Plasma endothelin-1 and nitric oxide correlate withligustrazine alleviation of pulmonary artery hypertension in patients of chronic cor pulmonale from high altitude plateau during acute exacerbation
En-zhi FENG, Sheng-yue YANG, Ning-xia HUANG, He YIN, Ying ZHANG, Zhong-xin TIAN
Center of Respiratory Medicine, the Fourth Hospital of PLA, Lanzhou Command, Xining 810007, China
Introduction
Pulmonary arterial hypertension (PAH) is the key pathologic event in the development of chronic pulmonary heart disease from chronic obstructive pulmonary disease (COPD). Current treatment strategies and research have focused on how to reduce pulmonary artery pressure and inhibit or reverse the pathological changes of pulmonary vascular tissues. Ligustrazine, or the chemical name teramethylpyrazine (TMP), is the active ingredient of Chinese medicine Ligusticum. Most scholars believe that TMP can reduce pulmonary artery pressure and vascular resistance in patients with pulmonary hypertension, and improve cardiac function whileexerting little effect on systemic blood pressure.Therefore, it is an effective selective pulmonary vasodilator [1-2]. Although the function of ligustrazine is thought to be mediated by the platelet regulation function of β-receptors in the brain [3-5],the exact mechanism is still not very clear.
Plateau area at a high altitude is a special lowpressure and hypoxic environment. People living in this environment usually have a baseline“hypoxic pulmonary hypertension”. そose who have pulmonary heart disease will suあer more severe form of PAH due to the hypoxia caused by pulmonary heart disease itself and the effect of hypoxic environment [5-6]. Therefore, strategies to reduce pulmonary artery pressure and inhibit or reverse the pathological changes of pulmonary vascular tissues become more critical in the treatment of chronic pulmonary heart disease in patients from highaltitude areas.
Endothelin-1 (ET-1) and nitric oxide (NO) are the most important pulmonary vascellum regulators.Abnormal expression of them play key roles in the pathogenesis of pulmonary hypertension associated with COPD. そerefore we supposed that ligustrazine alleviated the PAH may deal with them. So we try to verify weather ligustrazine is eあective in treating patients of COPD associated chronic cor pulmonale(CCP) in acute exacerbation at high altitude as it did at plain,and investigate the relationship of them.
Study subjects and Methods
Study subjects
Seventy patients from Qinghai-Tibet Plateau Area (at altitude 2 260-3 500 m) who were admitted into our hospital with COPD associated chronic pulmonary heart disease from December 2006 to December 2008 were recruited into this study. There were 46 males and 24 females, 50 — 76 years old, with the mean age of 57.8 ± 8.1 years. Diagnosis of pulmonary heart disease in acute exacerbation was made based on the diagnostic criteria established in the 1980 National Conference for Pulmonary Heart Disease.All subjects gave their informed consent, and the study protocol was approved by the Institutional Ethics Committee. The absence of other heart and lung diseases was confirmed by physical examination,electrocardiogram, echocardiography, chest X-ray,and pulmonary function tests.
Study designs
Patients were randomly divided into 2 groups: a
standard therapy group (control group, n=35) and a ligustrazine treatment combined with the standard treatment group (treatment group, n=35). There were 23 males and 12 females in the control group at age of 50 to 75 (mean 58. 1 ± 8.0) years. They received the standard anti-inflammatory treatment that included ceftriaxone (2.0 g), levofloxacin (0.2 g) and ambroxol (30 mg) twice a day by intravenous injection, and salbutamol (4.8 mg) 3 times a day by oral, in combination of continuous oxygenation(1-3 L/min). In the treatment group, there were 24 males and 11 females, aged 52 to 76 (mean = 57.5± 8.2) years. They all received the same standard treatment as the control group and additional I.V.infusion of 80 mg/100 ml ligustrazine (Sichuan SuLe Pharmaceutical) for 2 weeks. Before and at the end of two week treatment, all the patients were evaluated for clinic outcomes, and measured for arterial partial pressure of oxygen (PaO2), mPAP, and right ventricle size. Blood samples were also collected at the same time points for measurement of plasma levels of ET-1 and NO.
