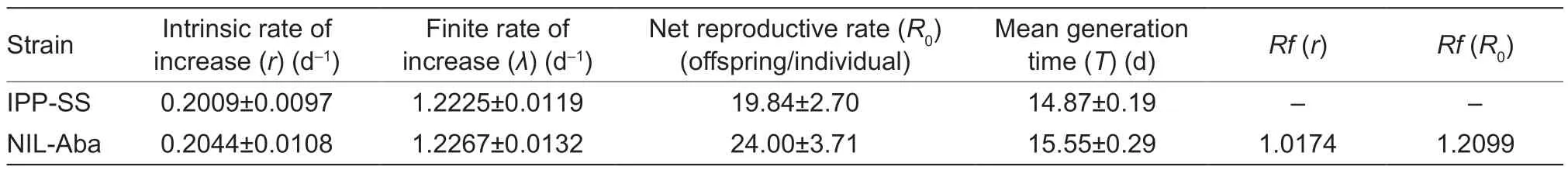
Lack of fitness cost and inheritance of resistance to abamectin based on the establishment of a near-isogenic strain of Tetranychus urticae
ZHANG Yan ,TlAN Tian ,ZHANG Kun ,ZHANG You-jun ,WU Qing-jun ,XlE WenGUO Zhao-jiangWANG Shao-li#
1 State Key Laboratory of Vegetable Biobreeding, Institute of Vegetables and Flowers, Chinese Academy of Agricultural Sciences,Beijing 100081, P.R.China
2 College of Agriculture, Yangtze University, Jingzhou 434025, P.R.China
3 Sanya Nanfan Research Institute, Hainan University, Sanya 572024, P.R.China
Abstract Many populations of the two-spotted spider mite,Tetranychus urticae Koch,have developed high levels of resistance to the pesticide abamectin in China and other countries.This study developed a near-isogenic line to understand better the inheritance,cross-resistance,and fitness costs associated with abamectin resistance in the field population of T.urticae in China.We introduced the trait that confers extremely high abamectin resistance in a field-collected population of T.urticae into a susceptible laboratory strain (IPP-SS) to generate an abamectin-resistant near-isogenic line (NIL-Aba).This process was carried out through multiple backcrossing to IPP-SS and via parthenogenesis and abamectin screening.Compared with IPP-SS,the NIL-Aba strain had a 25 147-fold resistance to abamectin and a high level of cross-resistance to bifenthrin (288.17-fold),an intermediate level to emamectin benzoate (42.57-fold),and low levels to bifenazate,chlorfenapyr,cyflumetofen,cyenopyrafen,and cyetpyrafen with resistance ranging from 3.18-to 9.31-fold.But it had no cross-resistance to profenofos.The resistance to abamectin in NIL-Aba was autosomal,incompletely dominant,and polygenic.Based on two sex life table parameters,no fitness cost was found in NIL-Aba.Establishing the NIL-Aba strain provides a reliable basis for an in-depth study of abamectin resistance in T.urticae.New information on toxicological characteristics and fitness cost should facilitate the management of abamectin resistance in field populations of T.urticae.
Keywords: two-spotted spider mite,abamectin resistance,near-isogenic strain,inheritance pattern,fitness cost
1.lntroduction
The two-spotted spider mite,TetranychusurticaeKoch,has caused substantial damage to fruits and vegetables(Van Leeuwenet al.2010;Wybouwet al.2019).Tetranychus urticae,whose control is dependent on pesticide applications,has evolved resistance to a variety of pesticides,including pyrethroids,organophosphates,and abamectin,because of its short life cycle,high fecundity,and parthenogenetic reproduction (Dermauwet al.2013;Xuet al.2021).
Abamectin is widely used for the management of major pests,including insects,mites,and nematodes.As an agonist of GABA receptors on neuromuscular cells,abamectin can disrupt neural signaling and cause paralysis and death (Wolstenholme 2010).Due to the heavy application of abamectin,T.urticaeand other phytophagous mites have developed high levels of resistance to abamectin in China (Xuet al.2018;Zhanget al.2022).The resistance ofT.urticaeto abamectin mainly involves target-site resistance and metabolic resistance (Liet al.2007;Van Leeuwenet al.2010;Rigaet al.2014;Van Leeuwen and Dermauw 2016;Xuet al.2020).Mutations in glutamate-gated chloride channels(GluCls) have been shown to contribute to abamectin resistance (for mutation G314D in GluCl1 and G326E in GluCl3) (Kwonet al.2010;Dermauwet al.2012;Mermanset al.2017) or to be associated with abamectin resistance (for mutation I321T in GluCl3) (Xueet al.2021) inT.urticae.The expression of genes that encode detoxification enzymes,including cytochrome-P450-monooxoygenases (P450s),glutathioneS-transferases(GSTs),and uridine diphosphate-glycosyltransferases(UGTs),is also predicted to be involved in abamectin resistance (Ahnet al.2014;Daneshianet al.2021;Xuet al.2021).Among detoxification enzymes,however,only the cytochrome P450 monooxygenaseCYP392A16has been found to directly contribute to abamectin resistance inT.urticaethroughinvitrometabolism experiment (Rigaet al.2014;Papapostolouet al.2022).
