Effects of 5-Azacytidine (AZA) on the Growth, Antioxidant Activities and Germination of Pellicle Cystsof Scrippsiella acuminata (Diophyceae)
WANG Zhaohui, ZHANG Jianneng, TANG Tao, ZHANG Yuning, and HU Ren
Effects of 5-Azacytidine (AZA) on the Growth, Antioxidant Activities and Germination of Pellicle Cystsof(Diophyceae)
WANG Zhaohui*, ZHANG Jianneng, TANG Tao, ZHANG Yuning, and HU Ren*
,,510632,
In this study, we investigated the effects of different concentrations of 5-azacytidine (AZA), a DNA methyltransferase in- hibitor, on the growth, antioxidant activities and germination of pellicle cysts of. The purpose of this study is to understand the toxic effects of AZA on marine microalgae, and to demonstrate the effect of DNA methyltransferase inhibitors on the germination of pellicle cysts. Results showed that AZA inhibited the growth ofsignificantly, and displaced a clear dose- dependent inhibition trend with the 96h EC50of 146.77μmolL?1(35.84mgL?1). Pellicle cysts ofwere less sensitive to AZA than the vegetative cells, and the EC50value of AZA to the germination of pellicle cysts ofwas 8.08mmolL?1(1.97gL?1). After exposed to AZA, the antioxidant activities inresponded rapidly and significantly. Among them, soluble pro- tein and superoxide dismutase (SOD) were more sensitive to AZA, and significant promotions occurred after exposed to 10μmolL?1AZA for 24h. Meanwhile, malondialdehyde (MDA) contents in algal cells did not change significantly after exposed to low concen- trations of AZA, but increased firstly and then decreased under high concentration of AZA. The glutathione (GSH) levels inincreased significantly under high concentrations of AZA, and remained unchanged at low concentrations of AZA. The results suggested that the enhanced protein level and SOD activity ofeliminated reactive oxygen species (ROS) to a certain ex- tent, and thus protected algal cells against damages of ROS caused by AZA.
5-azacytidine;; pellicle cysts; toxicity; superoxide dismutase; glutathione; malondialdehyde
1 Introduction
5-Azacytidine (AZA) is a DNA methyltransferase inhi- bitor as a deoxycytidine analogue. It can form an irrever- sible complex with DNA methyltransferase through cova- lent bond, and result in DNA demethylation during cell di-vision (Christman, 2002). AZA is commonly used in the cli-nical treatment of myelodysplastic syndrome and acute mye-loid leukemia (Annereau., 2014; Li., 2022). Stud- ies have confirmed that AZA has cytotoxicity at high con- centration (Betekhtin., 2018) and demethylation at lowconcentration (Li., 2017). In the plant kingdom, AZAreduces plant stem growth through DNA hypomethylation (Sano., 1990), affects somatic embryogenesis (Osorio- Montalvo., 2018; Nowicka., 2019; Chen., 2021) and flowering (Cheng., 2019; Zhang., 2020),and protects plants from pollution and environmental stress- es (Lu., 2016; Ogneva., 2019; Yan., 2021).
Microalgae are the primary producers of marine ecosys-tem, which play an important role in maintaining the stabi-lity of marine ecosystem. However, the studies on DNA me-thylation in algal cells are limited.has significantly different genome methylation le- vels at different stages of its life cycle, and the low fre- quency of methylation in the nuclear genome is in sharp contrast to the high frequency of methylation in the chlo- roplast genome (Lopez., 2015). About 6% of the ge- nome ofis intermittently me- thylated, and DNA methylation inis close- ly related to differential expression of algal cells under tran-scriptional silencing and adverse conditions such as sali- nity stress, temperature stress or nutritional restriction (Ve- luchamy., 2013). Recent study has shown that cell structure and reproductive cycle of the giant kelp () are controlled by DNA methylation, and there are significant differences in the degree of methylation at different stages of its life cycle (Fan., 2020).
DNA methyltransferase inhibitors affect the growth and life cycle of algal cells. Studies have shown that AZA can reduce the degree of methylation and inhibit the growth of algal cells (Bacova., 2019), inhibit the matching of ga-metes and hinder the development of the zygotes (Feng andChiang, 1984), and delay the cell cycle of algal cells (Ho., 2007). In addition, DNA methyltransferase inhibitorshave significant effects on antioxidant capacity of algal cells(Bacova., 2019), and inhibit the expression of anti-oxidant enzyme genes (Palsamy., 2014). However, the effect of DNA methylation inhibitors on dinoflagellates, animportant group of marine phytoplankton with complex life cycle and trophic types, is very limited (Lohuis and Miller, 1998; Ho., 2007).
Pellicle cyst formation is regarded as a temporary stra- tegy for many species of dinoflagellates to escape harsh or adverse environments (Garcés., 2002; Olli, 2004; Bra- vo., 2010). Pellicle cysts generally do not have a man- datory resting period and can germinate rapidly into vege- tative cells soon after incubated into favorable conditions, usually within several minutes to 24 hours (Olli, 2004; On- da., 2014; Shin., 2017).()is a widespread bloom species in the coastal and estuarine waters (Wang., 2007; Zin- ssmeister., 2011). It is easy to be cultured and is fea- sible to form resting and pellicle cysts under laboratory con- ditions (Guo., 2021), which made it a favorite orga-nism for laboratory studies. In this study, the effects of dif-ferent concentrations of AZA on the growth, antioxidant ac-tivities and germination of pellicle cysts of.were studied. This study is to understand the toxic effects of AZA on marine microalgae, and to demonstrate the ef- fects of DNA methyltransferase inhibitors on the germina- tion of pellicle cysts.
2 Materials and Methods
2.1 Algal Culture
was isolated from Daya Bay of the South China Sea, and cultivated in the Department of Ecology, Jinan University, China. Cultures were maintained in auto- claved (121℃, 20min) f/2 medium (Guillard, 1975) with- out silica (f/2-Si) in an illumination incubator with tempe- rature of 20℃±1℃, salinity of 30, and illuminance of 150μmol photon m?2s?1with a dark:light cycle of 12h:12h.
2.2 Chemicals
5-Azacytidine (CAS: 320-67-2, molecular weight: 244.20) was purchased from APExBIO (CAT#A1907, Houstom, TX, USA). Solubility is ≥13.55mgmL?1in H2O with ultra- sonic. Stock solution was prepared by diluting AZA in dis-tilled water with ultrasonic at a final concentration of 40mmolL?1.
