Enhancing boll protein synthesis and carbohydrate conversion by the application of exogenous amino acids at the peak flowering stage increased the boll Bt toxin concentration and lint yield in cotton
LIU Zhen-yu ,LI Yi-yang ,Leila.I.M.TAMBEL ,LIU Yu-ting ,DAI Yu-yang ,XU Ze ,LENG Xin-hua,ZHANG Xiang,CHEN De-hua#,CHEN Yuan#
1 Jiangsu Key Laboratory of Crop Genetics and Physiology/Co-Innovation Center for Modern Production Technology of Grain Crops, Yangzhou University, Yangzhou 225009, P.R.China
2 Agricultural Research Cooperation, Biotechnology and Biosafety Research Center, Khartoum 13314, Sudan
Abstract In Bacillus thuringenesis (Bt) transgenic cotton,the cotton boll has the lowest insecticidal protein content when compared to the other organs.The present study investigated the effects of amino acid spray application at the peak flowering stage on the cotton boll Bt toxin concentration and yield formation.Boll protein synthesis and carbohydrate conversion were also studied to reveal the fundamental mechanism.Three treatments (i.e.,CK,the untreated control;LA1,five amino acids;LA2,21 amino acids) were applied to two Bt cultivars of G.hirsutum (i.e.,the hybrid Sikang 3 and the conventional Sikang 1) in the cotton-growing seasons during 2017 and 2018.Amino acid spray application at the peak flowering stage resulted in an increase of 5.2–16.4% in the boll Bt protein concentration and an increase of 5.5–11.3%in the seed cotton yield,but there was no difference between the two amino acid treatments.In addition,amino acid applications led to increases in the amino acid content,soluble protein content,glutamate pyruvate transaminase (GPT)activity,glutamate oxaloacetate transaminase (GOT) activity,glucose content,fructose content and soluble acid invertase(SAI) activity.This study also found that Bt protein content,enhanced boll number and the weight of opened bolls were closely related to carbon and nitrogen metabolism.The Bt protein content had significant linear positive correlations with amino acid and soluble protein contents.Enhanced boll number had significant linear positive correlations with the GPT and GOT activities from 15–25 days after flowering (DAF).The weight of opened bolls from 55–65 DAF had a significant linear positive correlation with the SAI activity.These results indicate that the enhancement of boll protein synthesis and carbohydrate conversion by amino acid application resulted in a simultaneous increase in the boll Bt protein concentration and cotton lint yield.
Keywords: Bt cotton,boll insecticidal protein,protein synthesis,carbohydrate conversion
1.Introduction
The Bt cotton varieties which are registered and commercialized show more than 90% insecticidal ability in China;but that evaluation is based on the insect resistance of leaves,while the boll Bt insect resistance is only actually 10% of that in leaves,and the insecticidal effect is only about 40% for whole plant (Dong and Li 2007;Xuet al.2008;Sunet al.2016).The damage from cotton bollworm still occurs during the cotton growth seasons (Zhanget al.2019;Luet al.2022) in the cotton production regions of China.Therefore,enhancing Bt insect resistance,especially for boll insect efficacy,is more important for the control of bollworm.Many studies have shown that declining insect resistance with the development process results in a low insecticidal ability at the boll-setting stage in Bt cotton (Glenn 2011;Chenet al.2018);the lowest Bt efficacy expressed in the boll still causes boll abscission and yield loss due to bollworm(Chenet al.2005a,b);and the altered insect resistance is associated with the Bt toxin concentration (Gasser and Fraley 1989;Adamczyk and Meredith 2004;Shenet al.2010).In addition to the associations with the silencing of the Bt gene and physiological age (Xiaet al.2005;Wanget al.2009;Chenet al.2012a,b),the reduced Bt toxin content is also associated with protein synthesis and degradation (Zhanget al.2007;Chenet al.2017).After flowering,the protein synthesis ability decreases and the decomposition ability increases.The activities of glutamate pyruvate transaminase (GPT),glutamate oxaloacetate transaminase (GOT) and the key enzymes of amino acid synthesis decrease,while the activities of proteases and peptidases which decompose proteins increase.The content of the insecticidal protein in Bt cotton can be increased using either protein synthesis promoters to enhance protein synthesis or protease inhibitors to inhibit protein decomposition (Zhou 2021).In addition,the changes in Bt insecticidal protein concentrations in cotton leaves and bolls are closely related to the physiological and biochemical mechanisms of protein turnover and metabolism as well as the operation and distribution of nitrogen assimilation,such as amino acids in leaves.
The use of nitrogen regulation for enhancing the Bt toxin content by increasing protein synthesis has been investigated in our previous studies.Those studies found that nitrogen application under soil nitrogen deficit conditions can enhance the Bt protein concentrations of square and boll (Li 2019),while excessive nitrogen in the soil causes yield reduction due to the overgrowth of vegetative organs (Boquet and Breitenbeck 2000;Wiedenfeldet al.2009;Chenet al.2021b;Wanget al.2021),and it also causes ecological environmental pollution.The enhanced yield and Bt protein concentrations of the boll wall and seeds are detected after urea spraying at the boll development stage (Zhanget al.2021a;Zhouet al.2021b) and increased flower Bt toxin content also occurs due to amino acid application(Abidallhaet al.2017).Protein degradation and synthesis increase remarkably in flowers under the application of amino acids.The enhanced protein metabolism contributes to an increase in the protein concentration.As a part of the total soluble protein,the Bt protein in flowers also increases under the application of amino acids (Tambelet al.2019).The cotton boll is the yield organ and the first target of bollworm damage.However,the effects of amino acid spraying,as a way to address bollworm damage,on both boll Bt efficacy and the economic yield of Bt cotton have rarely been studied.
Therefore,in order to reduce the risks associated with pesticide use,and avoid an increase in nitrogen fertilizer use and its ecological damage,it is hypothesized that spraying exogenous amino acids during boll formation could simultaneously enhance the insecticidal efficacy and economic yield of Bt cotton.In this study,the possibility of simultaneously enhancing both boll Bt protein expression and yield formation by spraying amino acids was initially investigated and the associated mechanism was explored.
