Hypothalamic circuits and aging: keeping the circadian clock updated
Rosa Vázquez-Lizarraga, Lucia Mendoza-Viveros,2,3, Carolina Cid-Castro,2,3, Sareni Ruiz-Montoya, Erick Carre?o-Vázquez,Ricardo Orozco-Solis,2,*
Abstract Over the past century, age-related diseases, such as cancer, type-2 diabetes, obesity, and mental illness, have shown a significant increase, negatively impacting overall quality of life.Studies on aged animal models have unveiled a progressive discoordination at multiple regulatory levels, including transcriptional, translational, and post-translational processes, resulting from cellular stress and circadian derangements.The circadian clock emerges as a key regulator, sustaining physiological homeostasis and promoting healthy aging through timely molecular coordination of pivotal cellular processes, such as stem-cell function, cellular stress responses, and inter-tissue communication,which become disrupted during aging.Given the crucial role of hypothalamic circuits in regulating organismal physiology, metabolic control, sleep homeostasis, and circadian rhythms, and their dependence on these processes, strategies aimed at enhancing hypothalamic and circadian function,including pharmacological and non-pharmacological approaches, offer systemic benefits for healthy aging.Intranasal brain-directed drug administration represents a promising avenue for effectively targeting specific brain regions, like the hypothalamus, while reducing side effects associated with systemic drug delivery, thereby presenting new therapeutic possibilities for diverse age-related conditions.
Key Words: aging; astrocytes; cellular stress responses; circadian clock; hypothalamus; intranasal drug administration; metabolic control; nutrient sensor; SIRT1; sleep homeostasis
Introduction
Population studies show that life expectancy has increased by around 13–14 years in the last 50 years.However, studies conducted on women and men aged 60 years or older show a dramatic increase in diseases associated with the aging process, including cancer, neurodegenerative diseases,type 2 diabetes mellitus, and cardiovascular diseases.The aging process is characterized by a gradual loss of physiological, cellular, and molecular functions that leads to the development of various pathologies.Genetic components are involved in longevity, as different species display the different extents of longevity, a notion supported by the recent identification of specific polymorphism associated with aging (Orozco-Solis and Sassone-Corsi,2014a; Lin et al., 2021).This is explained in part by the way in which each organism is adapted and interacts with the environment to ensure survival,through complex gene-environment links, to ensure organismal homeostasis.Therefore, during aging these molecular and physiological mechanisms decay provoking an organismal disorganization and concomitantly, a plethora of agerelated diseases (López-Otín et al., 2023).Although aging is a natural process,many of the pathologies associated with this process can be reduced, a concept known as “healthy aging” (Orozco-Solis and Sassone-Corsi, 2014a).Studies carried out during the last 30 years have focused on understanding the physiological, cellular, and molecular mechanisms underlying aging phenotypes (Orozco-Solis and Sassone-Corsi, 2014a; Goodell and Rando,2015; Sen et al., 2016; He and Sharpless, 2017; López-Otín et al., 2023).These studies have identified different pathways that, when modulated, are capable of significantly reducing the effects of aging, including nutrient and metabolic sensors such as sirtuins (SIRTs), adenosine monophosphate-activated protein kinase (AMPK), mammalian target of rapamycin (mTOR), peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1a), circadian rhythms, epigenetic mechanisms, and cellular stress response mechanisms(Orozco-Solis and Sassone-Corsi, 2014a; López-Otín et al., 2023).While these evolutionary conserved mechanisms are ubiquitously present in all tissues and cells, their contribution the cellular and organismal homeostasis is specific.Therefore, taking into consideration the notion of integrons in aging (Dillin et al., 2014) and the observation that key tissues are able to influence the whole body homeostasis (Brown et al., 2013; Satoh et al., 2013; Zhang et al., 2013),it is conceivable that modulating these mechanisms in these key tissues would substantially improve the healthy aging in the whole organism.In this regard,the hypothalamic circuits play a crucial role in integrating and coordinating a wide range of physiological and metabolic processes (Figure 1A).Dysfunction of these circuits can give rise to disorders that are considered hallmarks of aging, including obesity, diabetes, sleep disorders, hypertension, and reproductive dysfunction.Importantly, the circadian clock, present in virtually every cell and tissue, is centrally controlled by the suprachiasmatic nucleus of the hypothalamus.The circadian clock contributes to homeostatic control by regulating various physiological processes, metabolism, behavior, endocrine function, and cellular pathways(Orozco-Solis and Sassone-Corsi, 2014a).Therefore, understanding how aging affects these pivotal circuits is essential for developing strategies to mitigate age-related declines in physiological and metabolic function.In this review, we summarize some of the mechanisms contributing to the detrimental effects of aging, with an emphasis on the role of the circadian clock at both central and peripheral levels.Furthermore,we delve into the critical role of silent mating-type information regulation 2 homolog 1 (SIRT1) as a nutrient sensor connecting metabolism with various key cellular and molecular processes that deteriorate during aging, such as circadian rhythms, cellular stress responses, and stem cell homeostasis.Finally, we analyze the impact of these processes on the hypothalamic control of organismal homeostasis and propose therapeutic strategies to enhance its function and, consequently, promoting healthy aging.
Search Strategy
We conducted a search in the PubMed and Google Scholar databases, using the following keywords: “aging”, “circadian clock”, “hypothalamus”, “SIRT1”,“metabolism”, “cellular stress response” and “intranasal drug administration”.We limited our search to articles published between 2010 and 2023 but also included relevant articles published before this period.
The Circadian Clock
Throughout evolution, organisms have adapted to the natural 24-hour daynight cycle and developed the ability to anticipate and regulate their biological processes accordingly.Circadian rhythms govern a wide array of physiological,behavioral, and metabolic functions.In mammals, the suprachiasmatic nucleus (SCN) located in the hypothalamus acts as the central pacemaker,synchronizing daily with light cues through the retino-hypothalamic tract.This synchronization extends to peripheral clocks in other brain regions and organs, through synaptic connections, autonomic innervations, and endocrine signaling.Additionally, non-photic inputs such as behavior, nutrition, exercise,and social interactions also influence the SCN’s activity (Orozco-Solis and Sassone-Corsi, 2014b).
At the molecular level, the circadian clock operates through a complex network of interrelated transcriptional-translational feedback loops.Central to this network are the transcription factors CLOCK and BMAL1, which form heterodimers and cyclically bind to specific promoter elements known as E-boxes, activating the expression of clock-controlled genes (CCGs).Among these CCGs, the proteins period 1–3 (Per 1–3) and cryptochrome 1–2 (Cry1–2) play a critical role in repressing the activity of CLOCK/BMAL1 through a negative feedback loop with a period of approximately 24 hours(Figure 1B; Aguilar-Arnal and Sassone-Corsi, 2013; Orozco-Solis and Sassone-Corsi, 2014b).Additionally, several CCGs encode transcriptional regulators such as D-box binding protein, thyrotroph embryonic factor, retinoic acidrelated orphan receptor alpha, and reverse erythroblastosis virus alpha and beta (REV-ERBα/β).These proteins bind to specific promoter elements and further contribute to the generation of circadian waves in gene expression.It is estimated that the circadian clock controls the rhythmic expression of approximately 10–30% of genes in a given cell (Aguilar-Arnal and Sassone-Corsi, 2013; Orozco-Solis and Sassone-Corsi, 2014b).However, recent studies suggest that many more genes may exhibit robust oscillations in response to nutritional and metabolic cues, indicating a broader role for the circadian system in modulating physiological processes (Eckel-Mahan et al., 2013;Orozco-Solis and Sassone-Corsi, 2014b).For example, a high-caloric diet induces a profound reprogramming in the circadian transcriptome and,consequently, in the metabolome in the liver (Eckel-Mahan et al., 2013).In this context, the circadian clock is intricately regulated by nutritional and hormonal cues, operating through dedicated signaling pathways.Seminal investigations have unraveled the impact of nutrient sensors and hormones on the circadian clock, highlighting the essential involvement of pathways such as SIRTs, AMPK, mTOR, and PI3K (Additional Table 1).These pathways orchestrate transcriptional responses mediated by key transcriptional regulators including FOXO1, X-box binding protein 1 (XBP1), ATF1, ChREBP,STAT3, pCREB, SF1, and others.Furthermore, posttranslational modifications on clock proteins, cellular energy sensors, and chromatin remodeling events contribute additional layers of regulation.
