Role of HCN channels in the nervous system: membrane excitability and various modulations
Xie-chuan WENG, Shao-jun LIU
Department of Neurobiology and State Key Laboratory of Proteomics, Beijing Institute of Basic Medical Sciences,Beijing 100850, China
Introduction
Hyperpolarization-activated and cyclic nucleotidegated channels encoded by HCN1-4 gene family are cation channels that opened when the membrane potential get hyperpolarized. HCN channels are permeable to both K+and Na+ions with the ratio about (3-5:1) when the reversal potential is around-30 mV[1]. Both extracellular Cl?and extracellular K+are required for HCN channels opening.Interestingly, HCN channels have a cyclic nucleotidebinding domain (CNBD) called the C-linker. The CNBD-C-linker can bind of cyclic AMP, cyclic GMP and cyclic CMP (cAMP, cGMP and cCMP),which can induce a rightward shift in the voltagedependence to facilitate HCN channel activation [2-4]. Thus HCN channels were also termed as funny current (If) or queer current (Iq) in the past. The role of HCN channels in the nervous system were closely linked with its electrophysiology features [5].In this review, we try to describe the physiological roles of HCN channels in the nervous system, related modulation factors as well as its future clinical implications as novel drug target.
そe HCN channel family
HCN channels were identified and characterized in the 1970s in sinoatrial node cells and neurons.HCN channels are tetrameric complexes consisting of four subunits (HCN1-HCN4), which share about 60% sequence identity with each other in mammals[6]. For every HCN channel subunit, it has six transmembrane segments which include six α-helices (S1-S6). A loop domain between the S5 and S6 helix forms the ion selectivity filter, and the positively charged S4 helix has the exact function as a voltage sensor (Fig.1)[7].
そe speeds of HCN channel activation are strongly voltage-dependent though the currents evoked from the four HCN channels are diあrent from each other according to their activation kinetics, which are HCN1 ? HCN2 ? HCN3 ? HCN4 for the speed.HCN channels are also distinct from each other intheir response to cCMP, cAMP and cGMP [2, 4].When activated, HCN2 and HCN4 can be shifted by around +15 mV, while HCN1 and HCN3 are weakly modulated by extracellular cAMP[4]. As we can imagine, the biophysical properties of the wild heteromeric HCN currents are determined by its composed single homomeric subunits. It is very likely that the formation of heteromeric channels is vital for the diversity of the native Ihrecorded in diあerent types of neurons.
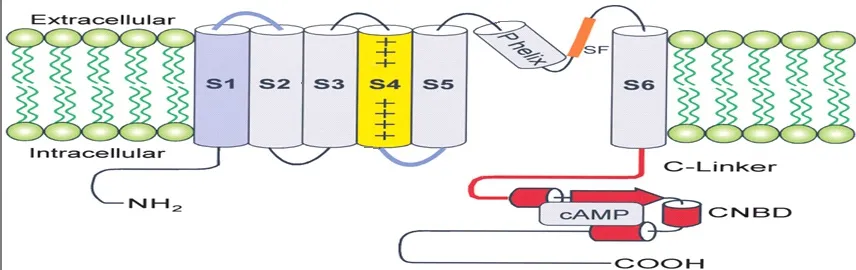
Fig. 1 A schematic drawing of the structure of a single HCN channel subunit, with the cyclic nucleotide binding site (Martin Biel, 2002). Cyclic nucleotides can speed up channel opening and activate the channel at more positive voltages.
Expression of HCN in the nervous system
Beside brain and heart, HCN channels are found to express in taste buds, pancreatic cells, testis,smooth muscle, inner ear and kidney [5, 8-12]. そe role and functions of HCN channels in these cell types remain unclear. In the brain, HCN1 isoform is expressed strongly in hippocampus, cerebral cortex and superior colliculus, while HCN2 is found in the most part of the brain [13]. Expression of HCN3 is found in hypothalamic nuclei, olfactory bulb,retinal cone and pedicles. HCN4 has been reported in basal ganglia, thalamic nuclei, olfactory bulb and fast-spiking interneuron of the rat spinal cord and hippocampus[10, 14] .