Heet al.(2009) found that the abamectin-resistant strains ofTetranychusmites have always been isolated from the field such that reference strains with the same or similar background were lacking.Due to the differences in genetic backgrounds between the reference and field-evolved resistant populations,determining the mechanism of resistance has been difficult.Additionally,it has been unclear whether the resistance resulted from specific resistance mechanisms or from differences in fitness (Zhuet al.2015).The abamectin-resistantTetranychuscinnabarinus,a sibling species ofT.urticae,was reported to have a fitness cost on cotton and bean hosts (Heet al.2004);however,it is not predicted to have similar fitness forT.urticaehaving abamectin resistance.Compared to the weaker resistance to abamectin forT.cinnabarinusin the field (Biet al.2016),there is a big difference in the development of abamectin resistance in the field populations ofT.urticae,which has evolved high resistance against abamectin in China and European countries (Xuet al.2018;Xueet al.2021).This suggests possible different fitness or other characteristics in theT.urticaeresistant strain.In addition,the fitness costs associated with pesticides are also influenced by the genetic background of the insect population,and theT.urticaefield population differs in the genetic background with the reference strain,so the determination of fitness costs is difficult and inaccurate forT.urticae.The near-isogenic resistant strain after multi-backcrosses has the same background as the susceptible strain,which facilitates the assessment of fitness costs and further studies on resistance (Zhuet al.2015).Therefore,in order to determine the fitness and other physiological,biochemical,and molecular properties related to resistance,it is essential to establish a resistant near-isogenic line to eliminate the complications caused by differences in genetic backgrounds.
In this study,we established an abamectin-resistant near-isogenic line (NIL-Aba) ofT.urticaeand evaluated its inheritance of abamectin resistance,cross-resistance with other pesticides,and fitness parameters.These results increase our understanding of abamectin resistance inT.urticaeand should provide guidance for the management of abamectin resistance in the field.
2.Materials and methods
2.1.Mites
An abamectin-susceptible laboratory strain (IPP-SS) was provided by Dr.Xu Xuenong of the Chinese Academy of Agricultural Sciences;at the outset of this study,IPP-SS had been kept for >10 years without any pesticide exposure.In the autumn of 2020,an abamectin-resistant field population,CP-BJ,was collected from chili plants in a greenhouse in Beijing,China.All mites were reared on disks of young leaves of kidney bean (PhaseolusvulgraisLinn,cv.Bifeng) in a growth chamber at (26±1)°C,(60±5)%relative humidity (RH),and a 16 h L:8 h D photoperiod.
2.2.Acaricides and chemicals
All of the tested chemicals were commercial formulations.They were provided by chemical companies as follows:abamectin 5% EC (Hebei Veyong Biochemical Co.,Ltd.,Hebei,China),emamectin benzoate 5% EC(Jinnonghua Pharmaceutical Co.,Ltd.,Shandong,China);bifenthrin 25 g L–1EC (Quzhou Xiangfeng Agricultural Products Co.,Ltd.,Zhejiang,China);profenofos 40% EC(Yongnong Biological Science Co.,Ltd.,Zhejiang,China);chlorfenapyr 240 g L–1SC (BASF Plant Protection Co.,Ltd.,Jiangsu,China);bifenazate 43% SC (Macdermid Technology Co.,Ltd.,Jiangsu,China);cyflumetofen 20%SC (Suzhou FMC Plant Protectant Co.,Ltd.,Jiangsu,China);cyenopyrafen 30% SC (Nissan Chemical Industries,Ltd.,Japan),cyetpyrafen 30% SC (Zhonghua Pesticide Chemical Research and Development Co.,Ltd.,Shenyang,China).
2.3.Toxicity bioassays
A leaf-dipping method was used to determine the toxicity of pesticides toT.urticaestrains,as previously reported(Xuet al.2018).Based on a preliminary experiment,each pesticide was diluted with distilled water,each concentration was represented by three replicates,and distilled water was used as the control.Leaf disks (2 cm in diameter) were immersed in each solution for 10 s and were dried in a fume hood.The disks were then placed with their abaxial sides up on 0.2% agar in plastic Petri dishes (3.5 cm in diameter,one disk per dish).A total of 25–30 active female adult mites were then placed on the leaf disk in each dish.
After 24 h,the mites were examined with a stereo microscope (SXZ-7;Olympus,Japan);mites were recorded as dead if they did not move after being gently touched with the tip of a brush.When the mortality of the control treatment did not exceed 20%,probit analysis with Polo Plus 2.0 Software (LeOra Software,Berkeley,CA,USA) was used to evaluate the following properties of the mortality of pesticide-treated mites: slope (mortality on pesticide concentration)±SE,LC50value and 95% fiducial limits,Chi-square value,and degrees of freedom (df).Resistance-fold (RF) values were calculated by dividing the LC50of resistant populations by the LC50of the IPP-SS strain.
2.4.Establishment of T. urticae NlL-Aba
As previously described forFrankliniellaoccidentalis(Yuanet al.2017) andBemisiatabaci(Wanget al.2020),NILAba ofT.urticaewas established by multiple backcrosses combined with abamectin screening (Fig.1).First,200 unfertilized females of the susceptible strain IPP-SS(SS♀) and 200 males of the resistant population CP-BJ(R♂) were selected and placed at a 1:1 ratio on clean leaf disks in a Petri dish for paired rearing.After 3 days,the adults were removed,and the eggs on the leaf disks were retained.One mite was selected per dish to obtain unmated female progeny when the eggs had developed into nymphs.Next,the male individuals F1P1(S♂+R♂)were obtained by parthenogenesis from the female mites and were then exposed to an appropriate concentration of abamectin in order to select for abamectin resistance.After selection,the F1P1(R♂) resistant males were backcrossed with the unfertilized susceptible adult female mites (SS♀) to generate offspring,i.e.,the BC1generation.After this procedure was repeated eight times,the unfertilized female adult mites (SR♀) of the BC8 generation were backcrossed with their male parent BC7P1(R♂) to produce offspring BC8F1(SR♀+RR♀+S♂+R♂).The NIL-Aba (RR♀+R♂) ofT.urticaewas obtained after additional abamectin screening.
