PHENOLIC ANTIOXIDANTS DETERMINATION IN FOOD ITEMS USING REVERSED-PHASE HPLC
Chen Xijin,Zhang Zhicai
(1.College of Urban Construction&Safety Engineering,Nanjing University of Technology,
Nanjing,210009,P.R.China;
2.School of Food Science and Biotechnology,Jiangsu University,Zhenjiang,212013,P.R.China)
INTRODUCTION
Natural and artificial antioxidants play important roles in inhibiting oil oxidation reaction of food.It is well known that too much oxidation reaction will cause adverse influence,producing unpleasant odors and harmful compounds including aldehydes,ketones and organic acids.Since natural antioxidants are generally unstable,food producers prefer artificial antioxidants including propyl gallate(PG),tertiary butyl hydroquinone(TBHQ),butylated hydroxyanisole(BHA)and butylated hydroxytoluene(BHT).Their structures are shown in Fig.1. Adding synthetic phenolic antioxidants(SPAs)to fat,cooking oil can inhibit oxidation reaction in production and deposit process,thus there have been more than 50 years for the use of SPAs in food industry[1-2].However,there exist problems in the use of SPAs that BHA and BHT are revealed to be harmful to liver and cause cancer[3-4].So,the use of SPAs in food is strictly regulated,though different countries have their different permitted limits.For example,the use of TBHQ is allowed in USA,but not in some European countries. Generally, SPA concentration of 100—200 ug/g in oil and fat is allowed,and this is the direct reason for the determination of SPAs in the food industry quality control process[5].
For the analysis of antioxidants, several different techniques have been used,including thin-layer chromatography (TCL)[6], gas chromatography(GC)[7],capillary electrophoresis(CE)[8]and stripping voltammetry[9],yet all of the method above may be interfered by the samples.With its advantages of high precision and sensitivity, the high performance liquid chromatography (HPLC)became the main technique for the analysis of SPAs in meat juice,dehydrated soups,meat gravy,dehydrated meat,dehydrated pet food, baked food, palm oil,potatoes and corn chips, popcorn, cheese,breakfast cereals,drink powder mixture and livers[10].In the related analysis,the recoveries of spiked PG,TBHQ and BHA are above 90%,though the recovery of BHT is only 75%—90%,which does not meet the analysis requirements.
Sample preparation is the experiment step before the HPLC separation,and it is a key point for the experiment.The solid phase extraction method is mainly used in antioxidant concentration and separation which is based on solubility principle.The liquid-liquid extraction method is widely used in multi-component solution separation of SPAs in food.So,the experiment is to set up a simple method for the analysis of SPAs.
1 MATERIALSAND METHODS
The reare 38 food samples including 16 kinds of cooking oil,16 kinds of bread spread and 6 kinds of bread cream.The 16 kinds of bread spread include 6 kinds of butter and 10 kinds of cheese.
PG(97%),TBHQ(97%),BHA(98%)and BHT(99%)are purchased from Aldrich Co.(WI,USA),and MeOH and ACN(HPLC grade)are purchased from Merck(Darmstadt,Germany).Glacial acetic acid and is opropanol are A.R.grade.Water is two times distilled water.
SPAs stock standard solution are prepared in MeOH/ACN(1∶1,in volume)with 500 mg/ml as the concentration,after being sha kened to a clear solution,sealed by aluminum foil,and stored at 4°C for one month.Before used in the HPLC analysis,the stock standard solutions are diluted with 1∶1(in volume)MeOH/ACN to be suitable concentrations.
The instrumentation used in the sample preparation process includes ultrasound bath(up 5200H)and vortex vibrator(VORTEX-6,Beijing Chuangbo Bio-Tech Co. Ltd.). The UV absorption spectra are performed using UV-visible spectrometer(Varian,Cary 50).
A sample(10.0 g)is extracted with MeOH/ACN in a corked flask(100.0 ml)for 15 min by shaking under high spread, and then is centrifuged at 3 500 r/min for 10 min. The supernatant is collected and cooled in the refrigerator for 1 h.The clear liquid obtained is injected directly into the HPLC system. The extraction process is shown in Fig 2.