2.3 Effects of AZA on the Growth and Antioxidant Activities of Algal Cells
The experiment was carried out in eighteen Erlenmeyer flasks with 300mL f/2-Si medium, including six AZA con- centrations (0, 10, 50, 100, 500, and 1000μmolL?1) and each concentration in triplicates. The initial cell densities were about 1.6×104cellsmL?1, and the experiment lastedfor 96h. Cell counting were performed every day duringthe experiment, and antioxidant activities were measured at 0, 24, 48, and 96h after exposed to AZA.
Cell counts were performed under an inverted micro- scope (Leica DMIRB) at a magnification of 200×. A split of 5mL culture was harvested in each flask, and then was centrifuged at 12000for 10min at 4℃ for the measure- ment of antioxidant activities. Antioxidant enzymes were extracted using 0.1mmolL?1sodium phosphate buffer (pH7.2–7.4) by sonication (Sonopuls Ultrasonic Homogenizer, Bandelin) for 10min in an ice bath. The cellular homoge- nate was then centrifuged at 35000for 10min at 4℃, and the supernatant was stored at ?20℃ for further analysis. All antioxidant activities including soluble protein, super- oxide dismutase (SOD), glutathione (GSH), and malondial- dehyde (MDA) were determined using the commercial test kits (Nanjing Jiancheng Biological Engineering Company, China) according to the manufacturer’s instructions.
2.4 Effects of AZA on Germination of Pellicle Cysts of Scrippsiella acuminata
Algae in the mid exponential stage were inoculated in darkness at 8℃. Large numbers of pellicle cysts were pro- duced within 48h. Cysts were randomly selected and cul- tured into the 96-well flat bottom microplates containing 200μL of f/2 medium with different concentrations of AZA (0, 10, 50, 100, 200, 500, 1000, 2000, 10000, and 20000μmolL?1). Each concentration treatment included thirty cysts, which were set in triplicates. The culture plates were incubated in an illumination incubator with the same con- ditions presented in Section 2.1.
Germination experiments lasted for 12 days. Cyst germi- nation was indicated by the presence of motile cells or an empty cyst wall (successful cyst germination). The presence of intact cysts represented the living cysts. Otherwise, the cysts were considered dead. Treatments were examined every day by an inverted microscope (Leica DMIRB). Theaccumulative germination rate is defined as the percentages of germinated cysts to the overall tested cysts. Meanwhile, dead cysts were counted, and the death rate of cysts was calculated.
2.5 Data and Statistics Analysis
Specific growth rate (, d?1) was calculated in 4 day test using the equation:
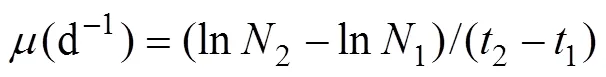
where1and2are the cell densities at day1and day2, respectively, and the maximum specific growth rate (max) within the four-day experiment was calculated.
The inhibition rate (IR) is defined as percentage of AZA inhibiting the growth rate:
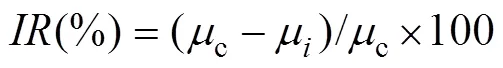
wherecis the growth rate of the control group,μis the growth rate in the test group.
The inhibition rate of cyst germination (IG) is defined as percentage of AZA inhibiting the accumulative germination rate of each test group:
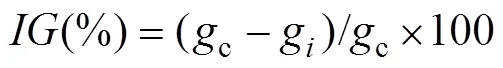
wherecis the growth rate of the control group,gis the growth rate in the test group.
Median effective concentration (EC50) was calculated from the dose-response regression equation using ‘logari- thmic-probit’, and 95% confidence limits were calculated by SPSS 28.0. Analysis of variance (ANOVA) was used to compare growth curves and germination rate under diffe- rent AZA concentrations. ANOVA analysis with post hoc(Tukey test) was used for multiple comparisons of the spe- cific growth rate and antioxidant activities. Statistical ana- lyses were performed using SPSS 28.0. The difference was considered significant when<0.05.
3 Results
3.1 Effects of AZA on the Growth of Scrippsiella acuminata
Effects of AZA on the growth ofwere il- lustrated in Fig.1. Algal cells in the control group grew quickly, and cell density reached 5.05×104cellsmL?1after 96h incubation with themaxvalue of 0.30d?1. AZA inhi- bited the growth ofsignificantly (<0.05 or<0.01), and displaced a clear dose-dependent inhibition trend. Cell densities in the test groups ranged between 2.55×104cellsmL?1and 3.39×104cellsmL?1, and themaxva- lues were 0.18–0.22d?1. The 96h EC50of AZA towas 146.77μmolL?1(35.84mgL?1), with 95% li- mitation of 141.46–152.08μmolL?1(34.56–37.17mgL?1).
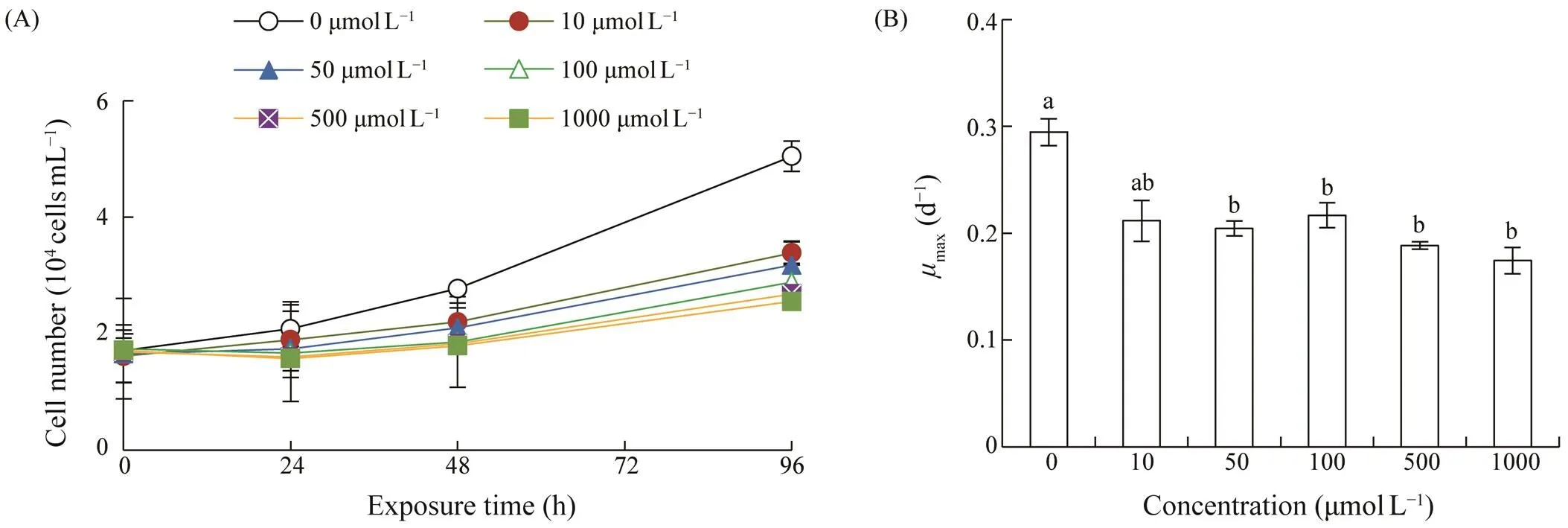
Fig.1 Growth of Scrippsiella acuminata under different concentrations of 5-azacytidine. (A), growth curve; (B), the maximum specific growth rate (μmax). Error bars indicate standard deviation (SD) of the triplicate test groups. Data bars carrying different letter designations (a, b) indicate significant differences between treatments (P<0.05, Tukey test), and the same and similar letter designations (e.g., a and ab, b and ab) denote no significant differences between treatments (P>0.05).