2.Materials and methods
2.1.Plant materials and field experiment design
Field experiments were conducted at Yangzhou University Farm,Jiangsu Province,China (32°30′N,119°25′E)from 2017 to 2018.In the two-year study,two Bt transgenic cotton cultivars were used,i.e.,Sikang 1 (S1,conventional) and Sikang 3 (S3,hybrid).Seeds were sown on 12 April 2017 and 13 April 2018 in a plastic lilliputian greenhouse.Seedlings were transplanted to the field on 18 May 2017 and 19 May 2018.The sandy loam soil (Typic fluvaquents,Entisols (USDA Soil Taxonomy))of the experimental field contained 21.6 g kg?1organic matter,as well as 108.2,20.7 and 83.5 mg kg?1available N-P-K in 2017;and 21.9 g kg?1organic matter,as well as 110.5,21.0 and 84.6 mg kg?1available N-P-K in 2018.Insecticides and plant growth regulator DPC (1,1-dimethyl piperidinium chloride,C7H16CIN) were applied following local cultural practices for high cotton yield.The K (120 kg ha?1KCl) and P (300 kg ha?1single superphosphate)were applied at the transplanting stage,and the same proportions of KCl and superphosphate were top-dressed at the early flowering stage.The N (300 kg ha–1urea) was applied before the transplanting stage (20%),at the early flowering stage (20%) and at the peak flowering stage(60%).The study was arranged with a split plot design,i.e.,the main plot treatment consisted of two cultivars(S1 and S3) and the subplot treatment consisted of three different amino acid treatments (i.e.,0 (CK),five (LA1)and 21 (LA2) types of amino acids) with a concentration of 20 mg kg?1.For LA1,the five selected amino acids were aspartic acid,glutamic acid,proline,methionine and arginine,which all have a significant effect on Bt protein content based on previous studies (Abidallhaet al.2017).For LA2,the 21 amino acids included aspartic acid,glutamic acid,proline,methionine,arginine,glycine,tyrosine,phenylalanine,histidine,serine,threonine,alanine,cysteine,valine,isoleucine,leucine,lysine,tryptophan,asparagine,ornithine and glutamine.The solutions were sprayed on the cotton plants at the peak flowering stage.The flowers at the first position of the fourth to sixth fruiting branches of the plants were labeled.The spraying position was the whole plant.Three replications were conducted and each plot was 6 m in length with four rows spaced 0.9 m apart.The exogenous amino acid (20 mg kg?1for each amino acid component)was sprayed with pneumatic spray bottles (fan-type nozzle),and each subplot was sprayed with 1 000 mL of the solution to ensure that the whole plant was moist.The sprays were conducted at 16:00 h in the afternoon during the peak flowering stage on 25 July 2017 and 27 July 2018 for the two-year experiment.
2.2.lnvestigation of boll number,boll weight and economic yield
The boll number of individual plants was recorded for the 10 plants in the center of the third row on days 20,25 and 30 after amino acid treatment,and the boll weight was obtained from 30 open bolls on days 55,60 and 65 after amino acid treatment.On the day when the bolls were 30% open,the retained boll number per plant was also obtained from 10 consecutive plants in the center two rows of each plot.The seed cotton yield per plot was determined by weighing the manually harvested seed cotton from the center four rows.Seed cotton was ginned for lint and the lint percentage was calculated as the ratio of lint weight to seed cotton weight.
2.3.Physiological measurements
Flowers were labeled when the amino acid was applied.Five bolls were collected at each timepoint from the first position of the 7th to 8th fruiting branches on 15,20 and 25 days after flowering (DAF).The bolls were thoroughly mixed before subsampling.
CryIAc protein contentThe Bt protein content in cotton boll extracts was determined by enzyme-linked immunosorbent assay (ELISA) (Chenet al.1997).Three subsamples (0.2 g fresh weight (FW)) were extracted from each boll using a standard 2-cm diameter paper ticket punch.Boll tissue extracts (~0.5 g) were prepared by homogenizing the frozen boll tissue in 2 mL extraction buffer (1.33 g Na2CO3+0.192 g DTT+1.461 g NaCl+0.5 g vitamin C dissolved in 250 mL distilled water).The contents were then transferred to 10-mL centrifuge tubes,and the residue was washed with 3 mL of the buffer which was also transferred to the centrifuge tubes.The contents in the tube were shaken by hand and then stored at 4°C for 4 h.After centrifugation at 10 000 r min–1for 20 min at 4°C,the supernatant was passed through a C18 Sep-Pak Cartridge (Waters,Milford,MA) and collected to determine the Bt protein content.The Cry1Ac level was quantified using a commercially available kit (Scientific Service,Inc.,China Agricultural University,China).Microtitration plates were coated with standard Cry1A insecticidal proteins and samples,and incubated at 37°C for 4 h.Antibodies were added to each well and the plates were incubated at 37°C for an additional 30 min.The antibodies against the Cry1A insecticidal protein were obtained as described by Weileret al.(1981).Horseradish peroxidase-labeled goat antirabbit immunoglobulin was added to each well and the plates were incubated at 37°C for 30 min.Finally,a buffered enzyme substrate (1,2-phenylenediamine)was added,and the enzymatic reaction was performed at 37°C for 15 min in the dark and then terminated using 3 mol L–1H2SO4.The absorbance was recorded at 490 nm.Cry1Ac protein concentrations were calculated from the ELISA data,as described by Weileret al.(1981).All other chemicals were supplied by Scientific Service,Inc.,China Agricultural University,China.
GPT and GOT activitiesThe boll samples (1.0 g) were homogenized in 5 mL of 0.05 mol L–1Tris-HCl (pH 7.2)and the mixture was centrifuged at 26 100×g for 10 min at 4°C.The supernatant was the crude extract,and it was used for the GOT and GPT activity assays.To obtain the GOT activity,0.2 mL of the supernatant was added to a mixture containing 0.5 mL of 0.8 mol L–1alanine in 0.1 mol L–1Tris-HCl (pH 7.5),0.1 mL of 2 mol L–1pyridoxal phosphate solution and 0.2 mL of 0.1 mol L–12-oxoglutarate solution.The reaction mixture was incubated at 37°C for 10 min,and 0.1 mL of 0.2 mol L–1trichloroacetic acid solution was added to terminate the reaction.Then,the fluorescent color intensity of the hydrazone in the water-saturated toluene was measured at 520 nm.The GOT activity was calculated in terms of pyruvate production based on pyruvate standards run simultaneously.The same procedure used for the GOT assay was followed to obtain the GPT activity,except that in the GPT assay,0.5 mL of 0.8 mol L–1alanine in 0.1 mol L–1Tris-HCl (pH 7.5) was used instead of 0.5 mL of 0.1 mol L–1buffered aspartate solution in the reaction mixture and the addition of aniline citrate was omitted(Thomas 1975).