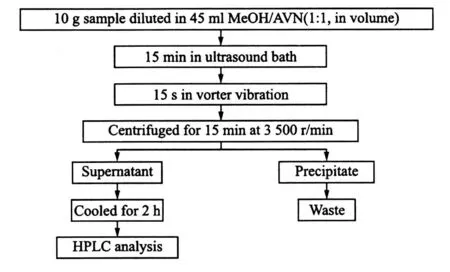
Fig.2 Scheme for determination of SPAs
The HPLC analyses are carried out on a Varian HPLC system (Agilent HPLC 120),consisting of an autosampler,a 240 Pro Star pump,a 320 Pro Star UV detector operated by CP-SCANVIEW version 6 software. HPLC separation is carried out on a particle size 250 mm×4.0 mm and Lichrospher column 5 um.The mobile phase is composed of water(1% HAC)as mobile phase A and acetonitrile(1% ACN)as mobile phase B. The analytical separation is performed using gradient elution.A segmented gradient of mobile phase Bis increased from 30%to 90% in 5 min,followed by ramping of mobile phase B to 100%in 4 min and is held constantly for 1—2 min.Then the mobile phase is filtered and degassed.The UV detection wavelength is set at 280 nm.The flow rate is maintained at 1.5 ml/min.After each experiment,the system is eluted with water/MeOH(20∶80,in volume)for 30 min.
2 RESULTSAND DISCUSSION
2.1 SPAs UV absorption spectra
The maximum absorption appears at 275,295,290 and 280 nm for PG,TBHQ,BHA and BHT respectively. So the HPLC detection wavelength is set at 280 nm.
2.2 HPLC condition
When the mobile phase is chosen as MeOH/ACN(1∶1,in volume)with flow rate of 1.0 ml/min,BHA and BHT are separated well,but not for PG and TBHQ.With mobile phase A as MeOH/ACN(1∶1,in volume),B as water/acetic acid(99∶ 1,in volume)of ratio A∶ B=98∶2(in volume)and flo wrate of 0.5 ml/min,PG and TBHQ still could not be separated.So,the gradient elution reported by Razali et al[11]is used with little adjustment,by using MeOH/ACN(1∶ 1,in volume)and water/HAC(99∶ 1,in volume)as mobile phase;or ACN and MeOH/ACN(1∶1,in volume)as mobile phase.The acidified water is used to prohibit ionization of hydroxyl in phenol compounds[12].
The two mobile phases both can give well separation.The second one is faster,so it is chosen in the experiment.Within only 8 min,PG,TBHQ,BHA and BHT are separated in sequence,which is in accordance with their polarity,as shown in Fig.3.
2.3 Linearity and detection limits
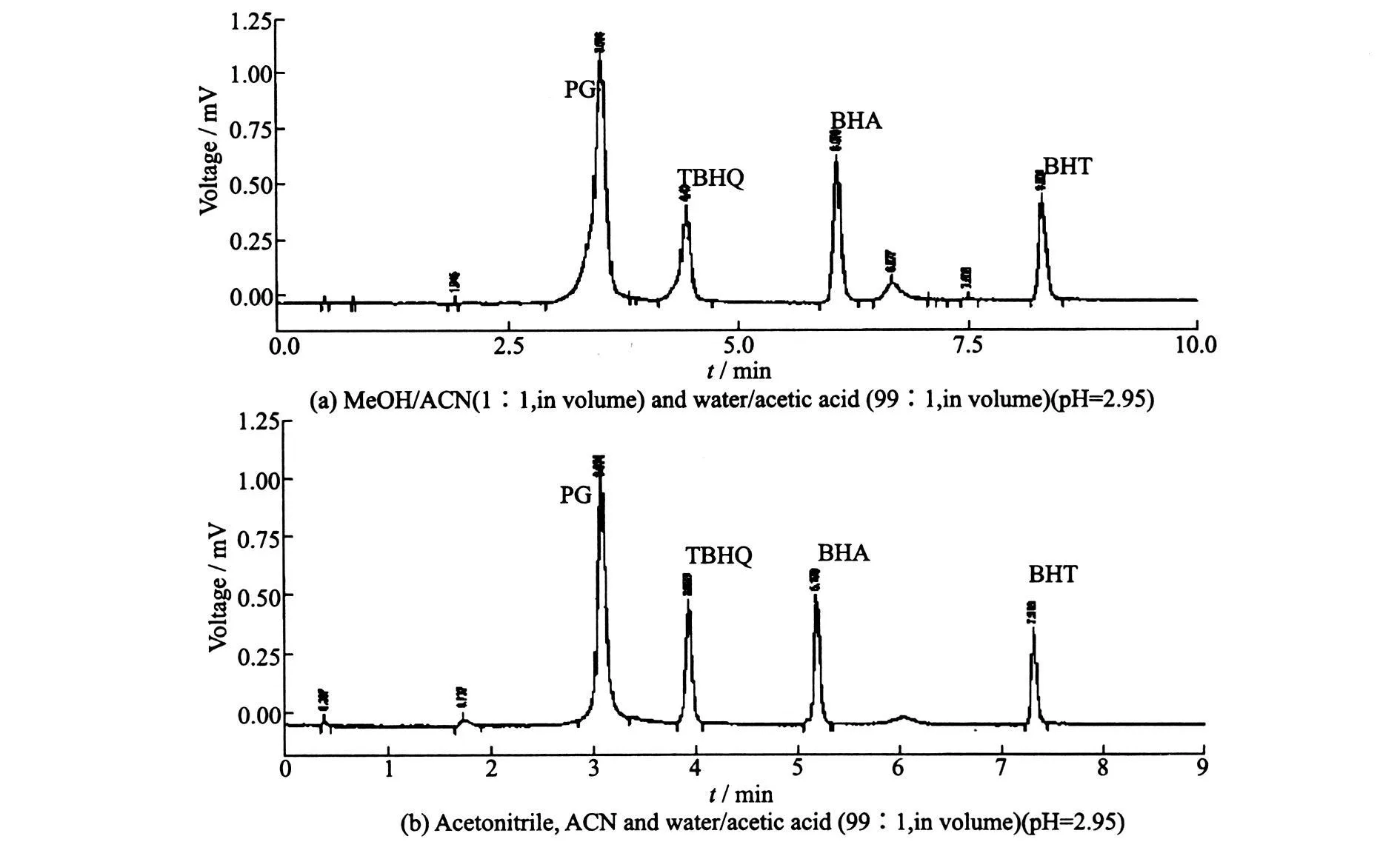
Fig.3 Chromatograms for gradient elution of SPA-freepalm olein sample spiked with 50 mg?l-1each SPA at flow rate of 1.5 ml?min-1