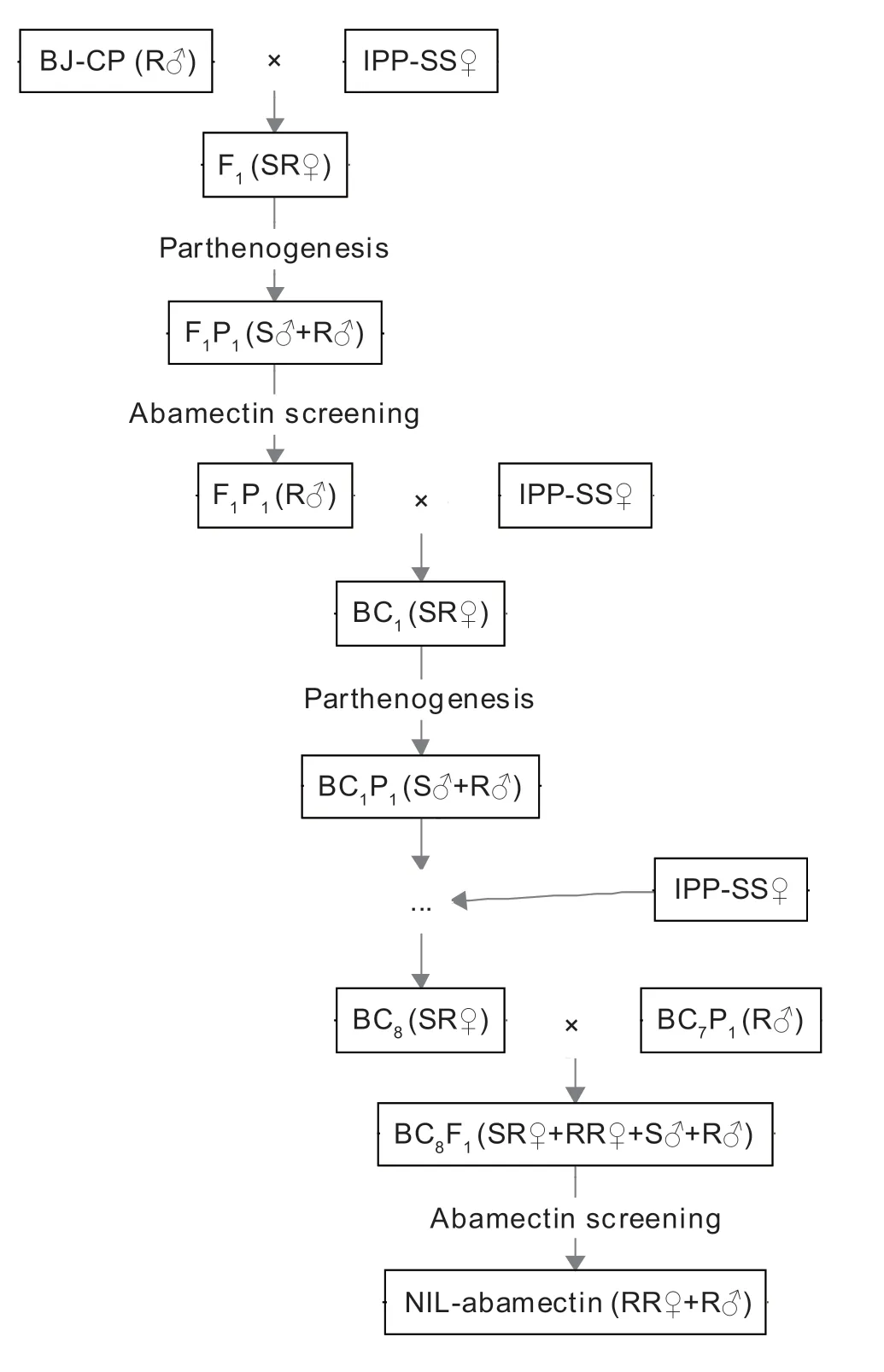
Fig.1 Construction of the near-isogenic Tetranychus urticae strain resistant to abamectin.