Free amino acid and soluble protein contentThe boll samples (0.8 g) were used for the extraction and analysis of the amino acid and soluble protein contents.The samples were added to 3 mL Milli-Q water.The mixture was stirred at 4°C and centrifuged at 800×g for 5 min;then,the supernatant was stored in ice.Next,the pellets were re-extracted twice and the resulting supernatants were pooled for analysis.The total free amino acid content was determined by the ninhydrin assay and the absorbance readings were converted to mg amino acid g–1FW using a glycine standard curve.
Glucose,fructose and sucrose contentsThe glucose,fructose and sucrose contents were extracted from 2 g dried leaves,which were ground and passed through a 1-mm sieve to obtain a fine powder.Next,0.2 g of the sample was placed into a 2-mL centrifuge tube with 1.0 mL of 80% ethanol and homogenized in a vortex for 1 min.Then,the tube was placed in a 70°C water bath for 90 min.Samples were then allowed to cool naturally at room temperature and centrifuged at 16 100×g and 25°C for 10 min.The resulting supernatant was placed in a new tube,and the final volume was adjusted to 1 mL by adding 80% ethanol.This supernatant was measured using the enzymatic method of Stitt (1989).A total of 130 μL buffer solution was added to each well of a 96-well plate,containing 200 mmol L–1imidazole,4 mmol L–1nicotinamide adenine dinucleotide,10 mmol L–1magnesium chloride,2 mmol L–1adenosine tri-phosphate,0.4 U of glucose-6-phosphate dehydrogenase,20 μL sample extract and 110 μL distilled water.
Glucose was measured by first taking the initial optical density (OD;A1) before adding the enzyme.Next,5 μL hexokinase (0.2 U) was added to each well and the peak OD was recorded after 5 min,which was taken as the final OD (A2).Then,fructose was measured by adding 5 μL phosphoglucoisomerase (0.6 U) and the peak OD was recorded.Finally,sucrose was measured by adding 5 μL invertase (50 U) and the peak OD was recorded.All these measurements were taken at 340 nm.ΔOD was calculated by subtracting the final OD from the initial OD (i.e.,A2–A1) and then dividing by 6.2 (i.e.,the nicotinamide adenine dinucleotide molar absorptivity)to obtain the absorbance value (μmol) of nicotinamide adenine dinucleotide-hydrogen per well,i.e.,ΔA=(A2–A1)/6.2.
Soluble acid invertase activitiesThree boll samples and their subtending leaves were analyzed for SAI activity(soluble acid invertase,EC 3.2.1.26).Each sample(0.5 g) was ground into powder using a mortar and pestle,and then placed into the 5 mL extraction solution containing 50 mmol L–1Hepes-NaOH buffer (pH 7.5),0.5 mmol L–1MgCl2,1 mmol L–1Na2EDTA,2 mmol L–1diethyldithiocarbamic acid,2.5 mmol L–1dithiothreitol,1%bovine serum albumin and 2% polyvinylpyrrolidone.The extract was centrifuged at 12 000×g for 20 min,and the SAI activity was determined by assaying the supernatant(Zahooret al.2017).
2.4.Statistical analysis
Experimental data were analyzed using SPSS.The significance of the differences between different treatments was tested with the least significant difference(P≤0.05),and the Pearson correlation coefficient was used to measure their correlations.
3.Results
3.1.Effects of exogenous amino acid application at the peak flowering stage on boll Bt toxin concentration and lint yield
The data in Table 1 show that the cultivars under the LA1 and LA2 treatments had enhanced boll Bt protein contents from 15–25 DAF.Compared to the control group(CK),the Bt toxin contents increased by 5.3–13.1% for cultivar S1 and by 9.2–16.4% for cultivar S3 in 2017.The LA2 treatment group exhibited higher boll Bt protein content than the LA1 treatment group,but no significant difference was found between LA1 and LA2.Similar experimental results were also obtained in 2018,with the Bt toxin contents enhanced by 5.2–11.8% and 6.8–13.4%for cultivars S1 and S3 under the amino acid treatments,respectively.There was no significant difference in the boll Bt protein contents between S1 and S3.
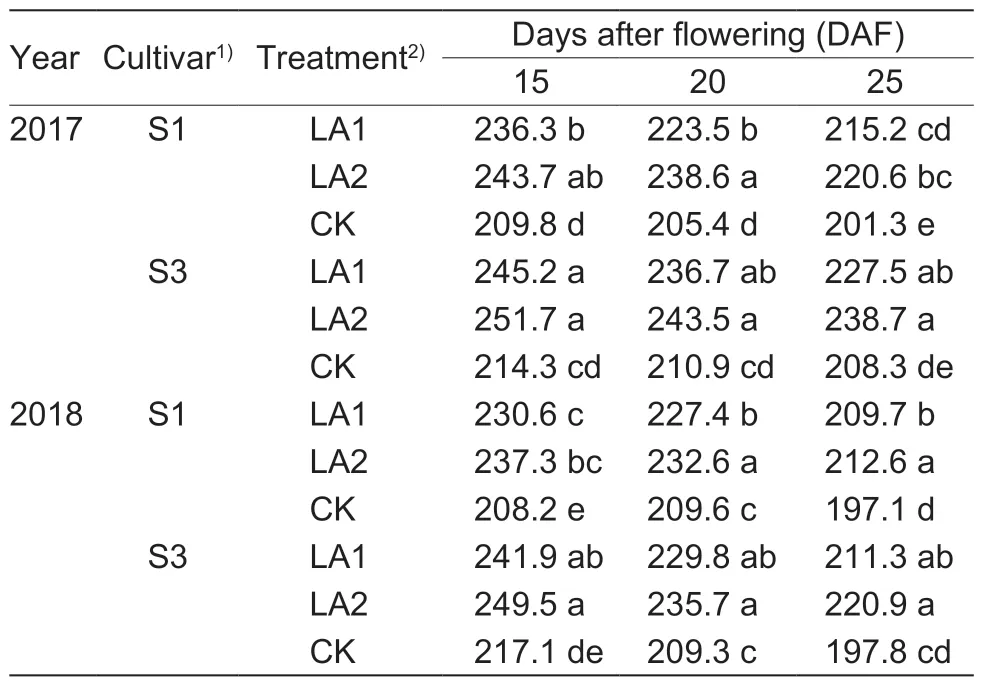
Table 1 Effect of the application of amino acids on boll Bt protein concentration (ng g–1 FW)
The application of amino acids also significantly improved both seed cotton yield and lint yield (Table 2).Compared to the control in 2017,the seed cotton yield and lint yield increased by 9.2–11.3% and 9.1–11.2% for cultivar S1,and by 5.8–8.3% and 8.4–9.7% for cultivar S3,respectively.Compared to the LA1 treatment group,the LA2 treatment group exhibited higher seed cotton and lint yield,without significant differences.Similar experimental results were also observed in 2018.
