Analysis on the cation distribution of MgxNi1-xFe2O4(x=0,0.25,0.5,0.75,1)using M¨ossbauer spectroscopy and magnetic measurement
Shiyu Xu(徐詩語), Jiajun Mo(莫家俊), Lebin Liu(劉樂彬), and Min Liu(劉敏),3,?
1College of Nuclear Science and Technology,University of South China,Hengyang 430074,China
2School of Physics,Huazhong University of Science and Technology,Wuhan 421001,China
3Zhuhai Tsinghua University Research Institute Innovation Center,Zhuhai 519000,China
Keywords: spinel structure,magnetic properties,ferrite,M¨ossbauer spectra
1.Introduction
Considerable attention has been given to spinel ferrite,the ferrite with the general formulaMFe2O4(Mare divalent ions, such as Mn2+, Ba2+, Mg2+, Co2+, Cu2+, Ni2+, Zn2+,Co2+).[1]Historically, it has been used in many fields due to its high resistivity,chemical stability,mechanical hardness,and reasonable cost,including in information storage systems,magnetic cores, magnetic fluids, microwave absorbers, and many others.[2,3]

NiFe2O4is an inverse spinel ferrite that is also a known soft ferrite.[12]Due to its high-frequency permeability, high resistance, and low dielectric loss, it is widely used in highfrequency electronics.[3]The electromagnetic properties of Ni ferrite can be improved by ion doping, making it more practical.[13]The magnetic moments of Fe3+ions and Ni2+ions are 5μBand 2μB, respectively.Based on the A-B superexchange interaction, NiFe2O4has a theoretical magnetic moment of 2μB.[14,15]
Ni-Mg ferrite is a widely used type of soft magnetic material, but the structure and electromagnetic properties of Mg ferrite are different from those of Ni ferrite.This is because Mg2+cations have a certain preference for octahedral sites,but this preference is weaker than that of Ni cations.[16]Also,Mg2+ions have a diamagnetic moment(0μB);therefore,the superexchange interaction with iron ions is different from that with Ni2+ions.[15]It is also important to note that the position preferences and ionic radii of Ni2+ions and Mg2+ions are also different,which affect the magnetic properties of Mg-Ni ferrite.In this way, Ni-Mg ferrite can be optimized for outstanding properties by changing the proportion of Ni2+ions and Mg2+ions.
We prepared Mg-Ni spinel ferrite materials,MgxNi1-xFe2O4(x=0, 0.25, 0.5, 0.75, 1), using the sol-gel method in this study.Tests were performed on samples using x-ray diffractometers,M¨ossbauer spectrometers,and vibrating sample magnetometers.Different properties were fitted and analyzed based on x-ray diffraction(XRD),M¨ossbauer spectra, and vibrating sample magnetometer (VSM)data, and the cation distribution and magnetic changes were determined.
2.Experimental techniques
2.1.Sample preparation
In this work, Mg-Ni spinel ferrites, MgxNi1-xFe2O4(x= 0, 0.25, 0.5, 0.75, 1), were prepared using the sol-gel method.The raw materials iron(III) nitrate nonahydrate Fe(NO3)3·9H2O, nickel(II) nitrate hexahydrate Ni(NO3)2·6H2O,magnesium(II)nitrate hexahydrate Mg(NO3)2·6H2O and citric acid C6H8O7were purchased from Macklin.A calculation was made to determine the amount of raw materials required for the experiment.One mole of spinel ferrite requires three moles of nitrate and three moles of citric acid.The desired quantities of reagents were dissolved in 150 ml of deionized water and heated at 80?C.Ammonia was gradually added to the compound solution while stirring to maintain a pH of 7-9.The solution was stirred for 5 h until it evaporated to produce a highly viscous gel.This gel was then baked at 225?C for 4 h,resulting in foamy ferrite powder.A dense ceramic metal oxide is formed by grinding the oxide into a fine powder, then calcining it at 1000?C for 9 h in a muffle furnace.Finally, the calcined metal oxide is ground in a mortar to obtain the finished product.[12]
2.2.Characterization of the samples
The XRD patterns of samples were recorded using a Siemens D500 diffractometer with Cuα(λ=1.5418 ?A) radiation, which is in a Bragg angle range (10?≤θ ≤80?).The patterns were analyzed using MDI-JADE software.The M¨ossbauer spectra were obtained at room temperature by a Seeco W304 M¨ossbauer spectrometer with a57Co/Rh source.Spectra were fitted using Mosswinn 4.0 software, and the most appropriate parameters were estimated.A magnetization curve (M-H) for the compounds was determined from VSM measurements at temperatures of 300 K and 5 K,respectively,and from-7 T to 7 T external magnetic field.
