A rat model of high altitude polycythemia rapidly established by hypobaric hypoxia exposure
Pei-bing LI, Hong-jing NIE, Wei LIU, Bing-nan DENG, Hui-li ZHU, Rui-feng DUAN, Zhao-li CHEN,Hai WANG
Key Laboratory of Military Environmental Medicine, Tianjin Institute of Health and Environmental Medicine, Tianjin 300050, China
Introduction
Chronic mountain sickness (CMS) affects people who are native or longtime residents of high altitude locations and usually begins insidiously in adult life [1]. It has been known for over a century that erythrocytosis occurs at high altitude, that is, a hallmark of altitude exposure is an increase in hemoglobin (Hb) concentration, initially due to a decrease in plasma volume and later due to an increase in red blood cells [2]. High altitude polycythemia (HAPC) is one common and serious pattern of CMS defined by the domestic scholars,which was mostly named Monge disease by abroad scholars[3]. It was caused by compensatory erythrocyte hyperplasia and followed with high blood viscosity and high resistance of blood flow.Although an increase of Hb concentration augments the blood oxygen carrying capacity, blood viscosity raises rapidly with Hb concentration above about 180 g/L. そen, a decrease in blood flow results in retained microcirculation, increased peripheral resistance and workload on the heart. そus, erythrocytosis of high altitude residents may lead to further hypoxia and into a “vicious cycle”. Persons who suあer from HAPC usually present headache, dizziness, cardiopalmus,insomnia, anergy, fatigue, local cyanosis, muscle and joint pain, dilation of veins, mental and physical fatigue, and cognitive impairment, etc,when their Hb concentration is higher than 210 g/L in men and 190 g/L in women [4,5]. So HAPC seriously affects the health of the residents at high altitude [6]. It was harmful for the health of people of high altitude native and immigration. Life quality was depressedfor light patients, and loss of employment mostly happened. Heavy patients would suffer serious microcirculation disturbance and right heart failure which would endanger life to death.
In spite of the fact that there are some achievement and advancement in the research of HAPC now, the pathogenesis of HAPC has not been fully elucidated,and the therapeutic measures of HAPC are merely restricted at symptomatic treatment with low effects[7]. Therefore, it’s urgent to explore HAPC pathogenesis and effective therapeutic measures for it. An successful experimental animal model is necessary for pathophysiological mechanism elucidation and medicine research. In order to resolve the questions mentioned above, we need to construct appropriate animal model simulating HAPC. To date,there are four main methods to construct animal models for HAPC, including highland spot model[8],EPO induction model[9], normobaric hypoxia model[10], integrated factors model of hypobaria and cold and cobalt chloride[11]. そese models have been widely applied in high altitude medical research and facilitate science advance, but there are some shortages to some extent. It is well known that HAPC is an idiopathic disease only in high altitude regions especially over 3 000 m altitude, and hypoxia is a prominent reason which induces HAPC. However,it is seldom used to construct HAPC model for simulating high altitude hypoxia in research[12].In the present study we intended to verify whether it is feasible to construct HAPC rat models for pathogenesis and drugs research by simulating single hypobaric hypoxia through appropriate animal and adjusted altitude height on the basis of previous reports.
Materials and Methods
Animals
A total of 48 male Wistar rats (provided by the animal breeding center of Institute of Health and Environmental Medicine) weighing (200±20)g were used in this study. The animal room was kept quiet, dry, and ventilative. Room temperature and humidity were maintained at (20±2)°C and 35%~50% with natural light. Rats were conventionally fed in cages and four rats in one cage with a commercial pelleted diet and tap water ad libitum. After 7 days of acclimation period, trace rats blood were collected from caudal vessel by puncturing rats tail with a sterilized syringe with a 30G thin needle and hemoglobin concentration were immediately determined colorimetrically by the cyan methaemoglobin method[13]. Then the rats were randomly divided into two groups of normal control group and hypoxia model group by both hemoglobin concentration and rats weight. All animal treatments were strictly in accordance with international ethical guidelines and the National Institutes of Health Guide concerning the Care and Use of Laboratory Animals, and the experimentswere carried out with the approval of the Committee of Experimental Animal Administration of the Institute.