2.5.lnheritance analyses
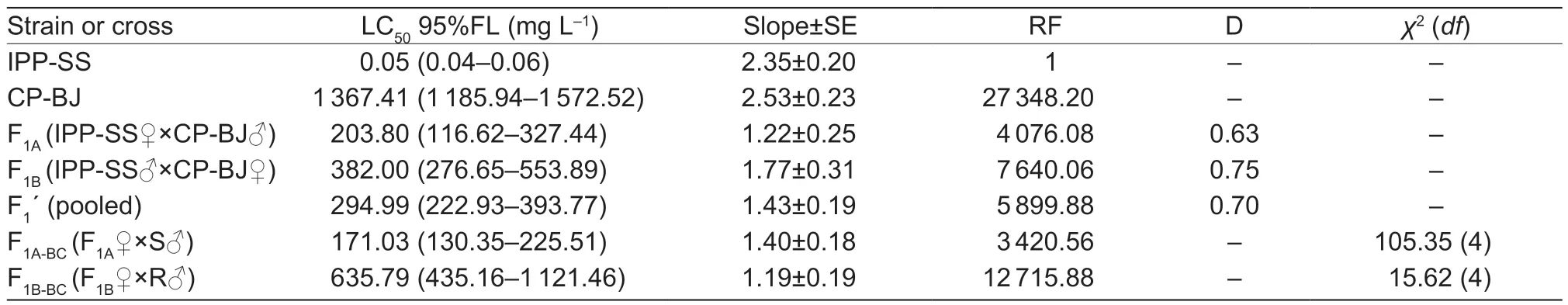
To determine how abamectin resistance is inherited inT.urticae,three groups (IPP-SS♀×CP-BJ♂,IPPSS♂×CP-BJ♀,and pooled) were crossed with the IPPSS strain and CP-BJ population.BecauseT.urticae is parthenogenetic,all genes of haploid males are inherited only from the maternal side.Therefore,the F2females generated from F1self-crossing offspring are equivalent to the backcross females.The toxicological responses to abamectin of F1and its offspring from the reciprocal crosses were determined (Hoyet al.1988).
The degree of resistance dominance (D) was calculated by the following formula:
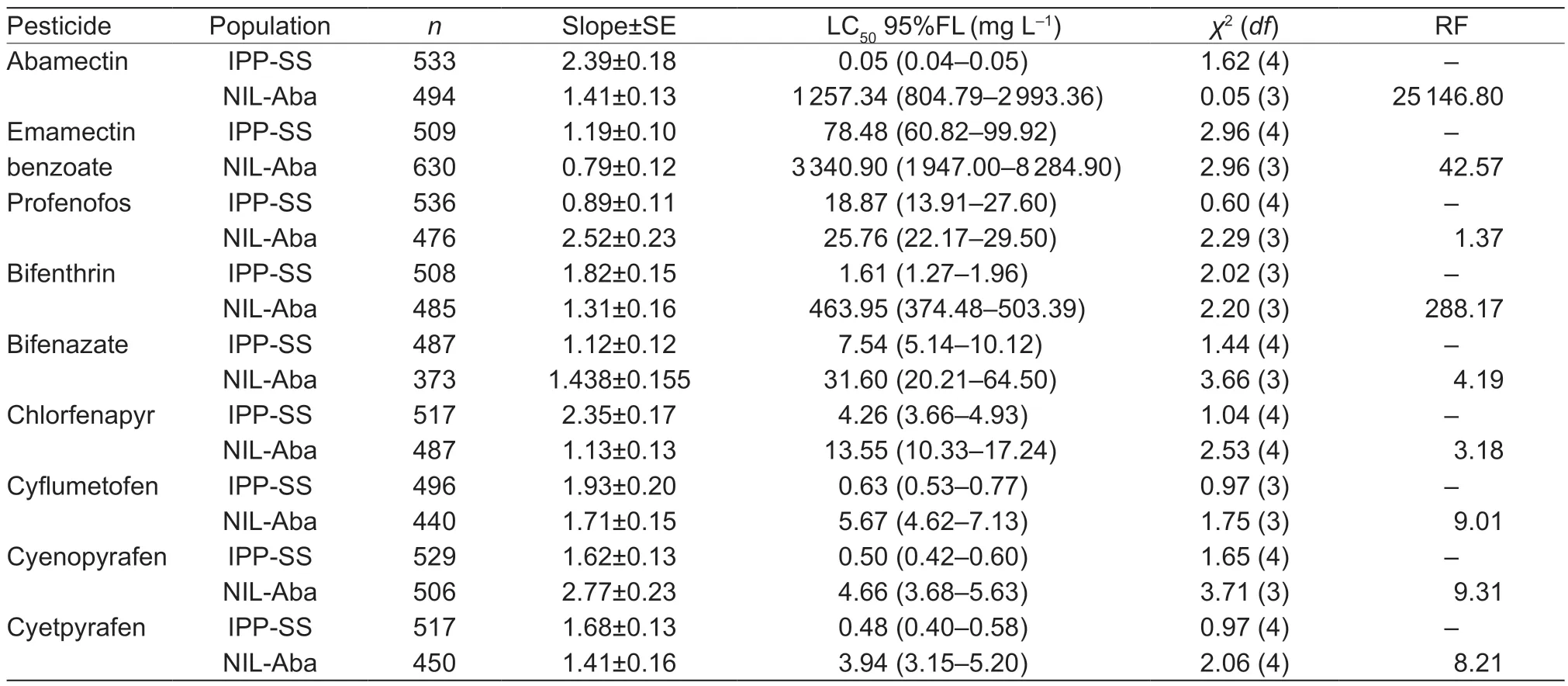
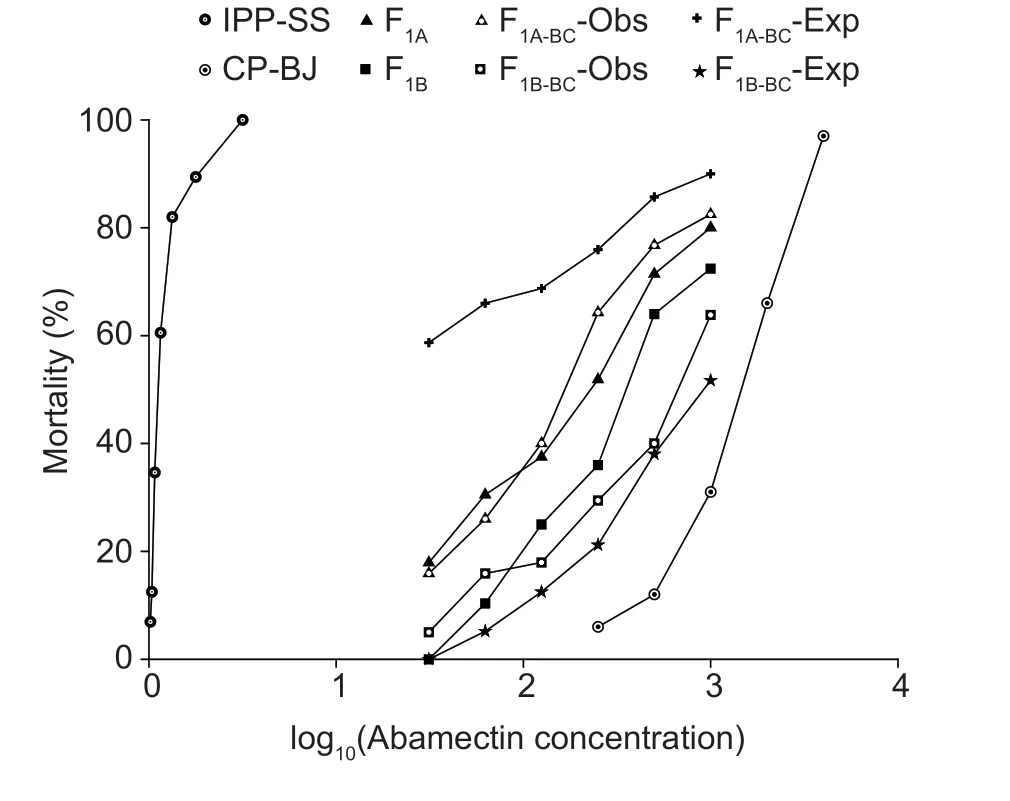
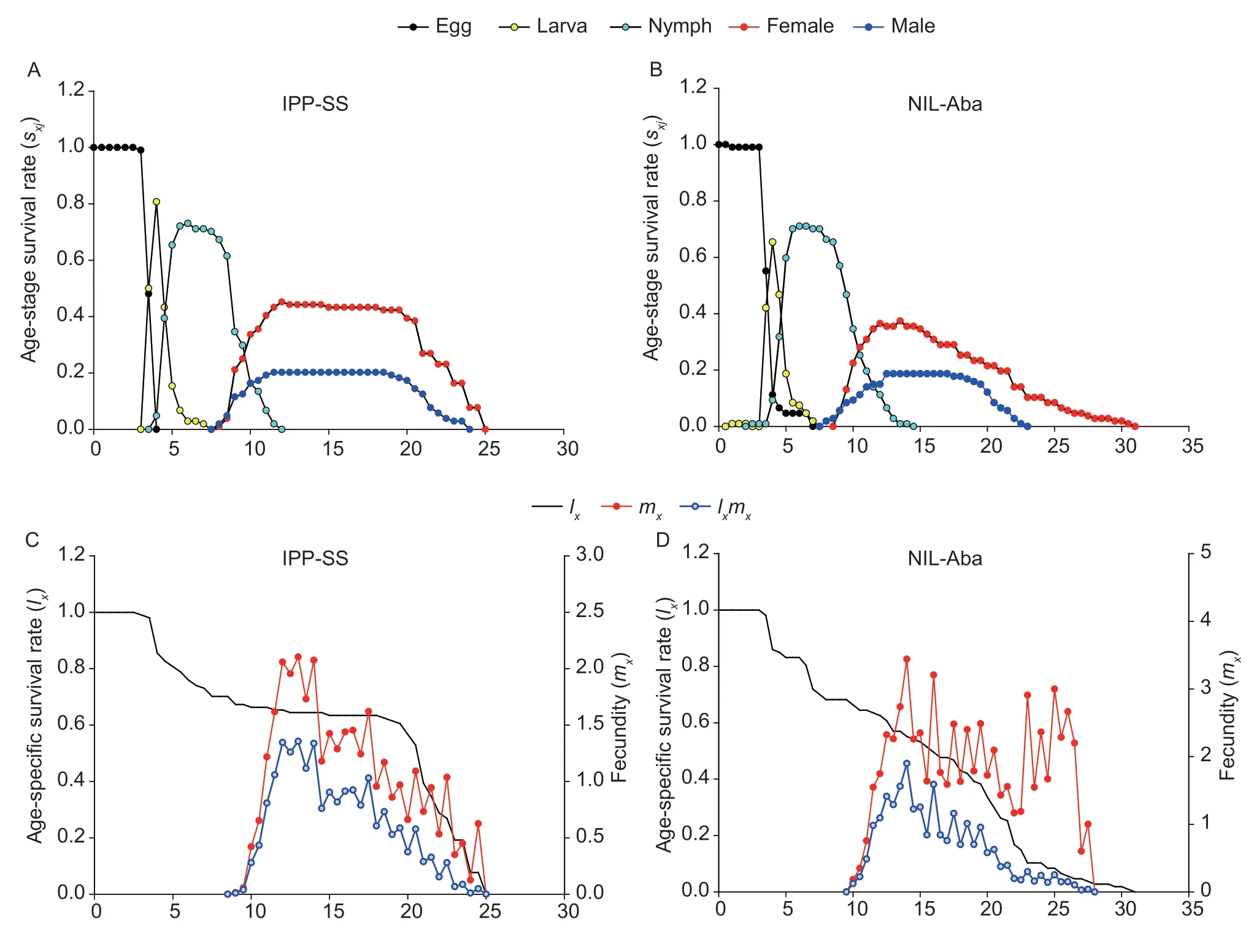
where F1,RR,and SS are the LC50values of heterozygous,CP-BJ,and IPP-SS strains,respectively.According to Stone (1968),if D=?1,the resistance is fully recessive;if ?1 The number of genes potentially involved in the resistance ofT.urticaeto abamectin was estimated based on the responses of back-cross progeny to abamectin by the Chi-square test and the goodness-offit between the concentration–response curve between the observed and the theoretical mortality.If there was no significant difference between the observed and expected concentration–response curves,the resistance was considered monogenic;otherwise,the resistance was considered polygenic (Tsukamoto 1983;Keena and Granett 1990).Expected and observedχ2values of the BC generation were also analyzed to estimate the number of genetic resistance factors.Chi-square goodness of fit was determined with the following formula (Tabashnik 1991): whereNiis the observed mortality in backcross hybrids at a particular dose,pis the expected mortality calculated from the Mendelian model (Georghiou and Taylor 1977),qis calculated as 1–p,nis the number of mites exposed at a particular dose,ris the number of treatment doses (r=6,in this study),andiis one of treatment dose (i=1,in this study).As noted earlier for concentration-response curves,a significant difference between observed and expectedχ2values indicated that the resistance was polygenic,and an insignificant difference between observed and expectedχ2values indicated that the resistance was monogenic (Keena and Granett 1990). As previously described (Xuet al.2019;Sunet al.2022),life tables were generated for the susceptible strain IPP-SS and the NIL-Aba.OneT.urticaefemale was placed on a leaf disk that sat on a sponge-filter paper in plastic Petri dishes;after one egg was deposited,the female was removed (usually in about 4 h).In this manner,a total of 120 eggs were obtained for each strain and kept under the previously indicated conditions.The status and mortality of each developmental stage were recorded every 12 h.After they matured,the males and females were mated,and the number of eggs laid by a single female and the number of surviving adult mites was recorded every 12 h until death. All life table data from NIL-Aba and IPP-SST.urticaestrains were analyzed using the TWOSEX-MSChart program (Chi and Liu 1985;Chi 1988,2022) to determine the following parameters: the age-stage-specific survival rate (sxj,wherexis age andjis stage),agespecific survival rate (lx),age-specific fecundity (mx),and population parameters (r,the intrinsic rate of increase;λ,the finite rate of increase;R0,the