Minimally Invasive Perventricular Device Closure of Ventricular Septal Defect: a Comparative Study in 80 Patients
Xin-chao Yang and De-bin Liu
Department of Cardiovascular Surgery, the First Clinical Hospital of Lanzhou University, Lanzhou 730000, China
IN recent years, the treatment of ventricular septal defect (VSD) has become more and more successful with cardiopulmonary bypass and percutaneous transluminal closure device, and has developed into a safe and effective method.1-4Along with the advances of technology, minimally invasive perventricular device closure of VSD has been widely and successfully conducted in some institutions in China.5-7From September 2011 to February 2013, we collected 40 patients who underwent perventricular closure of VSD via a substernal small incision, and another 40 patients who underwent the conventional surgical repair, aiming to compare the effect of minimally invasive perventricular device closure and that of the conventional surgical repair, and to evaluate the efficacy of minimally invasive perventricular device closure.
PATIENTS AND METHODS
Patient inclusion
From September 2011 to February 2013, all patients in the First Clinical Hospital of Lanzhou University fulfilling the following inclusion criteria were selected: (1) aged more than 1 year and less than 32 years; (2) body weight between 10 kg and 58 kg; (3) having isolated large VSD (2-14 mm) with left-to-right shunt but no significant pulmonary arterial hypertension. Patients with concomitant diseases were excluded from the study except the cases with small patent ductus arteriosus or atrial septal defect. Altogether 40 patients underwent perventricular closure (minimally invasive group), and another 40 patients receiving the conventional surgical repair of VSD were included as the surgical group.
VSD closure procedure
VSD closure procedures were performed with the patients under general anesthesia in supine position, with single-lumen tracheal intubation. Transesophageal echocar- diography (TEE) was performed to measure the exact size and location of VSD. After incision (3-5 cm) at the lower sternum, the pericardium was suspended and the right ventricular free wall was exposed, a purse string suture was placed on the right ventricular free wall. A guide wire was inserted through the ventricular septal defect to the left ventricle under TEE guidance, over which a modified delivery sheath was introduced to establish the delivery pathway. The device was advanced inside the short delivery sheath, and the left ventricular disk was deployed under TEE guidance by retracting the sheath. Once the left ventricular disk was confirmed by TEE, the right ventricular disk was deployed until the device was completely opened. If no atrioventricular or aortic valvular disturbance or residual shunt was detected, the sheath was pulled out of the right ventricle. TEE was performed again to detect and evaluate the residual interventricular shunt and observe the aortic valve bypass. The pericardium was partially closed, a chest tube was placed in the mediastinum, the sternum and chest wall was closed in the routine manner. After the operation, the patients were transferred to and observed in intensive care unit.
Statistical analysis
Statistical analysis was performed with StatsDirect software. Data are expressed as means±SD (range). Clinical parameters between the 2 groups were compared with independent samples t-test. Nominal variables of the 2 groups were compared using Fisher’s exact test. Complications were analyzed by Chi-square test. P<0.05 was considered statistically significant.
RESULTS
Clinical outcomes
The surgical group and the minimally invasive group did not show significant differences in success rate, cardiothoracic ratio, left ventricular ejection fraction, left ventricular end-diastolic diameter, left ventricular end-systolic diameter, left ventricular end-systolic volume, left ventricular end- diastolic volume, and left ventricular end-diastolic pressure (all P>0.05, Table 1).
In the minimally invasive group, the VSDs were successfully occluded in all patients but 1 (97.5%), who required immediate surgical conversion after the operation failed. TEE analysis revealed 5 cases of residual shunt (<2 mm), 6 arrhythmia, 7 conduction block, and 2 aortic and tricuspid incompetence (Table 2). There was no incidence of device embolization or occluder shift. The whole process took 58±21 minutes, the deployment of the device was finished in 35±15 minutes. The chest tube was removed 1-2 days after the operation. All the patients in this group were discharged no later than 7 days (Table 3). TEE images displayed normal cardiac function and proper position of the device without affecting the surrounding tissues.