Experiment method
Normal control group rats were bred in normoxia conditions and served as controls. Animals from hypoxia group were suffered hypoxia exposure for 8 hours per day at simulated 5 500 m high altitude in hypobaric chamber(the rats were placed into the hypobaric chamber, and decompressed at a speed of 15 m/s. The simulated altitude of 5 500 m was reached and maintained for 8 h before slowly returning to normal altitude at same speed). Rats general status were recorded in experiment duration.One subgroup of eight rats were separately taken out from normal control and hypoxia model group just after 2, 4, 12 weeks hypoxia exposure respectively.Rats were fasted for 16 h before blood collection. そe abdominal cavity was cut open following anesthesia with pentobarbitone to expose the abdominal aorta.A catheter (Surflow needle 20 gauge) was inserted into the aorta to collect naturally flowing blood.Blood samples were collected into anticoagulated tubes with appropriate amounts of dipotassium ethylenediamine tetraacetic acid (K2EDTA). All samples were analysed within 6 h of collection.
Indexes and examination methods
By means of an automatic hematology analyzer(BECKMAN COULTER, USA), three hematological parameters were measured: Hemoglobin concentration, hematocrit and red blood cell counts.Diluting and lysis buffers were used according to the manufacturers’ directions. Quantity control samples (4C Cell Control, Coulter Diagnostics) were run once daily at three levels and calibrations were performed biannually. Haemoglobin is measured by a patented cyanide-free method, RBC are counted by the impedance principle in modified veterinary use by increasing the red cell aperture bath current from 150 V to 200 V thereby decreasing the lower erythrocyte threshold to approximately 27 fl, and the hematocrit (HCT) are calculated by sofware. Whole blood viscosity readings were taken at low, medial and high shear rate of 1 s-1, 5 s-1and 30 s-1using a a cone-and-plate viscometer. そe levels of erythrocyte aggregation was measured in whole blood by the aggregometer, which was a rheoscopic apparatus composed of a densitometer head that detects light transmission changes in whole blood during the aggregation process that follows a disaggregating agitation. All measurements were taken at room temperature of 25°C.
Statistical analysis
All data in this study were expressed as mean ±SD, and analyzed with the SPSS 18.0 statistical package. A one-way ANOVA was used to determine the statistical significance. P<0.05 was considered statistically significant for all tests.
Results
Eあects of hypobaric hypoxia on general status and weight of rats
Afer 2 weeks, 4 weeks, 12 weeks hypoxia exposure,hypoxia model rats were all alive and showed dark purple in lip, tongue and ear mucous membrane compared with normal control, just as polyhaemia physiognomy of HAPC patients. そe weight of Rats in hypoxia model group had a decreased incline compared with that of rats in normal control group,but the diあerence had no statistical significance(Fig 1).
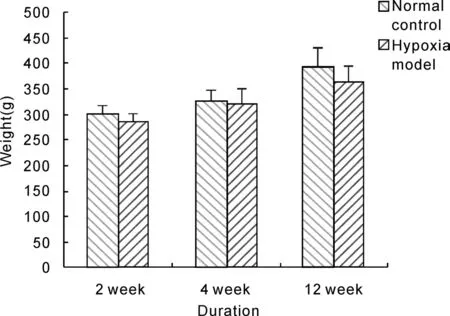
Fig. 1 Effect of hypobaric hypoxia on rats weight.
Eあect of hypobaric hypoxia on rats hemoglobin concentration, red blood cell count and hematocrit
Figure 2-4 showed that hemoglobin concentrations of rats in hypoxia model groups were above 210 g/L and significantly higher than that in normal control groups(P<0.01), it increased by about 39.9%, 36.4%and 42.2% after 2 weeks, 4 weeks and 12 weeks hypoxia exposure respectively, and rat hemoglobin concentrations of 4 weeks and 12 weeks duration hypoxia exposure were almost in one line with that of 2 weeks exposure. Red blood cell count and hematocrit of rats in hypoxia model groups were significantly increased compared with corresponding normal control groups after 2 weeks, 4 weeks and 12 weeks hypoxia exposure(P<0.01), and red blood cell count and hematocrit of 4 weeks and 12 weeks duration in hypoxia model groups were significantly higher than that of 2 weeks duration(P<0.01).These results indicated that hypobaric hypoxia could notably increase the levels of hemoglobin concentration, red blood cell count and hematocrit in model rats.