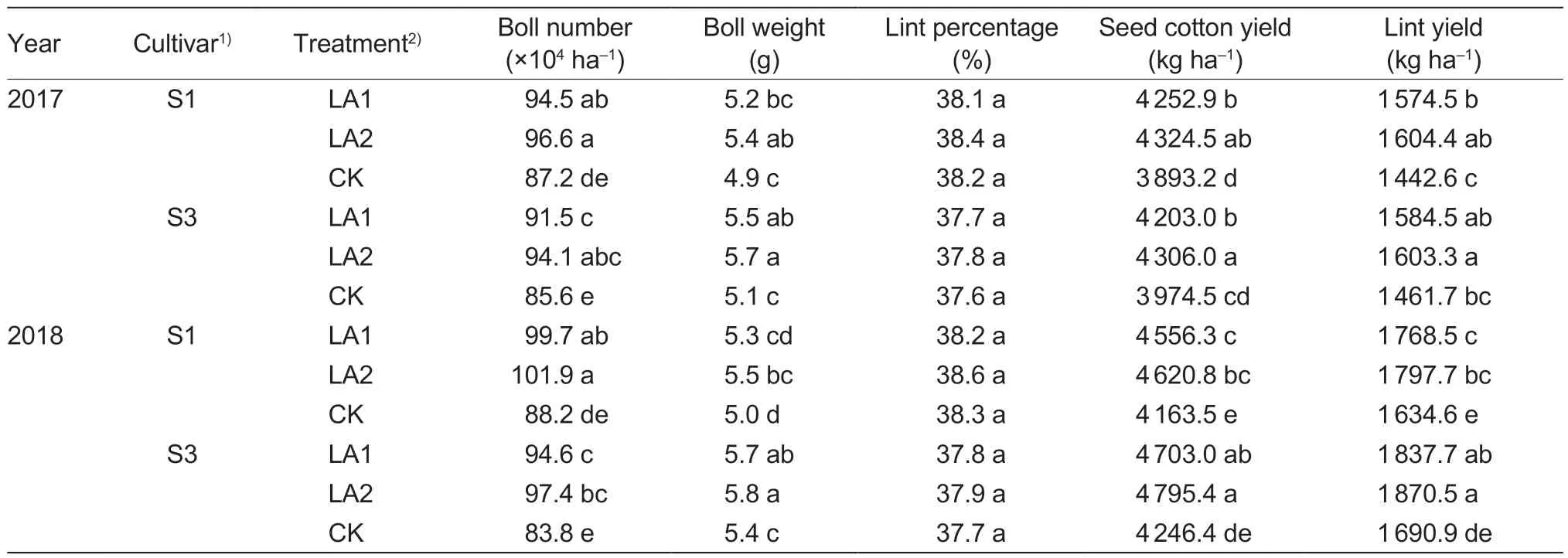
Table 2 Effects of the application of amino acids on yield and yield components
The yield formation further indicates that both boll number and boll weight increased under the amino acid treatments during the growing season (Table 3).The boll number increased from 2.2 to 4.9 boll/plant from 20–35 DAF after amino acid treatment,and the boll weight increased from 0.3 to 0.9 g from 55–65 DAF in the cotton-growing seasons during 2017–2018.Compared to the control,the LA1 and LA2 treatments significantly increased the boll numbers from 20–35 DAF and boll weights from 55–65 DAF.The LA2 treatment group had a higher boll number and boll weight than the LA1 treatment group,without significant differences.
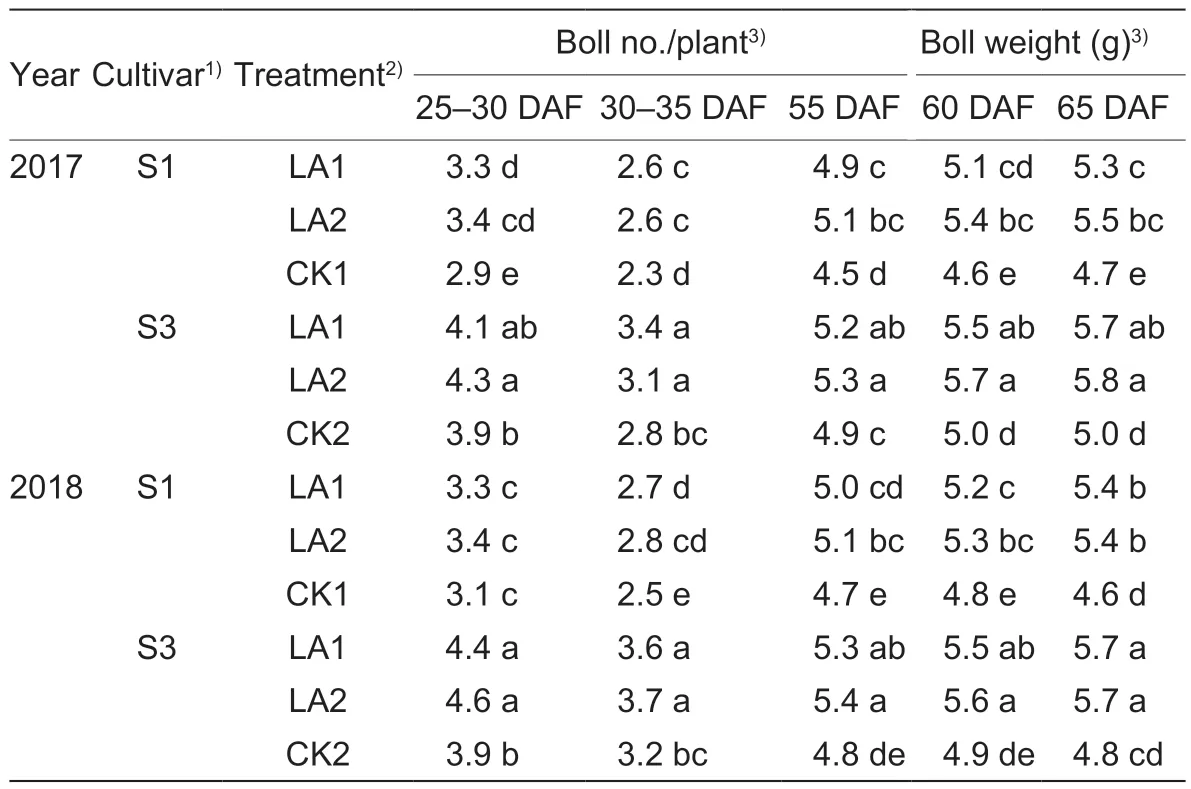
Table 3 Effects of the application of amino acids on yield formation in cotton
