Mixing planting proportions in a plantation aff ects functional traits and biomass allocation of Cunninghamia lanceolata and Phoebe bournei seedlings
Meiqin Zha 1,2 · Youzhi Han 1 · Xiangrong Cheng 2
Abstract Functional traits of trees are signif icantly associated with their adaptation strategies and productivity.However, the eff ects of species composition and mixing proportion on the functional traits of trees grown in mixed plantations have not been studied extensively. In this study,planting experiments (duration about seven months) were used to study variations in functional traits and biomass allocation of Cunninghamia lanceolata (Lamb.) Hook and Phoebe bournei (Hemsley) Yang seedlings in f ive diff erent mixes (0C:4P, 1C:3P, 1C:1P, 3C:1P, and 4C:0P). Total leaf area per seedling increased in each species as its respective proportion in the mixture decreased. However, the specif ic leaf area decreased for P. bournei under low percent composition, and the specif ic leaf area for C. lanceolata diff ered only marginally among the plantings. The net photosynthetic rates of the two species were higher in the mixed plantings than in their corresponding monocultures, whereas the transpiration rate, stomatal conductance, and instantaneous water use effi ciency were not diff erent among the plantings.The average root length and root surface area of C. lanceolata and P. bournei were higher in the mixed plantings than in their monocultures. Specif ically, root surface area of C.lanceolate and both root length and surface area of P. bournei increased signif icantly in the 1C:3P and 2C:2P mixed plantings. Leaf, stem, root, and total dry mass per seedling for C. lanceolata decreased with its increasing percent composition in the mixed plantings, while these variables varied less for P. bournei. The plasticity of biomass allocation was relatively low for both species. Total biomass per planting was higher in the mixed plantings than in the monocultures.Our study indicates that species composition and mixing proportion can considerably aff ect the functional traits of C.lanceolata and P. bournei. The increase in productivity in the mixed plantings may be partially attributed to low rates of competition between the two species, and future studies should examine the diff erent interspecies relationships. The results of this study can be used to improve plantation productivity and ultimately increase the sustainability of tree products and help to better understand the adaptation strategies of plant coexistence.
Keywords Mixing proportion · Tree species composition · Functional traits · Interspecif ci relationship ·Intraspecif ic relationship
Introduction
Plantations play important roles in providing timber and environment protection (Liu et al. 2018). In China, the total area covered by plantations is 79.54 million ha, accounting for 32.9% of the total forest area (Du et al. 2020). However,most plantations are managed as monocultures, often resulting in reduced productivity, biodiversity, and capacity for ecological services (Farooq et al. 2019a). Mixed plantations have certain advantages over monocultures, including higher biomass production, faster nutrient cycling, higher stability and recovery capacity, and increased diversity in ecological services (Xiang et al. 2015). Nevertheless, the advantages of mixed plantations largely depend on the species composition and the mixing proportions (Bauhus et al. 2004).
In recent years, studies have focused on the relationships between tree species composition and functional traits or productivity (Pretzsch et al. 2010; To?go et al. 2015). In mixed plantations, species composition has shown a substantial eff ect on plant functional traits, which may in turn aff ect stand productivity (Forey et al. 2016). For example,Bolte and Villanueva ( 2006) reported that specific root length (SRL) and specif ic root surface area (SRA) of European beech (Fagus sylvaticaL.) were signif icantly higher in mixed European beech and Norway spruce (Picea abies(L.)H. Karst.) plantations than in monocultures. However, Xiang et al. ( 2015) found no signif icant diff erences between mixed and pure stands in specif ic root length, specif ic root surface area, or f ine-root biomass of ring-cupped oak (Quercus glaucaThunb.) or of Japanese oak (Lithocarpus glaber(Thunb.) Nakai). Variations in functional traits in diff erent compositions are associated with species interactions (Lei et al. 2012). Additionally, the relationship between species composition and stand productivity is believed to mainly depend on the net eff ect of interaction between a given pair of species (Forrester 2014). Nevertheless, these interactions are often associated with many biotic and abiotic factors such as climate, soil fertility, tree age, functional diversity of the tree species, and availability of resources (Ammer 2019). For example, the growth characteristics ofEucalyptus globulusLabill. andAcacia mearnsiiDe Wild. in mixed plantations vary considerably with time, which indicates that the vector size and direction of plant interaction are aff ected by spatial and temporal dynamics of resource availability(Forrester et al. 2011). However, studies on the eff ects of tree species composition on productivity in mixed plantations mainly involve growth, nutrient cycles, biological productivity, and eco-economic benef its (Forrester et al. 2006; Piotto 2008; Chomel et al. 2014; Niccoli et al. 2021). Knowledge of the eff ects by which above- and below-ground interactions among diff erent species on functional traits and of how these interactions aff ect productivity are poorly understood(Roscher et al. 2011; Abakumova et al. 2016).
In the management of mixed plantations, determining the optimal mixing proportion is important to optimize stand productivity. Bauhus et al. ( 2000) reported that the highest stem volume and tree height occurred in a 50% southern blue gum(Eucalyptus globulus) and 50% black wattle (Acacia mearnsii)mixture. Ghorbani et al. ( 2018) demonstrated that 50% eastern cottonwood (Populus deltoidesMarsh.) and 50% Caucasian alder (Alnus subcordataC.A.Mey.) could provide economic and environmental benef its by increasing the stability and productivity of the stands. The mixing proportion changes the stand structure and may inf luence above- and belowground functional traits of trees (Qin et al. 2016). For example, Nunes et al. ( 2014) found that the specif ic leaf area of Douglas- f ir(Pseudotsuga menziesii(Mirb.) Franco) did not diff er signif icantly among three mixing proportions with sweet chestnut(Castanea sativaMill.) but was signif icantly higher in the 50:50 Douglas- f ir and sweet chestnut plantings compared with the monocultures. However, little information is available on the eff ects of mixing proportions on above- and below-ground functional traits of diff erent tree species.
Cunninghamia lanceolata(Lamb.) Hook. is a fast-growing evergreen coniferous species widely planted in southern China for its high timber productivity (Farooq et al. 2019b; Wu et al.2019). According to the 9th National Forest Resources Inventory,C. lanceolatacovers more than 11 million ha, which occupies almost 14.2% of the area of all plantations and 6.0%of the total forested area in China (Ma and Lin 2019). In recent years, southern China has established numerous mixed plantations ofC. lanceolataand broadleaved species with the aim of improving stand productivity and stability.Phoebe bournei(Hemsley) Yang is a typical evergreen broadleaf species widely distributed in subtropical China. It is commonly known as ‘wood with golden wire’ and can be used for high-quality furniture and handicrafts due to its brilliant color and remarkable durability (Zhang et al. 2021). As a valuable and protected broadleaf reforestation species,P. bourneiis often planted withC. lanceolatain mixed plantations (Wang et al. 2021; Zhang et al. 2021). However, the eff ects of various mixing proportions of the two species on their functional traits are poorly understood. Therefore, we aimed to study the eff ects of mixing species proportions on the functional traits and biomass allocation ofC. lanceolataandP. bourneiseedlings using planter experiments. Specif ically, the following research questions were addressed: (1) how do functional traits and biomass allocation of the two species vary with mixing species proportions? (2) what are the correlations between functional traits and biomass allocation in the diff erent planting? and (3) what are the possible physiological mechanisms underpinning the observed responses of functional traits and biomass allocation to the variation of mixing species proportions?