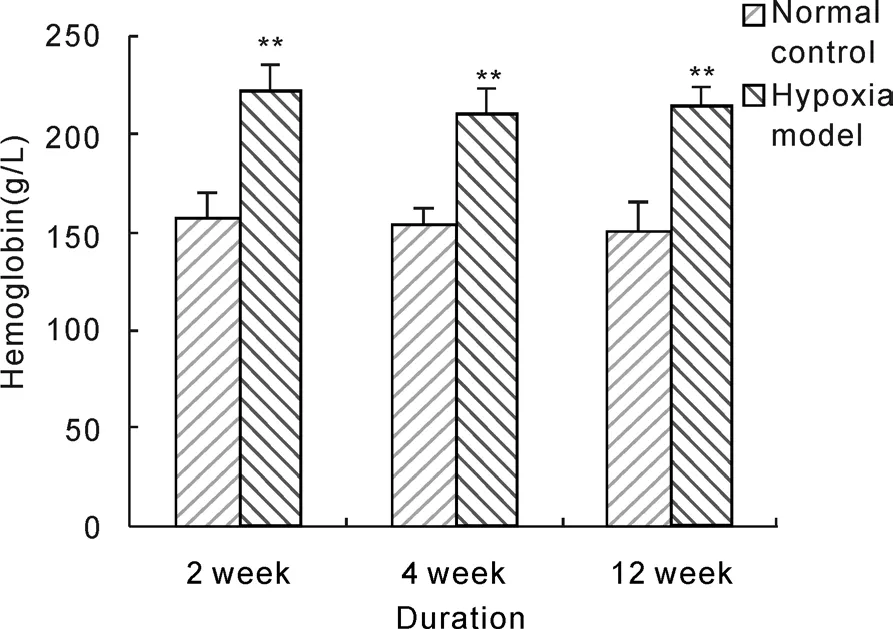
Fig. 2 Effect of hypobaric hypoxia on rats hemoglobin concentration. **P<0.01 vs normal control.
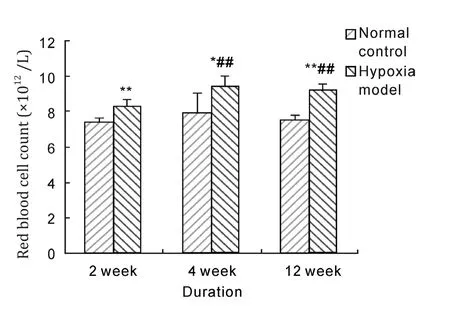
Fig. 3 Effect of hypobaric hypoxia on rats red blood cell count. *P<0.05, **P<0.01 vs normal control; ##P<0.01 vs 2 week hypoxia model.
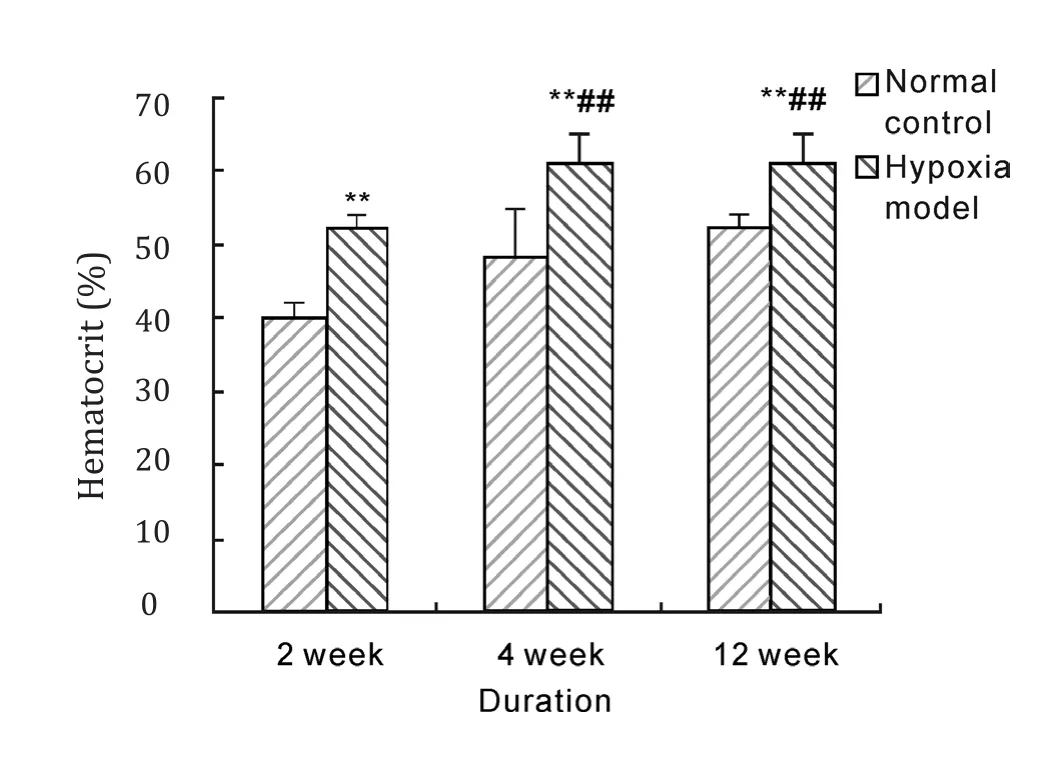
Fig. 4 Effect of hypobaric hypoxia on rats hematocrit.**P<0.01 vs normal control; ## P<0.01 vs 2 week hypoxia model.
Eあects of hypobaric hypoxia on blood viscosity of rats