3.Results and discussion
3.1.X-ray diffraction analysis
The XRD data of Mg-Ni spinel ferrite MgxNi1-xFe2O4(x=0, 0.25, 0.5, 0.75, 1) with different Mg2+ion contents are shown in Fig.1.The XRD patterns of the synthesized materials are shown in Figs.1(a)-1(e),respectively,and those of NiFe2O4(PDF:#97-005-2387)and MgFe2O4(PDF:#97-004-0679) in the PDF file are shown in Figs.1(f) and 1(g).The patterns of the materials prepared in the experiment are generally consistent with those of the standard JCPDS cards.Each sample showed identical peaks of planes (220), (331),(400),(422),(511)and(440).Therefore,the single-phase cubic spinel structureFdˉ3mis formed, and all the compounds are spinel ferrites with a face-centered cubic structure.[1]
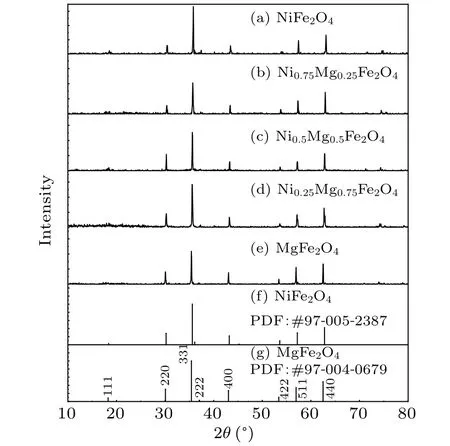
Fig.1.Powder XRD patterns of spinel MgxNi1-xFe2O4 (x=0, 0.25,0.5,0.75,1)powers.
Using the type of diffraction peak and intensity of the diffraction ray, it is possible to calculate the crystallite size(D),the lattice parameter(a),and cell volume of the samples.Based on the Scherrer equation,the crystallite size(D)of the samples was estimated by[17]
wherekis a constant(the value is usually 0.9),βis the half intensity width of the different peaks,λis the x-ray wavelength,andθis the angle of diffraction.
The lattice constanta(?A)is calculated from the Nelson-Riley correction and Bragg equation[18]
whereλis the x-ray wavelength,h,k, andlare the Miller indices,andθis the angle of diffraction.
Based on the calculated microcrystalline size(D),lattice parameter (a) and cell volume of the samples.The lattice constant increases from 8.3 ?A to 8.4 ?A, within the range of spinel ferrite lattice constants.Grain sizes range from 10 nm to 100 nm, which are larger nanoparticles.The cell volume ranges from 580 ?A3to 600 ?A3, which is within the normal range of Ni-Mg spinel ferrites.The preparation of Ni-Mg spinel ferrite appears to be successful.

Fig.2.The evolution of the lattice constant of the samples and standard lattice constants of NiFe2O4 and MgFe2O4.