3.2 Effects of AZA on Antioxidant Activities of Scrippsiella acuminata
Soluble protein were from 0.49 to 0.52ngcell?1in the control group without significant differences (>0.05) du- ring the experiment (Fig.2). Soluble protein showed a sig- nificant increase after the cells were exposed to AZA (<0.05 or<0.01), and generally presented a dose- and time-dependent increase. The highest protein levels in the test groups ranged between 0.56 and 1.31ngL?1, which were 1.47–2.29 times of those before exposure.
Other antioxidant activities were shown by cellular le- vel and content per mg protein. No significant differences occurred in the control group for all antioxidant activities during the 96h experiment (>0.05), and most of the an- tioxidant activities increased after AZA exposure (Figs.3–5). SOD activity showed a dose-dependent increase after exposure of AZA, and reached 1.21–1.60U(104cells)?1at 24h, and then remained an increase trend or maintained at high levels after 48h exposure (Fig.3A). SOD activity per 104cells decreased sharply at 96h exposure; however it wasstill significantly higher than that in the control group (<0.01). SOD activity per mg protein generally increasedaf- ter exposed to AZA with concentrations of 10, 50, and 1000 μmolL?1for 24h (<0.01). However, it did not increase under exposure of moderate concentrations of 100 and 500μmolL?1, and then SOD activity decreased to the levels low-er than the control with the extension of exposure time un- der all AZA concentrations (Fig.3B).
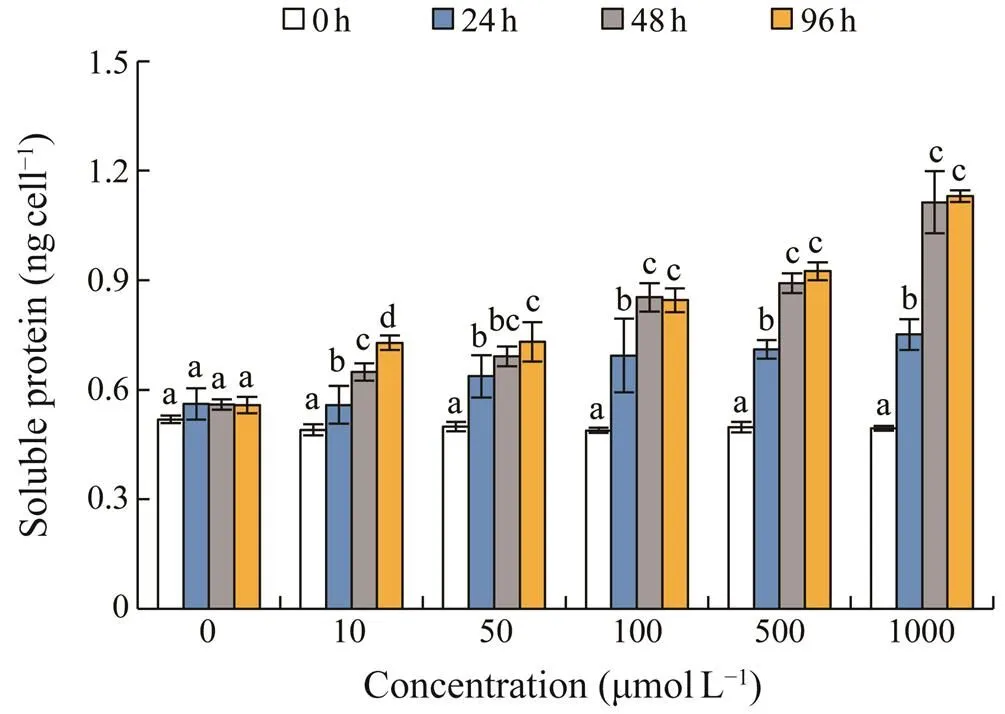
Fig.2 Changes in soluble protein in Scrippsiella acumina- ta under different concentrations of 5-azacytidine. Error barsindicate standard deviation (SD) of the triplicate test groups.Data bars carrying different letter designations at each con- centration indicate significant differences between exposure time (P<0.05, Tukey test), and the same and similar letter designations (e.g., b and bc, c and bc) denote no significant differences between different exposure time (P>0.05).
As shown in Fig.4A, the cellular GSH level in the control ranged between 19.8 and 20.9fmolcell?1. GSH increased significantly after exposed to high AZA concentrations (100–1000μmolL?1), and was not significantly enhanced under low AZA concentrations of 10 and 50μmolL?1. The high- est GSH contents generally occurred after 96h exposure of AZA, which were 1.21–2.09 times of those before treat- ment. GSH content per mg protein showed no significant increase after exposed to AZA, but generally decreased with the longer exposure time (Fig.4B).