Importantly, the inter-tissue communication between the peripheral and central clock is of vital importance in the control of homeostasis.For instance,peripheral clocks alone exhibit limited capacity to generate local rhythms and rely on extracellular circadian cues such as neurotransmitters, hormones,peptides, temperature cycles, and nutrients (Dyar et al., 2018; Koronowski and Sassone-Corsi, 2021), emphasizing the crucial role of circadian coordination across tissues for maintaining organismal homeostasis (Koronowski and Sassone-Corsi, 2021).This is supported by compelling evidence from tissuespecific loss-of-function and reconstitution experiments in clock-deficient mice (Greco et al., 2021; Petrus et al., 2022).For example, recent studies have shed light on the collaborative regulation of glucose homeostasis by the liver and muscle clocks, in coordination with feeding-fasting rhythms(Smith et al., 2023).The insulin secreted by the pancreas exerts its influence on the circadian clock through the IRS/IGF1-PI3K-mTOR axis, resulting in the upregulation of PER2 translation (Crosby et al., 2019).Additionally, glucagon activates BMAL1 expression in hepatocytes via CREB phosphorylation (Sun et al., 2015).
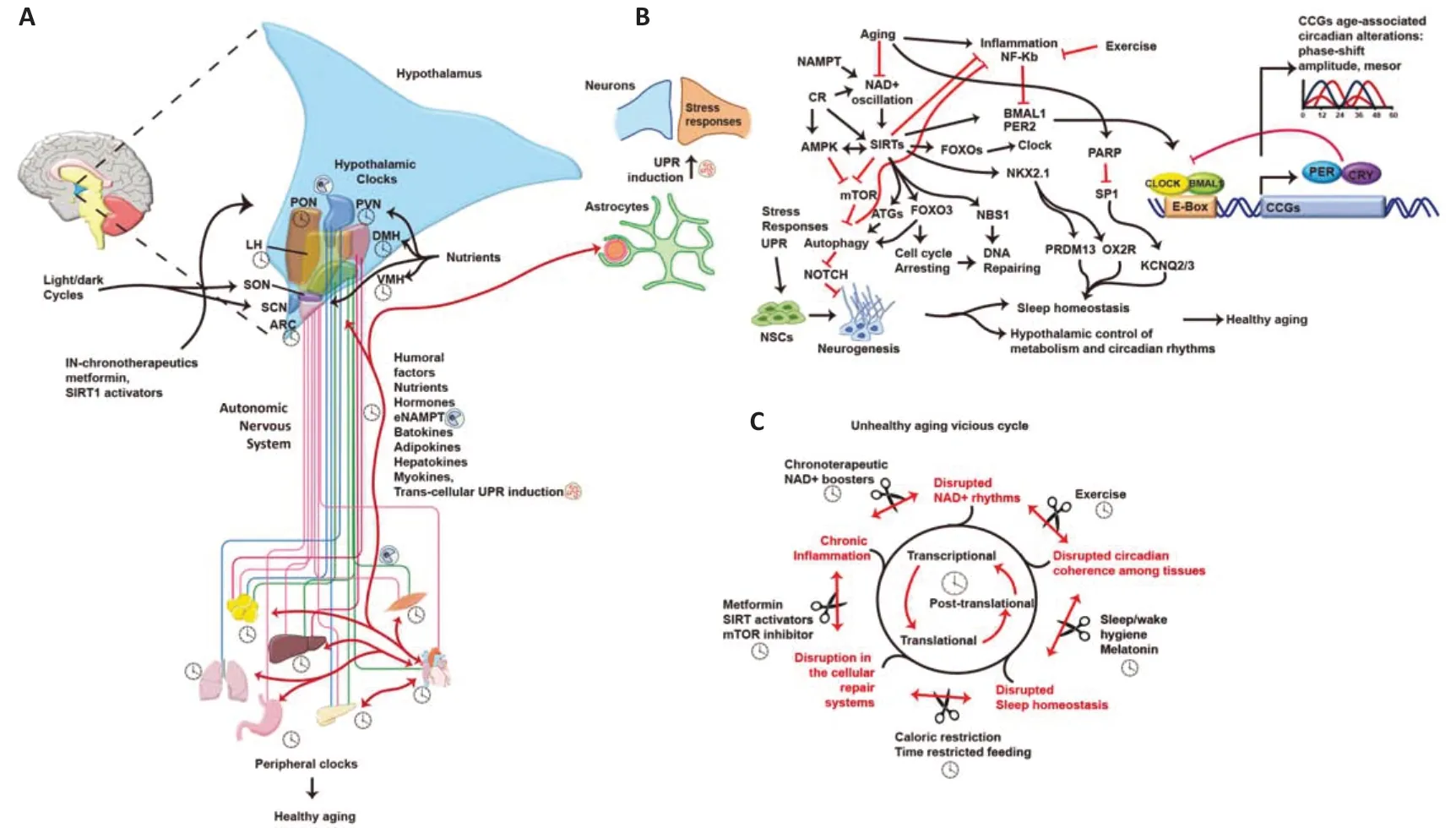
Figure 1 |Hypothalamus as a therapeutic target to reduce age-related diseases.(A) Hypothalamic nuclei and their clocks receive external and humoral signals, inducing neuronal and astrocytic responses that modulate peripheral tissues and clocks via the autonomous nervous system (ANS) and hormones.Circadian functional coherence between the hypothalamus and peripheral tissues is necessary to maintain organismal homeostasis and promote healthy aging.Intranasal (IN) drug administration might improve hypothalamic function.(B) Astrocytes and neurons interact to maintain hypothalamic functions,including appropriate cellular stress responses.Caloric restriction (CR) and exercise modulate metabolic sensors such as AMPK, MTOR, and SIRT1, ultimately inducing unfolded protein response (UPR), autophagy, cell cycle arrest, and DNA repair processes.These mechanisms support functional cellular processes like neuronal stem cells, neurogenesis,sleep homeostasis, hypothalamic metabolic control, and circadian rhythms.(C) During aging, accumulating alterations create a progressive vicious cycle that disrupts gene expression regulatory mechanisms, induces inflammation, and interferes with protective cellular responses, circadian coordination, sleep homeostasis, metabolic control, and more.However, specific therapies and chronotherapies aimed at improving hypothalamic functions, such as NAD+ boosters, CR, time-restricted feeding (TRF), melatonin, etc., may slow down this vicious cycle and promote healthy aging.Peripheral tissue figures were drawn by using pictures from Servier Medical Art.Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/).AMPK: AMP-activated protein kinase; ARC: arcuate nucleus; ATGs:autophagy-related; BMAL1: brain and muscle ARNT-like 1; CCGs: clock-controlled genes; CLOCK: circadian locomotor output cycles kaput; CR: caloric restriction; DMH: dorsomedial hypothalamus; Enampt: extracellular nicotinamide phosphoribosyltransferase; FOXO3: forkhead box O3; KCNQ2/3: potassium voltage-gated channel subfamily Q member 2/3; LH:lateral hypothalamus; MTOR: mammalian target of rapamycin; NAD: nicotinamide adenine dinucleotide; NAMPT: nicotinamide phosphoribosyltransferase; NBS1: nijmegen breakage syndrome 1; NFKB: nuclear factor kappa B; NKX2.1: NK2 homebox 1; NOTCH: notch receptor; OX2R: orexin 2 receptor; PARP: poly(ADP-ribose) polymerase; PER2: period circadian regulator 2; PON: preoptic nucleus; PRDM13: PR domain-containing protein 13; PVN: paraventricular nucleus; SCN: suprachiasmatic nucleus; SIRTs: sirtuins; SON: supraoptic nucleus;SP1: specificity protein 1; UPR: unfolded protein response; VMH: ventromedial hypothalamus.