net reproduction rate;andT,the mean generation time).The standard errors(SEs) of all these parameters were estimated by the bootstrap procedure with 100 000 bootstraps.Graphs were generated with SigmaPlot version 14.0 Software(Systat Software,San Jose,CA,USA).The relative fitness (Rf) values were calculated using the intrinsic rate of increase (r) and the net reproduction rate (R0). The concentration-response results for the IPP-SS strain,the CP-BJ population,and their F1progeny from reciprocal crosses are summarized in Table 1.LC50values of F1progeny did not significantly differ (based on the overlapping of 95% FL) among all three crossing groups,indicating that the resistant CP-BJ population inherited abamectin resistance in an autosomal manner.The values of degree of resistance dominance (D) for F1A,F1B,and F1′ derived from CP-BJ ranged from 0 to 1,indicating that the abamectin resistance of the CP-BJ population was incompletely dominant. Table 1 Bioassays of abamectin on susceptible IPP-SS and resistant CP-BJ strains of Tetranychus urticae and their F1 progenies from reciprocal crosses1) The NIL-Aba ofT.urticaewas obtained through the backcrossing and abamectin screening for 8 cycles,with the susceptible strain (IPP-SS) and abamectin-resistant field population (CP-BJ) ofT.urticae(Fig.1).Compared with the IPP-SS,the LC50of NIL-Aba strain was 1 257.34 mg L–1(Table 2),showing the resistant ratio of 25 146.80-fold,which was basically the same as resistant parent CP-BJ(resistant ratio 27 348.20) (Table 1). Table 2 Cross-resistance of the abamectin-selected NIL-Aba and the abamectin-susceptible IPP-SS strains of Tetranychus urticae1) To determine the number of genes involved in abamectin resistance,we subjected the females of back-crossed progeny to an abamectin toxicity test.The LC-P lines of the back-crossed progeny (F1A-BCand F1B-BC) had no obvious flat slope in the mortality rate of 50% and had slopes that differed from those of expected LC-P lines (Fig.2).The lack of plateaus suggested that the abamectin resistance inT.urticaemight be governed by more than one gene.In addition,the results of the Chisquare test showed that Σχ1A-BC2=105.35>Σχ0.012=13.277(df=4) and that Σχ1B-BC2=15.62>Σχ0.012=13.277 (df=4),which did not conform to the hypothesis of single-gene inheritance,indicating that the resistance ofT.urticaeto abamectin was polygenic.The log concentration-mortality analysis and Chi-square test strongly indicated thatT.urticaeinheritance of resistance to abamectin might be controlled by multiple genes. Fig.2 The observed and expected log concentration–mortality lines against abamectin for susceptible strain IPP-SS and resistant population CP-BJ of Tetranychus urticae and their reciprocal (F1A and F1B) and backcross progenies (F1A-BC-Exp,F1B-BC-Exp,F1A-BC-Obs and F1B-BC-Obs). Compared to IPP-SS,the NIL-Aba strain exhibited RF values of 288.17 to bifenthrin and 42.57 to emamectin benzoate,indicating the presence of cross-resistance(Table 2).The NIL-Aba strain also showed low crossresistance to bifenazate,chlorfenapyr,cyflumetofen,cyenopyrafen,and cyetpyrafen,with RF values ranging from 3.18 to 9.31.However,no cross-resistance to profenofos was observed in the NIL-Aba strain (Table 2). The life table parameters of NIL-Aba and IPP-SS were evaluated (Table 3;Fig.3),and the relative fitness of NILAba was determined (Table 4).The