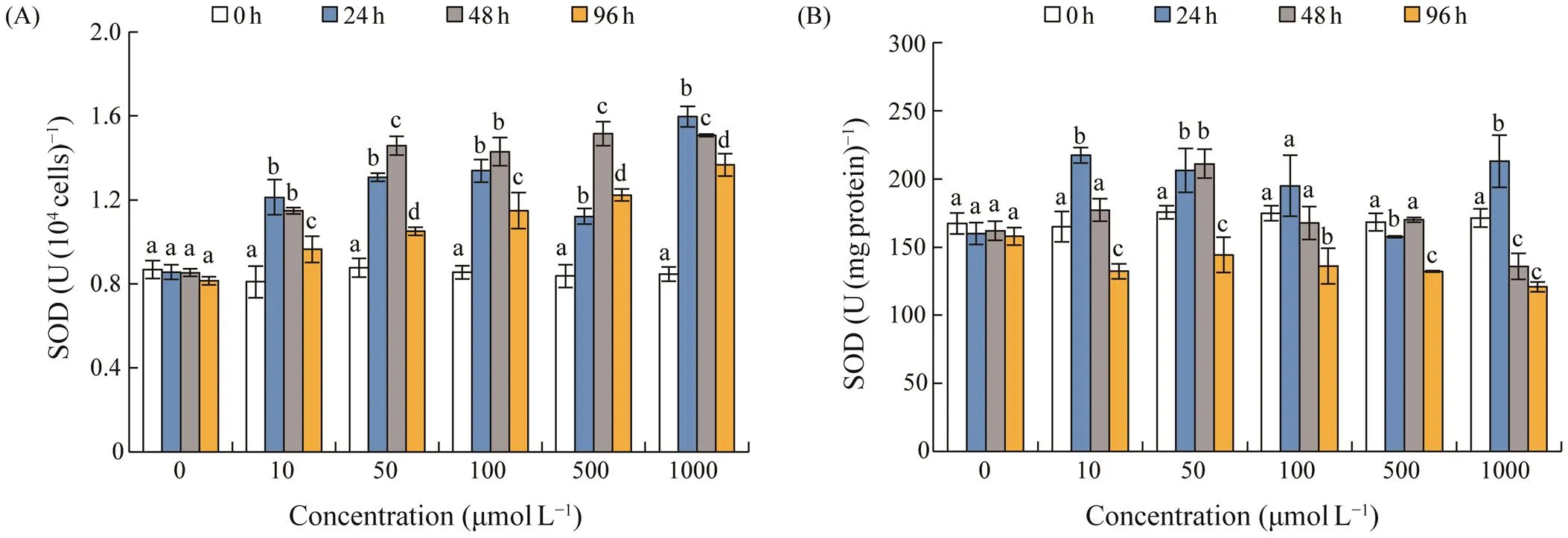
Fig.3 Changes in superoxide dismutase (SOD) activity in S. acuminata under different concentrations of 5-azacytidine. (A), SOD in U(104cells)?1; (B), SOD in U(mgprotein)?1. Error bars indicate standard deviation (SD) of the triplicate test groups. Data bars carrying different letter designations at each concentration indicate significant differences between exposure time (P<0.05, Tukey test), and the same letter designations denote no significant differences between different exposure time (P>0.05).
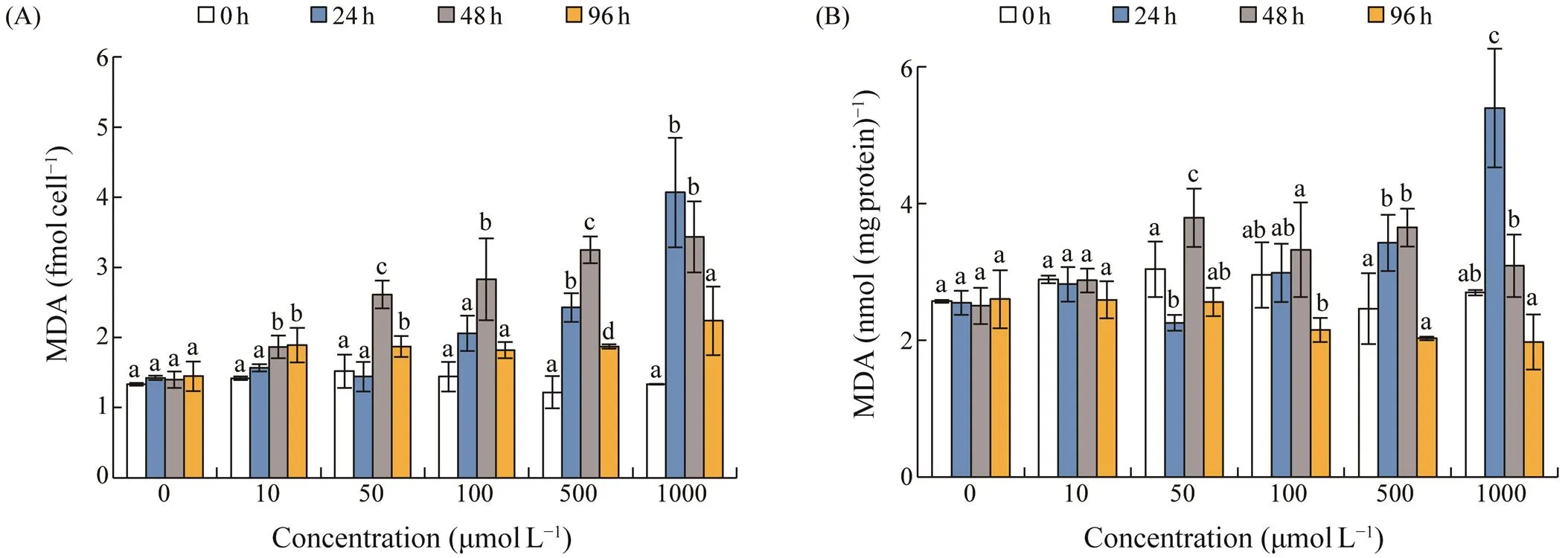
Fig.5 Changes in malondialdehyde (MDA) content in S. acuminata under different concentrations of 5-azacytidine. (A), MDA in fmolcell?1; (B), MDA in nmol(mgprotein)?1. Error bars indicate standard deviation (SD) of the triplicate test groups. Data bars carrying different letter designations (a, b) indicate significant differences between different treatments (P<0.05, Tukey test), and the same and similar letter designations (e.g., a and ab, b and ab) denote no significant differences between treatments (P>0.05).