In the neuron, HCN channels are expressed in dendrites, somas, and axon terminals[1]. The expression density of HCN channels on the neural surface is not same. In thalamic reticular neurons,HCN channels are expressed more strongly in distal dendrites than in somas, while they distribute almost evenly in thalamocortical relay neurons. In layer V pyramidal neurons of cerebral cortex of matured rats,HCN1 is expressed strongly in dendrites, whereas HCN2 distributed mainly in the soma [15].
Together with our recent researches, four types of HCN subunits are found to express in a variety of sensory neuron types especially in the dorsal root ganglia (DRGs) and spinal cord [5, 11, 16,17]. RNA detection and Immunocytochemistry revealed that HCN1 and HCN2 co-localize in normal rat DRGs, mainly in large neurons. HCN1 is the most abundant subtype, and the second seems to be HCN2. HCN1 and HCN2 expression were related with the conduction velocity (CV) of both nociceptors and non-nociceptors [11]. In addition,the expression of HCN2 were higher than HCN1 in muscle spindle afferents (MSAs) than in other types neurons in rat DRG[5], which was same as the previous report that MSAs have high HCN currents(Ih) . In C-nociceptors, low HCN1 and HCN2 were consistent with their low/undetectable Ih. In some C-LTMs neurons, HCN2 were even higher than in C-nociceptor though HCN2 in C-nociceptor plays a central role in the chronic pain [6, 11].
Important role of HCN in the chronic pain
Increasing evidences have shown that HCN channels play important roles in both neuropathic and inflammatory pain [5, 6, 11, 16, 18].
In vivo intracellular recordings studies from L4/L5 DRG neurons with hindlimb chronic inflammation model induced by complete Freund’s adjuvant(CFA) demonstrated that the inflammation-induced nociceptor hyperexcitability is maily associated with C- but not Aδ nociceptors, with increases in Ihamplitude/density as well as the proportion of Ihexpressing neurons[5]. Similar studies from other group also reported that HCN2 expression in small neurons increased 1 day after CFA-induced cutaneous inflammation, while it decreased 4 days in large neurons after CFA in the DRG. These evidences indicated that HCN2-expression change in pathologic condition altered the excitability of neurons and involved in chronic inflammatory pain[11].
Despite increases in Ih, HCN1 and HCN2 mRNA and protein were found to decrease after nerve injury while HCN3 or HCN4 seemed to have no upregulation. Thus, the increases in Ihdensity after nerve injury might be attributed to other factors except protein expression increase. These results showed the post-translational regulation of HCN function [1]. Focusing on Aδneurons rather than Aa/b neurons, Ihwas found large increases after nerve injury in a rodent model of foramenal stenosisinduced radiculopathy. In these rats, chronic direct DRG compression is created with inserting steel rods into the intervertebral foramina through the L4 and L5 spinal nerves and the behavioral and electrophysiological test is done in five to seven days.Deletion of the HCN2 subunit from nociceptive neurons abolishes heat-evoked inflammatory pain and all aspects of neuropathic pain, but acute pain sensation is unaffected [6, 18, 19]. Also, ZD7288, a selective HCN channels blocker, could remarkably reverse the symptom of chronic pain such as hypergesia, allodynia, and spontaneous foot lifting[1, 5]. そese works showed that HCN channels could contribute to the inflammatory and neuropathic pain, and suggested that selective blockers of HCN2 may have value as analgesics in the therapy of pain.
Involvement of HCN channels in brain disorders
As we know, the central nervous system (CNS) is a complicated network of cells with diverse electrical properties. Decreases or increases in the kinetics and magnitude of Ihin some specific neurons can have different inhibitory or excitatory impacts on brain function, which depends on the properties of the neuron and their roles in signal integration [20].In the brain, evidences has shown that the kinetics and expression of HCN channels are affected by some pathological disease such as epilepsy, absence seizures, and Parkinson’s disease, drug addiction,depression as well as some environmental stimuli [1,21-24]. For example, decrease in surface expression and channel current of HCN channel is observed in one hour after epilepticus rat models, but the total channel proteins expression, mRNA and voltage dependence of Ih were found to decrease several days later [17-18].