As it appears on the standard JCPDS cards (NiFe2O4:#97-005-2387;MgFe2O4: #97-004-0679),NiFe2O4has a lattice constant ofa=b=c=8.34577 ?A, and MgFe2O4has a lattice constant ofa=b=c=8.3998 ?A.Standard cards are compared with the calculated results of the experimental data for lattice constants.The lattice constants of the samples and the standard NiFe2O4and MgFe2O4are shown in Fig.2.It can be seen that the samples’lattice constants increase as the amount of Mg2+increases.The lattice constant of the samples increases with increasing Mg2+ion content since the radii of Ni2+ions and Mg2+ions at octahedral positions are 0.069 nm and 0.072 nm, respectively.[19]The lattice constants of synthesized NiFe2O4and MgFe2O4differ slightly from those of the standard JCPDS card.According to Msomiet al.’s[20]study on Co-Ni spinel ferrite,lattice constants have a relationship with annealing temperatures.The lattice constant first increases(200-700?C)and then decreases(700-1100?C)as the annealing temperature increases (200-1100?C).Thus, it is speculated that the higher annealing temperature may have caused the difference in lattice constants between the sample and the standard JCPDS cards.An increase in lattice constant is observed with changes in Mg ion content,which is consistent with the results reported by Rosnanet al.This implies successful Mg2+ion doping.[21]
3.2.M¨ossbauer spectroscopy analysis
Due to the strong preference of Ni2+ions for octahedral sites,two sextets were used to fit the M¨ossbauer spectrum data of the NiFe2O4sample.The results of the fitting and the associated parameters are shown in Table 1 and Fig.3(a).The obtained results indicate that the peak area of iron ions in the tetrahedral sites(IA)is 47.5%,while that in the octahedral sites(IB)is 52.5%.The positions of cations can be calculated byIAandIBusing the following formula:[22]
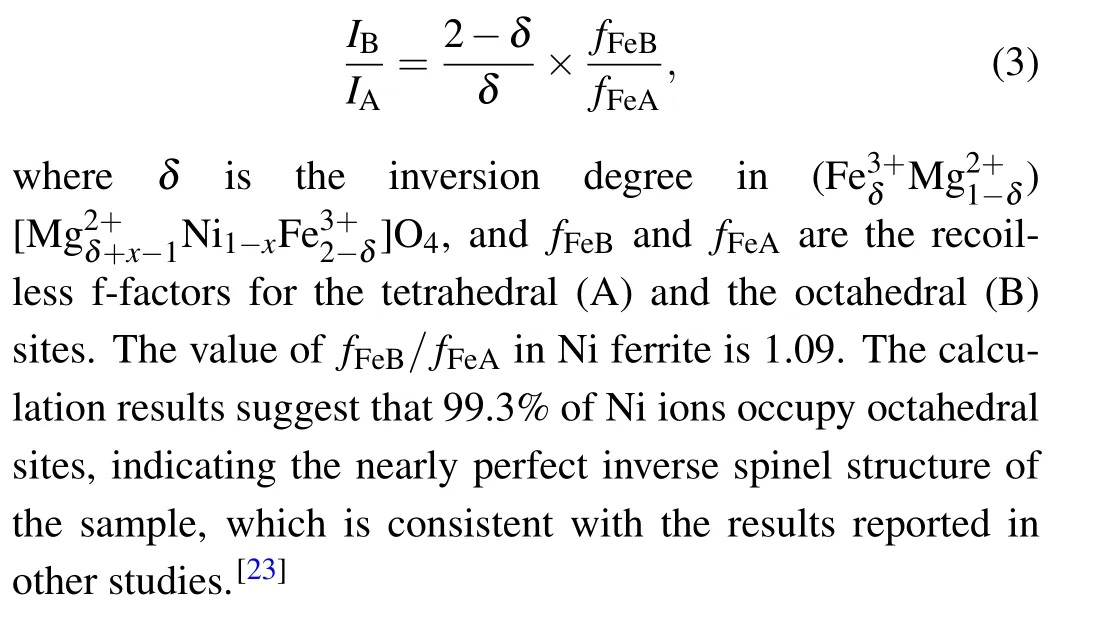
MgxNi1-xFe2O4(x=0.25,0.5,0.75,1)has a more complicated cation distribution due to the addition of Mg2+ions.Some approximations are introduced to simplify the calculation.For the octahedral sites of Fe3+ions, a sextet (labeled as sextet A) was used for fitting.For the tetrahedral sites of Fe3+ions,a study found that the dominant superexchange interaction for Fe3+ions at octahedral sites is the interaction between A-sites and B-sites: (A)-O-[B].Because it is 15-20 times stronger than the interaction between B-sites and Bsites, only the superexchange between B-sites and A-sites is considered.[23]Tetrahedral sites are considered to contain no nickel ions, since Ni2+ions prefer octahedral sites.Due to the trivalent Fe ions in the sample,the structure has extremely high symmetry, quadruple splitting (QS)=0 mm/s and isomer shift(IS)=0-0.5 mm/s.Many studies[24,25]have reported that the M¨ossbauer spectroscopic parameters of Fe ions at the B site are influenced by the six nearest tetrahedral sites of cations.Depending on the fraction of Mg2+ions at these tetrahedral sites surrounding the Fe ions, different sextets can be observed,denoted as B,B1,...,Bn,wherenis the number of Mg2+ions among the six nearest tetrahedral sites.The probability of observing a certain sextet at the octahedral siteP(n,a)can be expressed as a function of the number of Mg2+ions among the six nearest tetrahedral sites(n)using the following equation:
whereais the Mg2+ion content of the A-site.P(n,a)values lower than 5%contribute very little to M¨ossbauer spectra and can be omitted.[9]Some papers[16]defineaas the total amount of Mg ionsx, but many studies have shown that MgFe2O4is a disordered spinel ferrite and the value ofadoes not equalx.[19,26]Therefore, in this study, the fitting of the M¨ossbauer spectra for B-sites is not solely based on Eq.(4).M¨ossbauer spectrum data of MgxNi1-xFe2O4withx=0.25,0.5,0.75,and 1 were fitted starting from two sextets.The value of (1-IA)from the fitting was used as the actual Mg2+ion contentafor the A-sites, which was then substituted into Eq.(4).The probability given by Eq.(4)was compared with the fitted peak areas(Irel),and a set of better fitting results was chosen as the final results.This results in four, five, five, and six sextets in the samples withx=0.25,0.5,0.75,and 1,as shown in Fig.3 and Table 1.