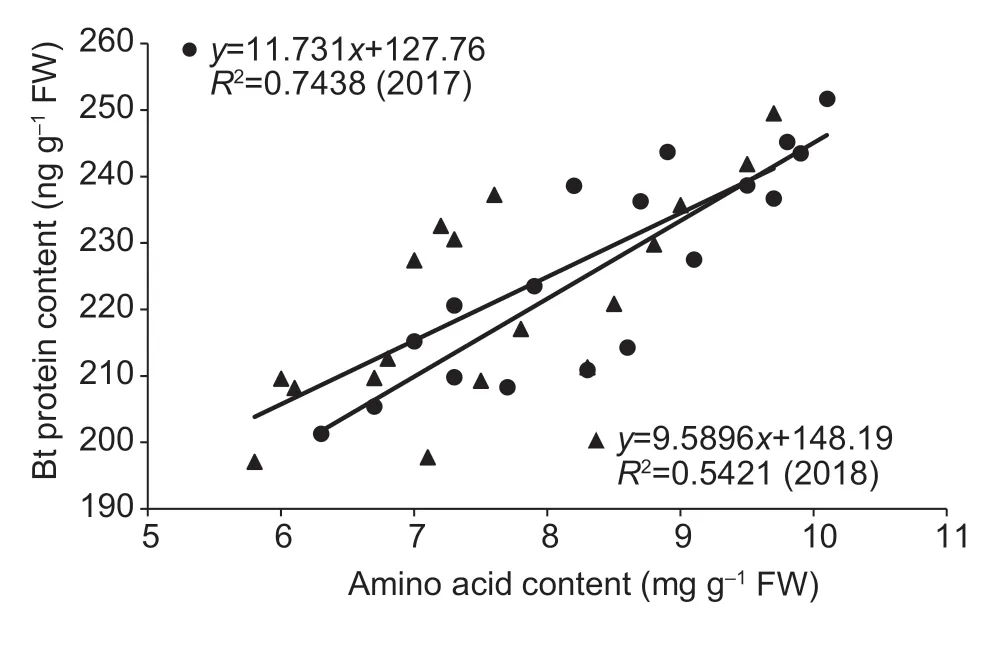
Fig.1 Linear correlations between boll Bt protein content and amino acid content at 15 to 25 days after flowering (DAF) in 2017 and 2018.
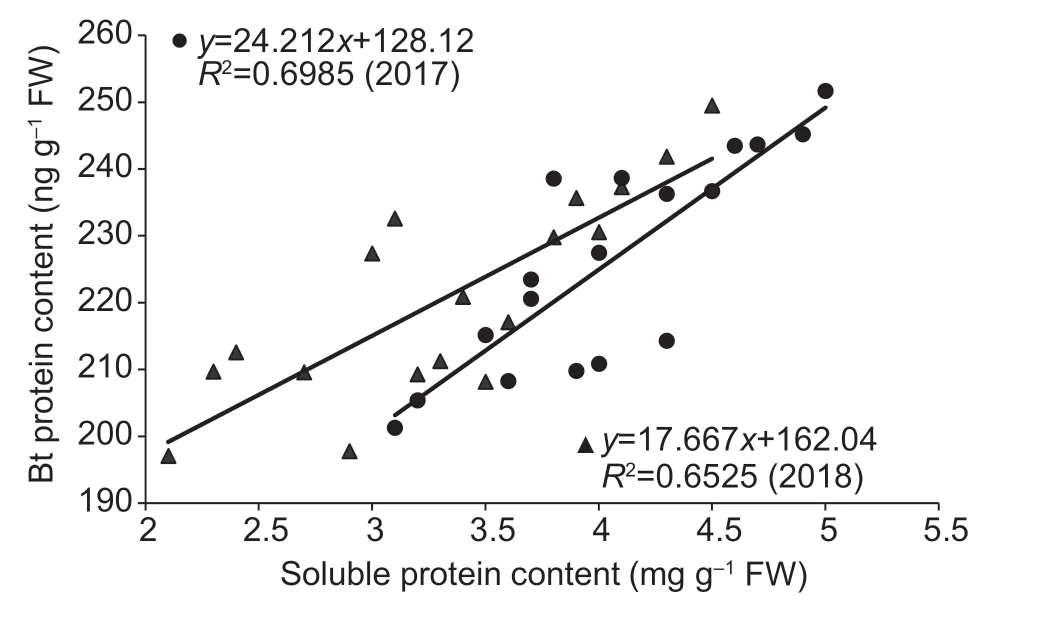
Fig.2 Linear correlations between boll Bt protein content and soluble protein content at 15 to 25 days after flowering (DAF)in 2017 and 2018.
3.2.Effects of amino acid spraying at the peak flowering stage on boll protein metabolism
Increased boll amino acid and soluble protein contents during 15–25 DAF were observed after amino acid spray application (Table 4).Compared to the untreated control in 2017,the boll amino acid contents were enhanced by 11.1–21.9% and 12.2–23.4% for S1 and S3,respectively,but without significant differences between the LA1 and LA2 treatments.Similar results were observed in 2018.