The liver circadian clock is also modulated by hepatic-secreted peptides or hepatokines.The hepatokine angiopoietin-related protein 8 (ANGPTL8) resets diurnal rhythms of hepatic clock and metabolic genes through PirB-mediated signaling, kinase phosphorylation, and transient activation of the core-clock gene PER1 (Chen et al., 2019).FGF21, another hepatokine, acts distantly,modulating circadian behavior by inhibiting AVP signaling in the SCN, thus influencing the central clock (Bookout et al., 2013).
In this regard, the serum response factor (SRF), a transcription factor stimulated by serum, plays a crucial role in synchronizing the central and peripheral clocks through the RHO-MRTF pathway.Moreover, activation of SRF regulates core-clock target genes PER2, CRY, and NFIL3 by binding to serum response elements within their promoters (Gerber et al., 2013; Xiong et al., 2022).Interestingly, SRF also plays a pivotal role in essential brain functions such as immediate early gene activation, neuronal cell migration,neurite outgrowth, synaptic plasticity, learning, and memory, and may contribute to SCN function (Kornhauser et al., 1996; Kn?ll and Nordheim,2009).However, the specific blood-borne factor and its receptor involved in the SRF signaling, as well as the circadian influence of the SRF on the central nervous system (CNS) remain elusive.
Therefore, it is intriguing to speculate that unidentified humoral factors may serve as synchronizers across various brain areas and tissues, thus ensuring the coordination of circadian rhythms.This represents an exciting avenue for further research.
The Circadian Clock in Aging
Circadian disruption has been associated with several age-related pathologies,including cancer, chronic inflammation, cardiovascular diseases, metabolic disorders, and mental illnesses (Hofman and Swaab, 2006; Kondratova and Kondratov, 2012; Orozco-Solis and Sassone-Corsi, 2014a).As individuals age,changes in the daily fluctuations of hormones like melatonin and cortisol,body temperature, and disruptions in sleep patterns can result in disturbances to the body’s natural cycles.These disruptions encompass reductions in both the amplitude and timing of these cycles (Carskadon et al., 1982; Orozco-Solis and Sassone-Corsi, 2014a; Figure 1B).
For instance, pioneering and current studies have established a causal link between the central clock function and the aging process.Grafting fetal SCN to an aged hamster model (known as taus/+) restored behavioral rhythmicity and extended lifespan (Hurd and Ralph, 1998).This finding is consistent with the observation that young and aged mice exhibit different rhythms in the gene expression of core-clock genes in the SCN (Chang and Guarente, 2013;Bonaconsa et al., 2014).Moreover, transplantation of the pineal gland from young to old mice or chronic melatonin administration at night has been shown to prolong lifespan, while the opposite effect was observed when the glands were implanted from old to young mice (Lesnikov and Pierpaoli, 1994).Additionally, melatonin administration has been found to improve metabolism in middle-aged rats, bringing it closer to that of young animals (Rasmussen et al., 1999).In addition to its circadian-related functions, melatonin also exhibits immunomodulatory, neuroendocrine, and antioxidant effects, all of which contribute to its anti-aging properties (Hardeland et al., 2003; Poeggeler,2005).In line with this, the strength of melatonin’s rhythmic patterns in the blood gradually diminishes as individuals age.Intriguingly, cultured fibroblasts treated with serum from older individuals exhibited a shorter circadian period and phase advance, suggesting that a circulating factor in older serum can alter cellular rhythms.Notably, this factor is not melatonin or cortisol, as the levels of these hormones were found to be similar in young and old blood donors (Pagani et al., 2011).Therefore, hormones and yet unknown serum factors may contribute to aging by disrupting circadian rhythms (Schwarz et al., 2023).However, further research is necessary to address open questions such as the identity of these factors, the circadian pattern in young/old individuals, their tissue source/target tissues, and the implications for healthy or unhealthy aging.
On this line, growing evidence has demonstrated that the loss of intracellular communication involving alterations in neuronal, neuroendocrine, hormonal signaling pathways, as well as serum factors secreted by adipose tissue, liver,heart, muscle, etc., or cell non-autonomous mechanisms, are disrupted during aging (Higuchi-Sanabria et al., 2020; Miller et al., 2020; Koronowski and Sassone-Corsi, 2021; López-Otín et al., 2023), contributing with the perturbation of the circadian communication between tissues, leading to the induction of age-associated pathologies (Koronowski and Sassone-Corsi,2021).
Furthermore, in aging, thousands of circadian genes undergo tissue-specific reorganization, as evidenced by circadian transcriptomic experiments conducted on various aged organisms, including mice, flies, and the human brain.Notably, there is also an age-related reduction in the number of rhythmic transcripts (Chen et al., 2016; Kuintzle et al., 2017; Sato et al.,2017; Solanas et al., 2017; Wolff et al., 2023).It has been postulated that with aging, the circadian clock may lose its predictive homeostasis, leading to an increased reliance on reactive pathways in response to stressors and altered communication between peripheral clocks (Sato et al., 2017; Wolff et al., 2023).For example, a recent study revealed disrupted liver rhythms in lipid metabolism during aging, characterized by a 4-hour phase-shift in the circadian expression of the transcription factor EGR1, which controls the expression of lipid metabolism genes such as Cidea and Per2.Strikingly,aligning the light-dark cycle with the liver oscillation of EGR1 in aged mice reversed the metabolic disturbances (Wu et al., 2023).Consistently,Adlanmerini et al.(2021) found that the circadian nuclear receptor REV-ERBα/β promotes metabolic adaptation to an obesogenic diet by modulating diurnal food intake and leptin sensitivity.Notably, mice lacking REV-ERBα/β in the SCN display an intrinsic circadian period of 21 hours, leading to a misalignment between internal and external time and resulting in metabolic disruptions.Remarkably, when the external light-dark cycles are adjusted to match the 21-hour period, the internal and external rhythms become synchronized again,reversing the metabolic effects (Adlanmerini et al., 2021).Along these lines,aligning the light-dark cycle with the 4-hour displaced rhythms in the liver of aged mice reverts metabolic disturbances (Wu et al., 2023).
Another interesting factor that might contribute to circadian coordination during aging is the steroid hormone estrogen.It is known that females have higher longevity compared to males, and this has been partly attributed to better energetic metabolism, neuroprotection, anti-oxidative responses,immunological and inflammatory responses, and sex-associated differences in stem cells (Dulken and Brunet, 2015; Suarez et al., 2023), all these processes modulated by the circadian clock (Aguilar-Arnal and Sassone-Corsi, 2011;Orozco-Solis and Sassone-Corsi, 2014a; Benitah and Welz, 2020; Nassan and Videnovic, 2022).For instance, estrogen signaling has been linked with circadian control (Hatcher et al., 2020).In line with this, recent observations have indicated that rhythmic gene expression exhibits sex-specific differences,with a more sustained pattern in females during aging (Noh et al., 2022;Talamanca et al., 2023).Interestingly, mice treated with a weak estrogen receptor, 17-alpha-estradiol (17aE2), robustly extend lifespan in male mice but not in females (Strong et al., 2016).Importantly, estrogen receptors alpha and beta are ubiquitously expressed in various tissues, including the brain,heart, lungs, liver, kidney, gastrointestinal tract, adrenals, bone, bladder,mammary gland, fallopian tube, ovary, uterus, prostate, and testis (Drummond and Fuller, 2010; Warner et al., 2017).Furthermore, the estrogen receptors Esr1 and Esr2 exhibit circadian expression, and interestingly, the oscillation of Esr1 is lost in the kidney and muscle of aged mice, while the oscillation of Esr2 is lost in muscle from aged mice (from reported data Aging Circadian Database) (Wolff et al., 2023).However, further research is needed to determine if females maintain stronger circadian synchrony among tissues during aging and how estrogen could couple the different clocks.
