirements for sample solubility, chemicals with diあerent physiochemical properties(hydrophobic or solid chemicals, complex mixture or formula) can be directly tested; v) reduction of experimental animal use.
However, there are still some disadvantages in using 3D-RHE models: i) lack of skin appendages like follicle,sweat glands, sebaceous glands; ii) only a single organ model, lacking the interaction with other organs; iii)address only acute toxic potential and not cumulative irritancy; iv) inferior barrier function, resulting in higher percutaneous absorption rate than native human skin; v)limited model suppliers and relatively high cost in China.Future goals further development of 3D-RHE model methods may include providing further validation data requested by the government to support global acceptance and regulatory approval; extendingin vitromodels to reflectin vivoexposure conditions (e.g. rinse-off); and development of testing protocols to reflect chronic exposure.
Acknowledgements
This project was supported by the National Natural Science Foundation of China (No. 51403006 and No.51203008) and the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry.
[1] L.Ma; S.Wei. Reviewing Safety Assessment of Cosmetics.China Cosmetics Academic Symposium 2004, 8-12.
[2] Pauwels M; Rogiers V. Safety Evaluation of Cosmetics in the EU: Reality and Challenges for the Toxicologist. Toxicology Letters 2004, 151(1), 7-17.
[3] C.X.Zhang; Z.J.Du; Z.Wang; Q.S.Zhang; X.M.Zhang.Application of Three-dimensional Reconstructed Human Skin Models in the Safety and Efficacy Evaluation of Cosmetics.China Cosmetics Review 2014, 2, 38-40.
[4] Gordon S; Daneshian M; Bouwstra J; Caloni F; etc. Non-Animal Models of Epithelial Barriers (skin, intestine and lung) in Research, Industrial Applications and Regulatory Toxicology. Alternatives to Animal Experimentation 2015,32(4), 327-378.
[5] S.J.Cheng; H.Jiao. Alternative Laboratory Animal Methods Principles and Applications. Beijing: Science Press 2010, 188-340.
[6] Z.Y.An. The Construction of Skin Model and Its Testing Application in Cosmeceutical Master Thesis of Agricultural University of Hebei Province 2013.
[7] Kandárová H; Hayden P; Klausner M; Kubilus J; Sheasgreen J. AnIn VitroSkin Irritation Test (SIT) Using the EpiDerm Reconstructed Human Epidermal (RHE) Model.Electrophoresis 2009, (29), 1366.
[8] Kaluzhny Y; Kandárová H; Hayden P; Kubilus J; d’Argembeau-Thornton L; Klausner M. Development of the EpiOcular?Eye Irritation Test (EpiOcular-EIT) for Hazard Identification and Labelling of Eye Irritating Chemicals in Response to the Requirements of the Cosmetics Directive and REACH Legislation. Alternatives to Laboratory Animals 2015, 43(2),101-127.
[9] Jirová D; Kejlová K; Bendová H; Ditrichová D; Mezulániková M. Phototoxicity of Bituminous Tars–correspondence between Results of 3T3 NRU PT, 3D Skin Model and Exprimntal Humandata. Toxicologyin Vitro2005, 19(7),931–934.
[10] Reus AA; Reisinger K; Downs TR, Carr GJ; Zeller A; Corvi R;Krul CAM;Pfuhler S. Comet Assay in Reconstructed 3D Human Epidermal Skin Models–investigation of Intra- and Inter-laboratory Reproducibility with Coded Chemicals.Mutagenesis 2013, 28 (6), 709–720.
[11] Bernhofer LP; Seiberg M; Martin KM. The Influence of The Response of Skin Equivalents Systems to Topically Applied Consumer Products by Epithelial Mesenchymal Interaction. Toxicologyin Vitro1999, 13: 219-229.
[12] Ministry of Public Health of the People's Republic of China.Safety and Technical Standards for Cosmetics 2015.
[13] Test No.404: Acute Dermal Irritation/Corrosion. OECD Guidelines for the Testing of Chemicals 2010, 1(4): 1-13.
[14] Test No.439:In VitroSkin Irritation: Reconstructed Human Epidermis Test Method. OECD Guidelines for the Testing of Chemicals 2010, 1(4): 1-18.
[15] Costin GE; Raabe H; Curren R.In VitroSafety Testing Strategy for Skin Irritation Using the 3D Reconstructed Human Epidermis. Romanian Journal of Biochemistry 2009,46(2), 165-186.
[16] Spiekstra SW; Dos Santos GG; Scheper RJ; Gibbs S. Potential Method to Determine Irritant Potencyin Vitro-Comparison of Two Reconstructed Epidermal Culture Models with Different Barrier Competency. Toxicologyin Vitro2009, 23(2), 349-355.
[17] Test No.492: Reconstructed Human Cornea-like Epithelium(RhCE) Test Method for Identifying Chemicals Not Requiring Classification and Labelling for Eye Irritation or Serious Eye Damage. OECD Guidelines for the Testing of Chemicals 2015, 1-27.
[18] Test No.437: Bovine Corneal Opacity and Permeability Test Method for Identifying Ocular Corrosives and Severe Irritants. OECD Guidelines for the Testing of Chemicals 2010,1(4), 1-18.