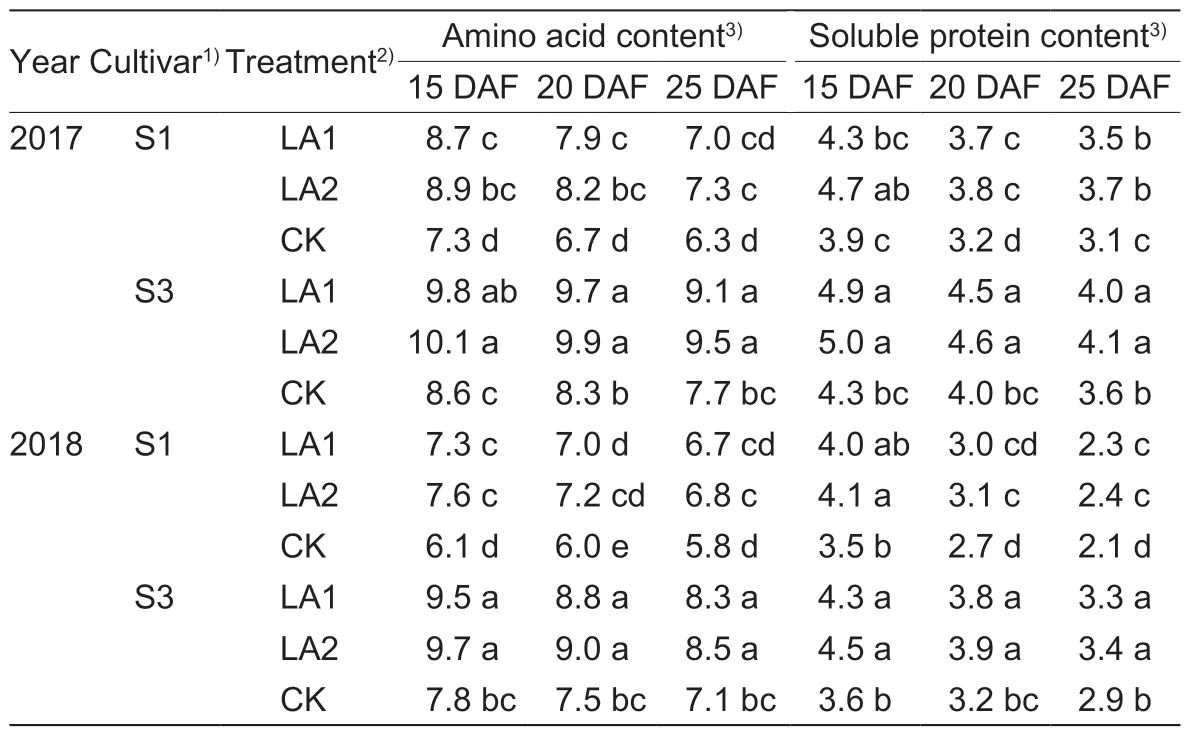
Table 4 Effects of the application of amino acids on boll amino acid and soluble protein content (mg g–1 FW)
The boll soluble protein content also increased with the amino acid application (Table 4).Compared to the control,the boll soluble protein contents were increased by 10.3–20.5% and 10.0–16.3% for S1 and S3 during 15–25 DAF in 2017,respectively,but without significant differences between the LA1 and LA2 treatments.The results in 2018 were similar to those in 2017.
Lower insect resistance usually occurs at the early boll development stage (20 DAF).There were significant linear positive correlations between boll Bt protein content and boll amino acid content in 2017 (r=0.862**) and 2018(r=0.736**),as well as between boll Bt protein content and soluble protein content in 2017 (r=0.836**) and 2018 (r=0.808**) during 15–25 DAF(Figs.1 and 2).
The boll GPT and GOT activities were enhanced under the LA1 and LA2 treatments in the cotton-growing seasons in the two-year study (Table 5).In 2017,the enzymatic activities of GPT and GOT were increased by 17.1–26.3% and 11.4–17.2% for S1,and by 10.5–17.6%and 11.7–19.5% for S3,respectively.No significant differences were found between the LA1 and LA2 treatments.Similar experimental results were obtained in 2018.
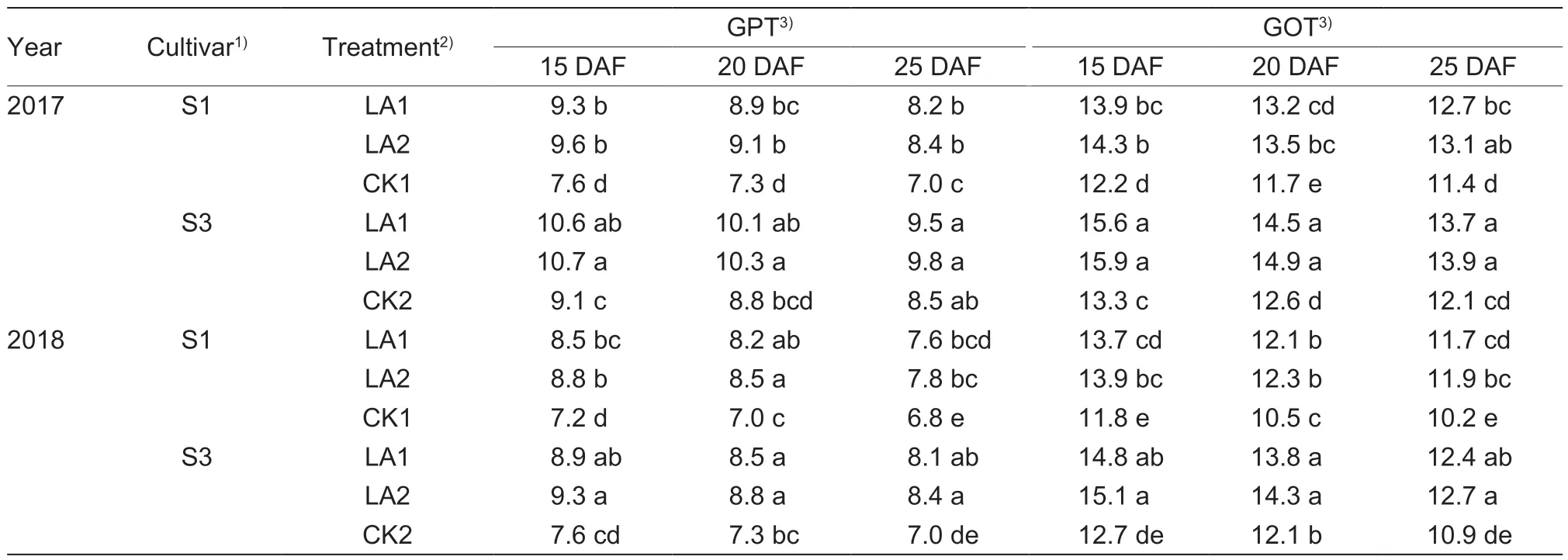
Table 5 Effects of the application of amino acids on boll glutamate pyruvate transaminase (GPT) and glutamate oxaloacetate transaminase (GOT) activities (μmol g–1 FW h–1)
The enhanced enzymatic activities of GPT and GOT were associated with increased boll number.A significant linear positive correlation was found between the boll number of individual plants and boll GPT activities during 20–35 DAF in 2017 (r=0.784**)and 2018 (r=0.758**),as well as between the boll number of individual plants and boll GOT activities during 20–35 DAF in 2017 (r=0.790**)and 2018 (r=0.909*) (Figs.3 and 4).