Finally, stem-cell function is crucial for tissue maintenance and regeneration,while during aging this capacity is reduced by a process known as “stem cell exhaustion” (López-Otín et al., 2023).Interestingly, during aging the circadian rhythms in gene expression in the stem cells are profoundly reprogrammed(Sato et al., 2017; Solanas et al., 2017; Wolff et al., 2023); aged epidermal stem cells maintain diurnal rhythms in cell division while losing their ability to temporally organize DNA replication to minimize UV exposure, thereby increasing the risk of DNA mutations.Similarly, aged muscular stem cells preserve rhythms in cellular functions related to cellular interactions within their niche to protect quiescent cells, but at the expense of losing rhythms in proteostasis-preserving functions like autophagy (Solanas et al., 2017).Likewise, in differentiated skeletal cells, rhythmic genes involved in autophagy lose temporal organization, impacting muscle maintenance such as myofibril assembly, while the vascular endothelial growth factor pathway gains rhythmicity (Wolff et al., 2023).Importantly, recent studies have highlighted the reciprocal regulation between chaperone-mediated autophagy and the circadian clock (Juste et al., 2021), and both processes decline with aging(Aman et al., 2021; Ulgherait et al., 2021).Collectively these data support the notion that chronotherapeutic strategies aiming to correct the internal cellular clock and/or to synchronize circadian rhythms among different tissues can reduce age-related diseases (Orozco-Solis and Sassone-Corsi, 2014a).
SIRT1: a Caloric Restriction Effector
As organisms age, their metabolic function tends to decline.This decline can be attributed to changes in energy metabolism, nutrient sensing, and metabolic signaling pathways.Importantly, recent research has shed light on the impact of environmental factors such as caloric restriction, exercise,chronotherapeutic interventions, and pharmacological strategies in improving metabolic control and reducing the effects of aging.In this regard, caloric restriction (CR) has emerged as a potent environmental intervention that delays the aging process in various organisms, including yeast, worms,flies, rodents, primates, and humans (Di Francesco et al., 2018).Notably,experimental protocols of CR often involve time-restricted feeding, serving as a nutritional zeitgeber.Interestingly, studies have shown that time-restricted feeding without caloric reduction alone can induce significant beneficial metabolic effects in various organisms, including flies, mice, and humans(Longo and Panda, 2016).In line with this, a recent study examined the individual contributions of each nutritional intervention on lifespan (Acosta-Rodríguez et al., 2022).The authors found that while CR itself (spreading out food throughout the entire 24 hours) extended lifespan by 10%, combining it with time-restricted feeding during the day extended lifespan by 20%,strikingly, night-restricted feeding extended lifespan by 35% (Acosta-Rodríguez et al., 2022).Interestingly, while feeding time has a weak influence on circadian gene expression, in aged mice, it strongly relies on natural feeding time, emphasizing the importance of feeding time in the regulation of metabolism during aging (Acosta-Rodríguez et al., 2022).
One of the key metabolites involved in the beneficial effects of CR is the nicotinamide adenine dinucleotide (NAD+).Interestingly it has been shown that the levels of NAD+decline during aging, while calorie restriction increases its levels (Gomes et al., 2013; Yoshino et al., 2018).NAD+can be synthesized de novo from tryptophan or through the salvage pathway.Importantly circadian clock regulates the synthesis of NAD+by controlling the circadian expression of the nicotinamide phosphoribosyltransferase (NAMPT) gene,which encodes the key rate-limiting enzyme in the NAD+salvage pathway.Moreover, there are two forms of NAMPT: intracellular NAMPT (iNAMPT)and extracellular NAMPT (eNAMPT).eNAMPT serves as an inter-tissue communication system that maintains NAD+homeostasis in the organism(Yoshida et al., 2019).In this regard, since the discovery in the early 2000s that CR delays aging and promotes metabolic benefits in part by activating the NAD+-dependent deacetylase SIRT1 (Cohen et al., 2004), it is now evident that SIRT1 possesses pleiotropic functions as an anti-aging effector in part by modulating the circadian clock via the deacetylation of its target proteins including the core-clock proteins PER2 and BMAL1 or the regulators FOXO1, PGC1a, P53, E2F1, STAT3, SCREBP1C, LXR, NFkB, etc.(Orozco-Solis and Sassone-Corsi, 2014a), as well as histone H3 K9/K14 at CCGs promoters,contributing to circadian chromatin remodeling through epigenetic mechanisms (Aguilar-Arnal et al., 2015).Indeed, it has been recently discovered that CR can rescue the age-dependent loss of cyclic global protein acetylation in the liver, with SIRT1 playing a pivotal role (Sato et al., 2017).
Moreover, SIRT1 is necessary for efficient DNA repair by modifying chromatin structure, allowing the recruitment of DNA repair factors such as NBS1(Oberdoerffer et al., 2008), and its overexpression or NAD+-mediated activation, triggers oxidative stress responses, in part by arresting the cell cycle via FOXO3 in mice (Brunet et al., 2004; Kobayashi et al., 2005; Lee et al., 2008).Interestingly these cellular protective processes are evolutionary conserved; in C.elegans, genetic or pharmacological restoration of NAD+prevents age-associated metabolic decline and promotes longevity via SIR-2.1 (a human SIRT1 ortholog)-mediated deacetylation of the FOXO1/DAF-16 transcription factor, thereby increasing the mitochondrial unfolded protein response (Mouchiroud et al., 2013).
Finally, SIRT1 has been widely implicated in the maintenance of stem-cell functions, including stemness, differentiation, and stress protection, slowing down the detrimental effects of aging.In neuronal, embryonic, hematopoietic,or adipose stem cells, SIRT1 induces autophagy, reducing oxidative stress and senescence (Libert et al., 2008; Ou et al., 2014; Liu et al., 2018, 2022).For instance, SIRT1 stimulates autophagy via mTOR inhibition and deacetylation of several autophagic proteins such as ATG5, ATG7, and ATG8 (Huang et al.,2015), which might induce anti-aging effects (Salminen and Kaarniranta, 2009;Chen et al., 2020).Moreover, SIRT1 activation promotes neurogenesis over self-renewal by BCL6-SIRT1-mediated deacetylation and repression of target genes in the NOTCH signaling pathway, similar to the effects of CR, stimulating neurogenesis and enhancing synaptic plasticity (Hornsby et al., 2016).Also,the SIRT1 activator SRT1720 improves metabolism and confers protection against physiological and cognitive disturbances in old mice fed a high-fat diet, partly through the inhibition of age-associated inflammation via NFKB inactivation, a condition known to inhibit autophagy (Kauppinen et al., 2013;Mitchell et al., 2014; Kang et al., 2015).Likewise, metformin activates AMPK,SIRT1 and inhibits mTORC1 pathways (Martin-Montalvo et al., 2013; Kulkarni et al., 2020) and induces neurogenesis (Wang et al., 2012; Tanokashira et al.,2018)
Therefore, anti-aging therapies aiming to increase SIRT1 activity are promising strategies to enhance healthy aging in part by improving the circadian clock function, by enhancing cellular anti-stress responses, and by reducing systemic inflammation.
The Hypothalamus: an Aging Clock Controlled by a Circadian Clock
Given their role in integrating external and peripheral information and coordinating various physiological processes such as growth, development,reproduction, metabolism, circadian rhythms, sleep regulation, endocrine control, body temperature, stress response, reproductive function, and autonomic nervous system, the hypothalamic circuits have been hypothesized to potentially impact the rate of aging (Li et al., 2012; Zhang et al., 2013,2017; Orozco-Solis and Sassone-Corsi, 2014a; Cavadas et al., 2016; Cai and Khor, 2019).These neural networks consist of multiple nuclei including the suprachiasmatic nucleus (SCN), arcuate nucleus (ARC), paraventricular nucleus (PVN), ventromedial hypothalamus (VMH), dorsomedial hypothalamus (DMH), and lateral hypothalamus (LH) (Figure 1A).They receive and process inputs from various sensory systems, responding to circulating hormones, nutrients, and neurotransmitters.These signals converge on key dedicated sensors and signaling pathways (Additional Table 1), which in turn induce chromatin modifications, transcriptional responses,and ultimately neuronal responses.Importantly, accumulating identification of new subpopulations of neurons within these nuclei, controlling very specific autonomic outputs in different tissues, stresses the notion that neural circuits involved in autonomic regulation are highly specialized and functionally diverse.This intricate integration enables precise regulation of the organism’s energetic demands and circadian control (Myers and Olson,2012; Pozo and Claret, 2018; Figure 1A).