The SPA standard mixture solution(0.1—1.0 mg/l)are injected into HPLC to obtain its sensitivity.The detection limits are obtained with signal noise ratio 3.The detection limit for PGis 0.3 mg/l.The calibration curres are linear over the range of 1.0—300 mg/l for all thefour SPAs.
2.4 Reproducibility study
The intra-day repeatability of the peak area is examined by injecting 50 mg/l SPAs mixture solution 5 times into the HPLCsystem.Relative standard deviation(RSD)for retention times and peak areas are 0.5%—1.6% and 1.1%—3.8%,respectively.The same method is used to obtain the reproducibility after 5 days,and it is found that RSD for retention times and peak areas are all below 4%.
2.5 Optimization of extraction condition
2.5.1 Recovery of cooking oil sample
Sample preparation is a very important process before HPLC analysis.The aim of the sample preparation process is to increase the analysis sensitivity by removing interfering matrix components and particulates and concentrating the analyses.
PG,TBHQ,BHA and BHT dissolved in isopropanol areadded in Malaysia refined palm oil which does not contain SPAs. The final concentrations for these SPAs are 50 and 200 mg/l for each.The solutions are mixed well to have all the SPAs dissolved.
Razali et al[11]used extraction with methanol method to analyze the same palm oil. But extaction with methanol does not suit well with the extraction of SPAs, especially for the extraction of BHT.This is why the paper studies extraction with MeOH/ACN(1∶1,in volume).The shortcoming of extraction with methanol is that the recoveries of PGand TBHQ are high,as the extraction of fat and protein are achieved simultaneously,but the recovery of BHT is low because of its low polarity[13]. According to report,ACN is a suitable ex tractant.Thus,in the experiment,MeOH/ACN is used. After extraction and ultrasound bath,the extracts are saved in refrigerator for 2 h.
The recoveries are obviously increased by using MeOH/ACN(1∶1,in volume)as extractant.The same effect is achieved by using ultrasound/vortex technique,especially for the extraction of BHT.The final results show that the recoveries for the four SPAs are 94.6%—108.3%.