It is interesting that sensory inputs are important to increase and keep the expression level for the HCN channels. Whiskers Deprivation can decrease the HCN channels expression in neural dendrites in the rat somatosensory cortex[7]. In addition,research showed that HCN2 was increased in CA3 while HCN1 and HCN2 were found to decrease in CA1 of hippocampus in the epilepsy model [25, 26].In the social stress model, Ihwas upregulated and ZD7288 could treat the depression very well [24].And in the thalamus relay neurons, HCN channels participate in regulating arousal and sleep states[14]. そese studies showed that HCN channels were widely involved in the brain disorders. However,mechanisms underlying the change of HCN channel expression still require further researches [26-30].
Regulation of HCN on the neural excitability
Underlying mechanism of HCN channels on the neuronal disease were directly related to its regulation on the neural excitability. For the HCN channels, their reversal potential are around -30 mV which are higher than normal threshold of neurons to generate the action potential. Thus, the HCN channels can aあect the membrane potential level near the resting membrane potential [7]. For example,when the HCN channel conductance was removed in the rat superior colliculus, the neuron could induce hyperpolarized membrane potential [1].
Beside action potential, HCN channel conductance could also decrease the EPSP amplitude and suppress the effect of inhibitory synaptic inputs by evoking depolarizing conductance [1, 6-7]. When HCN channels activate more and more, the kinetics and amplitude of postsynaptic potentials get shorter and smaller. In neocortical and hippocampal pyramidal neurons, HCN current density increases along with the distance from the soma, which can inhibit the location dependency of synaptic inputs [21]. Low frequency of repetitive EPSPs could be decreased as HCN channel conductance shortens both the decay time and rise time of the EPSPs. Meanwhile,synchronous synaptic inputs is selectively improved[7]. Thus, HCN channel currents influence spike timing, the generation of synchronous neuronal discharges and the slow frequency inputs to regulate the excitability of neurons though sometimes the fire rhythm didn’t change [7,32].
In addition, cAMP, cGMp and PIP2 can shif HCN channels to the depolarizing direction, which make HCN channels active near the resting membrane potential working as an excitatory factor[2,3,7].
Various modulations on HCN channels
HCN channels could be regulated by intracellular signaling molecules, which contain cAMP, cGMP,TRIP8b and PIP2 [1, 2, 33, 34]. そey regulate HCN channel activity in deferent ways such as binding to the CNBD and/or phosphorylating the CNBD.Voltage-dependent potassium channels can also aあect HCN channel activity [7]. そus, some functions of HCN channel currents can be determined by the balance of these modulator factors. Many neuromodulators in the dorsal root ganglion have found to enhance Ihsuch as PGE2, substance P,serotonin, and neuromedin U. Also, activation of calcineurin and inactivation of P38 mitogenactivated protein kinase (p38 MAPK) in the hyperpolarizing shift of voltage-dependence is reported [1, 35].Regulation of Ihmay be a novel mechanism for these neuropeptides and inflammatory related mediators to generate chronic pain symptoms.
Protein kinases and phosphatases also regulate HCN channels by direct dephosphorylation and phosphorylation of the channels, or by interact with other proteins [6, 35]. そey include SRC kinase, P38 mitogen-activated protein kinase, calcineurin and cGMP-dependent protein kinase 2. For example, SRC kinase can regulates Ihcurrents by phosphorylating tyrosine residues of HCN1, HCN2 and HCN4. P38 mitogen-activated protein kinase can upregulated HCN expression in hippocampal pyramidal neurons,while the phosphatase calcineurin can suppress HCN currents in hippocampal pyramidal neurons[1, 6].
In addition, the function of several proteins assembled with HCN channels is not clear. Further studies need to be done to make clear the roles of these proteins such as MIRP1, MINT2, and caveolin 3, filamin A as well as voltage-dependent channel potassium and the relationship with HCN channels.Evidence shows that they can work together in the same brain disorders [24].
Several low-molecular-weight factors including acidic lipids (phosphatidylinositol 4,5-bisphosphate,phosphatidic acid and arachidonic acid ), extraand intracellular concentrations of ion such as K+,H+, and Cl—can regulate HCN channel activity [6].For example, the activation of HCN channels could be shif by about +20 mV with phosphatidylinositol 4,5-bisphosphate. This regulation linked with some pathological process that HCN channel might be involved in such as the cell death or apoptosis in inflammatory or tissue acidosis when the extracellular environments are changed.