The disruption of these pathways within distinct hypothalamic nuclei leads to metabolic disturbances, causing alterations in specific behavioral and homeostatic responses in various target tissues (Additional Table 1).For instance, the deletion of SIRT1 in pro-opiomelanocortin neurons within the ARC results in heightened susceptibility to diet-induced obesity.This is attributed to reduced energy expenditure caused by diminished sympatheticmediated activation of brown adipose tissue (BAT) (Ramadori et al., 2010).Additionally, the elimination of SIRT1 in AGRP neurons leads to decreased electrical responses of AGRP neurons to ghrelin, resulting in reduced food intake, lean mass, fat mass, and body weight (Dietrich et al., 2010).In the VMH, SIRT1 is crucial for the adaptation of the central clock to feeding cues(Orozco-Solis et al., 2015).Interestingly, a recent study identified a SCNPVN (corticotropin-releasing factor neurons)-LH (hypocretin neurons) circuit in mice, in which SCN-GABAergic neurons increase corticotropin-releasing factor neuronal activity in the PVN during the night, thereby activating orexin neurons in the LH (Ono et al., 2020).
Notably, most of these studies attempting to elucidate the molecular mechanisms controlling energy balance by hypothalamic signaling pathways did not consider the timing of the day or the role of the molecular clock in this regulation.However, current research is starting to shed light on the role of the hypothalamic circadian clock in the control of metabolism, as initially established in peripheral tissues.For instance, the core-clock gene BMAL1 in Sf1 neurons of the VMH participates in the circadian control of energy expenditure through the circadian modulation of brown adipose tissue(Orozco-Solis et al., 2016).Remarkably, the circadian clock in the forebrain plays a pivotal role in aligning feeding rhythms with the light/dark cycle and controlling hepatic glucose production throughout the day-night cycle.This is achieved through the circadian coordination of essential biological processes,including insulin, glucagon, and FOXO signaling pathways (Cedernaes et al., 2019).Furthermore, in AGRP neurons, BMAL1 coordinates neuronal responses to circadian fluctuations in leptin levels and feeding conditions,thereby maintaining energy homeostasis (Cedernaes et al., 2019).Moreover as mentioned above the circadian nuclear receptor REV-ERBα/β in the SCN plays a pivotal role in the synchronization of light-dark cycles with the feeding cycles by the diurnal modulation of leptin sensitivity (Adlanmerini et al.,2021).
In this regard, the desynchronization between central and peripheral tissues occurring during aging might be due to a reduced capacity of the brain clocks to adapt to environmental zeitgebers.For instance, SIRT1 controls the central clock by activating BMAL1 and CLOCK through PGC1a and NAMPT.However,this mechanism decays during aging, resulting in a longer interval period,disrupted circadian activity, and reduced capacity to adapt to light inputs.Interestingly, overexpression of SIRT1 in the brain protects against these effects in aged mice (Chang and Guarente, 2013).Moreover, in young mice,the PVN, which sends and receives signals from the SCN, exhibits remarkable neuronal plasticity in response to changes in the light-dark photoperiod cycles.However, this plasticity is attenuated in aged mice, implying a potential role of epigenetic mechanisms in mediating this age-related effect (Pritchard et al., 2020).
Environmental aggressors can disrupt the function of hypothalamic pacemakers.For example, exposure to low-dose bisphenol A dysregulates neurogenesis in the SCN and induces long-lasting effects on circadian function.Notably, these effects can be maintained transgenerationally,likely mediated by epigenetic mechanisms (Nesan et al., 2021).Moreover,age-related changes in chromatin modifications have been associated with decreased expression of kisspeptin (Kiss1), as well as the core-clock genes Per1 and Per2, within the anteroventral periventricular nucleus of the hypothalamus.The anteroventral periventricular nucleus is involved in the KISS1-mediated activation of gonadotropin-releasing hormone and the subsequent release of luteinizing hormone from the anterior pituitary gland (Dai et al., 2022).Interestingly, KISS1 also plays a role in the circadian regulation of energy metabolism and sleep/wake cycles in the ARC, aiding in the coordination of metabolism and reproduction in females (Padilla et al.,2019).
Furthermore, SCN-mediated circadian control of hypothalamic orexin levels promotes sympathetic activity and hepatic glucose production via OX2R.Conversely, during the night, it inhibits glucose production via OX1R and parasympathetic activity.This circadian control is accompanied by the activation of endoplasmic reticulum (ER) stress responses in the liver.Importantly, the loss of orexin rhythms during aging disrupts the daily recovery from stress, leading to a chronic ER stress state and insulin resistance(Tsuneki et al., 2015).
These observations imply that similar to peripheral tissues, the circadian control of hypothalamic processes decays during aging, disrupting the diurnal coordination of diverse physiological processes.Consequently, this disruption leads to detrimental age-related disturbances in critical processes, such as metabolic control, stem-cell homeostasis, and sleep homeostasis.
Sleep Homeostasis: a Link between the Circadian Clock and Aging
Although the overall role of sleep remains unclear, it has been postulated that sleep has an early evolutionary origin and is associated with functions such as the restoration of brain energy metabolism (Albrecht and Ripperger,2018).More recently, sleep has been linked to cognitive processes associated with neuronal plasticity, which is characterized by an increased number of synaptic contacts during wakefulness and a reduction in basal levels during sleep (Diekelmann and Born, 2010; de Vivo et al., 2017).Importantly, sleep perturbation induces age-related pathologies; the combination of chronic sleep deprivation and disruption to the circadian rhythm, often experienced by individuals engaged in shift work, has a detrimental impact on glucose regulation and metabolism (Buxton et al., 2012; Depner et al., 2014).Also, poor sleep quality or chronic sleep deprivation promotes age-related neurodegenerative disorders including deficits in various brain functions, such as learning ability and decision-making, and has been associated with mental disorders such as depression and anxiety (Alhola and Polo-Kantola, 2007; Li et al., 2022b; Sato et al., 2022).
In this regard, a direct relationship has been observed between neuronal plasticity during the wakefulness stage and the subsequent depth of sleep, as determined by the NREM sleep phase.Intriguingly, acute sleep deprivation ASD (5 hours) has distinct effects on place cell representations,sleep architecture, and memory in young and old mice.As expected, young mice exhibit superior sleep homeostasis and cognitive performance under normal conditions compared to old mice.Surprisingly, ASD negatively impacts these functions in young mice, while it improves sleep microarchitecture and memory in old mice.This effect may be attributed to the ASD-induced enhancement in NREM sleep quality and an increase in spindle count during the sleep recovery period, countering the age-related sleep fragmentation observed under control conditions (Yuan et al., 2021).This agrees with the induction of dendritic spines, and the antidepressant effects of ASD (5–12 hours; Orozco-Solis et al., 2017; Gisabella et al., 2020), while chronic sleep deprivation (72 hours or ASD over several weeks) induces a reduction of hippocampal neurogenesis, and memory performance in adult mice (Owen et al., 2021).
Moreover, it has been discovered that hypothalamic neurogenesis contributes to sleep homeostasis.Young mice exhibit robust circadian fluctuations in wake/sleep cycles (wake/NREM/REM), and blocking hypothalamic neurogenesis in these mice results in an aging-like disruption of sleep homeostasis (Kostin et al., 2019).This highlights the importance of neurogenesis in maintaining the healthy regulation of sleep patterns,emphasizing the need for sufficient and quality sleep to support optimal cognitive and physiological processes.
Moreover, the reduction of cellular stress has emerged as a promising therapeutic approach for enhancing cellular and organismal function.Recent research has shown that targeting ER stress in the brain through systemic administration of the chemical chaperone sodium 4-phenyl butyrate can effectively reverse sleep fragmentation and cognitive deficits in aged mice(Hafycz et al., 2022).Additionally, mice lacking SIRT1, a key regulator involved in stress responses, exhibit similar sleep homeostasis alterations to those observed in aged mice.This effect is attributed to the reduction of SIRT1-mediated anti-stress responses and the detrimental effects of lipofuscin accumulation (Panossian et al., 2011; Prola et al., 2017; Wang et al., 2020a).SIRT1 has further been linked to the brain-derived neurotrophic factor (BDNF)expression via deacetylation of MeCPT2 in the hippocampus (Zocchi and Sassone-Corsi, 2012).Accordingly, exercise induces hippocampal BDNF a potent inducer of neuronal plasticity, via SIR1-dependent induction in PGC1a in response to lactate (El Hayek et al., 2019).