Cellular MDA levels were from 1.32 to 1.43fmolcell?1in the control group. MDA level increased significantly after exposed to AZA, and peaked after 24h or 48h exposure (Fig.5A). MDA content increased in a dose-dependent trend, and the highest level of 4.07fmolcell?1was observed at 1000μmolL?1AZA for 24h. The content of MDA per mg protein showed no significant increase under concentration of 10μmolL?1AZA, and increased significantly after 24h or 48h at concentrations >50μmolL?1(Fig.5B). The high- est level occurred at 1000μmolL?1AZA for 24h.
3.3 Effects of AZA on the Germination of Pellicle Cysts of S. acuminata
The germination rate and mortality rate of pellicle cysts ofunder different concentrations of AZA wereshown in Fig.6 and Fig.7, respectively. Cysts in all test groups began to germinate within 24h after incubation, andthe germination rate in the control group was 22.22% at 24h. AZA under concentrations of 10–100μmolL?1significant-ly stimulated cyst germination during the first 24h exposure (<0.05), and the germination rates ranged from 26.02% to 29.73%. While concentrations high than 100μmolL?1exerted inhibitory effects on cyst germination within 24h ex- posure, and the lowest germination rate of 3.25% was re- corded under concentration of 20mmolL?1. The accumu- lative germination rates increased gradually in all groups, and reached the maximum level within 4–7 days. The ma- ximum cumulative germination rate was 65.56% in the con-trol group, and the maximum cumulative germination ratesof all test groups were lower than that of the control group, ranging from 20.24% to 62.48%. The cumulative germina- tion rate decreased with the increase of AZA concentrations, while there was no significant difference between 10μmol
L?1AZA group and the control group (>0.05), and it was significantly lower under other concentrations of AZA (<0.01). The EC50value of AZA to the germination ofpellicle cysts was 8.08mmolL?1(1.97gL?1) with 95% limitation of 4.80–13.57mmolL?1(1.17–3.31gL?1).
Pellicle cysts died during the incubation if they did not germinate. Death began at the first day of incubation even in the control group, and the death rates increased rapidly at d2–d4 (Fig.7). At the end of the experiment (after 12 days), all pellicle cysts either germinated to vegetative cellsor lysed and died. The cumulative death rate in the controlgroup was 34.4%. The cumulative death rates in test groups were higher than that in the control group, and increased with the increase of AZA concentrations, ranging from 37.52% to 79.76%.
4 Discussion
AZA in concentrations of 10–1000μmolL?1showed sig- nificant inhibitory effects on the growth ofwith 96h EC50value of 146.77μmolL?1. Our previous studies showed that 96h EC50values of AZA on the growth ofandwere 1.6μmolL?1and 11.6μmolL?1, respectively (Zhao., 2022). AZA could significantly inhibit the growth ofandafter they were exposed to 10μmolL?1AZA for just one day (Bacova., 2019). These results suggested that the tolerances of algal cells to AZA varied greatly among species. Bothandare armored dinoflagellates with thick cell wall, which can reduce the quantity of AZA into the cells, and thus increase their tolerances to AZA. How- ever, those algal cells without thick cell wall, such as the naked dinoflagellateand the green algaeand, represented high sensitivi- ties to AZA. In contrast, AZA is less toxic to aquatic crus- taceans with the 48h EC50value of 230.1mgL?1(942.3μmolL?1) to(Lindeman., 2019).
DNA methylation is an important epigenetic mechanism participating in silencing genes. Plants regulate gene ex-pression through DNA methylation and demethylation, and thus adjust their growth and abilities to adapt to the envi- ronments (Sheldon., 1999; Ta?kin., 2017). Dino- flagellates have atypically large genomes with highly repe-titive genes, which are known for extensive methylation,permanently condensed chromosomes (Lin, 2011; Stephens., 2020). Methylation levels in dinoflagellate genomes are nonstatic and can be modulated by environmental con- ditions, and in turn may regulate the expressions of genes in algal cells (Lohuis and Miller, 1998; Yang., 2020). Cell cycle was delayed in dinoflagellateunder 200μmolL?1AZA (Ho., 2007). Differ- ent concentrations of AZA (10–1000μmolL?1) exhibited obvious inhibitory effects on the growth ofin our study, which may be caused by the DNA hypome- thylation induced by AZA, which prevents chromosome compaction, and thus influences the mitosis process and delays the cell cycle (Ho., 2007).
AZA also significantly affected the germination of the pellicle cysts of. Low AZA concentrations (10, 50 and 100μmolL?1) promoted the germination of cysts at the first day of incubation, while high AZA con- centrations inhibited the germination. Studies have shown that AZA can promote the flowering of Chinese cabbage and chrysanthemum instead of low temperature stimulation (Li., 2003; Wang., 2009). As a DNA methylation inhibitor, AZA may reduce the DNA methylation level of the pellicle cysts at low concentrations, and activate the ex- pression of some germination genes, and thus promote the cyst germination. However, with the extension of exposure time and the increase of exposure concentration, AZA in- hibited the germination and increased the mortality of the pellicle cysts. The toxicity of AZA to the germination of pel- licle cysts of(8.08mmolL?1) was 55 times higher than that to the growth of vegetative cells (146.77μmolL?1), indicating much higher resistance ability of pel- licle cysts than that of vegetative cells. Pellicle cysts are temporary resting stages formed by vegetative cells faced to sudden environmental stresses (Onda., 2014). The wall of pellicle cysts is composed of multilayer cellulose or the principal strengthening elements of the periphery of themotile stage cell (Bravo and Figueroa, 2014), which enables them better resist to harmful chemicals. AZA is a de-methy- lating agent used in the laboratory studies (Ogneva., 2019) and in some cases as a treatment for acute myeloid leukemia and myelodysplastic syndrome (Annereau., 2014; Li., 2022). Therefore it may result in few environmental contaminations. There are no reports on the con-tamination of AZA in natural seawater and sediment or othernatural environments up till now, it can be deduced that AZA concentrations in the environments should be far less than levels that can cause biological toxicity.