Future clinical implication of HCN channels
As HCN channels play important roles in modulation of neural excitability and have vital functions in the diseases mentioned above, and these functions can be changed by pharmacological modulation,HCN channels now has become a potential novel pharmacological targets for chronic pain and some brain disorders.
Interestingly, some drugs that have eあects on the HCN channels named “specific bradycardic agents”have already been studied for years for animal behaviors and human beings tests though the its exact pharmacological target was not clear at the beginning [1, 25]. For example, β-blockers could aあect HCN channels as they are sensitivite to regulate cAMP levels. Some local anesthetics such as lidocaine were proved to be eあective on pain therapy for many years and theirs targets have definitely thought to be sodium channels. However, systematic given lidocaine could be eあective on neuropathic pain with the plasma lidocaine concentrations of about 8 to 25 μmol/L, which is quite close to its inhibitory eあects on the HCN channels (IC5038.2 μmol/L) but lower than its IC50on sodium channels [6, 19, 38-41]. Other good examples are from a2-adrenergic receptor agonists and antagonists such as exmedetomidine and clonidine, and idazoxan. They have been used in clinic as analgesics for years, but now their eあects are also thought to be related to direct Ih inhibition or indirect eあects on Ih(cAMP modulation). そough it is still early to say their ability to treat pain are completely from blocking Ih currents, it is sure that substantial overlap do exist between the ability on a2-receptors and Ih currents [6, 41].
However, almost all of these bradycardic agents have no selective effects on HCN channels as they normally have effects on more than two HCN subunits. These non-selective agents include alinidine (ST567), ZD7288, ivabradine (S16257) and zatebradine (UL-FS49) [5, 42]. They have widely been used for animal test in tachycardia and pain therapy, but further studies still need to be done to make clear theirs eあects and safety on human being.
In conclusion, because of the important role and function of HCN channels on membrane excitability,and the various modulation factors involved in these physiological and pathological condition,HCN channels has become a novel promising drug targets for chronic pain as well as a number of brain disorders. Some well-known drugs such as lidocaine,clonidine and ZD7288 will provide us very good clue or lead structure for specific HCN subunits selective antagonist discovery. However, further studies about functions of HCN channels in the brain and its underlying mechanisms on modulation of the channels still need to be explored as for future clinical usage of HCN channel drugs.
Acknowledgements
Project was supported by the Beijing Municipal Natural Science Foundation (7142123).
1. Benarroch EE. HCN channels: function and clinical implications[J].Neurology, 2013, 80(3): 304-310.
2. Zong X, Krause S, Chen CC, et al. Regulation of hyperpolarizationactivated cyclic nucleotide-gated (HCN) channel activity by cCMP[J]. J Biol Chem, 2012, 287(32): 26506-26512.
3. Wu S, Gao W, Xie C, et al. Inner activation gate in S6 contributes to the state-dependent binding of cAMP in full-length HCN2 channel[J]. J Gen Physiol, 2012, 140(1): 29-39.
4. Zheng JH, Walters ET, Song XJ. Dissociation of dorsal root ganglion neurons induces hyperexcitability that is maintained by increased responsiveness to cAMP and cGMP[J]. J Neurophysiol, 2007, 97(1):15-25.
5. Weng X, Smith T, Sathish J, et al. Chronic inflammatory pain is associated with increased excitability and hyperpolarizationactivated current (Ih) in C- but not Adelta-nociceptors[J]. Pain,2012, 153(4): 900-914.
6. Emery EC, Young GT, McNaughton PA. HCN2 ion channels: an emerging role as the pacemakers of pain[J]. Trends Pharmacol Sci,2012, 33(8): 456-463.
7. Kase D, Imoto K. The Role of HCN Channels on membrane excitability in the nervous system[J]. J Signal Transduct, 2012, 2012:619747.
8. Matsuyoshi H, Masuda N, Chancellor MB, et al. Expression of hyperpolarization-activated cyclic nucleotide-gated cation channels in rat dorsal root ganglion neurons innervating urinary bladder[J].Brain Res, 2006, 1119(1): 115-123.