It is interesting to note that NREM sleep can be enhanced through brain stimulation, either by increasing the expression of BDNF, or directly by supplying BDNF to the brain (Faraguna et al., 2008).Moreover, genetic evidence supports the link between sleep, BDNF, and age-related pathologies.The BDNF val66met polymorphism affects the activity-dependent secretion of BDNF, which in turn impacts intracellular trafficking and memory formation(Egan et al., 2003).This polymorphism is also associated with lower sleep consolidation and reduced learning capacity (Bachmann et al., 2012;Gosselin et al., 2016; Grant et al., 2018).Interestingly, val66met carriers display higher adverse effects of age on memory performance (Kennedy et al., 2015).Moreover, this polymorphism is further associated with hypothalamic-mediated energy homeostasis in mice, contributing to adipose tissue pathophysiology, resulting in reduced circulating leptin levels and hypothalamic expression of BDNF.These effects, in turn, promote increased food intake and overweight (Ieraci et al., 2020; Xie et al., 2023).Also, the elimination of BDNF in the VMH and DMH resulted in hyperphagic behavior and obesity, while the administration of BDNF in the PVN increased energy expenditure and reduced body weight (Unger et al., 2007; Wang et al., 2007).
Additional mechanisms within the hypothalamus, linking sleep homeostasis,metabolism, and aging have been identified.Within the hypocretin neurons of the LH, the transcription factor Lhx9 plays a key role in regulating sleep/wake cycles by promoting sleepiness.Aged mice exhibit a phase-shift and reduction in the mesor, highlighting alterations in the circadian rhythm of Lhx9 activity (Dalal et al., 2013; Wolff et al., 2023).Moreover, hyperexcitability of hypothalamic hypocretin/orexin neurons during aging contributes to sleep instability, and this effect is associated with a reduced expression of KCNQ2/3 potassium channels (Li et al., 2022a).Importantly, the expression of this channel is activated by the transcription factor specificity protein 1 (SP1)(Li et al., 2022a).Intriguingly, mistimed sleep disrupts the transcriptional rhythms of SP1 as observed in blood samples from participants exposed to a 28-hour forced desynchrony, and its expression is reduced in senescent cells (Oh et al., 2007; Archer et al., 2014; Li et al., 2022a).Interestingly, the DNA binding capacity of SP1 is under negative modulation by the NAD+-dependent poly(ADP-Ribose) polymerase 1 (PARP1) (Zaniolo et al., 2007).PARP1 has been recognized as a competitor to SIRT1, as both genetic ablation and pharmacological inhibition of PARP1 have been shown to increase NAD+content and enhance SIRT1 activity.Notably, during the aging process, PARP1 expression is upregulated in response to DNA damage (Bai et al., 2011),contributing to the reduction of the expression of the SP1-target genes,including KCNQ2/3 and notably the core core-clock genes Bmal1 and Per3(Archer et al., 2014).
Besides, in the SCN, SIRT1 plays a crucial role in regulating the central clock by activating BMAL1 and CLOCK through PGC1a and NAMPT.However, this regulatory mechanism tends to deteriorate with age, leading to various agerelated effects.Notably, the overexpression of SIRT1 has been demonstrated to protect against aging-related effects such as the inability to re-entrain the central clock by zeitgebers (Chang and Guarente, 2013).In line with this, Satoh et al.found that overexpression of SIRT1 in the brain extends lifespan through a mechanism involving the interaction between SIRT1 and the transcription factor NK2 Homeobox 1 (NKX2-1), as well as the upregulation of Ox2r expression in the wake-promoting nuclei DMH and LH.Importantly, these animals exhibit improved sleep homeostasis, physical activity, and muscular mitochondrial function (Satoh et al., 2013).Moreover, the absence of PR/SET Domain 13 (Prdm13), a gene highly and selectively expressed in the DMH,replicates age-related alterations in sleep quality and adiposity.Remarkably,Prdm13 expression is upregulated during dietary restriction, exhibits a diurnal rhythm, and declines with aging (Tsuji et al., 2023).Given that Prdm13 is transcriptionally regulated by NKX2-1 it is strongly suggested that PRDM13 is a downstream target of SIRT1 signaling (Satoh et al., 2015; Tsuji et al., 2023).This process highlights an intriguing interplay: SIRT1 is intricately influenced by the circadian fluctuations in NAD+levels.Recent evidence has shown that NAD+levels in the hypothalamus undergo circadian oscillations, playing a pivotal role in regulating circadian behavior and metabolism.This regulatory mechanism involves the eNAMPT-NAD+-SIRT1-FOXO1-melanocortin pathway.Notably, fasting stimulates adipocyte secretion of eNAMPT, facilitated by SIRT1-mediated deacetylation of iNAMPT.This, in turn, promotes the biosynthesis of NAD+in the hypothalamus (Yoshida et al., 2019; Park et al.,2023).Finally, as reported in peripheral tissues (Yoshino et al., 2018) a recent work demonstrated that the NAD+levels in different hypothalamic regions including the ARC, VMH, and LH were reduced during aging (Johnson et al.,2023).Interestingly, increasing circulating eNAMPT by overexpressing NAMPT in adipose tissue or supplementing eNAMPT-containing extracellular vesicles,results in a significant increase in plasma eNAMPT and higher NAD+levels in the hypothalamus, hippocampus, pancreas, and retina.Remarkably, this intervention improves physical activity, sleep homeostasis, and lifespan in aged mice (Yoshida et al., 2019).Therefore, enhancing the protective effects of SIRT1 holds potential for improving sleep homeostasis and overall wellbeing.Furthermore, these data highlight the intricate link between sleep homeostasis and the circadian clock in the performance of different brain functions during aging, as well as the relevance of key cellular and molecular mechanisms implicated.
Hypothalamic Stress Response Mechanisms:“Upgrading the Firmware”
While aging is a multifaceted process, extensive research has elucidated various molecular, cellular, and systemic mechanisms that contribute to the aging process (López-Otín et al., 2023).Importantly, as previously discussed,organisms employ diverse mechanisms to counteract cellular aging and maintain homeostasis.However, our understanding of the impact of aging on the hypothalamic control of physiology and metabolism remains limited.Nonetheless, recent discoveries have unveiled intriguing connections between cellular processes and anti-stress mechanisms with the functional properties of hypothalamic circuits, shedding light on their roles in regulating organismal metabolic and physiological homeostasis during the aging process.
Since the early 2000s, the presence of hypothalamic neurogenesis in adult mice has been recognized and linked to long-term regulation of energy balance (Kokoeva et al., 2005; Bartkowska et al., 2023).On this line, the loss of hypothalamic stem-cell function has been shown to accelerate aging and reduce lifespan in mice (Li et al., 2012; Zhang et al., 2017; Xiao et al.,2020).Also, a high-fat diet and leptin deficiency have been shown to impair stem-cell function and decrease hypothalamic neurogenesis (McNay et al.,2012).Moreover, nuclear factor-κB impairs neuronal differentiation and induces obesity (Li et al., 2012) while neurogenesis in the hypothalamus can be induced by the anti-inflammatory cytokine interleukin-6 and exercise(Niwa et al., 2016; Bobbo et al., 2021; Kostin et al., 2021), resulting in weight reduction.Notably, interleukin-6, released by muscles during physical activity,crosses the blood-brain barrier and likely contributes to hypothalamic neurogenesis, similar to its effects in the hippocampus (Pedersen, 2019).Additionally, the ablation of NAMPT in neuronal stem cells has been implicated in the detrimental effects of aging (Stein and Imai, 2014).In this regard, the SIRT1 activator SRT1720 has been shown to improve metabolism and provide protection against physiological and cognitive disturbances in old mice fed a high-fat diet (Mitchell et al., 2014).This effect is partially attributed to the inhibition of age-associated inflammation through NFKB inactivation.Furthermore, SRT1720 may enhance hypothalamic neurogenesis, as observed in the study by Li et al.(2012).Therefore, the restoration of functional capacity in hypothalamic stem cells by triggering anti-stress responses through SIRT1 activation, as demonstrated in various stem-cell lines (Libert et al., 2008; Ou et al., 2014; Liu et al., 2018, 2022), holds potential for improving hypothalamic metabolic and endocrine functions.Indeed, caloric restriction,known to activate SIRT1, has also been shown to protect neural stem cells from age-related deficits in the hippocampal subventricular zone (Apple et al.,2019).