In the surgical group, 1 patient died of acute infection. Complications included 2 residual shunt (<2 mm), 1 atrioventricular block, 1 tricuspid incompetence, 2 bleeding, 1 arrhythmia and 8 other complications (Table 2). The procedure duration, intensive care unit stay, hospital stay, and postoperative recovery time were significantly shorter in the minimally invasive group than in the surgical group (all P<0.05), but the equipment and total costs were significantly higher in the minimally invasive group than in the surgical group (both P<0.05) (Table 3). All the 40 patients in the surgical group needed blood products (800±120 ml) (Table 3), among them 22 cases used whole blood.
Follow-up results
Up to the submission of this article, all the included patients have undergone more than 3 months of follow-up. In the minimally invasive group, the short-term outcomes were acceptable. One patient has persistent incomplete right bundle branch block, another has developed intra-procedural heart block, requiring permanent pacemaker to maintain normal cardiac function. In addition, one patient has persistent closure-related trace and mild aortic regurgitation. There were no other new complications. In the surgical group, four patients suffered major new complications, one died of severe gastroenteritis, one had incision infection, one pericardial effusion, one heart failure; three patients had persistent arrhythmia, one has persistent conduction anomalies. There were no other complications. TEE analysis showed good systolic and diastolic function, displaying no residual interventricular shunt or tricuspid regurgitation.
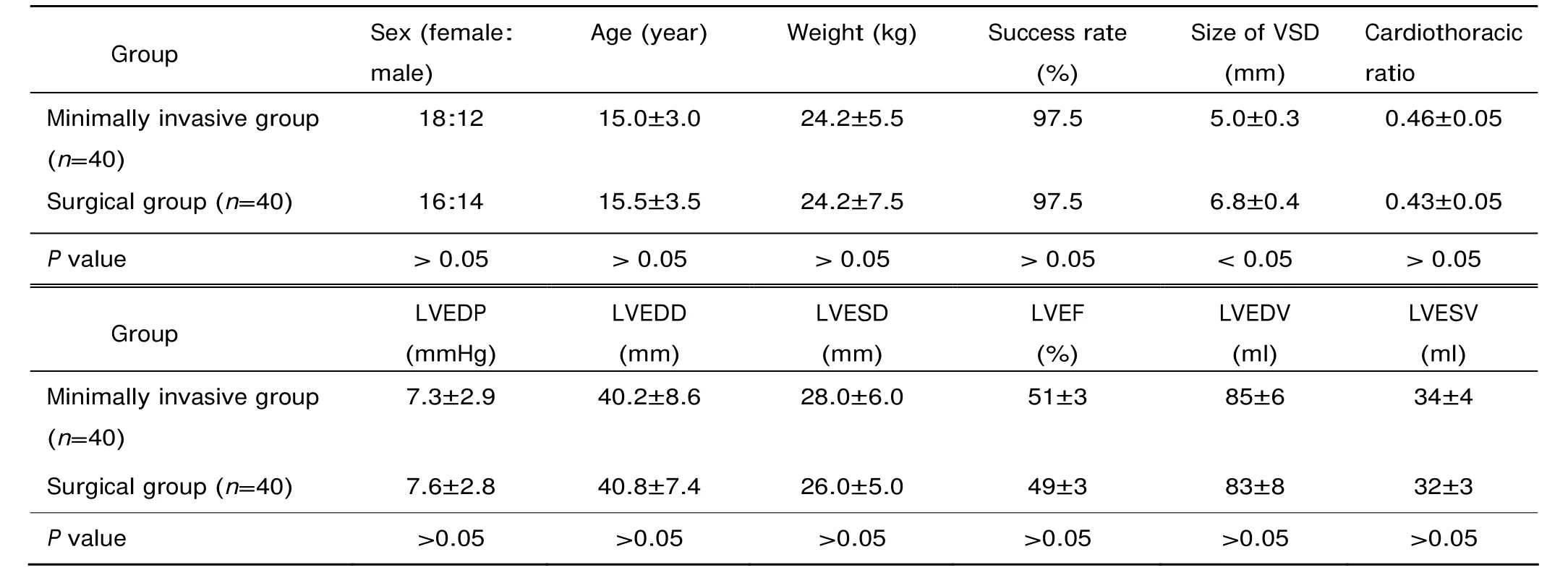
Table 1. The comparison of clinical indicators of the minimally invasive group and surgical group§
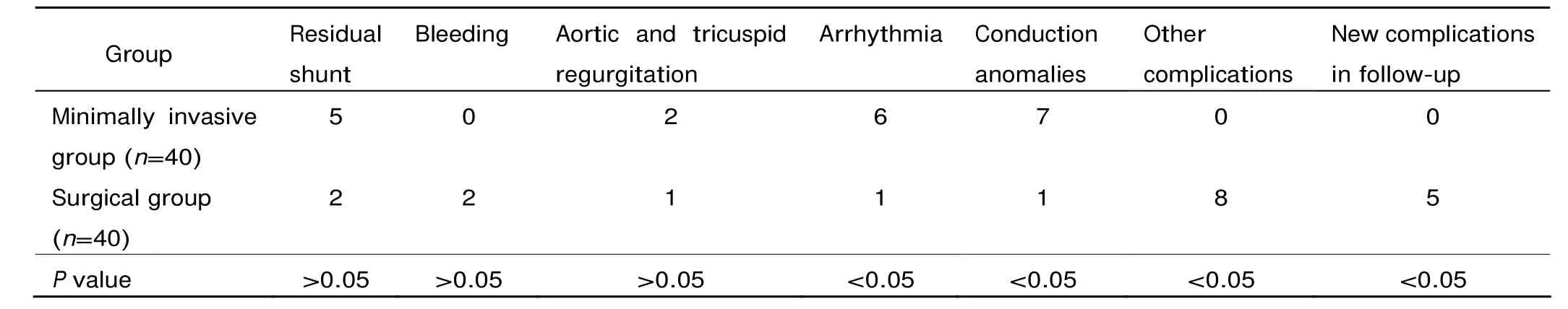
Table 2. Procedure-related complications of the minimally invasive group and surgical group (n)