There was substantial agreement between the fitted curve and the experimental data.The crystal blocking temperature is a size-dependent parameter.[27]When the blocking temperature of the crystal falls below test temperature,the thermal energy exceeds the anisotropic energy, and the samples display superparamagnetic behavior.It appears that the M¨ossbauer spectrum is a doublet or a collapse of the M¨ossbauer spectroscopy.The absence of these appearances suggests the synthesized sample has a large grain size,which is consistent with the XRD data.The values of the isomer shift are between 0.1 mm/s and 0.5 mm/s,with the B-site generally higher than the A-site,consistent with other studies.[26]
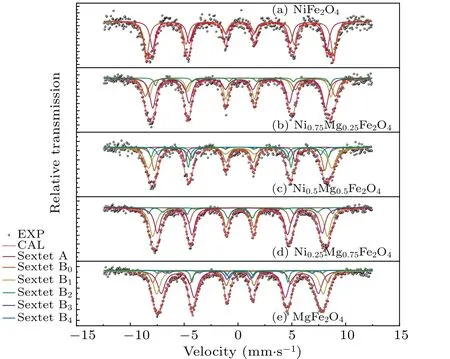
Fig.3.Room-temperature 57Fe M¨ossbauer spectra of spinel MgxNi1-xFe2O4 (x=0, 0.25, 0.5, 0.75, 1) powers.Here, EXP and CAL are experimental and computational M¨ossbauer data,respectively.

Table 1.M¨ossbauer parameters of spinel MgxNi1-xFe2O4 (x=0, 0.25, 0.5, 0.75, 1) powers.Here, IS is isomer shift, Γ is the Lorentzian linewidth,Heff is the hyperfine magnetic field,Irel is relative areas and cations at A and B sites.
Based on the relative areas of the tetrahedral siteIAand octahedral siteIB, we can calculate the distribution of Fe3+ions.As a result, it gives the cations at A and B sites of MgxNi1-xFe2O4(x=0, 0.25, 0.5, 0.75, 1), as shown in Table 1.As is evident, Ni2+ion content influences Mg2+ion proportions in tetrahedral sites.When Ni2+ions are abundant,Mg2+ions tend to occupy tetrahedral sites.Furthermore,the distribution of different cations causes different magnetic properties; therefore, it is possible to calculate the saturation magnetic moments of a whole series of samples.[28]The relationship between the saturation magnetic moment and the cation distribution can be calculated using the following formula for each chemical formula of the sample:[29]
where the notation{}represents the magnetic moment at the B site, and [] represents the magnetic moment at the A site.Here,μNiandμFeare 2μBand 5μB,respectively,andδis the inversion degree inThe calculation results are presented in Table 2 and Fig.4.CAL.With increasingx,the sample’s saturation magnetization first increases and then decreases.The trend of the variation of the saturation magnetization is similar to the trend of the doping non-magnetic ion Zn in CoFe2O4.[30]When the value ofxis between 0.25 and 0.5, the results of the M¨ossbauer spectroscopy show a significant number of Mg ions located in the tetrahedral position, which is consistent with previous studies:[19]Ni2+ions prefer octahedral positions more than Mg2+ions, resulting in more non-magnetic Mg2+ions occupying tetrahedral positions.Consequently, the total magnetic moment of tetrahedral sites decreases,and the saturation magnetic moment difference between tetrahedral and octahedral sites becomes greater.Asxreaches 0.5, the saturated magnetic moment reaches its maximum value.Afterwards, with the continued increase in Mg2+ions,the non-magnetic Mg2+ions occupying octahedral sites increase and the Ni2+ions decrease,leading to a rapid decrease in the total saturated magnetic moment within octahedral sites.The difference in the saturation magnetic moment between A and B sites decreases,which makes the saturation magnetic moment of the samples drop continuously.A sample’s saturation magnetic moment ceaselessly decreases before its Ni2+ion content reaches zero.The evolution of the saturated magnetic moment and Mg2+ion contentxclearly demonstrates the correlation between structure and magnetic properties.