After hypoxia exposure of 2 weeks, 4 weeks and 12 weeks duration, whole blood viscosity with low,middle and high shear rate in model groups were significantly higher than those in normal control group (P<0.05, P<0.01). And whole blood viscosity of 12 weeks duration was significantly increased compared with that of 2 weeks duration in hypoxia model group (P<0.05, P<0.01). These results suggested that hypobaric hypoxia could notably raise blood viscosity of rats.
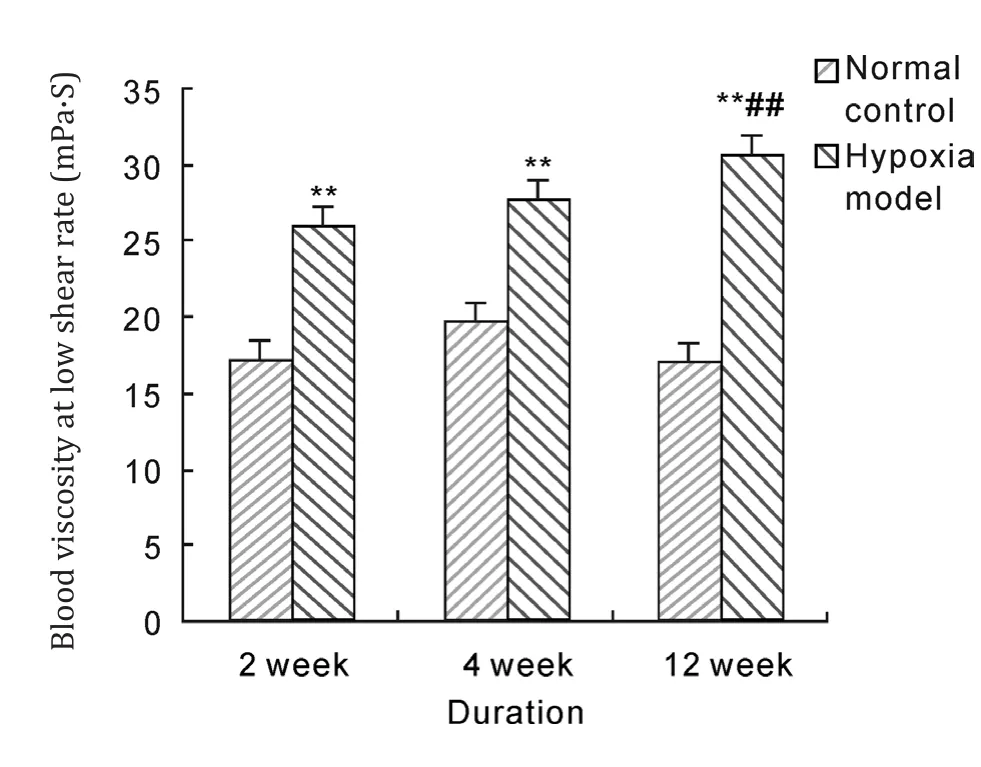
Fig. 5 Effects of hypobaric hypoxia on rats blood viscosity of low shear rate. **P<0.01 vs normal control; ## P<0.01 vs 2 week hypoxia model.