Plasma levels of ET-1 and NO
Patients were fasted overnight, and 2 ml blood was drawn into a tube with anticoagulant. After centrifugation at a low-temperature, plasma was transferred into a new tube and stored in -30°C freezer. Level of plasma ET-1 was determined by radioimmunoassay using a kit provided by the East Asian Institute of Immunity (Beijing, China)according to the manufacture’s instructions. Plasma NO level was determined based on the nitrate reductase method using a kit purchased from Jingmei Biological Engineering (Shenzhen, China) according to the manufacturer’s instructions.
Cardiovascular and pulmonary functional parametersそe right ventricular pre-ejection time (RVPEP) and pulmonary artery flow acceleration time (AT) were measured in the pulmonary artery flow spectrum using a color Doppler echocardiography (Compaq RT-6800). RVPEP is the time from the start of ECG Q wave to the start of systolic point in the spectrum;AT is the time from start of the systolic point in the pulmonary artery flow spectrum to the peak velocity.mPAP (mmHg) = 42.1 (RVPEP / AT) -15.7. The right ventricular diameter (RV) and right ventricular outflow tract diameter (RVOT) were measured by standard methods using a color Doppler echocardiography (Compaq RT-6800). Arterial oxygen (PaO2) was measured by a blood gas analyzer(Opti-II type) at 30 min afer stopping oxygenation.
Evaluation of clinical outcomes
Clinical outcomes in response at the end of the treatment were evaluated based on the standard response criteria for acute exacerbation of pulmonary heart disease established in the 2nd National Conference on Pulmonary Heart Diseases in 1977. そe criteria for clinical outcomes are defined as the following. Cough, sputum, dyspnea and pulmonary rales are the key clinical symptoms of the disease. When they are disappeared or the severity reduced by more than 80%, it indicates a good“Clinical Control”; when symptoms are significantly mitigated or severity reduced by more than 50%, it is considered as “Significant Improvement”; general ease of symptoms are considered “Improvement”,but when symptoms are not improved or even become worse, it is considered “No Improvement”.Total clinical efficiency is therefore defined as the percentage of all cases qualified for “Clinical Control”,“Significant Improvement” and “Improvement”.
Statistical analysis
Data obtained from this study were subjected to statistical analysis using the SPSS17.0 software.Data are presented as mean ± SD. The t test was used to compare data before and after treatment,and those between the two patient groups. Pearson linear correlation analysis was used to analyze the relationship between two variables, and χ2test was used to compare eきcacy rate.
Results
Demographics and clinical data
Patients randomly divided into the two groups showed no significant difference in their sex ratio,age, baseline levels of ET-1 and NO, and all other physiological parameters (all P> 0.05, Tab. 1).
Correlation of plasma ET-1 level with NO, PaO2, mPAP,RVOT, and RV values
When the data before treatment from both groups were combined, Pearson linear correlation analysis showed that plasma level of ET-1 was positively correlated with values of mean pulmonary arterial pressure (mPAP), outflow tract of right ventricle(RVOT), and right ventricle (RV) (r = 0.710, 0.853 and 0.766, respectively; P = 0.000 for all), but negatively with levels of NO and PaO2(r = -0.823 and-0.752, respectively; P = 0.000 for both).
Clinic outcomes
Based on the criteria, the total efficacy in the treatment group was 97.1%, while that in the control group was 77.1%. そe diあerence between two groups was significant (P<0.05, Tab. 2), demonstrating the eきcacy of ligustrazine.
Changes in the plasma levels of ET-1 and NO, and other physiological parameters
Before and after treatment, plasma ET-1 and NO levels, mPAP, right ventricular size, and PaO2were measured in both groups. After treatment, plasma ET-1, mPAP, and RVOT were decreased significantly,while NO and PaO2were increased significantly (P<0.01 vs pre-treatment, for all parameters) in both groups but the changes in the ligustrazine treatment group were more obvious compared with those in the control group (P <0.01 vs the control group for all parameters, Tab.3).