[19] Test No.438: Isolated Chicken Eye Test Method for Identifying Ocular Corrosives and Severe Irritants. OECD Guidelines for the Testing of Chemicals 2010, 1, 1-18.
[20] European Union Reference Laboratory for Alternatives to Animal Testing (EUAL ECVAM): Validation & Regulatory Acceptance: Topical Toxicity: Eye Irritation.
[21] Test No.460: Fluorescein Leakage Test Method for Identifying Ocular Corrosives and Severe Irritants. OECD Guidelines for the Testing of Chemicals 2012, 1, 1-16.
[22] Kaluzhny Y; Kandárová H; d’Argembeau-Thornton L;Kearney P; Klausner M. Eye Irritation Test (EIT) for Hazard Identification of Eye Irritating Chemicals Using Reconstructed Human Cornea-like Epithelial (RhCE) Tissue Model. Journal of Visualized Experiments 2015 (102), e52979-e52979.
[23] Pfannenbecker U; Bessou-Touya S; Faller C; Harbell J; etc.Cosmetics Europe Multi-laboratory Pre-validation of the EpiOcularTM Reconstituted Human Tissue Test Method for the Prediction of Eye Irritation. Toxicologyin Vitro2013,27(2), 619-626.
[24] Alépée N; Bessou-Touya S; Cotovio J; etc. Cosmetics Europe Multi-laboratory Pre-validation of the SkinEthic?Reconstituted Human Corneal Epithelium Test Method for the Prediction of Eye Irritation. Toxicologyin Vitro2013,27(5), 1476-1488.
[25] Test No.432:In Vitro3T3 NRU Phototoxicity Test. OECD Guidelines for the Testing of Chemicals 2010, 1(4), 1-15.
[26] Y D Zou; Y P Cao; H Y Zheng; X R Yang; etc. Phototoxicity Test of Chemicals Based on Tissue Engineered Skin. Journal of Clinical Rehabilitative Tissue Engineering Research 2009,13(11), 2147-2150.
[27] J H Lee; J E Kim; B J Kim; Cho KH.In VitroPhototoxicity Test Using Artificial Skin with Melanocytes. Photodermatology Photoimmunology & Photomedicine 2007, 23(2-3), 73-80.
[28] Lelièvre D; Justine P; Christiaens F; etc. The EpiSkin Phototoxicity Assay (EPA): Development of anin VitroTiered Strategy Using 17 Reference Chemicals to Predict Phototoxic Potency. Toxicologyin vitro2007, 21(6), 977-995.
[29] Y B Lu; R F Zhang; S S Xu; etc. A Reconstructed Epidermis Model of Chinese Han People Used forin VitroTesting. China Journal of Pharmacology and Toxicology 2013, 27(s1), 310-310.
[30] Park YH; Jeong SH; Yi SM; etc. Analysis for the Potential of Polystyrene and TiO2 Nanoparticles to Induce Skin Irritation,Phototoxicity, and Sensitization. Toxicologyin Vitro2011,25(8), 1863–1869.
[31] Bernard FX; Barrault C; Deguercy A; etc. Development of a Highly Sensitivein VitroPhototoxicity Assay Using the SkinEthicTM Reconstructed Human Epidermis. Cell Biology and Toxicology 2000, 16(6), 391-400.
[32] Pfuhler S; Kirst A; Aardema M; etc. A Tiered Approach to the Use of Alternatives to Animal Testing for the Safety Assessment of Cosmetics: Genotoxicity. A COLIPA analysis.Regulatory Toxicology and Pharmacology 2010, 57 (2–3),315–324.
[33] Kirkland D; Aardema M; Henderson L; Müller L. Evaluation of the Ability of a Battery of Threein VitroGenotoxicity Tests to Discriminate Rodent Carcinogens and Non-carcinogens:I. Sensitivity, Specificity and Relative Predictivity. Mutation Research 2005, 584, 1–256 .
[34] Pfuhler S; Fautz R; Ouedraogo G; etc. The Cosmetics Europe Strategy for Animal-free Genotoxicity Testing: Project Status Up-date. Toxicologyin Vitro.2014, 28, 18-23.
[35] T.Hu; Kaluzhny Y; Mun GC; etc. Intralaboratory and Interlaboratory Evaluation of the EpiDermTM 3D Human Reconstructed Skin Micronucleus (RSMN) Assay. Mutation Research 2009, 673(2), 100-108.
[36] Mun GC; Aardema MJ; Hu T; etc. Further Development of the EpiDerm 3D Reconstructed Human Skin Micronucleus(RSMN) Assay. Mutation Research/Genetic Toxicology and Environmental Mutagenesis 2009, 673(2), 92-99.
 China Detergent & Cosmetics2016年2期
China Detergent & Cosmetics2016年2期
- China Detergent & Cosmetics的其它文章
- Synthesis of Monoglycerides with Cinnamomum Burmannii Seeds Oil and Its Application in Moisturizing Cream
- New Application of a Series of Preservatives Derived from Amino Acid for Cosmetic Products
- Problems and Suggestions for Domestic Non-special Use Cosmetics Record Filing in China
- Patent Protection of Daily Chemical Industry in China
- New Trends of Comsmetic Regulations in China
- Development of Household Care Chemical Industry in Pakistan