Eあects of hypobaric hypoxia on erythrocyte aggregation index of rats
Aggregation index is ratio with whole blood reduction specific viscosity of 10 s-1divided by that of 40 s-1, and big value means powerful erythrocyte aggregation, inversely small value means weak aggregation. Erythrocyte aggregation index of rats in model groups were significantly increased compared with that in normal control groups after 2 weeks,4 weeks and 12 weeks hypoxia exposure (P<0.05,respectively). And aggregation index of 12 weeks duration was significantly higher than that of 2 weeks duration in model group (P<0.05).
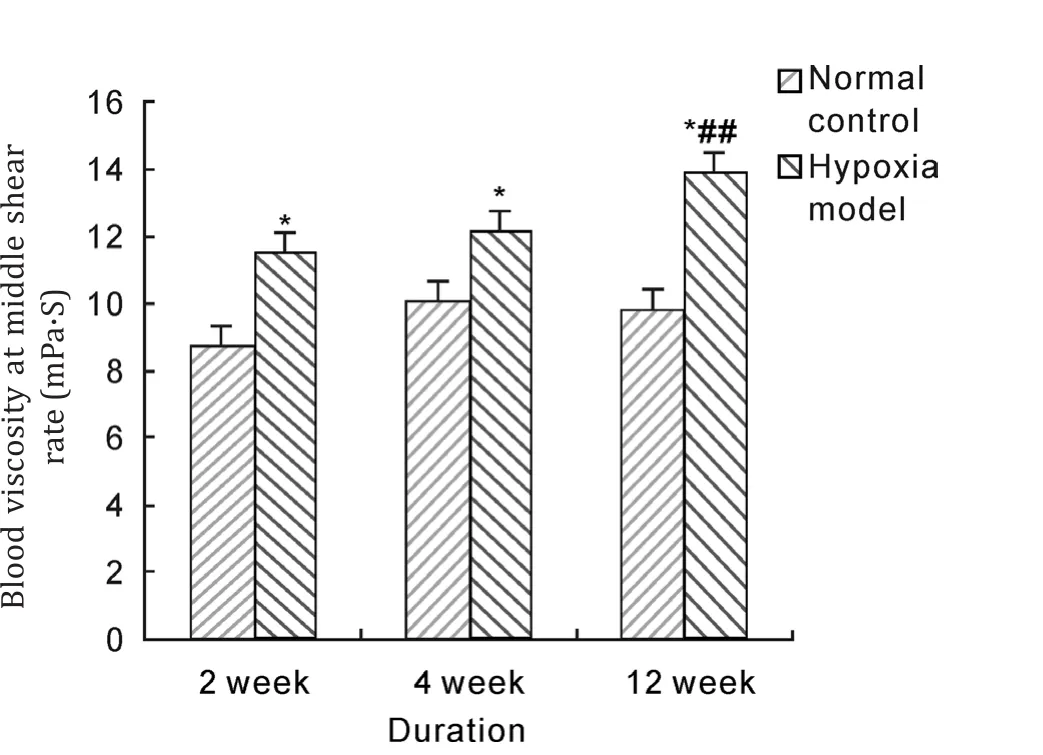
Fig. 6 Effects of hypobaric hypoxia on rats blood viscosity of middle shear rate. *P<0.05 vs normal control; ##P<0.01 vs 2 week hypoxia model.