Materials and methods
Study site
The experiment was carried out at the Hushan Tree Nursery (30°3′ N, 119°57′ E, elevation 35 m a.s.l.) in Fuyang,Zhejiang Province, China. The Hushan Tree Nursery is in a typical subtropical, humid monsoon climate with an average annual temperature of 16.4 °C. The total annual sunshine at the study site is 1816 h, and the average annual precipitation is 1441.9 mm. The frost-free period is 252 d. The 18 m × 6 m experimental greenhouse was 3 m high and covered with transparent plastic f ilm.
Plant materials and experimental design
Two tree species,C. lanceolataandP. bournei, were selected based on their wide cultivation and multiple functions. For each species, 100 one-year-old seedlings with similar heights and diameters were randomly divided into two groups of 50 seedlings each. One group was randomly selected for planting experiments, and the other group used for initial traits and biomass measurements according to Shen et al. ( 2019). The results are shown in Table 1.
The study consisted of the following plantings, each describing the composition of a single pot: fourP. bourneiseedlings (0C:4P), oneC. lanceolataand threeP. bourneiseedlings (1C:3P), twoC. lanceolataand twoP. bourneiseedlings (2C:2P), threeC. lanceolataand oneP. bourneiseedlings (3C:1P), and fourC. lanceolataseedlings (4C:0P)(Fig. 1). Each planting was replicated f ive times, resulting in total 25 pots.Yellow-red soil was collected from a natural forest in Hushan Nature Reserve, air-dried and sieved through a 1 × 1 cm mesh screen to remove stones and plant material.Organic manure and sand were purchased from the same horticulture company, and mixed with the soil at a ratio 7:2:1 (soil:manure:sand). Mixed soil (20 kg) was placed into plastic pots (external diameter: 48 cm, internal diameter: 41.5 cm, and height: 31 cm, with four drainage holes at the bottom). The initial chemical properties of the soil(Table 2) were analyzed according to Bao ( 2000). On March 18, 2019, the two species were planted at 20 cm × 20 cm spacing in each pot according to the f ive experimental plantings described above. Weeds in the pots were periodically removed and the seedlings watered every f ive days.
Photosynthetic gas exchange measurements
The photosynthetic gas exchange parameters of the two species were measured with a Li-6400XT portable photosynthetic system (Li-6400XT, Li-Cor, USA). A standard 2 cm × 3 cm leaf chamber and 2 cm × 6 cm needle leaf chamber were used to measure leaves ofP. bourneiandC. lanceolata, respectively. During each measurement, air temperature (Ta, °C) at 25 °C - 28 °C, and relative humidity (RH, %) at 58 - 62%, by the Li-6400XT portable photosynthesis system. CO2concentration in the sample chamberwas 400 μmol mol -1 , and a f ixed f low rate of 500 μmol s -1 was used. Light intensities were controlled using artif icial lighting with blue and red LED sources and were set at 1000 μmol m -2 s -1 for photosynthetically active radiation.All measurements were taken between 9:00 and 11:00 am September 17-19, 2019. In each planting pot, one seedling was selected for each species and three healthy, mature leaves selected for measurement. Net photosynthetic rate(Pn, μmol m -2 s -1 ), transpiration rate (Tr, mmol m -2 s -1 ),and stomatal conductance (Gs, mmol m -2 s -1 ) were recorded for 2 min after stable photosynthesis was established. The instantaneous water use effi ciency (WUE, μmol m -1 ) was calculated as net photosynthetic rate/transpiration rate.
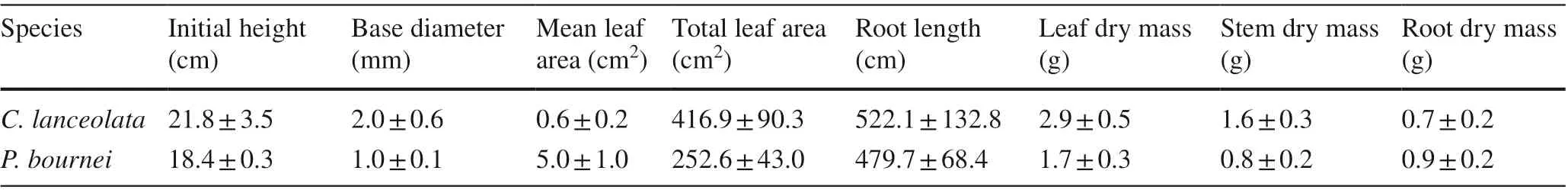
Table 1 Initial traits and biomass of Cunninghamia lanceolata and Phoebe bournei seedlings
Fig. 1 Mixtures used in the experiment. 0C:4P: four Phoebe bournei seedlings; 1C:3P: one Cunninghamia lanceolata and three P. bournei seedlings;2C:2P:two C. lanceolata and two P. bournei seedlings;3C:1P: three C. lanceolata and one P. bournei seedlings;and 4C:0P:four C. lanceolata seedlings

Table 2 Chemical properties of soil
Morphological trait measurements
All seedlings were harvested October 25, 2019, seven months after potting. Seedlings were cut at the stem and divided into leaves and stems. Roots were washed with water to remove soil, placed into labeled bags, and temporarily stored within 3 h on ice in a cooler.
A subsample of 20 healthy, mature leaves of each species per pot in each planting was randomly selected and pooled as a mixed sample for leaf area, thickness and saturated fresh weight measurements. The leaf thickness, excluding main and large secondary veins, was measured using a micrometer. The leaves were recut under deionized water to remove the petiole or rachis and were kept in deionized water at 4 °C for 12 h in darkness. They were then dried to remove any surface water and weighed to determine their saturated fresh weight (Paula and Pausas 2006; Qin and Shangguan 2019).Mean leaf area (MLA, cm 2 ), total leaf area (TLA, cm 2 ), root length (RL, cm), root surface area (RSA, cm 2 ), and average root diameter (ARD, mm) were determined using rootscanning equipment (WinRHIZO, Canada) and leaf and root image analysis software. All leaf and root samples were oven-dried at 60 °C to a constant mass and weighed. Specif ic leaf area (SLA, cm 2 g -1 ) was calculated as leaf area (LA,cm 2 ) divided by its dry mass (LM, g). Leaf dry matter content (LDMC, g g -1 ) was calibrated as dry mass per saturated fresh mass. Specif ic root length (SRL, cm g -1 ) and specif ic root area (SRA, cm 2 g -1 ) were the ratios between root length(RL) and root dry mass (RM, g), and between root surface area (RSA) and RM, respectively. The leaf morphological traits (MLA, TLA, SLA, LT, LDMC), root morphological traits (RL, RSA, SRL, SRA, ARD), biomass and its allocation ratio [LM, RM, stem dry mass (SM, g), total dry mass per seedling (TMPS, g), leaf-mass ratio (LMR), stem-mass ratio (SMR), root-mass ratio (RMR), and root-shoot ratio(R/S, def ined as dry weight of root biomass divided by dry weight of shoot biomass)] were reported as average values in each seedling, and total biomass per planting (TMPP, g)was reported as an average per planting.