Discussion
Pulmonary vascular endothelial dysfunction plays an important role in the pathogenesis of PAH associated with COPD. It can be induced by hypoxia or certain NO products, and then cause contraction of endothelium-derived vascular vessels and disorder of relaxation factors. This contractile response [7]together with other growth factors can promote pulmonary vascular remodeling and the formation of PAH [5,8]. Endothelial cells release vasodilators such as the strong vascular relaxing factor NO that can relax smooth muscle cells and pulmonary vascular vessels. NO can also inhibit ET synthesis and regulate pulmonary vasoconstriction induced by catecholamines and prostaglandin F2a, thereby reducing pulmonary vascular resistance [9]. Studies confirmed that patients with COPD associated PAH[10] and smokers [11] display reduced expression of monoxide synthase (NOS) in pulmonary artery endothelial cells, resulting in reduced production of endogenous NO and pulmonary vasoconstriction.Moreover, NO has anti-proliferative eあect. Decreased NO levels result in the increased expression of growth factors, and enhanced pulmonary vascular cell proliferation [11].
ET-1 is a bioactive peptide of 21 amino acids that promotes vessel contraction and vascular smooth muscle cell proliferation. Chronic hypoxia can stimulate pulmonary vascular and bronchial epithelial cells, and increase the expression and release of ET-1, which in turn causes pulmonary vasoconstriction and vascular smooth muscle cell proliferation, increases pulmonary vascular resistance, ultimately leading to pulmonary hypertension [12]. Recent studies [13] reported that plasma ET-1 level was significantly higher in patients with acute exacerbation of chronic pulmonary heart disease, and it was negatively correlated to PaO2.Chronic hypoxia can damage endothelial cells,decrease NOS activity, reduce NO synthesis and release, increase pulmonary vasoconstriction, and promote pulmonary hypertension and pulmonary heart disease. Patients with COPD associated hypoxemia and/or PAH show increased exhaled breath condensate and/or circulating ET-1 levels[10, 14]. Exhaled breath condensate fluid or blood concentration of ET-1 is significantly correlated with pulmonary artery systolic pressure or mean pressure[14]. ET-1 participates in the hypoxic pulmonary vascular cell contraction by inhibiting calciumactivated potassium and ATP dependent potassium channels.
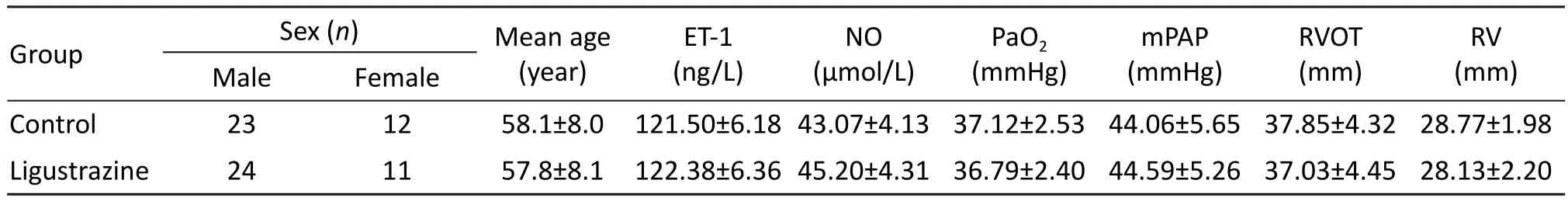
Tab. 1 Clinical data of the study subjects before treatment.