Among different tissues, maintaining an appropriate functional proteome is crucial for ensuring successful cellular homeostasis and healthy aging(López-Otín et al., 2023).This is achieved through diverse mechanisms, such as regulating protein translation, facilitating correct protein folding through chaperones, implementing quality control systems, and employing protein degradation pathways.It is worth noting that while these cellular mechanisms are present in different cells and tissues, the functional outcomes they produce often vary depending on the tissue type (Sala et al., 2017).For example, the thermogenic function of brown adipose tissue relies on the upregulation of proteasomal activity, ensuring the maintenance of functional proteins.This process is mediated by the ER-localized transcription factor NRF1 (Bartelt et al., 2011).In the liver, the ubiquitin ligase UBE3C targets the pro-autophagy protein VPS34 for proteasomal degradation, thus inhibiting autophagy.However, under ER-stress conditions, TRABID stabilizes VPS34,promoting proteostasis and liver metabolism.Intriguingly, this mechanism is diminished in liver steatosis, leading to a further decline in cellular proteostasis.
In this regard, stem cells heavily rely on proteostasis to maintain a robust state of pluripotency, which enables them to differentiate into various cell types (Guan et al., 2013; Llamas et al., 2020).The loss of autophagy leads to activated oxidative metabolism, resulting in accelerated differentiation,reduced stemness, and diminished regenerative potential of hematopoietic stem cells (HSCs) (Ho et al., 2017).Indeed during aging the activity of chaperone-mediated autophagy decreases, while genetic or pharmacological activation of chaperone-mediated autophagy can effectively restore the functionality of aged mice and human HSCs, improving the regulation of lipid metabolism and ensuring an adequate energy supply for HSCs (Dong et al., 2021).Notably, autophagy acts as a potent inhibitor of inflammation,a condition implicated in pancreatic disorders (Smith et al., 2018) and agerelated hypothalamic dysfunction (Cai and Khor, 2019).In this regard, in the CNS and HSCs, the activation of FOXO3 induces the expression of its target genes, which are involved in the regulation of autophagy, maintaining the integrity and functionality of neuronal stem cells (Warr et al., 2013; Audesse et al., 2019; Dong et al., 2021).
In line with this, recent research has provided evidence that the proper regulation of organismal energy balance relies on hypothalamic autophagy.The disruption of autophagic genes, such as Atg7 or Atg12, specifically in POMC neurons, results in metabolic disturbances (Coupé et al., 2012;Malhotra et al., 2015).Similarly, autophagy plays a role in the control of food intake and energy balance in AgRP neurons (Kaushik et al., 2011),while the absence of Atg7 in SF1 neurons of the VMH diminishes leptin sensitivity (Coupé et al., 2021).Notably, the autophagy process and the ERstress/UPR are intimately interconnected (Li et al., 2008; Mendoza-Viveros et al., 2023).Therefore, it is not surprising that reducing ER stress in the hypothalamus has been shown to improve metabolic control.For example,the induction of hypothalamic ER stress, whether through ceramide, highfat diet, or pharmacological interventions, leads to sympathetic inhibition and a reduction in BAT-mediated thermogenesis, while the overexpression of the chaperone GRP78/BIP in the VMH enhances BAT activity, improves leptin and insulin sensitivity, and decreases body weight (Contreras et al., 2014,2017).Notably, in this nucleus, estradiol induces BAT activity by reducing both AMPK activity and ER-stress levels (Martínez de Morentin et al., 2014;González-García et al., 2018).Therefore, coordinated transcriptional and cellular responses are essential in these processes, which are triggered by ERstress sensors, including inositol-requiring enzyme 1, PRKR-like ER-kinase, and transcription factor 6.This leads to the splicing of XBP1, generating the active isoform XBP1s.XBP1s, in turn, upregulates the expression of ER chaperone proteins, folding enzymes, protein degradation molecules, and autophagy proteins, facilitating the adaptive response to ER stress (Kishino et al., 2017;Liu et al., 2017).Interestingly, the induction of Xbp1 in POMC neurons has been shown to be protective against diet-induced obesity, improving insulin and leptin sensitivity.Notably, the activation of Xbp1 in POMC neurons also triggers the Xbp1-stress axis in the liver through a cell-nonautonomous mechanism (Williams et al., 2014).The precise mechanism underlying this process remains largely unknown; however, it has been proposed that extracellular microvesicles may transport unfolded proteins, miRNAs, and other factors, thereby initiating this response in distant tissues (Frakes and Dillin, 2017; Galluzzi et al., 2018; Frakes et al., 2020).
In the brain, astrocytes constitute the predominant cell type among glial cells in various regions.These cells have multiple functions, including information processing, signal transmission, neuronal regulation, synaptic plasticity, and neuronal protection through the release of gliotransmitters (Suzuki et al.,2011).In the hypothalamus astrocytes sense nutrients and hormones through the insulin leptin signaling, they co-regulate glucose sensing and feeding behavior (Kim et al., 2014; García-Cáceres et al., 2016; Varela et al., 2021).In the PVN, astrocytes participate in the controls of energy expenditure, insulin sensitivity, and glucose metabolism, while obesity disrupts astrocyte-neuronal communication in the PVN contributing to metabolic dysfunction (Herrera Moro Chao et al., 2022).
Aged astrocytes exhibit a decline in neuroprotective capacity (Pertusa et al.,2007) and adopt a reactive neuroinflammatory phenotype known as A1,compromising their neuroprotective and neuromodulatory functions (Clarke et al., 2018), rendering the brain more susceptible to neurodegenerative diseases.Also, hypothalamic astrocytes show decreased expression of thrombospondin, which could contribute to alterations in synaptic and dendritic spine morphology (Boisvert et al., 2018).Additionally, cultured aged hypothalamic astrocytes display reduced release of BDNF and GDNF, as well as decreased expression of LepRb, accompanied by an increase in TGFb(Santos et al., 2018).Also, ApoE–/–mice, which develop atherosclerosis, fatty acid metabolism disturbances, and Alzheimer’s disease, exhibit hypothalamic inflammation, activated glial cells (microglia and astrocytes), and cognitive decline when fed a high-fat diet.However, these effects can be reversed through swimming exercises and dietary control, which upregulate SIRT1 and GnRH expression while inhibiting nuclear factor-κB signaling (Wang et al., 2020b).Moreover, the activation of SIRT1 by resveratrol has been shown to mitigate the inflammatory response induced by LPS in astrocytes via adenosine signaling (Bobermin et al., 2019).
Interestingly, it has been recently reported a mechanism in which exocytosismediated secretion of eNAMPT by astrocytes contributes to the induction of autophagy and therefore, neuronal protection in a model of ischemic stroke injury (Deng et al., 2022).Moreover, in depressed mice, fluoxetine also exerts its antidepressant effects via a neurotransmitter-independent mechanism inducing the autophagy flux in the hippocampal astrocytes (Shu et al., 2019).In the hypothalamus, acute high-fat diet (HFD) or intralipid infusion leads to increased astrocytic expression of tumor necrosis factor-α, while prolonged exposure to HFD for 8 weeks results in suppressed tumor necrosis factor-α levels accompanied by upregulated neuroprotective and proautophagic factors HSF70 and CNTF in the ARC.Interestingly, at 20 weeks, tumor necrosis factor-α expression is induced again (Dalvi et al., 2017).Similarly, at the onset of HFD, there is an induction of inflammatory signaling, astrogliosis,and HSP72 expression, while these responses decay over 7 days on HFD(Thaler et al., 2012), suggesting that neuronal protective mechanisms initially limit the HFD-induced damage.Interestingly the increase in gliosis was also found within the mediobasal hypothalamus of obese patients as revealed by magnetic resonance imaging (Thaler et al., 2012).These findings provide further evidence supporting the role of astrocytic stress responses in the regulation of hypothalamic-mediated metabolic control.Importantly, under conditions of prolonged cellular stress, such as aging or chronic inflammation induced by obesity, these mechanisms within the astrocytes and neurons can be surpassed, resulting in apoptosis and significantly compromising the proper functioning of the hypothalamus and other brain areas (Sierra et al.,2007).