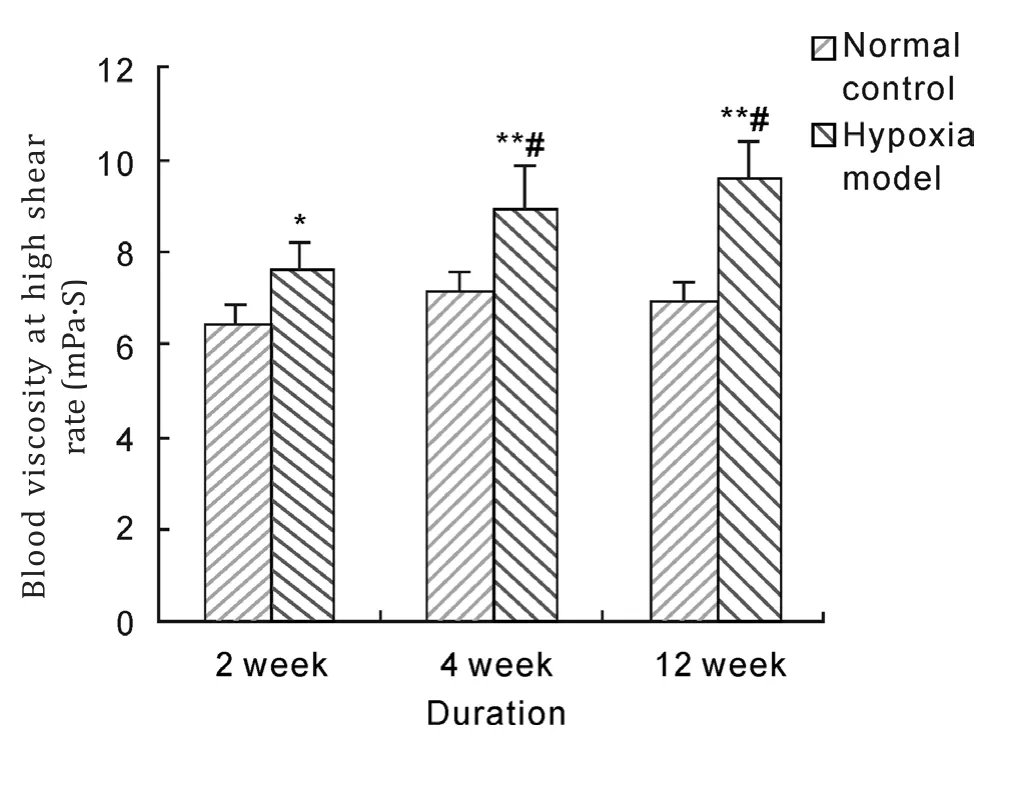
Fig. 7 Effects of hypobaric hypoxia on rats blood viscosity of high shear rate. *P<0.05, **P<0.01 vs normal control; #P<0.05 vs 2 week hypoxia model.
Discussion
HAPC was a kind of chronic mountain sickness.There were some obvious epidemiologic features for HAPC. It would mostly happen above 3 000 m altitude, and its incidence rate would increase with the height rise. そere were higher incidence of HAPC in immigration people than altitude native, and this disease would happen after immigration to altitude only for several months. The course of disease was a chronic process which was related to gender and work intensity, and there was higher incidence for male than female. Previous study showed that HAPC model were not successful when rats were housed in an altitude chamber resembling a high altitude of 5 000 m for 6 h daily for 40 consecutive day[11]. According to the epidemiologic features,we selected male Wistar rats as experiment animal and simulated 5 500 m altitude hypoxia exposure for 8h daily to construct HAPC model. The results showed that mucous membrane were dark purple in the lip, tongue and ear of model rats, which were just as polyhaemia physiognomy of HAPC patients,and hemoglobin concentration raised to 221 g/L with 39.9% increase afer simple 2 week hypobaric hypoxia exposure. Continued hypoxia exposure for 4 weeks and 12 weeks, hemoglobin concentration would be still in similar level. According to occupational high altitude disease diagnostic criteria(GBZ92-2008)and chronic mountain sickness Qinghai diagnostic criteria[14], diagnosis of HAPC was hemoglobin concentration above 210 g/L for male adult, and some studies considered it as diagnosis criteria of animal HAPC model because of similar hemoglobin normal range and hypoxia signs between rats and human. Thus, our research results documented that hemoglobin concentrations of rats in hypoxia exposure model were raised beyond the criteria value only after hypoxia exposure for 2 weeks, and maintained stable for 12 week experimental duration.そis demonstrated that hypobaric hypoxia exposure simulating 5 500 m high altitude for 8 hours daily could rapidly and validly construct HAPC rat model.