Statistical analyses
All data were tested for normality and homogeneity of variance (Levene’s test) before statistical analyses, and when necessary, were log-transformed. Diff erences in morphological and physiological characteristics and biomass ofC.lanceolataandP. bourneiin the diff erent planting mixtures were determined by one-way analysis of variance (ANOVA)and Duncan’s multiple range test in IBM SPSS 25.0 software(P< 0.05).
Principal components analysis (PCA) was used to explore the variation in functional traits and biomass allocation in the diff erent mixtures, and Pearson’s correlation analysis was used to evaluate their relationships. PCA was conducted using the ggplot2 (Version 3.3.5; Wickham 2021), ggrepel(Version 0.9.1; Slowikowski et al. 2021), plyr (Version 1.8.6;Wickham 2020), and vegan (Version 2.5-7; Oksanen et al.2020) packages. Pearson’s correlation analysis used the corrplot (Version 0.84; Wei and Simko 2017) package in R statistical software.
The diff erence in functional traits and biomass allocation ofC. lanceolataandP. bourneibetween the planting mixtures was tested using permutational multivariate analysis of variance (PERMANOVA) in the vegan package (ADONIS function) in R 3.6.3 (Anderson 2005).
Results
Variations in leaf functional traits
Several leaf morphological traits ofC. lanceolataandP.bourneivaried signif icantly among the diff erent plantings(Table 3). ForC. lanceolata, the mean leaf area (MLA)and specif ic leaf area (SLA) did not diff er signif icantly(P> 0.05), whereas the total leaf area (TLA) and leaf thickness (LT) were higher in the mixed plantings than in the 4C:0P planting. The leaf dry matter content (LDMC) ofC.lanceolatawas the highest in the 2C:2P mixed planting and lowest in the 1C:3P mixed planting. ForP. bournei, the mean leaf area, total leaf area, and leaf thickness were signif icantly higher in the mixed plantings than in the 0C:4P planting(P< 0.05), whereas, the specif ic leaf area decreased with the decrease in the proportion ofP. bourneiin the species composition (P< 0.05). The leaf dry matter content showed no signif icant diff erence among the plantings (P> 0.05).
Net photosynthetic rates (Pn) ofC. lanceolataandP.bourneiwere higher in the mixed plantings than in their corresponding monocultures, especially in the 2C:2P planting, while transpiration rates (Tr), stomatal conductance(Gs) levels, and instantaneous water use effi ciency (WUE)of the two species were not signif icantly diff erent among the plantings (P> 0.05, Table 4).
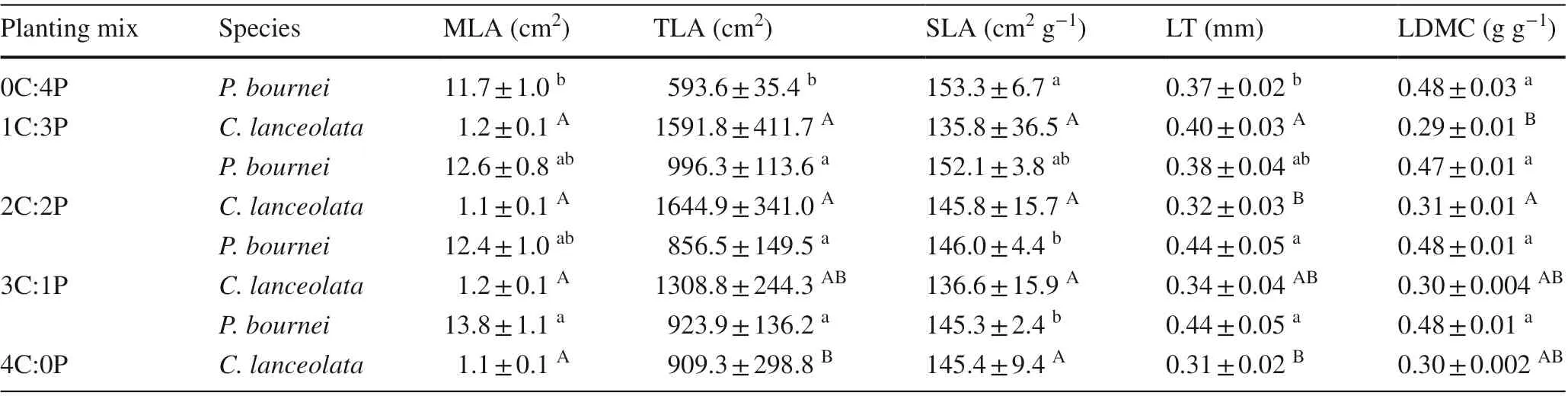
Table 3 Mean values of the leaf morphological traits of Cunninghamia lanceolata and Phoebe bournei in the diff erent plantings
Variations in root functional traits
Root morphological traits of the two species differed among the plantings (Table 5). ForC. lanceolata, root length (RL) and root surface area (RSA) were higher in the mixed plantings than in the pureC. lanceolataplanting and highest in the 2C:2P planting. Additionally, specific root area (SRA) was highest in the 2C:2P mixed planting. Specific root length (SRL) and average root diameter (ARD) showed no differences between the 4C:0P and mixed plantings (P> 0.05). ForP. bournei,root length and root surface area were significantly higher in the 1C:3P and 2C:2P mixed plantings than in the pureP. bourneiplanting (P< 0.05). In addition, specific root length and root areas were not significantly different among the plantings (P> 0.05). Average root diameter was significantly higher in the mixed plantings than in the pureP. bourneiplanting (P< 0.05), with the highest value observed in the 1C:3P mixed planting.
Biomass allocation
Leaf dry mass (LM), stem dry mass (SM), root dry mass(RM), and total dry mass per seedling (TMPS) of the two species were aff ected by the diff erent planting combinations(P< 0.05) (Fig. 2). ForC. lanceolata, these values were higher in the mixed plantings than in the pureC. lanceolataplanting. Leaf dry mass, stem dry mass, and total dry mass per seedling in the mixed plantings decreased with an increase in the proportion ofC. lanceolata, although this pattern was not observed for root dry mass. ForP. bournei,leaf dry mass, stem dry mass, root dry mass, and total dry mass per seedling were also signif icantly higher in the mixed plantings than in the pureP. bourneiplanting (P< 0.05),and all four values were the highest in the 1C:3P planting.However, the leaf dry mass, stem dry mass, root dry mass,and total dry mass per seedling did not diff er signif icantly between the mixed plantings (P> 0.05).
Total biomass per planting (TMPP) was higher in the mixed plantings than in the monocultures (Fig. 3). Specif ically, total biomass in the 1C:3P, 2C:2P, and 3C:1P mixedplantings was 29.3%, 37.4%, and 38.9%, respectively, higher than in the pureC. lanceolataplanting, and 121.2%, 135.1%,and 137.7% higher than in the pureP. bourneiplanting.