total immature period was significantly longer for NIL-Aba than for IPP-SS,which was manifested in three stationary periods showing 0.27,0.10,and 0.51 d longer than IPP-SS,respectively.The average lifespans of females and males were shorter for NIL-Aba than IPP-SS,whereas the average total fecundity was higher for NIL-Aba than IPP-SS (Table 3).The curves ofsxjwere similar for NIL-Aba and IPP-SS,but the survival rates for larvae,nymphs,and females were higher for IPP-SS than for NIL-Aba,whereas the NIL-Aba females could grow up for more than one month,longer than IPP-SS.Thelxwas substantially lower for NIL-Aba than for IPP-SS;however,themxandlxmxwere considerably higher for NIL-Aba,indicating that fecundity was higher for NIL-Aba (Fig.3). Table 4 Life table parameters of a susceptible laboratory strain (IPP-SS) and an abamectin-resistant near-isogenic line (NIL-Aba)strain of Tetranychus urticae1) Fig.3 The age-stage survival rate (sxj) (A and B),age-specific survival rate (lx),fecundity (mx),and net maternity (lxmx) (C and D)of a susceptible laboratory strain (IPP-SS) and an abamectin-resistant near-isogenic line (NIL-Aba) strain of Tetranychus urticae. Moreover,the life table parametersr,λ,R0,andTwere higher for NIL-Aba than for IPP-SS.The fitness values of NIL-Aba relative to IPP-SS were close to 1 (calculated onrand calculated onR0),indicating that there was no fitness cost or fitness advantage for NIL-Aba (Table 4). To study insecticide or stress resistance,researchers have often compared near-isogenic lines that differ substantially in resistance but not in other properties.For amphigenetic insects,such asSpodoptera exigua(Cheet al.2015) andPlutellaxylostella(Wanget al.2021),backcrosses or recombination are commonly used to generate the NILs.For parthenogenetic insects,such as thrips (Yuanet al.2017) and whiteflies (Wanget al.2020),researchers have obtained NILs by multiple backcrosses and parthenogenesis.In the current study,we took advantage of parthenogenesis and used abamectin selection to obtain resistant males (such as F1P1) ofT.urticae.For insects with bisexual reproduction,however,three steps are needed to generate resistant males: selfing,insecticide selection,and selection of surviving males (Wanget al.2021).In the current study,eight rounds of backcrossing and abamectin screening generated the NIL-Aba strain ofT.urticae,which had much higher abamectin resistance than the CP-BJ field population.We found that the inheritance of abamectin resistance inT.urticaewas autosomal,incompletely dominant,and polygenic.Our methods were similar to those in previous studies,which showed that the inheritance of abamectin resistance was completely dominant inHaemonchuscontortus(Le Jambreet al.2000) and incompletely dominant inP.xylostella(Puet al.2010).Our results are also consistent with previous reports that chlorpyrifos resistance inT.urticaeand cyetpyrafen resistance inT.cinnabarinusare autosomal,incompletely dominant,and polygenic (Ay and Yorulmaz 2010;Sunet al.2022).ForT.cinnabarinus,however,inheritance of resistance was previously found to be incompletely recessive against abamectin (Heet al.2009),which might help explain why abamectin resistance has seldom been reported in field populations ofT.cinnabarinus(Biet al.2016).The incomplete dominance of abamectin resistance inT.urticaein this study may also explain the rapid evolution