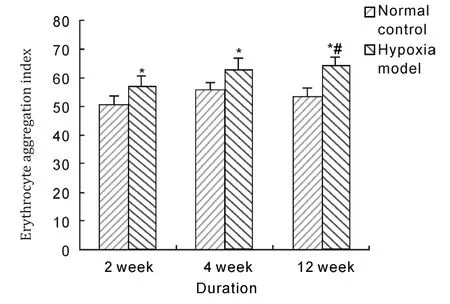
Fig. 8 Effects of hypobaric hypoxia on erythrocyte aggregation index of rats. *P<0.05 vs normal control; #P<0.05 vs 2 weeks duration.
When people entered high altitude and hypoxia circumstance, red blood cell would proliferate and hemoglobin would increase as a normal physiologic reaction, and this would promote oxygen transport and relief tissue anoxia. However, if compensatory hyperplasia of erythrocytes was excess, organism hypoxia would not relieve and microcirculation disturbance would develop, organism hypoxia became aggravated and induced a series of serious complication. How to judge whether erythrocyte proliferation induced by altitude hypoxia was physiologic compensatory hyperplasia or hyperproliferation? Some studies considered that we should make a judgement by hematocrit[15,16].If hematocrit was lower than 60%, erythtocyte proliferation would be physiologic and beneficial to body. If hematocrit was beyond 60%, erythrocyte proliferation would be excessive and harmful to body.From the results of this research, red blood cell count and hematocrit were significantly raised compared with normal control when hypoxia exposure reached 2 weeks. Further rise of red blood cell count and hematocrit happened when hypoxia exposure reached 4, 12 weeks and hematocrit was beyond 60%.そese results were in accordance with human HAPC development, suggesting that HAPC rat model could be successfully constructed by simple hypobaric hypoxia.
HAPC was a disease happening only in high altitude and one of its main features was excessive erythrocyte proliferation. Hyperplasia of erythrocyte would induce blood hyperviscosity and deterioration of erythrocyte function[17]. そus, hemorheology of HAPC patients, which showed thickness, sticking,gathering and coagulating character, caused blood flow slowing and attenuated erythrocyte function of oxygen uptaking and releasing. Meanwhile,tissue factors were actived by bypoxia and thrombosis would be easily formed when blood was in the hypercoagulable state. Thrombosis would further aggravate organism hypoxia and induce microcirculation disturbance and blood vessel embolism. This was main pathopoiesis and disability of HAPC. Blood viscosity was constituted by haemocyte and plasma, while viscosity caused by erythrocyte was highly more than plasma. So erythrocyte viscosity was the main factor which actually affected blood viscosity for HAPC. From the results of this research, whole blood viscosity with low, middle, high shear rate and erythrocyte aggregation index of rats in model group were significantly higher than those in normal control when exposed to hypoxia for 2 weeks, 4 weeks, and 12 weeks, and showed more duration more rise.These were in accordance with the development of HAPC and proved that it was feasible for constituting HAPC rat model through hypoxia exposure from another wayside.
HAPC pathogenesis was complicated and was not fully understood. So several animal models had been used in high altitude medical research and each model was not fully perfect to some extent,such as necessarily arriving highland for highland spot model, improper pathogenesis for EPO induction model and normobaric hypoxia model,with replicative time too long or influencing factor too complex for integrated factors model. From thepresent study, hypobaric hypoxia exposure that simulated high altitude 5 500 m for 8 hours per day could cause excessive increasing of hemoglobin, red blood cell count, hematocrit, whole blood viscosity with low, middle, high shear rate, and erythrocyte aggregation index in model rats. These proved that HAPC rat model was successfully established and hypoxia exposure was a convenient, rapid, stable,valid way to constitute HAPC model for pathogenesis and drugs research.
Acknowledgements
This work was supported by grants from National New Drug Research and Development of Key Project(2011ZXJ09105-05B) and the National Natural Science Foundation (81171870, 81471812).
1. León-Velarde F, Villafuertea FC, Richalet JP. Chronic mountain sickness and the heart [J]. Prog Cardiovasc Dis,2010, 52(6): 540-549.