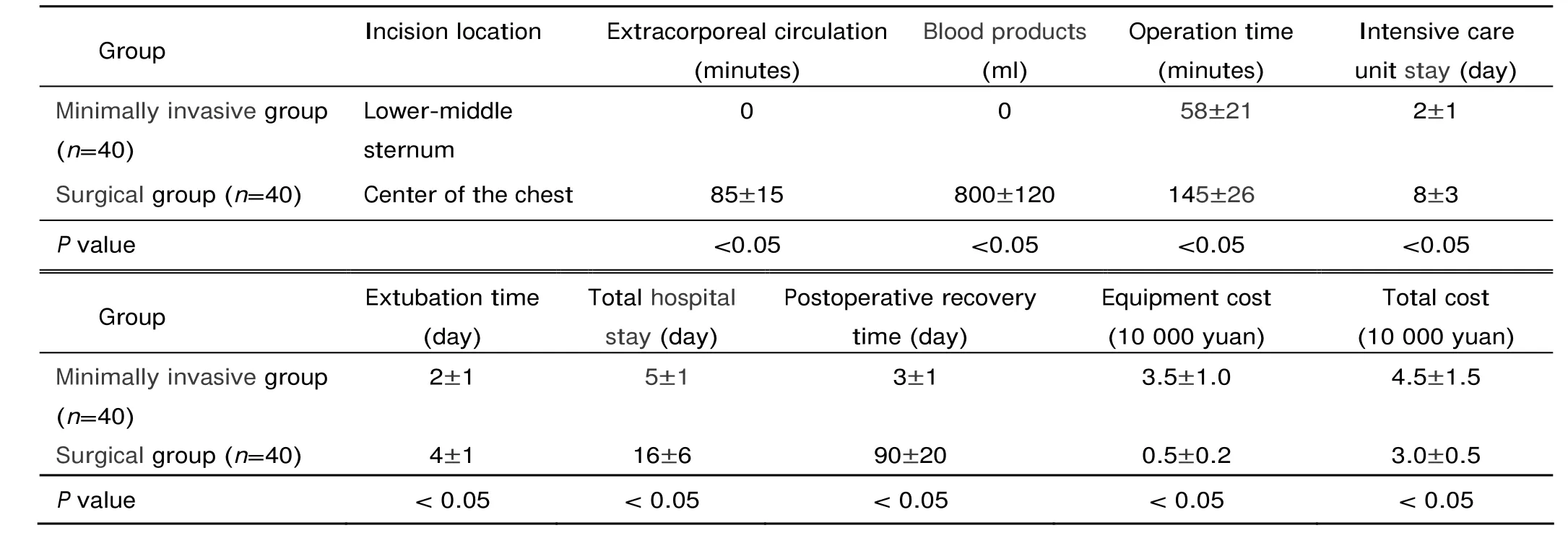
Table 3. The comparison of clinical outcomes of the minimally invasive group and surgical group§
DISCUSSION
Since the minimally invasive perventricular device closure of VSD was successfully applied in animal models,8-10this technology has been constantly reported in clinical settings.11-13The results of this study reflect some advantages of minimally invasive perventricular device closure of VSD in comparison with the conventional surgical repair. One advantage is that it can avoid the use of cardiopulmonary bypass. It could also shorten recovery time and reduce the incidence of complications. Other adv- antages of periventricular closure have been reported, including simple operation, effectiveness, and safety,5,14acceptable short-term follow-up outcomes15and satisfactory esthetic effect.16In addition, this technology has more accurate device positioning because the whole process could be monitored and guided by TEE, which can ensure the safety of the procedure. Once a patient is considered not suitable for this procedure or the device closure fails, the surgeons can immediately convert to traditional surgical repair. More importantly, this technology could be applied for periatrial closure of atrial septal defect17-19and patent ductus arteriosus,20,21even those close to the aortic valve. However, this new technology still has its own drawbacks and limitations. For instance, the residual leakage rate and procedure cost are high, the follow-up time is short and the incidence of complete heart block is unacceptable.22Further studies with larger cohorts and longer-term follow-up are necessary to evaluate the long-term results of minimally invasive perventricular device closure of VSD.
This comparative study has its limitations, namely small sample size, short follow-up, and lack of randomization. Despite these shortcomings, this study demonstrates the feasibility and advantages of minimally invasive perven- tricular device closure of VSD over traditional surgical repair. Certainly, long-term follow-up is also necessary to confirm the validity of this new technique.