Fig.4.The experimental (EXP) and computational (CAL) saturation magnetic moment of spinel MgxNi1-xFe2O4 (x=0,0.25,0.5,0.75,1)powers.
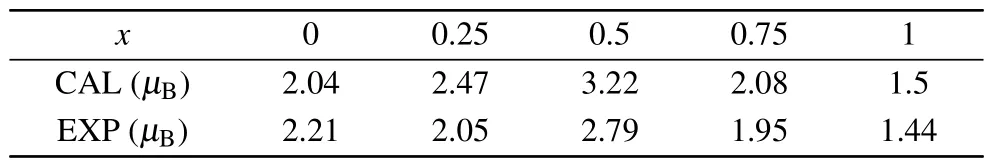
Table 2.The experimental and computational saturation magnetic moment of spinel MgxNi1-xFe2O4 (x=0,0.25,0.5,0.75,1)powers.
3.3.Vibrating sample magnetometer analysis
Data on the magnetization of the compounds were collected using VSM and are plotted in Figs.5 and 6.The data show typical ferromagnetic behavior at 5 K and 300 K, suggesting that the Curie temperature of the sample is higher than 300 K.The maximum saturation magnetization(Ms)was calculated using the following equation by using the hysteresis loop:[31]
whereσsis the saturation magnetization intensity in the hysteresis loop at 5 K, andMxis the relative molecular weight of the material.The calculated results are shown in Table 2 and Fig.4.EXP.As the Mg ion content increases, the saturation magnetization decreases from 2.21μBto 2.04μB,then increases to 2.79μB,and finally decreases to 1.44μB.The experimental and computational saturation magnetic moments of the compounds are compared in Fig.4.It is evident that both sets of data show a similar change trend,even though the theoretical data is higher than the experimental data when Mg and Ni ions coexist.The discrepancy between the experimental and theoretical data atx=0,0.25,0.5,and 0.75 may arise from the incomplete consideration of the distribution of Ni ions.Some Ni2+ions may occupy tetrahedral sites,especially when the concentration of Ni2+ions decreases.Some Ni2+ions in octahedral sites may potentially exchange with Mg2+ions in tetrahedral sites.As a result,the magnetic moment at tetrahedral sites increases,while the magnetic moment at octahedral sites decreases, leading to a smaller total magnetic moment.
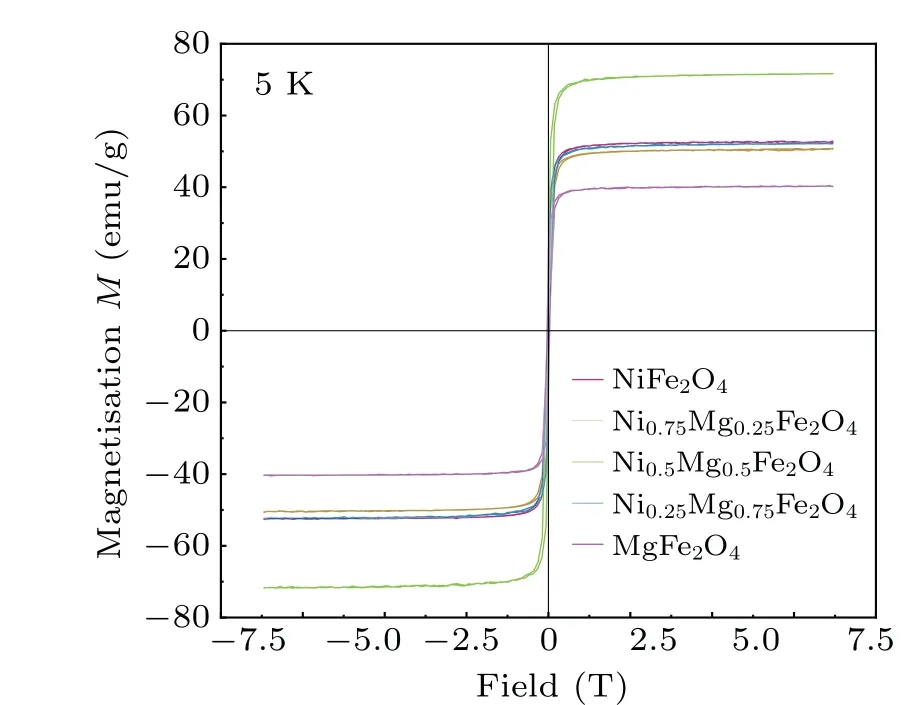
Fig.5.Magnetic hysteresis (M-H) loops of spinel MgxNi1-xFe2O4(x=0,0.25,0.5,0.75,1)powers at 5 K.