2.5.2 Recoveries of bread spread and cheese samples
The recovery experiment of bread spread and cheese is done with the same recovery method described above,and the recovery of BHT from the bread spread is found to be very low.So,increase the timefor ultrasound bath from 15 min to 25 min,vortex time from 15 s(1 200 r/min)to 5 min 4 min(1 400 r/min)or 1 min(1 600 r/min),thus the recoveries of the four SPAs from bread and cheese can beincreased.
The recoveries of bread spread and cheese samples spiked with SPAs 200 mg/l are listed in Table 1,found with the optimized extraction condition.Table 1 shows that the recovery of BHT recovery is similar with the values found by other research groups(for example,Rafecas et al[14]found the value to be 87% with ACN/propanol as extractant, and Kaˇro vicová &ˇSimko[10]found the value to be 74%with ACN as extractant).Our experiment shows that with MeOH/ACN(1∶1,in volume)as extratant and after ultrasound bath and vortex vibration,the extraction rate can be increased. Also,refrigerator storing can help to precipitate fat material,and all of these techniques can simplify the samples which can increase the HPLCcolumn life term.
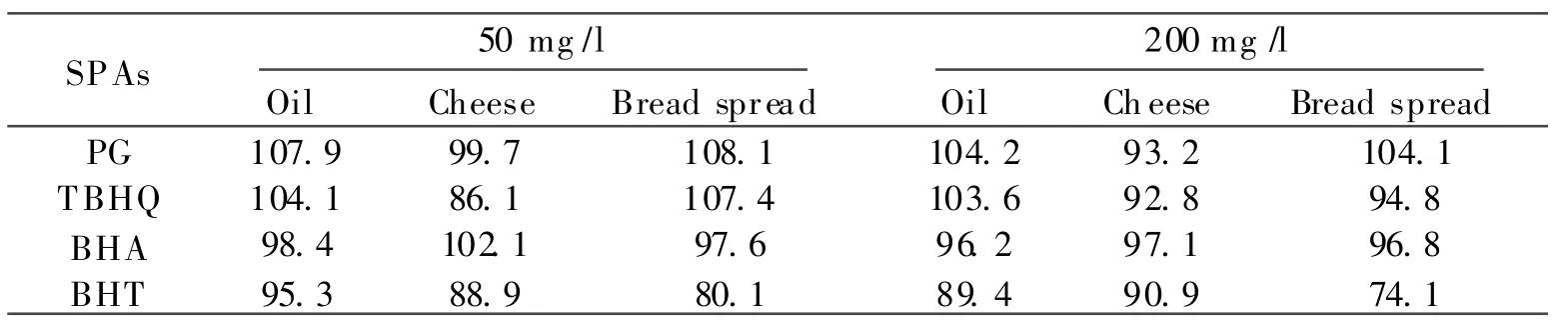
Table 1 Comparison of recoveries of SPAs in food products(n=3)
2.5.3 Sample analysis
The details of the foods containing SPAs are listed in Table 2.The samples are analyzed with the optimized extraction condition. The SPA peaks are identified by retention time,and this method is proved by spiked sample. The determination is achieved by external standard method using linear regression.
Sixteen cooking oils are analyzed,and the results show that most samples do not contain SPAs except for two samples containing 88.9 mg/kg BHT and 20.2 ng/kg TBHQ,as shown in Table 2.Among 16 bread spread samples,7 samples are found to contain BHT(14.4—175.0 mg/kg)and 8 samples are found to contain BHA(5.2—103.9 mg/kg),as shown in Table 2.All the food items containing SPAs are from Malaysia,which contain BHA or BHT,or both.No SPA is found in imported bread spread samples.No PG or TBHQ is found in any bread spread samples.No SPA is found in 6 cheese samples.The values of SPA amount found are below 200 mg/kg which is the Malaysia maximum permitted levels.The food produer marks the related SPAs containing food about the antioxidant, but not about the kinds and amounts.
The results also show that there is a high absorption at 3.5(R. T.) in each the chromatograms of the cheese samples except for 1 cheese sample,and the peak may correspond to preservatives,such as sorbic acid or benzoic acid.This is proved by injection of sorbic acid and benzoic acid samples dissolved in MeOH/ACN(1∶1,in volume)into the HPLC system under the same condition,and the results show that the peaks of sorbic acid and benzoic acid are at 3.5 min(R.T.),which means that most cheese samples contain preservatives.