2. Wu TY, Liu FY, Ouzhou-Loubu, et al. A genetic adaptive pattern-low hemoglobin concentration in the Himalayan highlanders[J]. Chin J Appl Physiol, 2013, 29(6): 481-493.
3. West JB. High-altitude medicine[J]. Am J Respir Crit Care Med, 2012, 186(12): 1229-37.
4. Winslow RM, Monge CC. Hypoxia, polycythemia, and chronic mountain sickness[M]. Baltimore (Md): Johns Hopkins University Press, 1987.
5. Leo′n-Velarde F, Maggiorini M, Reeves JT, et al. Consensus statement on chronic and subacute high altitude diseases.High Alt Med Biol, 2005, 6(2): 147—157.
6. Julian CG, Vargas E, Gonzales M, et al. Sleep-disordered breathing and oxidative stress in preclinical chronic mountain sickness (excessive erythrocytosis) [J]. Respir Physiol Neurobiol, 2013, 186(2): 188-196.
7. Rivera-Ch M, León-Velarde F, Huicho L. Treatment of chronic mountain sickness: critical reappraisal of an old problem[J]. Respir Physiol Neurobiol, 2007, 158(2-3): 251-265.
8. Jin GE, Yun HX, Ma L, et al. Establishment of an animal model of high altitude polycythemia and analyses of its physiological functional responses to hypoxia[J]. J Clin Rehabil Tissue Eng Res, 2009, 13(24): 4713-4716.
9. Wu XW, Yang YF, Wu Y, et al. Eあects of Qing-xue granules on immune function in rats of secondary polycythemia[J].Beijing J Tradit Chin Med, 2010, 29(11): 874-876.
10. Duan L, Xing YP, Duan RF, et al. Establishment of a rat model of high altitude polycythemia[J]. J Prev Med Chin PLA, 2012, 30(1): 5-7.
11. Jiang P, Xu GZ, Jia SN, et al. Study on the experimental methods for constituting model of rats high altitude polycythemia[J]. J Tibet Med, 1996, 17(4): 19-21.
12. Wu WB, Meng XL, Zhang Y, et al. Effect of tibetan compound prescription of Xinsanguo decoction on plateau erythrocythemia rats[J]. J Guangzhou Univ Tradit Chin Med, 2010, 27(5): 492-494.
13. Drabkin DL, Austin JH. Spectrophotometric studies:ii. preparations from washed blood cells; nitric oxide hemoglobin and sulさemoglobin[J]. J Biol Chem, 1935, 112:51—65.
14. Experts Group of Chronic Mountain Sickness in International Institution of High Altitude Medicine. Qinghai diagnostic criteria of chronic mountain sickness (Published by Sixth Annual International Academic Conference of High Altitude Medicine and Hypoxia Physiology)[J]. J Qinghai Med Coll, 2005, 26(1): 3-5.
15. Wu TY, Chen QH, Li Y, et al. Pathophysiology of high altitude excessive polycythemia: I. A study of optimal hematocrit[J]. Chin J High Alt Med, 1998, 8(2): 1-4.
16. Swenson ER. Normal exercise capacity in chronic mountain sickness: how high can the hematocrit go without consequence[J]? Chest, 2012, 142(4): 823-825.
17. Pichon A, Connes P, Quidu P, et al. Acetazolamide and chronic hypoxia: eあects on haemorheology and pulmonary haemodynamics[J]. Eur Respir J, 2012, 40(6): 1401-1409.
- 中國應(yīng)用生理學(xué)雜志的其它文章
- Pathophysiological changes in mitochondria of mammalian exposed to hypoxia at high altitude
- A dysfunction of CD4+ T lymphocytes in peripheral immune system of Parkinson’s disease model mice
- Microvesicles derived from hypoxia/reoxygenation-treated human umbilical vein endothelial cells impair relaxation of rat thoracic aortic rings
- Stimulation of endothelial non-neuronal muscarinicreceptor attenuates the progression of atherosclerosis via inhibiting endothelial cells activation
- Effect of acclimation training on physiological changes in a randomized controlled trial in hot-humid environment
- Scene-trait coping style of military rescuers in Wenchuan earthquake