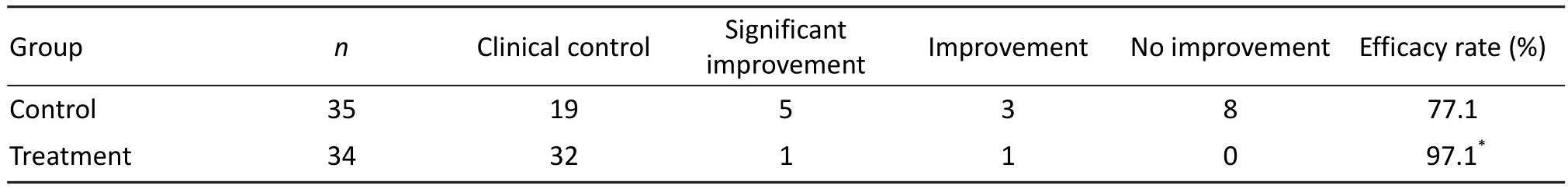
Tab. 2 Clinical outcomes of two treatment groups.

Tab. 3 Comparison of the panel of parameters in the two groups before and after treatment (n=35).
Due to the special low-pressure environment,people living in high-altitude area suあer more severe form of chronic pulmonary heart disease and PAH.Our results showed that, during acute exacerbation of pulmonary heart disease caused by the COPD,plasma ET-1 and mPAP are significantly higher,and NO and PaO2are significantly lower in patients from the plateau area than those from the plain area [15-18]. We also showed that ET-1 is positively correlated with mPAP, RVOT, and RV, but negatively correlated with NO and PaO2. These data indicate that ET-1 synthesis and release increase and NO synthesis and release decrease in those patients of chronic pulmonary heart disease in high altitude area under the influence of the severe hypoxemia. This obvious dysfunction of vessel contraction / relaxation factors may play an important role in the increased pulmonary artery pressure and the enlarged right ventricle.
Up to date, the treatment for PAH secondary to COPD is not ideal. Alveolar hypoxia is the main cause of increased pulmonary vascular resistance and mPAP in COPD patients, so long-term oxygenation is used to improve alveolar hypoxia caused mPAP.Chaouat [19] and Barbera [20] et al reported that long-term oxygen therapy can stabilize or reduce the progress of PAH. The longer the duration of oxygen therapy each day, the better the pulmonary hemodynamics improves, but mPAP rarely returns to the normal level. Studies have shown that inhalation of NO can improve pulmonary hemodynamics and hypoxemia in patients with COPD [19], but other studies also showed that inhaled NO can aggregate hypoxemia by inhibiting hypoxic pulmonary vasoconstriction and causing the imbalance of ventilation / perfusion [10]. Phosphodiesterase 5 inhibitor sildenafil has vasodilator effect through enhancing the function of cGMP. It has shown a good therapeutic effect on idiopathic PAH (IPAH)[17], but its effects on COPD are rarely reported and inconsistent. Alp et al [1] found that sildenafil significantly reduced mPAP and increased 6 minute walking distance in patients with COPD. However,Rietema [21] reported that sildenafil treatment did not improve cardiac output and exercise tolerance. ET-1 receptor antagonist bosentan may be effective and safe in the treatment of IPAH and thromboembolic PAH, but it cannot improve the pulmonary artery pressure, exercise capacity, and lung function of COPD patients. On the contrary, it decreased PaO2, increased alveolar-arterial oxygen diあerence, causing deterioration in the quality of life[21]. Animal studies have shown that simvastatin can inhibit cigarette smoke-induced emphysema and pulmonary vascular remodeling, suppressing PAH [22]. Recently, a randomized study by Lee et al.showed that pravastatin therapy can reduce systolic pulmonary artery pressure, improve dyspnea score,and prolong exercise time in patients of COPD with PAH, and its therapeutic eあect may be mediated by inhibiting the synthesis of ET-1 [23]. It seems that inhibition of ET-1 synthesis/release together with enhancement of NO synthesis/release may be the key to the prevention and treatment of pulmonary hypertension secondary to chronic pulmonary heart disease in high altitude areas because these events not only control the airway inflammation and correct hypoxemia and hypercapnia, but also help restore the equilibrium of the activities of these molecules.