In plants, including microalgae, the electron transport ac- tivities in chloroplasts, mitochondria and plasma membranesinevitably leak electrons onto O2during photosynthesis and respiration, resulting in the production of reactive oxygen species (ROS) (Gao., 2017). ROS production is usual-ly low and is balanced by the ROS scavenging system (Pow- les, 1984). However, both abiotic and biotic stresses are known to cause excess accumulation of ROS, which may result in the damage of intracellular biological macromole-cules, such as proteins, nucleic acids, and lipids,. (Mit- tler, 2002). AZA has reported to influence the activities of antioxidant enzymes and the content of non-enzymatic an- tioxidants in algal cells (Bacova., 2019). Soluble pro- tein ofincreased significantly and showed a dose- and time-dependent increasing trend within the 96h exposure of AZA in this study (Fig.2). The results suggest- ed that algal cells produced more protein and enzymes to protect themselves against AZA damage. Furthermore, AZA may reduce the level of genomic DNA methylation in al- gal cells (Christman, 2002), then enhance the expression of protein synthesis genes, and leads to the increase of pro- tein level. Soluble protein in algal cells showed a dose-de- pendent increasing trend under other environmental stress- es as well, such as heavy metals and pesticides (Zutshi., 2008; Wang., 2011).
SOD is one of the most important antioxidant enzymes to resist ROS damage among the antioxidant enzymes inalgal cells, and is generally considered as the first line of de- fense against ROS (Alscher., 2002). Significant in-vcreases in SOD activity were recorded inun- der different concentrations of AZA in this study (Fig.3), indicating that algal cells enhanced antioxidant capacity byincreasing SOD activity to protect against AZA damage. Meanwhile, the MDA contents did not change significant- ly after exposed to low concentrations of AZA; however, they increased firstly and then decreased under high con- centration of AZA (Fig.5). It is well-known that MDA is aproductof lipidperoxidation(Al-Rashed., 2016), and the increasing of MDA levels indicates the damage of cy- tomembrane through exposure to pollutants (Gao., 2017). Our results suggested that high SOD activity under low AZA concentration (10μmolL?1) scavenged ROS and protected cell membrane from ROS damage. However, when algal cells were exposed to high AZA concentrations (>10μmolL?1), even high SOD activity could not quench ROS generated by AZA stress, and resulted in high MDA level (Fig.5). As the extension of exposure time, high SOD activity protected cells from persistent ROS damage, and MDA content thus showed a downward trend. The results suggested that SOD might be a key antioxidant defense inagainst AZA stress.
GSH is one of the most important components of the an- tioxidant defense systems in cells (Mishra., 2006; Gao., 2017). In the present study, the GSH level inincreased significantly under high concentrations ofAZA, but remained unchanged at low concentrations (Fig.4). Our results agreed with those found by Bacova. (2019),which GSH levels inanddid not show significant changes after exposed to 10μmolL?1AZA for five days. These re- sults suggest that the sensitivity of GSH to AZA-inducedoxidative stress are lower than those of other antioxidant activities.
The antioxidant activities (SOD, MDA and GSH) were represented by both cellular level and activity per mg pro- tein in this study. Results showed that cellular antioxidant activities were more sensitive, which better reflected theresponse of algal cells to AZA. The activity per mg proteinwas influenced by the content of soluble proteins, and thus led to the decline of antioxidant activities under high AZA concentrations due to the increase of protein content. There- fore, it is reasonable to apply cellular antioxidant activities in evaluating the response of cells to environmental stresses.
5 Conclusions
The present study studied the antioxidant responses ofunder different concentrations of DNA me- thylation inhibitor AZA, and the responses of algal cellsto AZA depended on both dose and exposure time. AZA showed a dose-dependent inhibitory effect on the growth of, with the 96h EC50of 146.77μmolL?1.Pellicle cysts ofwere less sensitive to AZAthan the vegetative cells. AZA at concentrations of 10–100μmolL?1promoted the germination of pellicle cysts ofat the beginning of incubation however, the cu-mulative germination rates of all exposed groups were low- er than that of the control group (without AZA addition). The EC50of AZA to the germination of pellicle cysts ofwas 8.08mmolL?1. After exposed to AZA, the antioxidant activities incells responded ra- pidly and significantly. Among them, soluble protein and SOD were more sensitive to AZA, and significant promo- tion occurred after exposed to 10μmolL?1AZA for 24h. While concentrations above 100μmolL?1could significant- ly promote the increase of GSH and MDA contents. The results suggested that algal cells produced more proteins and enzymes (including SOD) to protect cells against AZA damage. Furthermore, our results demonstrated that cellu- lar antioxidant activities better reflected the response of al- gal cells to AZA, and should be more reasonable to be ap- plied in evaluating the response of cells to environmental stresses.
Acknowledgement
This work was supported by the National Natural Sci- ence Foundation of China (No. 42076141).
Al-Rashed, S. A., Ibrahim, M. M., El-Gaaly, G. A., Al-Shehri, S., and Mostafa, A., 2016. Evaluation of radical scavenging sys- tem in two microalgae in response to interactive stresses of UV- B radiation and nitrogen starvation.,23 (6): 706-712, DOI: 10.1016/j.sjbs.2016.06.010.
Alscher, R. G., Erturk, N., and Heath, L. S., 2002. Role of super- oxide dismutases (SODs) in controlling oxidative stress in plants., 53 (372): 1331-1341, DOI: 10.1093/jexbot/53.372.1331.
Annereau, M., Desmaris, R., Micol, J. B., Lazarovici, J., Chenal-lier, C., Saada, V.,., 2014. Results of treatment with 5-aza- cytidine in patients treated for myelodysplastic syndrome or se- condary acute myeloid leukemia associated with a concomitant active cancer., 32 (15S): e18029, DOI: 10.1200/jco.2014.32.15_suppl.e18029.
Bacova, R., Klejdus, B., Ryant, P., Cernei, N., Adam, V., and Hu- ska, D., 2019. The effects of 5-azacytidine and cadmium on global 5-methylcytosine content and secondary metabolites in the freshwater microalgaeand., 55 (2): 329-342, DOI: 10.1111/jpy.12819.
Betekhtin, A., Milewska-Hendel, A., Chajec, L., Rojek, M., No- wak, K., Kwasniewska, J.,., 2018. 5-Azacitidine induces cell death in a tissue culture of., 19 (6): 1806, DOI: 10.3390/ijms19061806.
Bravo, I., and Figueroa, R. I., 2014. Towards an ecological un- derstanding of dinoflagellate cyst functions., 2 (1): 11-32, DOI: 10.3390/microorganisms2010011.
Bravo, I., Figueroa, R. I., Garcés, E., Fraga, S., and Massanet, A., 2010. The intricacies of dinoflagellate pellicle cysts: The ex- ample ofcysts from a bloom-recurrent area (Bay of Baiona, NW Spain)., 57 (3-4): 166-174, DOI: 10. 1016/j.dsr2.2009.09.003.