9. Kouranova EV, Strassle BW, Ring RH, et al. Hyperpolarizationactivated cyclic nucleotide-gated channel mRNA and protein expression in large versus small diameter dorsal root ganglion neurons: correlation with hyperpolarization-activated current gating[J]. Neuroscience, 2008, 153(4): 1008-1019.
10. Ramakrishnan NA, Drescher MJ, Khan KM, et al. HCN1 and HCN2 proteins are expressed in cochlear hair cells: HCN1 can form a ternary complex with protocadherin 15 CD3 and F-actinbinding filamin A or can interact with HCN2[J]. J Biol Chem,2012, 287(45): 37628-37646.
11. Acosta C, McMullan S, Djouhri L, et al. HCN1 and HCN2 in Rat DRG neurons: levels in nociceptors and non-nociceptors, NT3-dependence and influence of CFA-induced skin inflammation on HCN2 and NT3 expression[J]. PloS one, 2012, 7(12): e50442.
12. Kim YH, Holt JR. Functional contributions of HCN channels in the primary auditory neurons of the mouse inner ear[J]. J Gen Physiol,2013, 142(3): 207-223.
13. Xiao J, Nguyen TV, Ngui K, et al. Molecular and functional analysis of hyperpolarisation-activated nucleotide-gated (HCN) channels in the enteric nervous system[J]. Neuroscience, 2004, 129(3): 603-614.14. Abbas SY, Ying SW, Goldstein PA. Compartmental distribution of hyperpolarization-activated cyclic-nucleotide-gated channel 2 and hyperpolarization-activated cyclic-nucleotide-gated channel 4 in thalamic reticular and thalamocortical relay neurons[J].Neuroscience, 2006, 141(4): 1811-1825.
15. Antal M, Papp I, Bahaerguli N, et al. Expression of hyperpolarization-activated and cyclic nucleotide-gated cation channel subunit 2 in axon terminals of peptidergic nociceptive primary sensory neurons in the superficial spinal dorsal horn of rats[J]. Eur J Neurosci, 2004, 19(5): 1336-1342.
16. Yao H, Donnelly DF, Ma C, et al. Upregulation of the hyperpolarization-activated cation current after chronic compression of the dorsal root ganglion[J]. J Neurosci, 2003, 23(6):2069-2074.
17. Takasu K, Ono H, Tanabe M. Spinal hyperpolarization-activated cyclic nucleotide-gated cation channels at primary afferent terminals contribute to chronic pain[J]. Pain, 2010, 151(1): 87-96.
18. Du L, Wang SJ, Cui J, et al. そe role of HCN channels within the periaqueductal gray in neuropathic pain[J]. Brain research, 2013,1500: 36-44.
19. He C, Chen F, Li B, et al. Neurophysiology of HCN channels: From cellular functions to multiple regulations[J]. Pro Neurobiol, 2014,112: 1-23.
20. Brown SM, Dubin AE, Chaplan SR. そe role of pacemaker currents in neuropathic pain[J]. Pain Pract, 2004, 4(3): 182-193.
21. Albertson AJ, Williams SB, Hablitz JJ. Regulation of epileptiform discharges in rat neocortex by HCN channels[J]. J neurophysiol,2013, 110(8): 1733-1743.
22. Saito Y, Inoue T, Zhu G, et al. Hyperpolarization-activated cyclic nucleotide gated channels: a potential molecular link between epileptic seizures and Abeta generation in Alzheimer’s disease[J].Mol Neurodegener, 2012, 7: 50.
23. Kitchigina V, Popova I, Sinelnikova V, et al. Disturbances of septohippocampal theta oscillations in the epileptic brain: reasons and consequences[J]. Exp Neurol, 2013, 247: 314-327.
24. Friedman AK, Walsh JJ, Juarez B, et al. Enhancing depression mechanisms in midbrain dopamine neurons achieves homeostatic resilience[J]. Science, 2014, 344(6181): 313-319.
25. Shah MM, Huang Z, Martinello K. HCN and KV7 (M-) channels as targets for epilepsy treatment[J]. Neuropharmacology, 2013, 69: 75-81.