Although Ligustrazine or TMP has been widely used for the treatment of pulmonary hypertension,the results on its pharmacological mechanism are inconsistent. Peng et al [24] found that low concentrations of TMP increases NO release by activating exogenous L-arginine in pulmonary artery endothelial cells. Cao et al [25] observed that TMP can suppress ET-1 release during acute hypoxia in anesthetized dogs. On the other hand, Liu et al [26]studied the effects of different concentrations of TMP on isolated pulmonary arterial and bronchial artery rings, and showed that TMP could expand pulmonary artery but had no eあect on ET-1. In the present study, TMP treatment showed better eきcacy than the standard treatment. In response to both the standard and TMP treatments, plasma NO levels and PaO2significantly increased while plasma ET-1, mPAP, RVOT, and RV significantly decreased,and TMP treatment significantly enhanced all these beneficial changes. The standard treatment for PAH is to use antibiotics, antiasthmatic,expectorant, and oxygenation. These treatments can help regain the balance of vascular contraction/relaxation factors and achieve an effective infection control, correction of hypoxemia and hypercapnia,decreased vasoconstrictor, and increased vasodilator factors. Since treatment with TMP significantly augmented the eきcacy of the traditional treatment,it is therefore reasonable to speculate that TMP may act selectively on pulmonary blood vessels. By decreasing ET-1 synthesis and release, and increasing NO synthesis and release, TMP may help to restore the balance of ET-1 and NO activities, and achieve the observed clinic efficacy on PAH. Therefore, our data demonstrate an important clinical value of TMP in the treatment of pulmonary heart disease in high altitude areas.
Acknowledgements
This study was supported by the Key scientific and technological project in Qinghai Province, China(2006-N-143).
1. Alp S, Skrygan M, Schmidt WE, et al. Sildenafil improves hemodynamic parameters in COPD-an investigation of six patients[J]. Pulm Pharmacol そer, 2006, 19(6): 386-392.
2. Stolz D, Rasch H, Linka A, et al. A randomised, controlled trial of bosentan in severe COPD[J]. Eur Respir J, 2008,32(3): 619-628.
3. Chen XY,Huang CH,Chen SN,et al.Study of tethamethylyrazine in treating in patients with chronic cor pulmonale at acute exacerbation stage[J]. J Clin Pulm Med,2007, 7(12): 706-707.
4. Xu YJ, Zhu L. Study on the protective effects of on acute lung injuty[J]. Clin Med China, 2006, 13: 916-917.
5. Wang MJ, Feng GX, Zhang P, et al. Eあects of intraccerebralventricular injection of TMP on pulmonary arteral pressure in rats[J]. J Yunyang Med Coll, 2000, 4: 217-218.
6. Zheng SJ, Dai Y. Effects of inhaled ligustrazine on pulmonary hypertension and platelet function in patients with COPD at acute exacerbation stage[J]. J Clin Exp Med,2006, 5: 157- 158.
7. Yang SY, Shen JL, GuoZY, et al. The relationship between change of serum basic fibroblast growth factor level and pulmonary arteral pressure and its intervention in patients with chronic cor pulmonale at high altitude areas[J]. Chin J Clin Med, 2008, 15(1): 47-49.
8. Yang SY, Ma ZQ. An evaluation of the acid-base disorders and diagnostic criterion of respiratory failure in chronic cor pulmonale in xining[J]. Chin J Tuberc Respi Dis, 1991, 16:175-177.
9. An J, Xing J, Jin Z. Antion mechanism endorhelin-1 on pulmonary arteries of canis familiarisex vivo[J]. Chin J Gerontol, 2007, 27: 1548-1550.
10. Li ZB. Research progresses of treatment of pulmonary hypertension[J]. World Clin Drugs, 2004, 25: 163-167.
11. Prie S, Stewart DJ, Dupuis J. Endothelin A receptor blokade improves nitric oxide-mediated vasodilation in monocrotaline-induced pulmonary hypertention[J].Circulation, 1998, 97(21): 2169-2174.