Chen, R. Z., Chen, X. H., Huo, W., Zheng, S. Z., Lin, Y. L., and Lai, Z. X., 2021. Transcriptome analysis of azacitidine (5-AzaC)-treatment affecting the development of early somatic em- bryogenesis in longan., 96 (3): 311-323, DOI: 10.1080/14620316.2020. 1847695.
Cheng, Y. H., Peng, X. Y., Yu, Y. C., Sun, Z. Y., and Han, L., 2019. The Effects of DNA methylation inhibition on flower de- velopment in the dioecious plant., 10 (2): 173, DOI: 10.3390/f10020173.
Christman, J. K., 2002. 5-azacytidine and 5-aza-2’-deoxycytidine as inhibitors of DNA methylation: Mechanistic studies and theirimplications for cancer therapy., 21 (35): 5483-5495, DOI: 10.1038/sj.onc.1205699.
Fan, X., Han, W. T., Teng, L. H., Jiang, P., Zhang, X. W., Xu, D.,., 2020. Single-base methylome profiling of the giant kelpreveals significant differences in DNAmethylation to microalgae and plants., 225 (1): 234-249, DOI: 10.1111/nph.16125.
Feng, T. Y., and Chiang, K. S., 1984. The persistence of mater- nal inheritance indespite hypomethylation of chloroplast DNA induced by inhibitors., 81 (11): 3438-3442, DOI: 10. 1073/pnas.81.11.3438.
Gao, Q. T., Wong, Y. S., and Tam, N. F. Y., 2017. Antioxidant re- sponses of different microalgal species to nonylphenol-induced oxidative stress., 29 (3): 1317-1329, DOI: 10.1007/s10811-017-1065-y.
Garcés, E., Masó, M., and Camp, J., 2002. Role of temporary cysts in the population dynamics of(Di- nophyceae)., 24 (7): 681-686, DOI: 10.1093/plankt/24.7.681.
Guillard, R. R. L., 1975. Culture of phytoplankton for feeding marine invertebrates. In:. Smith, W. L., and Chanley, M. H., eds., Springer, Boston, MA, 29-60.
Guo, X., Wang, Z. H., Liu, L., and Li, Y., 2021. Transcriptome and metabolome analyses of cold and darkness-induced pellicle cysts of., 22 (1): 526, DOI: 10.1186/s12864-021-07840-7.
Ho, P., Kong, K. F., Chan, Y. H., Tsang, J. S. H., and Wong, J. T. Y., 2007. An unusual S-adenosylmethionine synthetase gene from dinoflagellate is methylated., 8 (1): 87, DOI: 10.1186/1471-2199-8-87.
Li, M. L., Zeng, G. W., and Zhu, Z. J., 2003. Analysis of effects of 5-azficytidine on promoting flowering in non-heading Chi- nese cabbage., 29 (3): 287-290 (in Chinese with English ab- stract).
Li, X. F., Wang, W., Zhang, X., and Wu, Y., 2022. Azacitidine anddonor lymphocyte infusion for patients with relapsed acute mye-loid leukemia and myelodysplastic syndromes after allogeneic hematopoietic stem cell transplantation: A meta-analysis., 12: 949534, DOI: 10.3389/fonc.2022.949 534.
Li, X. F., Xia, Z. Y., Tang, J. Q., Wu, J. H., Tong, J., Li, M. J.,.,2017. Identification and biological evaluation of secondary me-tabolites from marine derived fungi–sp. SCSIOW3, cultivated in the presence of epigenetic modifying agents., 22 (8): 1302, DOI: 10.3390/molecules22081302.
Lin, S. J., 2011. Genomic understanding of dinoflagellates., 162 (6): 551-269, DOI: 10.1016/j.res mic.2011.04.006.
Lindeman, L. C., Thaulow, J., Song, Y., Kamstra, J. H., Xie, L., Asselman, J.,., 2019. Epigenetic, transcriptional and phe- notypic responses in two generations of Daphnia magna ex- posed to the DNA methylation inhibitor 5-azacytidine., 5 (3): 1-12, DOI: 10.1093/eep/dvz016.
Lohuis, M. R., and Miller, D. J., 1998. Light-regulated transcrip- tion of genes encoding peridinin chlorophyllproteins and the major intrinsic light-harvesting complex proteins in the dino- flagellateHulburt (Dinophycae): Changes in cytosine methylation accompany photoadaptation., 117 (1): 189-196, DOI: 10.1104/pp.117.1.189.
Lopez, D., Hamaji, T., Kropat, J., Hoff, D. P., Morselli, M., Rub- bi, L.,., 2015. Dynamic changes in the transcriptome and methylome ofthroughout its life cycle., 169 (4): 2730-2743, DOI: 10.1104/ pp.15.00861.
Lu, Y. C., Feng, S. J., Zhang, J. J., Luo, F., Zhang, S., and Yang, H., 2016. Genome-wide identification of DNA methylation pro- vides insights into the association of gene expression in rice exposed to pesticide atrazine., 6: 18985, DOI: 10.1038/srep18985.
Mishra, S., Srivastava, S., Tripathi, R. D., Kumar, R., Seth, C. S., and Gupta, D. K., 2006. Lead detoxification by coontail (L.) involves induction of phytochela- tins and antioxidant system in response to its accumulation., 65 (6): 1027-1039, DOI: 10.1016/j.chemosphere. 2006.03.033.
Mittler, R., 2002. Oxidative stress, antioxidants and stress tole- rance., 7 (9): 405-410, DOI: 10.1016/ S1360-1385(02)02312-9.
Nowicka, A., Juzon, K., Krzewska, M., Dziurka, M., Dubas, E., Kopec, P.,., 2019. Chemically-induced DNA de-methy- lation alters the effectiveness of microspore embryogenesis in triticale., 287: 110189, DOI: 10.1016/j.plantsci. 2019.110189.
Ogneva, Z. V., Suprun, A. R., Dubrovina, A. S., and Kiselev, K. V., 2019. Effect of 5-azacytidine induced DNA demethylation on abiotic stress tolerance in., 55 (2): 73-80, DOI: 10.17221/94/2018-PPS.
Olli, K., 2004. Temporary cyst formation of(Dinophyceae) in natural populations., 145(1): 1-8, DOI: 10.1007/s00227-004-1295-9.