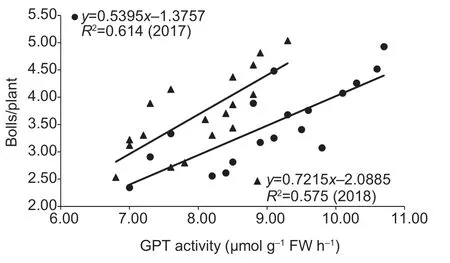
Fig.3 Linear correlations between boll number and glutamate pyruvate transaminase (GPT) activity at 15 to 25 days after flowering (DAF) in 2017 and 2018.
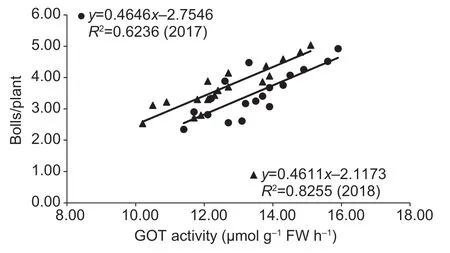
Fig.4 Linear correlations between boll number and glutamate oxaloacetate transaminase (GOT) activity at 15 to 25 days after flowering (DAF) in 2017 and 2018.
3.3.Effects of amino acid spraying at the peak flowering stage on boll carbohydrate conversion
The boll glucose and fructose contents increased with the amino acid treatment at the peak flowering stage (Table 6).The amino acid treatment groups showed significant differences from the untreated control.In 2017,the glucose content was enhanced by 8.1–13.6% and 5.9–15.9%for S1 and S3,respectively,but without significant differences between the LA1 and LA2 treatments.Similarly,the fructose contents increased by 13.6–17.9% and 14.3–18.3% for S1 and S3,respectively.Similar results were observed in 2018.
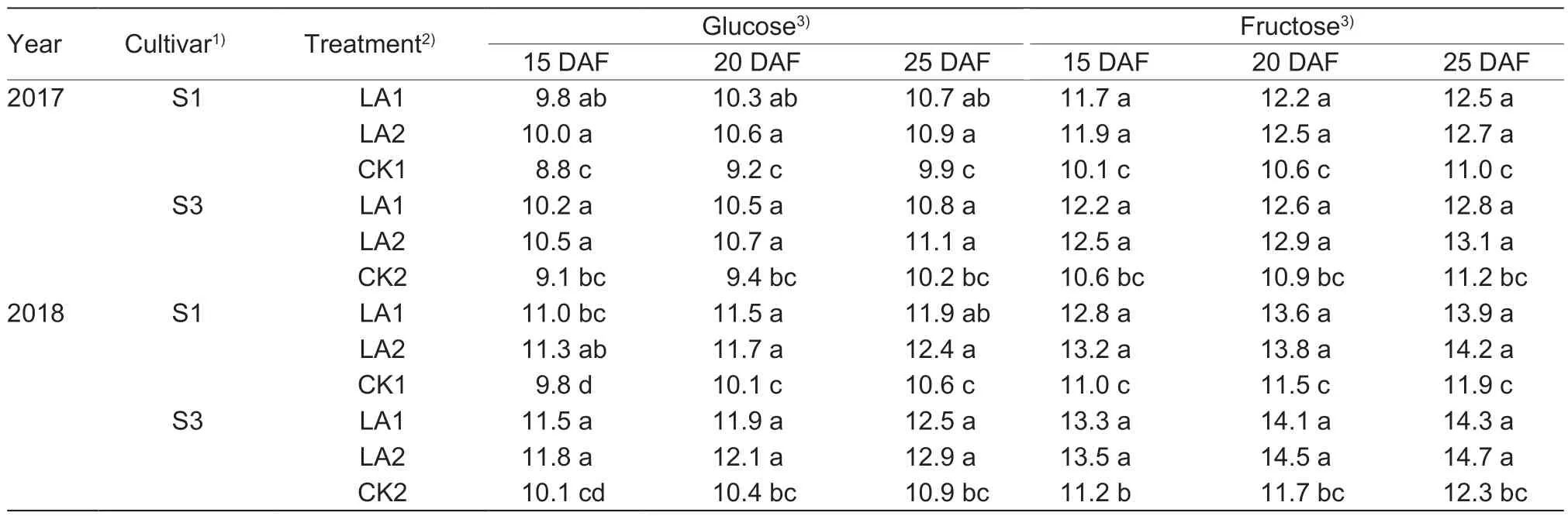
Table 6 Effects of the application of amino acids on boll glucose and fructose contents (mg g–1 DW)
The boll SAI activity increased remarkably with amino acid spraying at the peak flowering stage (Table 7).Compared to the control,the enzymatic activities were enhanced by 7.6–13.7% for S1 and by 11.7–20.7% for S3 in 2017,and they were increased by 9.8–15.9% for S1 and by 12.6–19.4% for S3 in 2018.However,there were no significant differences between the LA1 and LA2 treatment groups.Similar results were observed in 2018.There were significant linear positive correlations between the weights of opened boll during 55–65 DAF and the SAI activities in 2017(r=0.890**) and in 2018 (r=0.730**) (Fig.5).
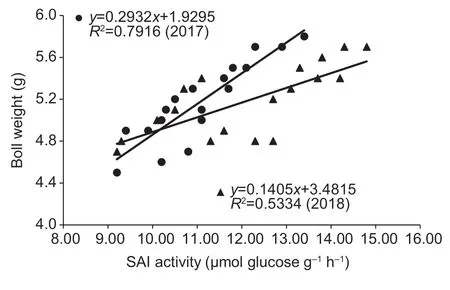
Fig.5 Linear correlations between soluble acid invertase (SAI)activity and opened boll weight at 55 to 65 days after flowing(DAF) in 2017 and 2018.