of resistance to abamectin in its field populations.It follows that careful resistance management is required to avoid selecting for abamectin resistance inT.urticae. In addition to the inheritance mode,the number of genes involved affects the level of pesticide resistance in pests(Maet al.2017).In insects and mites,polygenic pesticide resistance is more common and more difficult to avoid than monogenic resistance because the heterozygotes are also resistant to chemical pesticides (Kliot and Ghanim 2012).Polygenic resistance in mites has been commonly reported for several pesticides,including spirodiclofen(Van Pottelbergeet al.2009),cyflumetofen (Fenget al.2018),and abamectin (Heet al.2009;this present study).Such polygenic resistance might help explain why field populations ofT.urticaein China have developed extremely high abamectin resistance (Tanget al.2014;Zhanget al.2022).Previous studies confirmed that both mutation G314D in GluCl1 and G326E in GluCl3 are correlated with abamectin resistance inT.urticae(Kwonet al.2010;Dermauwet al.2012;Mermanset al.2017);these mutations were also determined in NIL-Aba strain obtained in this study,and the frequencies of G314D and G326E were 97.61 and 65.52% (data not shown),differing with the resistant parent CP-BJ with 98.15 and 100%,respectively (data not shown).In addition to the point mutations inGluClgenes (Kwonet al.2010) and the overexpression ofCYP392A16(Rigaet al.2014),other P450s also have been predicted to be involved in abamectin resistance inT.urticae(Xuet al.2021).IPM management strategies are clearly needed to prevent further development of abamectin resistance inT.urticae. Cross-resistance is common between chemical agents that belong to the same group of insecticide (Gormanet al.2010).In the current study,the NIL-Aba strain had an RF value of 42.57 to emamectin benzoate compared to the susceptible IPP-SS strain,indicating an intermediate level of cross-resistance.This result is consistent with previous studies ofS.exigua(Cheet al.2015) andP.xylostella(Puet al.2010).It is reasonable because abamectin and emamectin benzoate have similar modes of action and structures.We also found a high level of cross-resistance to bifenthrin in the NIL-Aba strain.The latter result is not surprising,given thatMuscadomesticawith resistance to pyrethroid was reported to have cross-resistance to abamectin,which appeared to be based on MFO-mediated metabolism and decreased cuticular penetration (Jeffrey 1989).The NIL-Aba strain also showed low or no crossresistance to bifenazate,chlorfenapyr,cyflumetofen,cyenopyrafen,cyetpyrafen,and profenofos.Therefore,these pesticides might be useful for rotations with abamectin forT.urticaecontrol in the field. Fitness costs always accompany the development of pesticide resistance and are important to consider when managing pesticide resistance.For many insects,fitness costs in resistant individuals mainly involve prolonged immature developmental time,reduced larval survival rate,or decreased fecundity (Fuet al.2018;Gulet al.2019;Wanget al.2022).In the current study,the NIL-Aba strain ofT.urticaewith high resistance to abamectin had no obvious fitness cost based on both the intrinsic rate of increase (r) and the net reproduction rate (R0).Moreover,NIL-Aba had a longer immature stage,a shorter adult lifespan,and a higher fecundity than the IPP-SS strain;this is