Onda, D. F. L., Lluisma, A. O., and Azanza, R. V., 2014. Develop-ment, morphological characteristics and viability of temporary cysts ofvar.(Dinophy- ceae)., 49 (3): 265- 275, DOI: 10.1080/09670262.2014.915062.
Osorio-Montalvo, P., Sáenz-Carbonell, L., and De-la-Pe?a, C., 2018. 5-Azacytidine: A promoter of epigenetic changes in the quest to improve plant somatic embryogenesis., 19 (10): 3182-3182, DOI: 10. 3390/ijms19103182.
Palsamy, P., Bidasee, K. R., and Shinohara, T., 2014. Valproic acid suppresses/Keap1 dependent antioxidant protection through induction of endoplasmic reticulum stress and1 promoter DNA demethylation in human lens epithelial cells., 121: 26-34, DOI: 10.1016/j.exer. 2014.01.021.
Powles, S. B., 1984. Photoinhibition of photosynthesis induced by visible-light., 35 (1): 15-44, DOI: 10.1146/annurev.pp.35.060184.000311.
Sano, H., Kamada, I., Youssefian, S., Katsumi, M., and Wabiko, H., 1990. A single treatment of rice seedlings with 5-azacy- tidine induces heritable dwarfism and undermethylation of ge- nomic DNA., 220 (3): 441-447, DOI: 10.1007/BF00391751.
Sheldon, C. C., Burn, J. E., Perez, P. P., Metzger, J., Edwards, J. A., Peacock, W. J.,., 1999. TheMADS box gene: A repressor of flowering in arabidopsis regulated by vernaliza- tion and methylation., 11 (3): 445-458, DOI: 10.1105/tpc.11.3.445.
Shin, H. H., Li, Z., Yoon, Y. H., Oh, S. J., and Lim, W. A., 2017. Formation and germination of temporary cysts ofMargalef (Dinophyceae) and their ecolo- gical role in dense blooms., 66: 57-64, DOI: 10.1016/j.hal.2017.05.002.
Stephens, T. G., Gonzalez-Pech, R. A., Cheng, Y. Y., Mohamed, A. R., Burt, D. W., Bhattacharya, D.,., 2020. Genomes of the dinoflagellateencode tandemly repeat- ed single-exon genes with adaptive functions., 18: 56, DOI: 10.1186/s12915-020-00782-8.
Ta?kin, K. M., ?zbilen, A., Sezer, F., Hurkan, K., and Gunes, S., 2017. Structure and expression of DNA methyltransferase genes from apomictic and sexualspecies., 67: 15-21, DOI: 10.1016/j.compbiol chem.2016.12.002.
Veluchamy, A., Lin, X., Maumus, F., Rivarola, M., Bhavsar, J., Creasy, T.,., 2013. Insights into the role of DNA methy- lation in diatoms by genome-wide profiling in., 4: 2091, DOI: 10.1038/ ncomms3091.
Wang, Z. C., Nie, L. J., and He, Y. X., 2009. The effect of 5- azacytidine to the DNA methylation and morphogenesis cha- racter of chrysanthemum duringgrowth., 36 (12): 1783-1790 (in Chinese with English abstract).
Wang, Z. H., Nie, X. P., Yue, W. J., and Li, X., 2011. Physiolo- gical responses of three marine microalgae exposed to cyper- methrin., 27 (10): 563-572, DOI: 10. 1002/tox.20678.
Wang, Z. H., Qi, Y. Z., and Yang, Y. F., 2007. Cyst formation: An important mechanism for the termination of(Dinophyceae) bloom., 29 (2): 209-218, DOI: 10.1093/plankt/fbm008.
Yan, T., Li, X., Cao, Y., Wu, B. Y., Wang, J., and Zhang, M. M., 2021. Effects of 5-azacytidine on drought yolerance of rice with high expression of C4-PEPC., 36 (4): 96-107 (in Chinese with English abstract).
Yang, F., Li, L., and Lin, S. J., 2020. Methylation pattern and expression dynamics of methylase and photosystem genes un- der varying light Intensities in(Symbio- diniaceae)., 56 (6): 1738-1747, DOI: 10. 1111/jpy.13070-20-045.
Zhang, Y. X., Si, F. H., Wang, Y. Y., Liu, C. Y., Zhang, T., Yuan, Y. C.,., 2020. Application of 5-azacytidine induces DNA hypomethylation and accelerates dormancy release in buds of tree peony., 147: 91-100, DOI: 10.1016/j.plaphy.2019.12.010.
Zhao, J. G., Tang, T., Zhang, J. N., Guo, H., and Wang, Z. H., 2022. Studies on growth and antioxidant responses of two di- noflagellates species under exposure to decitabine., 17 (3): 468-476 (in Chinese with Eng- lish abstract).
Zinssmeister, C., Soehner, S., Facher, E., Kirsch, M., Meier, K. J. S., and Gottschling, M., 2011. Catch me if you can: The taxo- nomic identity of(F. Stein) A.R.Loebl. (Thoracosphaeraceae, Dinophyceae)., 9 (2): 145-157, DOI: 10.1080/14772000.2011.586071.
Zutshi, S., Choudhary, M., Bharat, N., Abdin, M. Z., and Fatma, T., 2008. Evaluation of antioxidant defense responses to lead stress in-339., 44 (4): 889-896, DOI: 10.1111/j.1529-8817.2008.00542.x.
(November 8, 2022;
December 31, 2022;
March 20, 2023)
? Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2023
E-mail: twzh@jnu.edu.cn
E-mail: thuren@jnu.edu.cn
(Edited by Qiu Yantao)
 Journal of Ocean University of China2023年6期
Journal of Ocean University of China2023年6期
- Journal of Ocean University of China的其它文章
- Improving Yolo5 for Real-Time Detection of Small Targets in Side Scan Sonar Images
- Wave Radiation by a Floating Body in Water of Finite Depth Using an Exact DtN Boundary Condition
- Underwater Acoustic Signal Noise Reduction Based on a Fully Convolutional Encoder-Decoder Neural Network
- Revisiting the Seasonal Evolution of the Indian Ocean Dipole from the Perspective of Process-Based Decomposition
- Assessment of Storm Surge and Flood Inundation in Chittagong City of Bangladesh Based on ADCIRC and GIS
- Contraction of Heat Shock Protein 70 Genes Uncovers Heat Adaptability of Ostrea denselamellosa