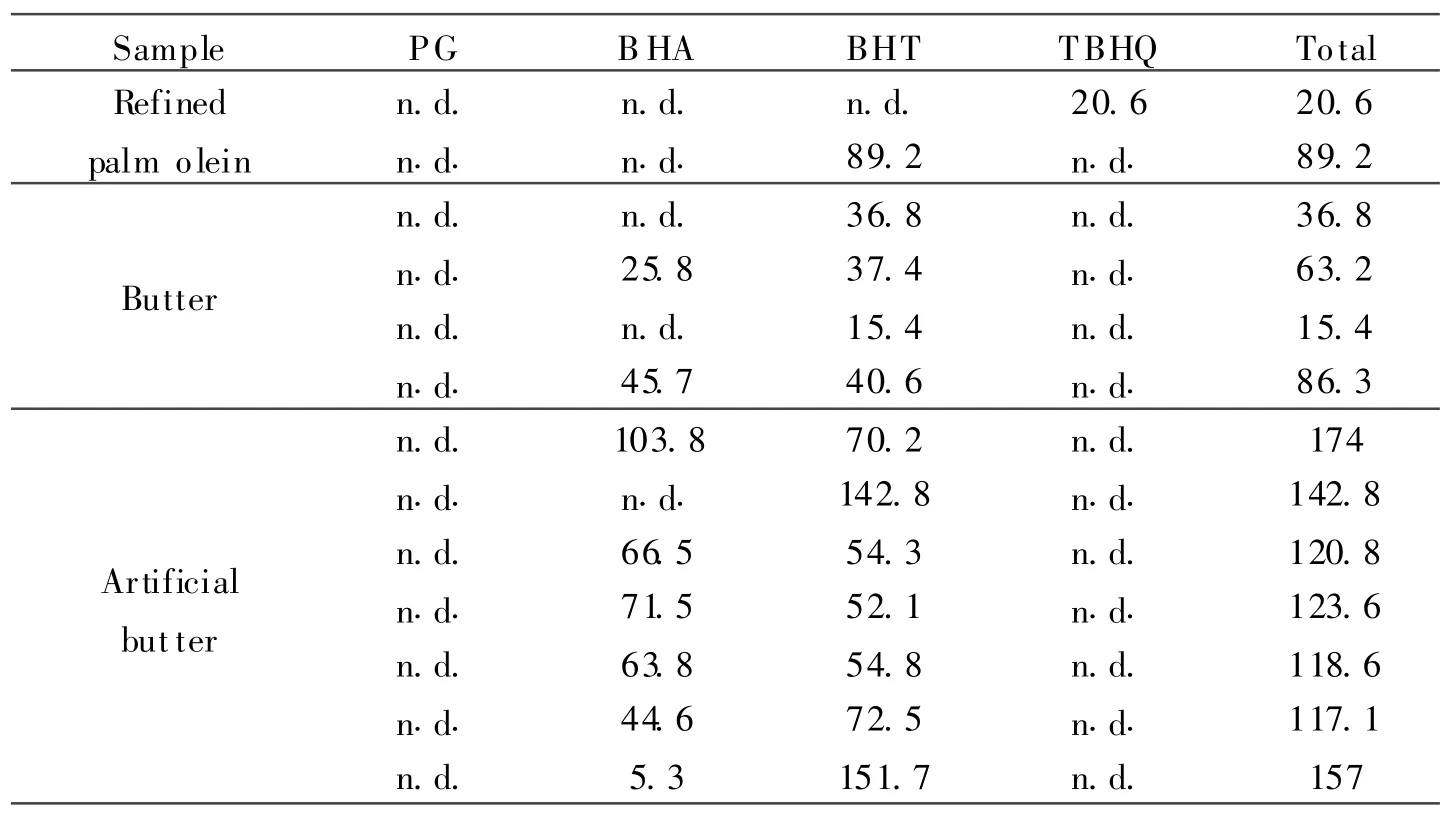
Table 2 Levels of SPAs f ound in SPA-positive food items
3 CONCLUSION
Analysis of SPAs is an important task in executing food safety laws.Using liquid-liquid extraction with MeOH/ACN as extractant can increase the recoveries of SPAs from oil,bread spread and cheese samples.Ultrasound bath and vortex vibrator can increase recoveries. Our extraction method uses less organic solvent compared with previous studies[7,14].12.5% of the oil samples contained SPAs,while no cheese samples contain SPAs. 68.7% bread spread samples contain SPAs (14.4—75.0 ng/kg)though the amo-unt is bellow the permitted level of 200 mg/kg.