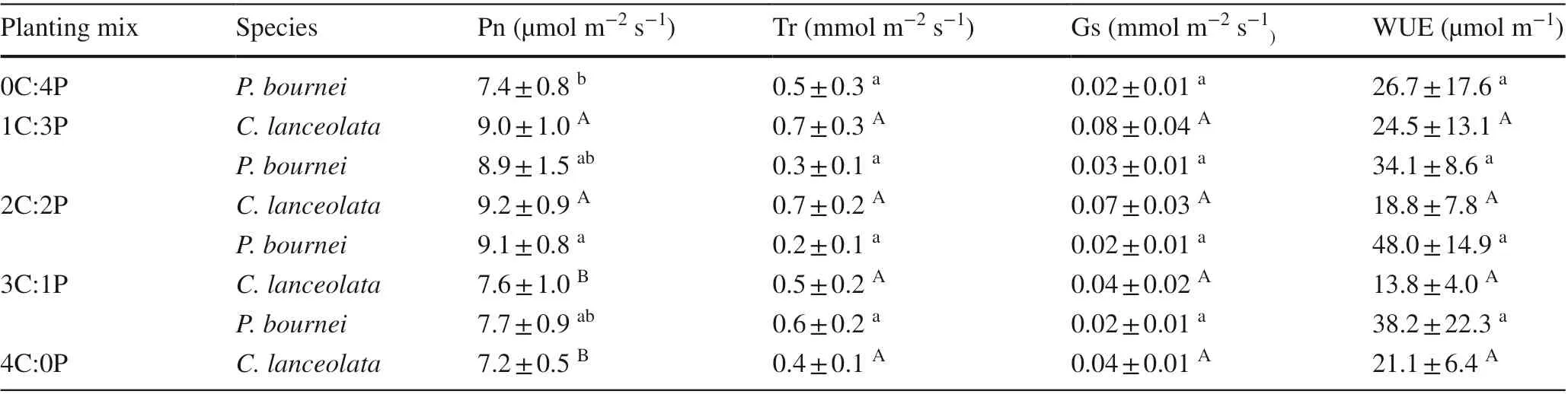
Table 4 Mean values of leaf photosynthetic traits of Cunninghamia lanceolata and Phoebe bournei in the diff erent plantings
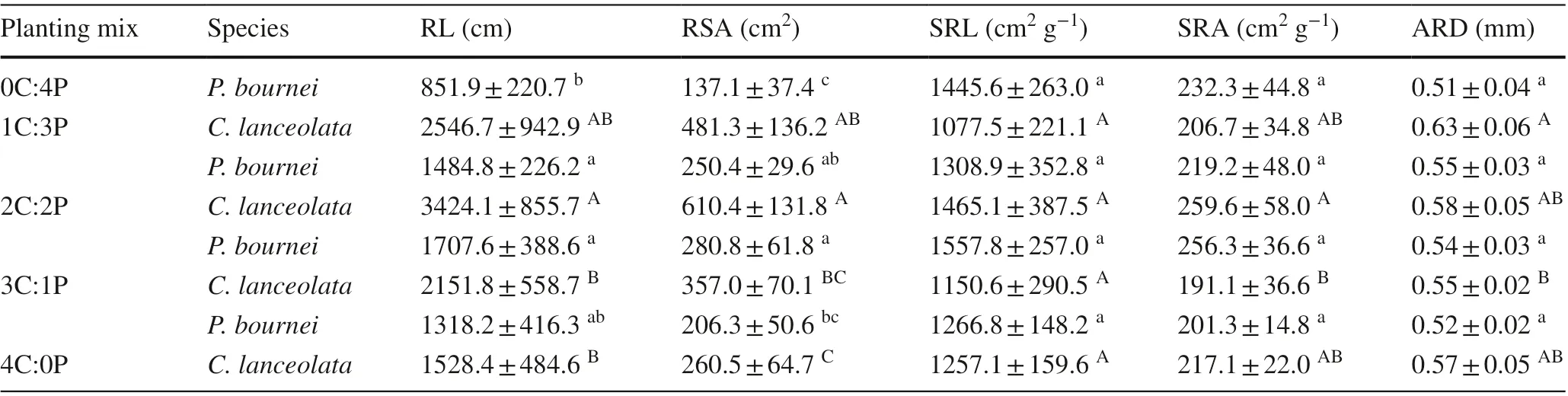
Table 5 Mean values of root morphological traits of Cunninghamia lanceolata and Phoebe bournei in the diff erent plantings
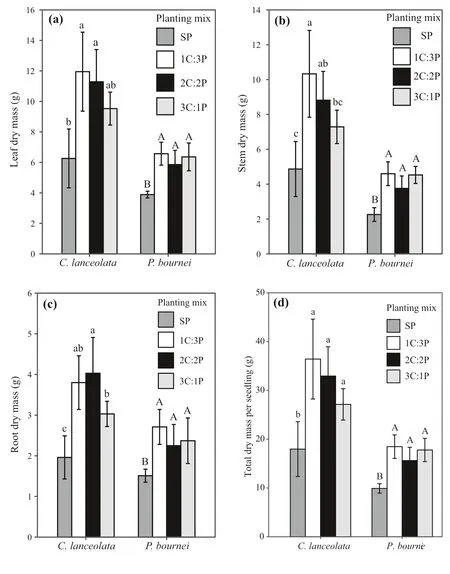
Fig. 2 Eff ects of diff erent mixed species proportions on a leaf, b stem, c root,and d total biomass per seedling for Cunninghamia lanceolata and Phoebe bournei. Error bars indicate the standard deviation of the mean ( n = 5). Diff erent letters represent signif icant diff erences among the plantings at the P < 0.05 level,with lowercase letters for C. lanceolata,and capital letters for P. bournei. Notes:SP: four C. lanceolata or four P. bournei seedlings; 1C:3P: one C. lanceolata and three P. bournei seedlings; 2C:2P:two C.lanceolata and two P. bournei seedlings;and 3C:1P: three C. lanceolata and one P.bournei seedlings
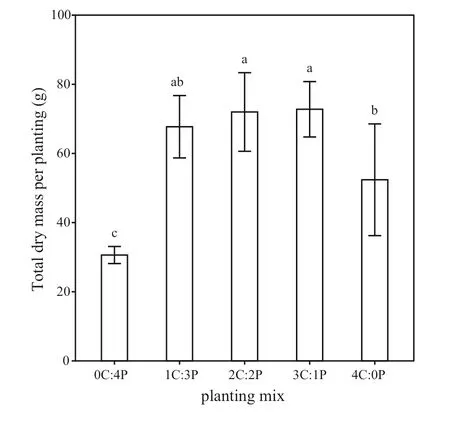
Fig. 3 Eff ects of diff erent mixed species proportions on total biomass per planting for Cunninghamia lanceolata and Phoebe bournei.Data are mean ± SD. Diff erent lowercase letters indicate signif icant diff erences among the plantings at the P < 0.05 level
For bothC. lanceolataandP. bournei, mixed plantings had no significant effect on the leaf mass ratio (LMR),root mass ratio (RMR), or root/shoot ratio (R/S) (P> 0.05)but inf luenced the shoot mass ratio (SMR).C. lanceolatawhich was the highest in the 1C:3P group compared with the other three plantings. The stem mass ratio ofP. bourneiwas higher in all mixed plantings than in the pureP. bourneiplanting Table 6 .