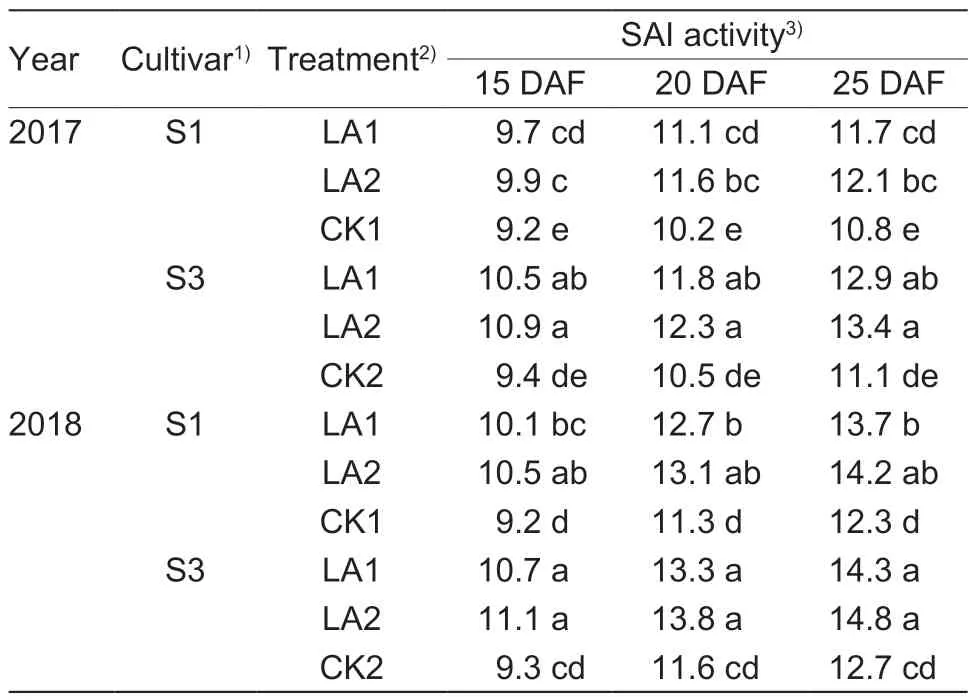
Table 7 Effects of the application of amino acids on boll soluble acid invertase (SAI) activity (μmol glucose g?1 h?1)
4.Discussion
4.1.The application of exogenous amino acids at the peak flowering stage can improve both boll Bt toxin concentration and lint yield
The expression of Bt toxin is closely related to nitrogen metabolism (Liuet al.2019;Chenet al.2021a),and an increased Bt toxin concentration in Bt cotton with nitrogen application has been previously reported.Under nitrogendeficient conditions,the Bt protein contents of squares and cotton seeds significantly increase with increasing nitrogen application (Zhanget al.2017,2021;Chenet al.2019,2021a,b;Zhang Xet al.2021;Zhang Zet al.2021).At the squaring and bolling stages,exogenous amino acid application results in a significant increase in the leaf Bt toxin content (Abidallhaet al.2017),and similar results have been reported in flowers (Tambelet al.2019).However,a high-dose nitrogen application results in excessive vegetative growth (Pettigrew and Adamczyk 2006;Zhouet al.2021a),which reduces lint yield due to boll abscission.Therefore,developing methods to enhance both boll Bt toxin concentration and lint yield has become an important goal in Bt cotton production.In the present study,the exogenous application of amino acids at the peak flowering stage increased both boll Bt protein concentration and lint yield under the condition of conventional soil nitrogen application,and there was no significant difference in the boll Bt protein content between the treatment with five amino acids (asparticacid,glutamic acid,proline,methionine and arginine)and the treatment with 21 amino acids.The increase in lint yield was associated with increases in boll number and boll weight,which result from improvements in carbohydrate and nitrogen metabolism (Aliet al.2019;Maet al.2019).In addition,the increased boll amino acid content benefits flower bud differentiation,which results in raising the boll number,and the increases in the boll glucose and fructose contents enhance boll weight (Kuaiet al.2014;Zhang Zet al.2021;Liuet al.2022).The results of this study indicate that lint yield and Bt insect resistance could be simultaneously improved with amino acid application at the peak flowering stage under conventional soil nitrogen application,and the application of the five amino acids which are the main components of Bt protein is sufficient to improve both boll insect resistance and lint yield.These results will be further verified in cotton production.
4.2.The enhanced protein synthesis and carbohydrate conversion by exogenous amino acid application both contributed to increasing the Bt protein concentration and lint yield
Bt protein expression shows a close relationship with nitrogen metabolism.Chenet al.(2005a,2007) reported that introducing Bt genes enhances leaf nitrogen metabolism,while reducing boll nitrogen metabolism intensity.Foliar urea can enhance protein synthesis of the boll wall and cotton seeds (Zhang Xet al.2021;Zhouet al.2021b).The findings in the present study are consistent with these reported results.Furthermore,this study found that amino acid application at the peak flowering stage could also enhance the carbohydrate conversion capability of cotton bolls,boll SAI activities,and glucose and fructose contents,which is beneficial to boll development and lint yield formation.These results are further verified by the positive linear correlations of boll number with both glucose and fructose contents and the correlation of boll weight with the SAI activity.Due to the amino acid application at the peak flowering stage,enhanced boll protein synthesis causes an increase in Bt toxin content.Then,the increased boll carbohydrate conversion intensity causes an increase in lint yield.Therefore,the increased intensities of both boll protein synthesis and carbohydrate conversion may represent the main physiological mechanism for the simultaneous improvement of both Bt insect resistance and lint yield in Bt cotton.
5.Conclusion
This study shows that the application of exogenous amino acids at the peak flowering stage can enhance the Bt toxin concentration of cotton bolls and lint yield under a conventional soil nitrogen application in Bt cotton.Higher boll number and boll weight,as well as increased boll Bt protein contents,were observed under two different amino acid treatments.The experimental results show that the enhanced boll amino acid content,soluble protein content,GPT activity and GOT activity increased the boll number per plant and Bt protein synthesis from 15–25 DAF.The increases in boll glucose and fructose contents,as well as the SAI activity,improved the weight of opened bolls from 55–65 DAF.These findings indicate the beneficial effects of amino acid application on both yield formation and Bt toxin concentration.This study shows that exogenous amino acid application at the peak flowering stage can enhance both yield potential and insect resistance in Bt cotton,thereby reducing the use of pesticides and protecting the ecological environment.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31901462 and 31671613),the Natural Science Foundation of Jiangsu Province,China (BK20191439),the Postgraduate Research &Practice Innovation Program of Jiangsu Province,China(KYCX22_3508) and the Priority Academic Program Development of Jiangsu Higher Education Institutions,China (PAPD).
Declaration of competing interest
The authors declare that they have no conflict of interest.
 Journal of Integrative Agriculture2023年6期
Journal of Integrative Agriculture2023年6期
- Journal of Integrative Agriculture的其它文章
- ldentification of two novel linear epitopes on the p30 protein of African swine fever virus
- Uncertainty aversion and farmers’ innovative seed adoption:Evidence from a field experiment in rural China
- Ensemble learning prediction of soybean yields in China based on meteorological data
- Increasing nitrogen absorption and assimilation ability under mixed NO3– and NH4+ supply is a driver to promote growth of maize seedlings
- Significant reduction of ammonia emissions while increasing crop yields using the 4R nutrient stewardship in an intensive cropping system
- Maize straw application as an interlayer improves organic carbon and total nitrogen concentrations in the soil profile: A four-year experiment in a saline soil