1. Yang R, Sheng Y, Cao K, et al. Transcatheter closure of perimembranous ventricular septal defect in children: safety and efficiency with symmetric and asymmetric occluders. Catheter Cardiovasc Interv 2011; 77:84-90.
2. Zhao PJ, Yu ZQ, Gao W, et al. Efficacy of the transcatheter closure of perimembranous and muscular ventricular septal defects with the Amplatzer duct occluder II. Zhonghua Xin Xue Guan Bing Za Zhi 2012; 40:817-20.
3. Koneti NR, Penumatsa RR, Kanchi V, et al. Retrograde transcatheter closure of ventricular septal defects in children using the Amplatzer Duct Occluder II. Catheter Cardiovasc Interv 2011; 77:252-9.
4. Koneti NR, Sreeram N, Penumatsa RR, et al. Transcatheter retrograde closure of perimembranous ventricular septal defects in children with the Amplatzer duct occluder II device. J Am Coll Cardiol 2012; 60:2421-2.
5. Li F, Chen M, Qiu Z, et al. A new minimally invasive technique to occlude ventricular septal defect using an occluder device. Ann Thorac Surg 2008; 85:1067-71.
6. Zhu D, Gan C, Li X, et al. Perventricular device closure of perimembranous ventricular septal defect in pediatric patients: technical and morphological considerations. Thorac Cardiovasc Surg 2013; 61:300-6.
7. Zhang GC, Chen Q, Chen LW, et al. Transthoracic echo- cardiographic guidance of minimally invasive perventricular device closure of perimembranous ventricular septal defect without cardiopulmonary bypass: initial experience. Eur Heart J Cardiovasc Imaging 2012; 13:739-44. 8. Amin Z, Danford DA, Lof J, et al. Intraoperative device closure of perimembranous ventricular septal defects without cardiopulmonary bypass: preliminary results with the perventricular technique. J Thorac Cardiovasc Surg 2004;127:234-41.