Correlations of functional traits with tree species mixture
The two species showed similar relationships between functional traits and biomass variables (Fig. 4). Total leaf area was signif icantly correlated with the root length (RL),root surface area (RSA), leaf dry mass (LM), stem dry mass (SM), root dry mass (RM), and total dry mass per seedling (TMPS). The root length and root surface area had strong positive correlations with the leaf dry mass,stem dry mass, root dry mass, and total dry mass per seedling. However, the two species showed diff erent correlations between functional traits and biomass allocation. ForC. lanceolata, the leaf dry matter content had a signif icant positive correlation with root traits (Fig. 4 a), and there were positive correlations between net photosynthetic and transpiration rates. ForP. bournei, total leaf area was positively correlated with net photosynthetic rates and stomatal conductance (Fig. 4 b). Leaf dry mass, stem dry mass,root dry mass, and total dry mass per seedling were also positively correlated with net photosynthetic rates.
The functional traits of leaves and roots, as well as biomass allocation forC. lanceolataandP. bourneiamong the diff erent plantings were further analyzed by PCA (Fig. 5). ForC. lanceolata, the f irst two components explained 96.0% of the total variance (58.6% and 37.4%,respectively, Fig. 5 a), and the total leaf area (TLA), root length (RL), and specif ic root length (SRL) contributed most to variation in the mixed plantings. The variation characteristics of the functional traits were signif icantly diff erent in the 1C:3P and 2C:2P mixtures compared to the 4C:0P monoculture (P< 0.05, Table 7). ForP. bournei, the f irst two PCA components of functional traits accounted for 97.4% of the variability in the data (76.0% and 21.4%,respectively, Fig. 5 b), and the total leaf area, root length and specif ic root length also varied signif icantly. The functional traits showed signif icant diff erences between 1P:3Cand 2P:2C mixtures (P< 0.05), and between 1P:3C, 2P:2C,3P:1C, and 0C:4P mixtures (P< 0.05, Table 7).
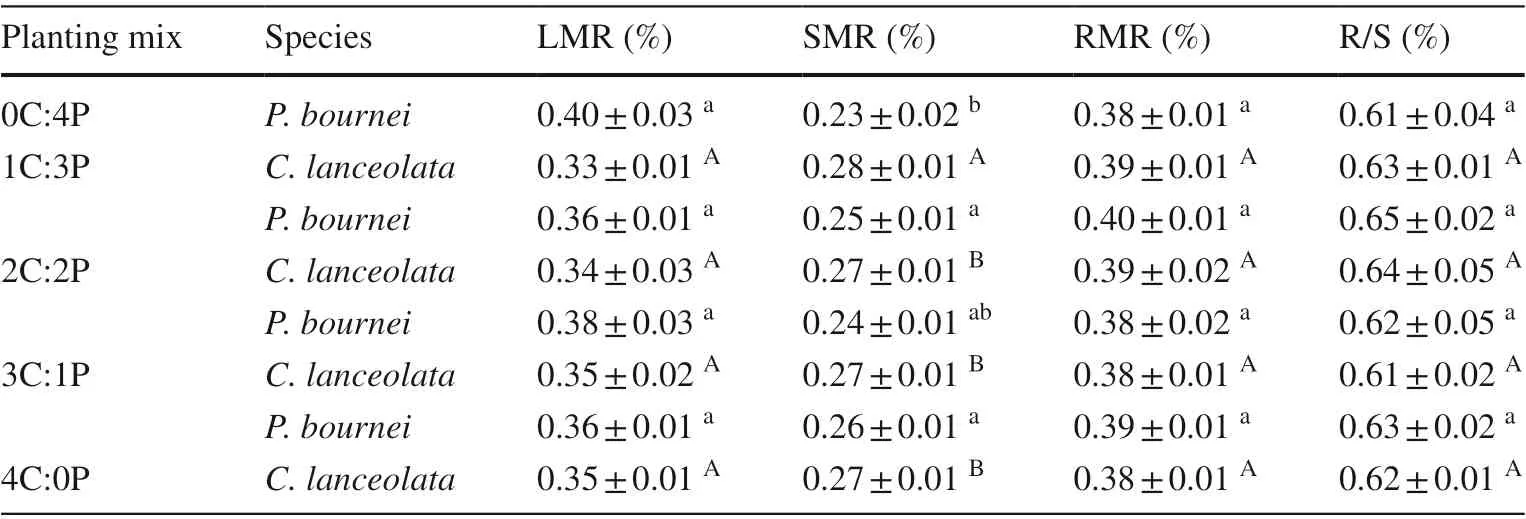
Table 6 Mean values of leaf mass, stem mass, and root mass ratios, and root /shoot ratios of Cunninghamia lanceolata and Phoebe bournei in the diff erent plantings
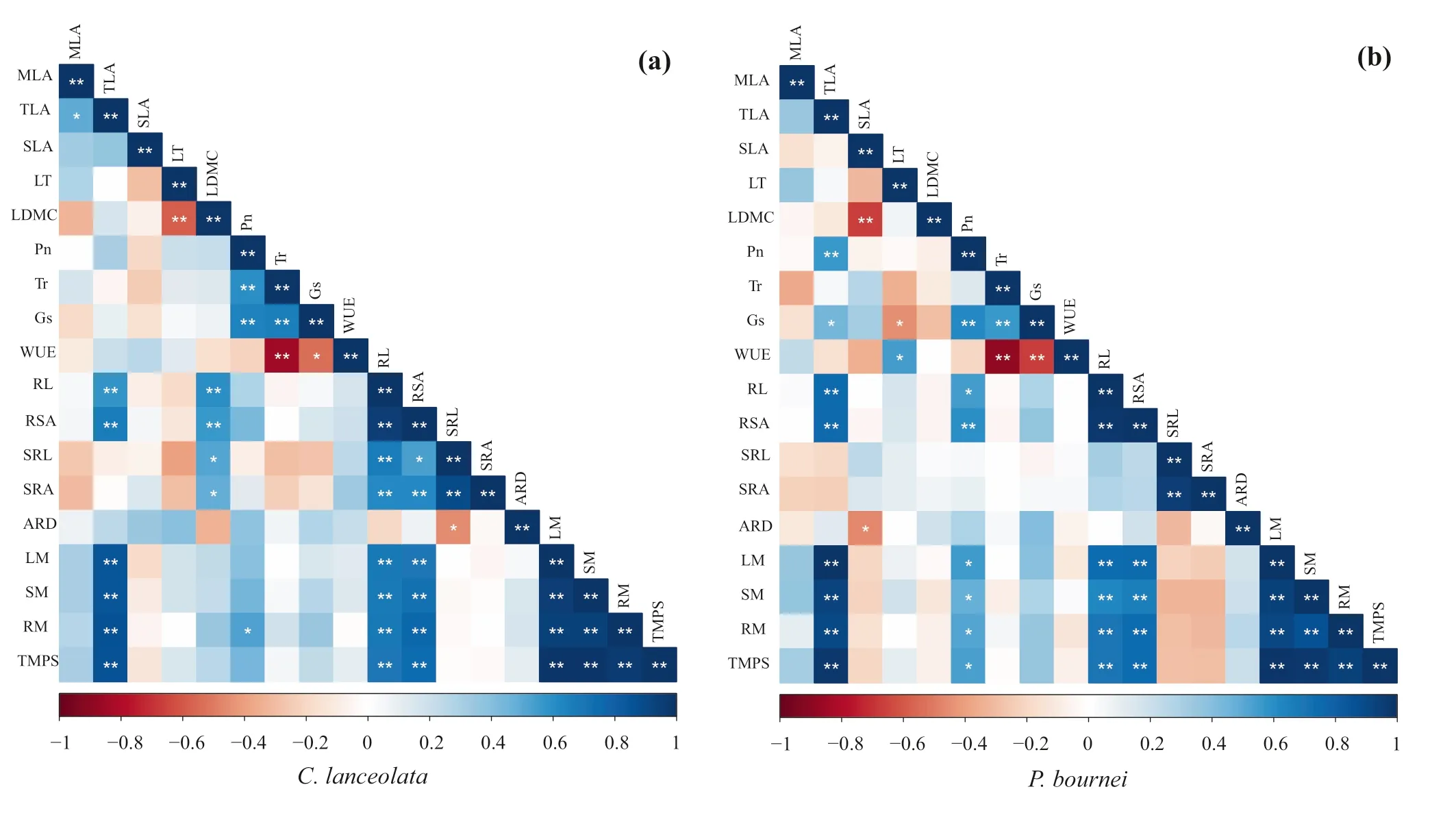
Fig. 4 Correlation analysis of functional traits and biomass allocation of a Cunninghamia lanceolata and b Phoebe bournei in the different plantings; **a highly signif icant correlation at P < 0.01 (bilateral), * a signif icant correlation at P < 0.05 (bilateral), n = 5. Notes:MLA: Mean leaf area, TLA: Total leaf area, SLA: Specif ic leaf area,LT: Leaf thickness, LDMC: Leaf dry matter content, Pn: Net photosynthetic rate, Tr: Transpiration rate, Gs: Stomatal conductance,WUE: Instantaneous water use effi ciency, RL: Root length, RSA:Root surface area, SRL: Specif ic root length, SRA: Specif ic root area,ARD: Average root diameter, LM: leaf dry mass, SM: Stem dry mass,RM: Root dry mass, TMPS: Total dry mass per seedling
Discussion
Eff ects of mixing proportion on leaf morphological and physiological traits