[1] Formanek Z,Kerry J P,Higgins F M,et al.Addition of synthetic and natural antioxidants to atocopheryl acetate supplemented beef patties:Effects of antioxidants and packaging on lipid oxidation[J].Meat Science,2001,58:337-341.
[2] McCarthy T L,Kerry J P,Kerry J F,et al.Evaluation of the antioxidant potential of natural food/plant extracts as compared with synthetic antioxidants and vitamin E in raw and cooked pork patties[J].Meat Science,2001,57:45-52.
[3] Grice H C. Safety evaluation of butylated hydrotoluene (BHT) in the liver, lung and gastrointestinal tract[J]. Food and Chemical Toxicology,1986,24:1127-1130.
[4] Wichi H P. Enchanced tumpr development by butylated hydroxyanisole (BHA) from the perspective of effect on forestomach and oesophageal squamous epithelium [J]. Food and Chemical Toxicology,1988,26:717-723.
[5] Noguera-OrtíJ F, Villanueva-Caman~as R M,Ramis-Ramos G.Direct injection of edible oils as microemulsions in a micellar mobile phase applied to the liquid chromatographic determination of synthetic antioxidants[J].Analytica Chimica Acta,1999,387:127-134.
[6] Ragazzi E,Veronese G.Quantitative analysis of phenolic compounds af ter thin-layer chromatography separation[J].Journal of Chromatography,1973,77:369-375.
[7] Gonzá lez M, Gallego M, Valcá rcel M. Gas chromatographic flow method for the preconcentration and simultaneous determination of antioxidant and preservative additives in fatty foods[J].Journal of Chromatography A,1999,848:529-536.
[8] Boyce M C,Spickett E E.Separation of food grade antioxidants(synthetic and natural)using mixed micellar electrokinetic capillary chromatography[J].Journal of Agricultural and Food Chemistry,1999,47:1970-1975.
[9] Guanghan L, Yu W, Leiming Y, et al.Determination of ascorbic acid in fruits and vegetables by stripping voltammetry on a glassy carbon electrode[J].Food Chemistry,1994,51:237-239.
[10]Kaˇrovic ováJ,ˇSimko P.Review:determination of synthetic antioxidants in food by high-performance liquid chromatography [J]. Journal of Chromatography A,2000,882:271-281.
[11]Razali I, Norhaya H, Norasimah A S.Determination of antioxidants in palm oil products by high performance liquid chromatography[J].Elaeis,1997,9:25-33.
[12]López M,MartínezF,Del Valle C,et al.Analysis of phenolic constituents of biological interest in red wines by high-performance liquid chromatography[J].Journal of Chromatography A,2001,922:359-363.
[13]Page B D, Charbonneau C F. Liquid chromatographic determination of seven antioxidants in dry food[J].Journal of the Association of Official Analytical Chemists,1989,72:259-265.
[14]Rafecas M,Guardiola F,Illera M,et al.Liquid chromatographic determination of phenolic antioxidants in bakery products[J]. Journal of Chromatography A,1998,822:305-309.
 Transactions of Nanjing University of Aeronautics and Astronautics2011年2期
Transactions of Nanjing University of Aeronautics and Astronautics2011年2期
- Transactions of Nanjing University of Aeronautics and Astronautics的其它文章
- APPROXIMATION OF INTERVAL BEZIER SURFACES
- STUDY ON OPTIMIZATION OF HIGH PERFORMANCE CONCRETE ADMIXTURES
- ANALYSISOF UN-COINCIDE COORDINATE ERROR IN SINGLE-AXISROTATING FIBER OPTIC STRAPDOWN INERTIAL NAVIGATION SYSTEM
- SI-INSPIRED ENERGY AWARE QoSROUTING TREE FOR WSN
- NOVEL APPROACH TO LOCATOR LAYOUT OPTIMIZATION BASED ON GENETIC ALGORITHM
- COMPENSATION CONTROL OF REAL-TIME UNBALANCE FORCE FOR ACTIVE MAGNETIC BEARING SYSTEM