12. Hida W, Tun Y, Kikuchi Y, et al. Pulmonary hypertension in patients with chronic obstructive pulmonary disease:recent advances in pathophysiology and management[J].Respirology, 2002, 7(1): 3-13.
13. Peinado VI, Pizarro S, Barbera JA. Pulmonary vascular involvement in COPD[J]. Chest, 2008, 134(4): 808-814.
14. McMurray JJ, Ray SG, Abdullab I, et al. Plasma endothelin in chronic heart failure[J]. Circulation, 1992, 85(4): 1374-1379.
15. Yang ZQ, Gu YQ. Cheges of Plasma endothelin and atrial natriuretic factor in patients with chronic cor pulmonale[J].Chin J Hemorh, 2001, 11: 83-84.
16. Carratu P, Scoditti C, Maniscalco M, et al. Exhaled and arterial levels of endothelin-1 are increased and correlate with pulmonary systolic pressure in COPD with pulmonary pertention[J]. BNC Pulm Med, 2008, 8: 20
17. Cheng DY, Chen WB, Xiao XR, et al. そe change of bFGF level and its relationship to pulmonary arterial pressure in patients with cor pulmonale[J]. J West China Univ Med Sci,2002, 4: 501-503.
18. Wang LY, Zhang HY, Ye Y, et al. Theraoeutic effect of hypoxic hypertension in chronic cardiopulmonary disease[J]. J New Chin Med, 2010, 5: 18-19.
19. Chaouat A, Naeije R, Weizenblum E. Pulmonary hypertension in COPD[J]. Eur Respir J, 2008, 32: 1371-1385.
20. Barbera JA, Peinado VI, Santos S. Pulmonary hypertension in chronic obstructive pulmonary disease[J]. Eur RespirJ,2003, 21: 892-905.
21. Rietema H, Holverda S, Bogaard HJ, et al. Siildenafil treatment in COPD does not affect stroke volume or exercise apacity[J]. Eur Respir J, 2008, 31: 759-764.
22. Lee JH, Lee DS, Kim EK, et al. Simvastatin inhibits cigarette smoking-induced emphysema and pulmonary hypertension in rat lungs[J]. Am J Respir Crit Care Med, 2005, 172(8):987-993.
23. Lee TM, Chen CC, Shen HN, et al. Effects of pravastatin on functional capacity in patients with chronic obstructive pulmonary disease and pulmonary hypertension[J]. Clin Sci(Lond), 2009, 116: 497-505.
24. Peng W, HucksD, Priest RM, et al. Ligustrazine induced endotheliumdependent relaxation in pulmonary arteries via an NO-mediated and exogenous L-aiginine-dependent mechanism[J]. J Br Pharmacol, 1995, 119(5): 1063-1071.
25. Cao WB, Zeng ZP, Zhu YJ, et al. Effects of tetramethylpyrazine,a chinese medicine,on plasma endothlin-1 levels during acute pulmonary hypoxia in anesthetized dog[J]. J Cardiovasc Pharmacol, 1998, 31: 456-462.
26. Liu SF, Cai YN, Evans TW, et al. Ligustrazine is a vasodilatar of hunan pulmonary and chronic obstructive pulmoerg EC. Polyunsaturater fatty acids improve exercise capacity in bronchial arteries[J]. Euro J Pharmacol, 1990, 191(3): 345-450.
 中國(guó)應(yīng)用生理學(xué)雜志2014年6期
中國(guó)應(yīng)用生理學(xué)雜志2014年6期
- 中國(guó)應(yīng)用生理學(xué)雜志的其它文章
- Children’s exercise capacity at high altitude in Tibet
- Hypoxic preconditioning: effect, mechanism and clinical implication (Part I)
- Role of HCN channels in the nervous system: membrane excitability and various modulations
- How to deal with cerebral palsy in 21st century
--A new epoch in clinic treatment - Hematological parameters in high altitude residents:Tibetan natives versus Han migrants
- A rat model of high altitude polycythemia rapidly established by hypobaric hypoxia exposure