9. Amin Z, Berry JM, Foker JE, et al. Intraoperative closure of muscular ventricular septal defect in a canine model and application of the technique in a baby. J Thorac Cardiovasc Surg 1998;115:1374-6.
10. Amin Z, Gu X, Berry JM, et al. New device for closure of muscular ventricular septal defects in a canine model. Circulation 1999;100:320-8.
11. Bacha EA, Cao QL, Starr JP, et al. Perventricular device closure of muscular ventricular septal defects on the beating heart: technique and results. J Thorac Cardiovasc Surg 2003;126:1718-23.
12. Maheshwari S, Suresh P, Bansal M, et al. Perventricular device closure of muscular ventricular septal defects on the beating heart. Indian Heart J 2004;56:333-5.
13. Zeng XJ, Sun SQ, Chen XF, et al. Device closure of perimembranous ventricular septal defects with a minimally invasive technique in 12 patients. Ann Thorac Surg 2008;85:192-4.
14. Xing Q, Pan S, An Q, et al. Minimally invasive perventricular device closure of perimembranous ventricular septal defect without cardiopulmonary bypass: multicenter experience and mid-term follow-up. J Thorac Cardiovasc Surg 2010;139:1409-15.
15. Gan C, Lin K, An Q, et al. Perventricular device closure of muscular ventricular septal defects on beating hearts: initial experience in eight children. J Thorac Cardiovasc Surg 2009;137:929-33.
16. Pan S, Xing Q, Cao Q, et al. Perventricular device closure of doubly committed subarterial ventral septal defect through left anterior minithoracotomy on beating hearts. Ann Thorac Surg 2012;94:2070-5.
17. Majunke N, Bialkowski J, Wilson N, et al. Closure of atrial septal defect with the Amplatzer septal occluder in adults. Am J Cardiol 2009;103:550-4.
18. Vaidyanathan B, Simpson JM, Kumar RK. Transesophageal echocardiography for device closure of atrial septal defects: case selection, planning, and procedural guidance. JACC Cardiovasc Imaging 2009;2:1238-42.
19. Seca L, Ca??o R, Silva J, et al. Intracardiac echocardiography imaging for device closure of atrial septal defects——a single-center experience. Rev Port de Cardiol (English Edition) 2012;31:407-12.
20. Hongxin L, Wenbin G, Zhu M, et al. New minimally invasive technique of perpulmonary device closure of patent ductus arteriosus through a parasternal approach. Ann Thorac Surg 2012;93:862-8.
21. Tao K, Zhu D, An Q, et al. Perventricular device closure of patent ductus arteriosus: a secondary chance. Ann Thorac Surg 2012;93:1007-9.
22. Predescu D, Chaturvedi RR, Friedberg MK, et al. Complete heart block associated with device closure of perimembranous ventricular septal defects. J Thorac Cardiovasc Surg 2008;136:1223-8.
 Chinese Medical Sciences Journal2014年2期
Chinese Medical Sciences Journal2014年2期
- Chinese Medical Sciences Journal的其它文章
- Serum Levels of Interleukin-1 Beta, Interleukin-6 and Melatonin over Summer and Winter in Kidney Deficiency Syndrome in Bizheng Rats△
- Expression of Peptidylarginine Deiminase 4 and Protein Tyrosine Phosphatase Nonreceptor Type 22 in the Synovium of Collagen-Induced Arthritis Rats△
- Lipocalin-2 Test in Distinguishing Acute Lung Injury Cases from Septic Mice Without Acute Lung Injury△
- False Human Immunodeficiency Virus Test Results Associated with Rheumatoid Factors in Rheumatoid Arthritis△
- Arachnoiditis Ossificans of Lumbosacral Spine: a Case Report and Literature Review
- Ketamine Abuse-Induced Obstructive Nephropathy and Kidney Injury