26. Poolos NP. Hyperpolarization-activated cyclic nucleotide-gated(HCN) ion channelopathy in epilepsy. In: Noebels JL, Avoli M,Rogawski MA, et al. Jasper’s basic mechanisms of the epilepsies[M].Bethesda (MD): National center for biotechnology information(US), 2012.
27. Ji RR. Recent progress in understanding the mechanisms of pain and itch[J]. Neurosci Bull, 2012, 28(2): 89-90.
28. Greene D, Kang S, Kosenko A, et al. Adrenergic regulation of HCN4 channel requires protein association with beta2-adrenergic receptor[J]. J Biol Chem, 2012, 287(28): 23690-23697.
29. Marcelin B, Lugo JN, Brewster AL, et al. Diあerential dorso-ventral distributions of Kv4.2 and HCN proteins confer distinct integrative properties to hippocampal CA1 pyramidal cell distal dendrites[J]. J Biol Chem, 2012, 287(21): 17656-17661.
30. Marcelin B, Liu Z, Chen Y, et al. Dorsoventral differences in intrinsic properties in developing CA1 pyramidal cells[J]. J Neurosci, 2012, 32(11): 3736-3747.
31. Marni F, Wu S, Shah GM, et al. Normal-mode-analysis-guided investigation of crucial intersubunit contacts in the cAMP-dependent gating in HCN channels[J]. Biophy J, 2012, 103(1): 19-28.
32. Scicchitano P, Carbonara S, Ricci G, et al. HCN channels and heart rate[J]. Molecules, 2012, 17(4): 4225-4235.
33. Xu X, Marni F, Wu S, et al. Local and global interpretations of a disease-causing mutation near the ligand entry path in hyperpolarization-activated cAMP-gated channel[J]. Structure,2012, 20(12): 2116-2123.
34. Brager DH, Lewis AS, Chetkovich DM, et al. Short- and long-term potentiation in CA1 neurons from mice lacking the h-channel auxiliary subunit TRIP8b[J]. J Neurophysiol, 2013, 110(10): 2350-2357.
35. Wilkars W, Liu Z, Lewis AS, et al. Regulation of axonal HCN1 traきcking in perforant path involves expression of specific TRIP8b isoforms[J]. PloS one, 2012, 7(2): e32181.
36. Postea O, Biel M. Exploring HCN channels as novel drug targets[J].Nat Rev Drug Discov, 2011, 10(12): 903-914.
37. Hammelmann V, Biel M, Zong X, et al. The cGMP-dependent protein kinase II is an inhibitory modulator of the hyperpolarization-activated HCN2 channel[J]. PLoS One, 2011,6(2): e17078.
38. Weng XC, Fu HJ, Li Y, et al. HCN2 plays an important role in depression[J]. Society for Neuroscience, Washington, USA, 2014.
39. Postea O, Biel M. Exploring HCN channels as novel drug targets[J].Nat Rev Drug Discov, 2011, 10(12): 903-914.
40. Wu X, Liao L, Liu X, et al. Is ZD7288 a selective blocker of hyperpolarization-activated cyclic nucleotide-gated channel currents[J]? Channels (Austin), 2012, 6(6): 438-442.
41. Boehlen A, Kunert A, Heinemann U. Eあects of XE991, retigabine,losigamone and ZD7288 on kainate-induced theta-like and gamma network oscillations in the rat hippocampus in vitro[J]. Brain Res,2009, 1295: 44-58.
42. Emery EC, Young GT, Berrocoso EM, et al. HCN2 ion channels play a central role in inflammatory and neuropathic pain[J].Science, 2011, 333(6048): 1462-1466.?
- 中國應用生理學雜志的其它文章
- Children’s exercise capacity at high altitude in Tibet
- Hypoxic preconditioning: effect, mechanism and clinical implication (Part I)
- How to deal with cerebral palsy in 21st century
--A new epoch in clinic treatment - Hematological parameters in high altitude residents:Tibetan natives versus Han migrants
- A rat model of high altitude polycythemia rapidly established by hypobaric hypoxia exposure
- Plasma endothelin-1 and nitric oxide correlate withligustrazine alleviation of pulmonary artery hypertension in patients of chronic cor pulmonale from high altitude plateau during acute exacerbation