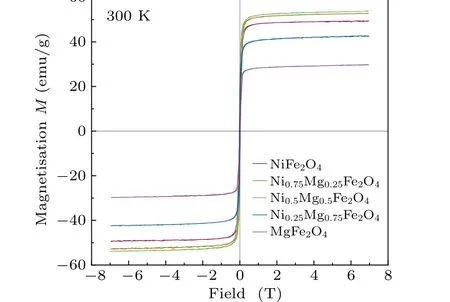
Fig.6.Magnetic hysteresis (M-H) loops of spinel MgxNi1-xFe2O4(x=0,0.25,0.5,0.75,1)powers at 300 K.
4.Conclusions
In this work, MgxNi1-xFe2O4(x=0, 0.25, 0.5, 0.75, 1)spinel ferrite materials were successfully prepared using the sol-gel method, and their structure and magnetic properties were analyzed.Based on the results,it can be concluded that the structure and magnetism of the samples are affected by the Mg2+ion contentx.Extensive XRD analysis showed that the sample remained a cubic spinel structureFdˉ3mwith an increase in Mg2+ion contentx.Because the Mg ion radius of octahedral sites is larger than the Ni ion radius, the crystallite size (D), lattice parameter (a), and cell volume gradually increase.According to the M¨ossbauer spectrum analysis, the distribution ratio of Fe3+ions between octahedral sites and tetrahedral sites changes with a change in Mg2+ion contentx.Cations are distributed differently in different materials,resulting in different magnetic properties.M¨ossbauer spectral analysis data are used to calculate the saturation magnetization of the sample.The results indicate that with the change in Mg2+ion contentx, the saturation magnetization of the sample first increases from 2.04μBto 3.22μB,and then decreases to 1.5μB.Based on theM-Hcurve at 5 K and 300 K, it is demonstrated that the sample is a ferromagnetic at room temperature.With an increase in Mg2+ion content, the saturation magnetization decreases from 2.21μBto 2.04μB, then increases to 2.79μB,and finally decreases to 1.44μB.Experimental data and data calculated by structure show the same evolution trend,and the error may be caused by an incomplete consideration of Ni ion distribution.
Acknowledgements
Project supported by the National Natural Science Foundation of China (Grant No.11447231), the National Undergraduate Innovation and Entrepreneurship Training Program Support Projects of China,the Natural Science Foundation of Hunan Province,China(Grant No.2020JJ4517),the Research Foundation of the Education Bureau of Hunan Province,China(Grant Nos.19A434, 19A433, and 19C1621), and the Opening Project of the Cooperative Innovation Center for Nuclear Fuel Cycle Technology and Equipment, University of South China(Grant Nos.2019KFY10 and 2019KFY09).
- Chinese Physics B的其它文章
- Diamond growth in a high temperature and high pressure Fe-Ni-C-Si system: Effect of synthesis pressure
- Multi-channel generation of vortex beams with controllable polarization states and orbital angular momentum
- Calibration of quantitative rescattering model for simulating vortex high-order harmonic generation driven by Laguerre-Gaussian beam with nonzero orbital angular momentum
- Materials and device engineering to achieve high-performance quantum dots light emitting diodes for display applications
- From breather solutions to lump solutions:A construction method for the Zakharov equation
- Complete population transfer between next-adjacent energy levels of a transmon qudit