Leaves convert light and CO2into chemical energy via photosynthesis (Cochrane et al. 2016). Leaf functional traits can ref lect the responses and adaptation strategies of plants to changes in biotic and abiotic factors (Qin and Shangguan 2019). Our results indicate that leaf functional traits diff er between broadleaf (P. bournei) and coniferous (C. lanceolata) species in response to mixed composition. The mean leaf area (MLA), total leaf area (TLA), and leaf thickness(LT) ofP. bourneiwere higher in the mixed plantings than in the 0C:4P monoculture, while the specif ic leaf area (SLA)increased proportionally with increased percent composition in the diff erent plantings (Table 3). The total leaf area and leaf thickness ofC. lanceolatawere also higher in the mixed plantings than in the 4C:0P monoculture, although there were no signif icant diff erences in the mean leaf area and specif ic leaf area between the monoculture and mixed plantings (Table 3). Qin et al. ( 2016) demonstrated that the mean leaf area and specif ic leaf area of black locust (Robinia pseudoacaciaL., a deciduous broadleaf) and Siberian elm (Ulmus pumilaL., a deciduous broadleaf) were higher in mixed plantings than in monocultures in a f ield experiment in northwest China. The specif ic leaf area of Siberian elm decreased marginally with increasing mixing proportion. In another study, Qin and Shangguan ( 2019) found that the mean leaf area and specif ic leaf area of Masson’s pine (Pinus massonianaLamb., a conifer) did not diff er signif icantly between monocultures and mixed plantations.These diff erent responses may be related to diff erences in plant growth type (deciduous broadleaf, conifer) (Qin and Shangguan 2019). Additionally, we found that the leaf dry matter content (LDMC) showed little variation among the diff erentC. lanceolataandP. bourneiplantings. Similarly,Qin et al. ( 2016) observed that the leaf dry matter content of black locust and Siberian elm did not show signif icant changes among plantations. However, Qin and Shangguan( 2019) reported that the leaf dry matter content of Masson’s pine was signif icantly lower in mixed plantations than inmonocultures. In addition, Benavides et al. ( 2019) observed that, compared with pure stands, the leaf dry matter content was higher in boreal and Mediterranean forests, while the opposite occurred in temperate forests. Therefore, the variation in leaf dry matter content in mixed stands largely depends on environmental conditions (moisture levels and temperature) and/or competition for soil resources in more diverse communities (Grossiord et al. 2014; Jucker et al.2014). In this study, the growth of seedlings was not limited by nutrients or water in any of the plantings, and accordingly, there was no signif icant diff erence in leaf dry matter content between the monocultures and mixed plantings. Net photosynthetic rates (Pn) ofC. lanceolataandP. bourneidecreased with their increasing mixing proportion, although other variables related to leaf morphology and photosynthesis showed less variation for the two species. Qin et al.( 2016) found that net photosynthetic rates, transpiration rates (Tr), and stomatal conductance (Gs) of black locust increased with higher mixing and were the lowest in monocultures. In addition, they found that photosynthetic variables for Siberian elm varied less among diff erent planting mixtures. These diff erent results may be because the mixing proportions can considerably aff ect photosynthetic variables,although these eff ects depend on the species. Moreover,these f indings may also be due to diff erent environmental conditions.