consistent with previous results obtained for NIL strains ofSpodopterafrugiperda(Padovezet al.2022),Laodelphaxstriatellus(Zenget al.2022),andCryptolestes ferrugineus(Singarayanet al.2021).However,abamectin resistance-related fitness costs have been found inT.cinnabarinusandP.xylostella,with negative effects on biological performance and population parameters(Heet al.2004;Wang and Wu 2014).In addition,fitness advantages in terms of increased fecundity were observed in fenpropathrin-resistantT.cinnabarinusand cyetpyrafenresistantT.urticaestrains (Liuet al.2016;Sunet al.2022).Such results also help explain why the abamectin resistance inT.urticaehas been relatively stable during the past eight years of research by our laboratory (Tanget al.2014;Zhanget al.2022). We generated NIL-Aba ofT.urticaethrough multiple backcrossing and abamectin screening.The inheritance of abamectin resistance in the NIL-Aba strain was autosomal,incompletely dominant,and polygenic.The NIL-Aba strain ofT.urticaehad a high level of crossresistance to bifenthrin and emamectin benzoate and a low level of cross-resistance to some newly developed pesticides,suggesting that pesticides must be carefully rotated to remain effective.In addition,lack of fitness cost was confirmed to be associated with abamectin resistance in the NIL-Aba strain.These findings help explain why abamectin resistance inT.urticaehas developed rapidly and stably in the field.The results also provide a foundation for further research on the mechanisms of abamectin resistance inT.urticae. Acknowledgements We gratefully acknowledge help from Prof.Hsin Chi,Department of Entomology,National Chung Hsing University,Taiwan of China,for his kindness of providing TWOSEX-MSChart Software and reviewing this manuscript.This research was funded by the National Natural Science Foundation of China (32072458),the earmarked fund for China Agriculture Research System(CARS-25),the Beijing Key Laboratory for Pest Control and Sustainable Cultivation of Vegetables,China,and the Science and Technology Innovation Program of the Chinese Academy of Agricultural Sciences (CAAS-ASTIPIVFCAAS). Declaration of competing interest The authors declare that they have no conflict of interest.2.6.Life table assay and fitness cost
3.Results
3.1.lnheritance of abamectin resistance in T.urticae
3.2.Establishment of NlL-Aba in T.urticae
3.3.Genetic inheritance of abamectin resistance
3.4.Cross-resistance of the T.urticae NlL-Aba strain
3.5.Fitness cost of the NlL-Aba strain

4.Discussion
5.Conclusion
 Journal of Integrative Agriculture2023年6期
Journal of Integrative Agriculture2023年6期
- Journal of Integrative Agriculture的其它文章
- ldentification of two novel linear epitopes on the p30 protein of African swine fever virus
- Uncertainty aversion and farmers’ innovative seed adoption:Evidence from a field experiment in rural China
- Ensemble learning prediction of soybean yields in China based on meteorological data
- Increasing nitrogen absorption and assimilation ability under mixed NO3– and NH4+ supply is a driver to promote growth of maize seedlings
- Significant reduction of ammonia emissions while increasing crop yields using the 4R nutrient stewardship in an intensive cropping system
- Maize straw application as an interlayer improves organic carbon and total nitrogen concentrations in the soil profile: A four-year experiment in a saline soil