Intranasal Drug Administration: a Promissory Route to Hypothalamic-Targeted Geroprotective Drugs
Given that the blood-brain barrier restricts the absorption of most drugs into the CNS, intranasal (IN) drug administration offers several advantages.This route exploits the anatomical connection between the nasal cavity and the CNS without crossing the blood-brain barrier.Moreover, IN administered drugs bypass drug elimination by the liver, gastrointestinal tract, and kidney filtration, thereby improving its pharmacokinetic/pharmacodynamic profile,reducing systemic exposure, and minimizing side effects (Erd? et al., 2018;Mignani et al., 2021).Therefore, this route has garnered significant interest in the field of drug delivery systems (Mignani et al., 2021).For example,the recently U.S.Food and Drug Administration-approved intranasal spray esketamine (Spravato?) has shown effectiveness in alleviating depressive symptoms in treatment-resistant depression patients (Sato et al., 2022; Zaki et al., 2023).
Moreover, the IN administration of hormones such as insulin, vasopressin,oxytocin, leptin, and glucagon has also been the subject of investigation.IN insulin, for instance, has been associated with improved metabolic control,including reduced food intake, weight loss, and enhanced glucose metabolism in rodent models.Interestingly, it has also demonstrated the potential to enhance cognitive performance, leading to clinical trials being established for Alzheimer’s disease treatment (Hallschmid, 2021a).Additionally, IN glucagon,used to treat insulin-induced severe hypoglycemia in diabetic patients, has shown comparable efficacy to intramuscular administration and has received U.S.Food and Drug Administration approval as intranasal glucagon powder(BAQSIMI?) (Lowe and Trujillo, 2020).
Intriguingly, preclinical studies on intranasal leptin have shown reductions in food intake and body weight in rats.Moreover, in mice with diet-induced obesity and sleep-disordered breathing, intranasal leptin has demonstrated the reduction of upper airway obstruction and hypoventilation during sleep,whereas intraperitoneal leptin did not exhibit respiratory effects.Importantly,this activates leptin signaling within hypothalamic and medullary centers,modulating respiratory motor neurons (Berger et al., 2019).
Pharmacological inhibition of the phosphatases PTP1B and TCPTP (which negatively regulate leptin and insulin signaling) by IN administration of claramine and RU486 reinstates leptin and insulin sensitivity in the ARC.This,in turn, promotes a negative energy balance and weight loss in obese mice.Notably, this approach also avoids the side effects associated with systemic inhibition of these enzymes, including inflammation, leukemia development,and autoimmune diseases (Dodd et al., 2019).
Studies on intranasal metformin have primarily been conducted in animal models.For instance, in pharmacologically induced Alzheimer’s disease models, mice treated with daily doses of 200 mg/kg of either intranasal or oral metformin for 4 weeks exhibited similar cognitive improvements(Kazkayasi et al., 2022).Additionally, oral metformin administration has been associated with antidepressant effects, improved cognitive function in depressed patients, and protective effects against dementia in individuals with type 2 diabetes (Campbell et al., 2018).For instance, metformin induces an increase in life-span in mice (Martin-Montalvo et al., 2013), and a reduced risk of developing cardiovascular disease and cognitive deterioration.Therefore, clinical trials are envisaged to analyze its geroprotective effects in a large cohort of patients (Kulkarni et al., 2020).
Though the efficacy of intranasal drug administration depends on various factors, including drug physicochemical properties, permeability characteristics, and enzymatic degradation, the development of new delivery technologies, such as drug coupling with nanoparticles, nanogels, powders,lipid nanostructures, and liposomes, will pave the way for the development of efficient hypothalamus-targeted geroprotective drugs (Campisi et al., 2019;Xu et al., 2020).
Concluding Remarks
Despite the advances in medicine over the past century, there has been a significant increase in the morbidity of non-infectious diseases, including cancer, type-2 diabetes, obesity, and mental illness.This concerning trend has been associated with a decline in overall quality of life.Recent extensive data from studies on aged animal models provide substantial evidence supporting the presence of progressive discoordination at multiple levels, including transcriptional, translational, and post-translational, in thousands of genes.This discoordination arises as a consequence of cellular stress responses,which give rise to an escalating vicious cycle fueled by the accumulation of internal and environmental stressors over time.As a result, the precise coordination needed to regulate the optimal timing of a protein’s presence for its integration within complex functional networks becomes disrupted.This disruption amplifies the detrimental effects on the cellular capacity to restore functional homeostasis at the molecular, cellular, and tissue levels.These mechanisms contribute significantly to the development of age-related disorders.
Moreover, as aging encompasses a multifaceted process that impacts all cells and tissues, the elucidation of pivotal molecular and cellular mechanisms underlying aging offers promise for the development of effective antigerontological therapies.Importantly, the circadian clock has been implicated in nearly every cellular and physiological process.Recent evidence highlights the crucial role of circadian coherence across tissues in sustaining optimal physiological homeostasis and promoting healthy aging.Consequently, the development of novel strategies to enhance circadian function, including pharmacological and non-pharmacological approaches (such as nutritional interventions and improvements in sleep homeostasis), holds promise as potential therapies to mitigate the detrimental effects of aging.Importantly,the targeted modulation of key cellular anti-aging defenses at the appropriate circadian timing holds the potential for maximizing the beneficial effects while minimizing potential side effects (Kaur et al., 2013; Escalante-Covarrubias et al., 2023).
Given that the hypothalamic circuits orchestrate a growing number of physiological and cellular processes in peripheral tissues, selectively addressing specific pathways and cellular processes within this brain region can enhance hypothalamic function.The resulting improvements in hypothalamic function would then have a systemic impact, exerting amplified and beneficial effects at the organismal level.
Finally, the advancement of intranasal brain-directed drug administration represents a promising strategy for targeting specific brain regions like the hypothalamus.Clinical and preclinical studies have demonstrated encouraging therapeutic outcomes through the intranasal administration of various substances for the treatment of diverse age-related conditions,such as depression, Alzheimer’s disease, and metabolic disorders.Therefore,this approach has the potential to not only effectively target the brain but also reduce potential side effects associated with systemic drug delivery(Hallschmid et al., 2004; Lee et al., 2020; Espinoza et al., 2021; Hallschmid,2021a, b; Roque et al., 2021; Kazkayasi et al., 2022; Sato et al., 2022;Stepochkina et al., 2022; Yao and Kendrick, 2022).
Acknowledgments:We thank members of the Orozco-Solis laboratory for helpful discussions and insights.
Author contributions:Manuscript design: RVL, LMV, CCC, SRM, and ECV;manuscript conceptualization and writing: ROS.All authors approved the final version of the manuscript.
Conflicts of interest:The authors declare no conflict of interests.
Data availability statement:All data relevant to the work are included in the article or uploaded as Additional files.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional file:
Additional Table 1: Metabolic sensors and transcriptional regulators that participate in the hypothalamic control of metabolism, the circadian clock and aging.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Does MgSO4 protect the preterm brain? Dissecting its role in the pathophysiology of hypoxic ischemic encephalopathy
- Exosomes derived from microglia overexpressing miR-124-3p alleviate neuronal endoplasmic reticulum stress damage after repetitive mild traumatic brain injury
- On implications of somatostatin in diabetic retinopathy
- Rebuilding insight into the pathophysiology of Alzheimer’s disease through new blood-brain barrier models
- The functions of exosomes targeting astrocytes and astrocyte-derived exosomes targeting other cell types
- Post-transcriptional mechanisms controlling neurogenesis and direct neuronal reprogramming