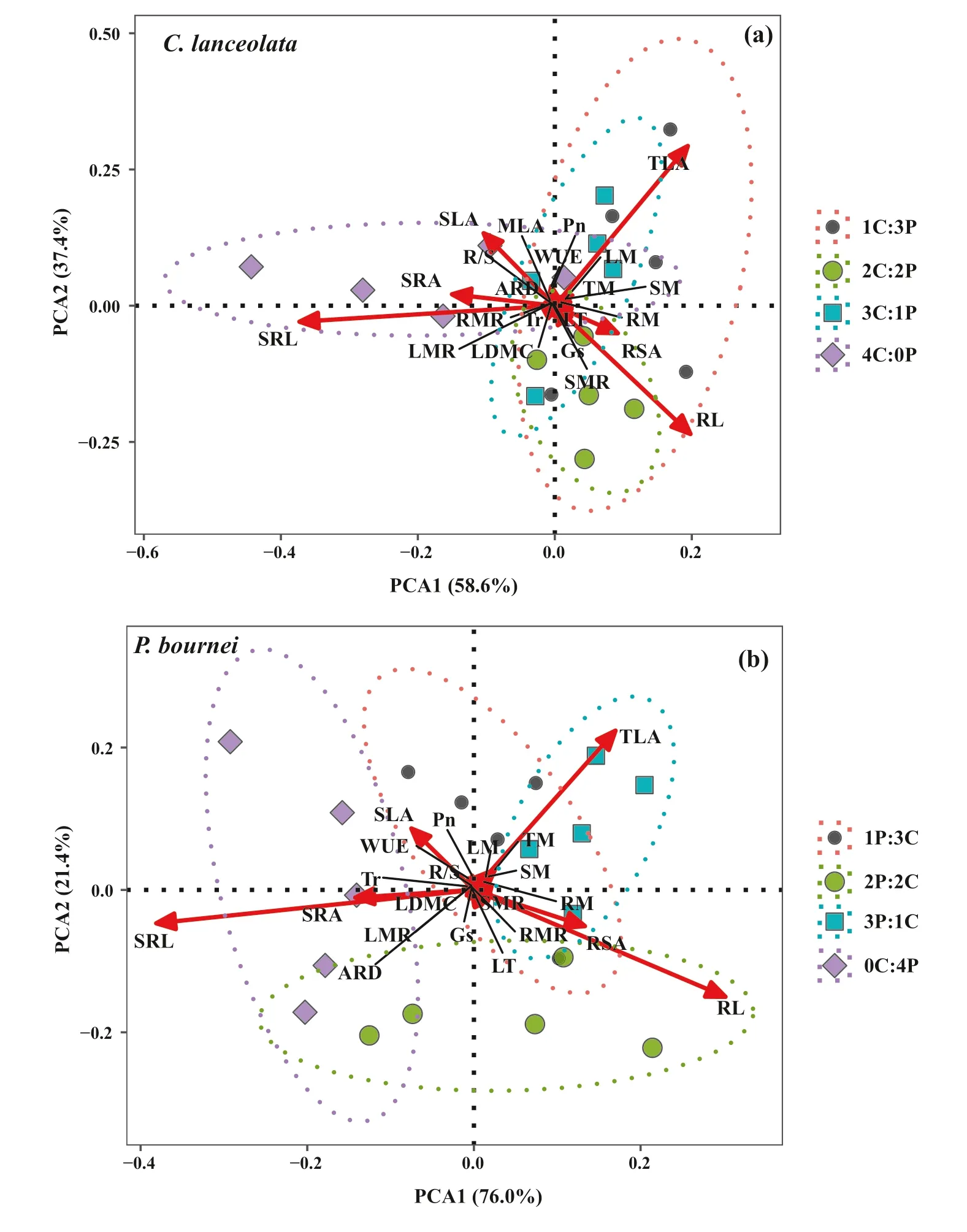
Fig. 5 Principal component(PCA) analysis of functional traits and biomass allocation of a Cunninghamia lanceolata and b Phoebe bournei in the diff erent plantings. n = 5. 0C:4P: four P. bournei seedlings; 1C:3P or 3P:1C: one C. lanceolata and three P. bournei seedlings;2C:2P or 2P:2C: two C. lanceolata and two P. bournei seedlings; 3C:1P or 1P:3C: three C.lanceolata and one P. bournei seedlings; 4C:0P: four C. lanceolata seedlings. Notes: MLA:mean leaf area, TLA: total leaf area, SLA: specif ic leaf area,LT: leaf thickness, LDMC:Leaf dry matter content, Pn:Net photosynthetic rate, Tr:Transpiration rate, Gs: stomatal conductance, WUE: instantaneous water use effi ciency, RL:root length, RSA: root surface area, SRL: specif ic root length,SRA: specif ic root area, ARD:average root diameter, LM: leaf dry mass, SM: stem dry mass,RM: root dry mass, LMR: leaf mass ratio, SMR: stem mass ratio, RMR: root mass ratio,R/S: root/shoot ratio
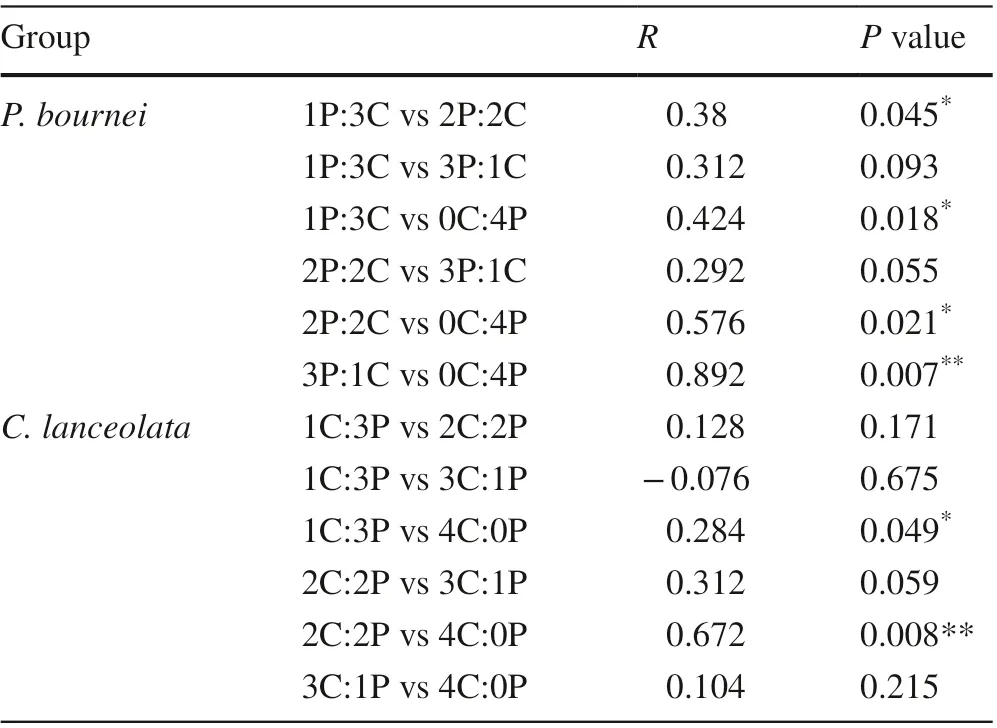
Table 7 Permutational multivariate analysis of variance (PERMANOVA) signif icance test for diff erences in functional traits and biomass allocation of Cunninghamia lanceolata and Phoebe bournei in the diff erent plantings
Eff ects of mixing proportion on root morphological traits
Roots are critical for the acquisition of water and mineral nutrients (Bardgett et al. 2014) and their functional traits can be used to infer resource acquisition strategies and mediate plant feedback with ecosystem functions (Bardgett et al.2014; Iversen 2014). Our results showed higher root lengths(RL) and root surface area (RSA) in the mixed plantings than in the monocultures, with the highest values in the 2C:2P planting (Table 5). This suggests that mixed plantings promote the capacity of both species to successfully exploit soil resources. Madsen et al. ( 2020) suggested that tropical tree roots extend further in mixed stands than in monocultures. Xiang et al. ( 2015) similarly found that the f ine root densities of Masson’s pine and Japanese oak were higher in mixed plantings than in monocultures. Conversely, Bolte and Villanueva ( 2006) reported that the f ine root densities of European beech and Norway spruce were lower in mixed than in pure stands. These results indicate that the inf luence of species composition on root functional traits are complex, and variations may be due to plasticity in response to species, stand composition, stand age, and interactions with neighbors (Shu et al. 2018). Our results may be attributed to lower interspecif ic competition compared to intraspecif ic competition (Table 4). In addition, the results also indicate that the specif ic root length (SRL) and specif ic root area(SRA) ofC. lanceolataandP. bourneiwere unaff ected by diff erent mixing proportions if both species were present(Table 5). Shu et al. ( 2018) observed that the specif ic root length ofP. massonianawas signif icantly higher in mixedP.massonianaandCinnamomum camphora(L.) J. Presl. plantations than in corresponding monocultures. The variations of specif ic root length under diff erent species composition may result from interactions between the species planted.However, Cheng et al. ( 2020) noted that the absorption of soil resources during the growth stage of seedlings may be improved by extending the root system rather than by forming thinner roots with a higher specif ic root length. In this study, the absence of signif icant diff erence in the specif ic root length and specif ic root area may be attributed to consistent soil conditions, including suffi cient water supply and high concentrations of nutrients. Furthermore, these results may be due to the short experimental period, which could have been insuffi cient to identify the competitive eff ect on soil resource utilization in the diff erent plantings.
Link between biomass allocation and functional traits
Biomass is an important indicator for evaluating the productivity of ecosystems. A positive relationship between species richness and productivity is universal (simova et al. 2013). Some studies have found that higher productivity occurs in mixed-species plantations compared with monocultures (Bielak et al. 2014; Pretzsch and Schutze 2016; Steckel et al. 2019). Our results also showed higher biomass in the mixed plantings than in the monocultures(Fig. 2), which may be attributed to increased benef its and reduced competitive interactions (Ammer 2019). For example, Bauhus et al. ( 2000) showed that superior productivity in mixed plantations of southern blue gum and black wattle may be the result of canopy stratif ication and improved nitrogen supply to the southern blue gum through nitrogen f ixation by black wattles. This study also found that the biomass ofC. lanceolatadecreased with increased proportion, and the biomass ofP. bourneivaried less in the three mixtures with no signif icant diff erences observed in total biomass (Fig. 2). Bauhus et al. ( 2004) reported that stem volume and biomass were the highest in a 50% black wattle and 50% southern blue gum mixed plantation. Forrester et al. ( 2004) found that volumes and aboveground biomass of southern blue gum and black wattle were signif icantly higher in mixtures than in monocultures at both the tree level and at the stand level after three to four years. Nevertheless,Santos et al. ( 2016) demonstrated that the overall biomass production ofEucalyptus urograndishybrids (Eucalyptus grandis×Eucalyptus urophylla) and brown salwood (Acacia mangiumWilld.) planted at a ratio of 1:1 was equal to or lower than that of theE. urograndismonocultures. The eff ect of diff erent mixing proportions on productivity mainly depends on the balance between positive and negative interactions among species (Forrester et al. 2004). Our results may be related to the complementary utilization of aboveand belowground resources by the two species in the mixed plantings and the suffi cient supply of soil nutrients and water during the experiment. Biomass allocation can ref lect the ability of plants to obtain resources. According to the optimal allocation theory, plants increase investment in organs that can obtain restricted resources (Baraloto et al. 2010).Our results demonstrate that the mixing proportion had no signif icant eff ect on biomass allocation forC. lanceolataandP. bournei. This may also be related to the relatively short experimental period or low plasticity in individual seedlings during the early growth stage.
Numerous studies have found that plants do not adapt to their environment by simply changing a single trait, but instead respond to and survive environmental changes by altering many functional traits of diff erent organs (Freschet et al. 2010). In this study, the total leaf area (TLA), root length (RL), and root surface area (RSA) were positively correlated with the leaf dry mass (LM), stem dry mass (SM),root dry mass (RM), and total dry mass per seedling (TMPS)for both species (Fig. 4), indicating that the variations in biomass depended on above- and belowground resource acquisition. Generally, higher biomass in mixed plantations than in monocultures is associated with complementarity interactions. These may include a higher light interception as a result of aboveground niche separation, or from vertical belowground niche segregation, in which the roots of one species shift to a deeper soil prof ile to explore higher volumes (Bauhus et al. 2004; Bolte and Villanueva 2006).Therefore, in this study, the increase in biomass of the two species in the mixtures may be due to increase plasticity differences in certain functional traits caused by interspecif ic complementary interactions. Overall, signif icant positive correlations between functional traits of leaves and roots,and biomass production and its allocation inC. lanceolataandP. bourneiamong diff erent plantings not only indicate multi-trait convergent adaptation, but some functional traits also showed diff erent responses to diff erent mixing proportions. In general, the correlations between plant functional traits are closely related to the site conditions of the habitat,phenotypic plasticity of plant traits, and physiological characteristics of the species themselves (McCarthy and Enquist 2007). Therefore, there were diff erences in the relationships between functional traits and biomass allocation ofC. lanceolataandP. bourneigrown in the diff erent plantings.
Conclusion
Variations in functional traits and biomass allocation ofC.lanceolataandP. bourneiwere studied in diff erent mixture proportions. These functional traits showed diff erent responses to species composition mixtures. Overall, the mixed species plantings had positive eff ects on seedling growth due to lower interspecif ic competition. The increase in the biomass of the two species in the mixed plantings was likely due to increases in the aboveground (total leaf area) and belowground (root length and specif ic root length)resource exploitation. The variation in interspecif ic functional traits in mixed plantations may change with increasing stand age for species with strong interspecif ic competition.Future research should focus on the relationships between growth, stand structure, and interspecif ic functional traits.Our results indicate that the mixing proportion considerably aff ects functional traits and biomass allocation of seedlings and that determining the appropriate mixing proportions is crucial to enhance productivity. Mixtures of species should be adopted instead of monocultures as this can increase productivity and limit some of the negative ecological eff ects associated with monocultures.
 Journal of Forestry Research2022年6期
Journal of Forestry Research2022年6期
- Journal of Forestry Research的其它文章
- Intraspecif ic leaf morphological variation in Quercus dentata Thunb.: a comparison of traditional and geometric morphometric methods, a pilot study
- Mapping the potential distribution suitability of 16 tree species under climate change in northeastern China using Maxent modelling
- Correction to: Eff ects of active molecules of Korean pine seed on rodent health and implications for forest regeneration
- Seed, germination, and seed-reserve traits diff er along an altitudinal gradient
- Analysis of the chloroplast genomes of four Pinus species in Northeast China: Insights into hybrid speciation and identif ication of DNA molecular markers
- Genetic variation and superior provenances selection for wood properties of Larix olgensis at four trials
