A phase II study of neoadjuvant chemotherapy followed by organ preservation in patients with muscle-invasive bladder cancer
Chinn Bu Drhm ,Nrenr Kumr ,*,Sntosh Kumr ,Arun Elngovn ,Buhi Singh Yv ,Rvimohn S.Mvuuru ,Anupm Ll ,Prmo K.Gupt ,Rkesh Kpoor
a Department of Radiotherapy & Oncology,PGIMER,Chandigarh,India
b Department of Urology,PGIMER,Chandigarh,India
c Department of Radiodiagnosis,PGIMER,Chandigarh,India
d Department of Biostatistics,PGIMER,Chandigarh,India
KEYWORDS Bladder preservation;Neoadjuvant chemotherapy;Radiotherapy;Muscle-invasive bladder cancer
Abstract Objective:Conservative approaches in muscle-invasive bladder cancer(MIBC)have been evolved to avoid aggressive surgery,but are limited to elderly,frail,and patients medically unfit for surgery.Our study aimed to assess the response rate of neoadjuvant chemotherapy(NACT)before radiotherapy(RT)in MIBC patients.
1.Introduction
Bladder cancer is the 11th most common cancer worldwide.It is responsible for about 2.1% of all cancer-related deaths and is the 4th leading cause of cancer-related mortality in elderly males over 80 years of age[1].In India,it is the 18th common cancer with a relative frequency of 1.8%and accounts for 1.42%of all cancer-related mortality[2].Neoadjuvant chemotherapy(NACT)followed by radical cystectomy(RC)along with bilateral pelvic lymph node dissection is the current treatment of choice in muscle-invasive bladder cancer(MIBC)[3,4].After the surgery,patients have to live with an artificial bladder or an ileal conduit which could significantly hamper the quality of life(QoL)and body image.Although continent urinary diversions and nerve sparing minimally invasive surgical techniques have been used to substitute bladder function,a considerable portion of patients still remain unsatisfied[5].Therefore,preservation of native bladder function has become paramount while maintaining similar oncological outcomes.Five-year survival rates after RC were 50%-60% when the disease was pathologically confined to bladder(pT2),but overall survival(OS)dropped to 10%-50% with extravesicular extension(≥pT3)[6].The local recurrence and distant failure rates in pT2 were 3%-4% and 10%-25% whereas in pT3/pT4 were 11%-16%and 20%-35%[7,8].The survival rate in MIBC depends on the presence or absence of distant microscopic metastasis at the time of local therapy and will not be affected by changing the modality of local therapy[9].Therefore,the rationale for using NACT before local treatment is to decrease the local tumor bulk,eradicate potential micrometastatic disease,and maintain the best QoL without compromising survival.Methotrexate,vinblastine,adriamycin,and cisplatin(MVAC),and gemcitabine-cisplatin(GC)are the accepted NACT regimens in MIBC[10,11].
Conservative approach using maximum transurethral resection of bladder tumor(TURBT)followed by radiotherapy(RT)has been an alternative to surgery.However,studies have shown that RT alone is inferior to surgery in terms of local recurrence and OS[12].Systemic chemotherapy has been incorporated in concurrent,neoadjuvant and adjuvant settings to improve outcomes.Numerous phase II studies using trimodality therapy(TMT)(TURBT,concurrent chemoradiation[CCRT],and cystectomy as salvage for local recurrence)showed results comparable to cystectomy series with 5-year survival rates of 50%-63%,and majority of these patients maintained their bladder function[13,14].Despite inferior results with TMT during the initial period and lack of direct prospective comparison between RC and TMT,currently,large retrospective series,propensity-matched analysis,and accumulated prospective data have proven more commensurate outcomes[15-18].The Selective bladder Preservation Against Radical Excision(SPARE)trial from United Kingdom attempted to compare RC(n=25)with TMT(n=20)after three cycles of NACT in node-negative MIBC patients.The trial was powered to evaluate feasibility and non-inferiority of TMT with RC in patients who responded to NACT,but was closed earlier due to poor target accrual rates.OS was not significant between two groups and bladder preservation was 73% in patients who received RT according to per protocol analysis,but failed to draw firm conclusions on clinical outcomes[19].Advanced Bladder Cancer Meta-analysis Collaboration group has shown 5%OS benefit with NACT regardless of local therapy[9]and current treatment guidelines have endorsed that the NACT followed by RC as the preferred approach in MIBC.However,modern TMT approaches commonly do not include the NACT.Recently a phase II study using NACT before RT reported showed similar outcomes compared to RC[20,21].We conducted this study to look for response rate and toxicity to NACT with GC regimen in MIBC.We aimed to assess the possibility of bladder preservation in patients achieving complete response(CR)and good partial response(PR)with this GC regimen.
2.Patients and methods
The prospective phase II study was conducted between November 2013 and November 2015.Our hypothesis was that NACT followed by RT is as effective as surgery in addition to having the advantage of bladder preservation.A sample size of 50 was estimated empirically based on the previously published literature;during the study period,we had assessed 58 patients for eligibility but only 44 patients were entered into the study after completion of written informed consent.However,four patients were excluded from the analysis because they breached the treatment protocol.Patient screening and selection have been summarized in the study flowchart(Fig.1).The trial was approved by institute ethics committee and registered in the Clinical Trial Registry of India(CTRI,http://ctri.nic.in/Clinicaltrials/login.php) with CTRI/2017/08/009318.Previously untreated patients of histologically provenmuscle-invasive transitional cell carcinoma(T2-T4a,N0,M0 according to American Joint Committee on Cancers[AJCC]7th edition 2010 Staging)of the urinary bladder were included in the study.Patients with non muscleinvasive bladder carcinoma or metastatic disease and those with Karnofsky performance status(KPS)<70,hemoglobin<9 g/dL,white blood cell count<3000/mL,platelet count<100 000/mL,deranged liver and kidney function test,previous RT to the pelvis,history of other malignancy,platinum-hypersensitivity,and those with significant comorbidity(uncontrolled hypertension,diabetes mellitus,coronary artery disease,and renal and liver failure)were excluded from the study.
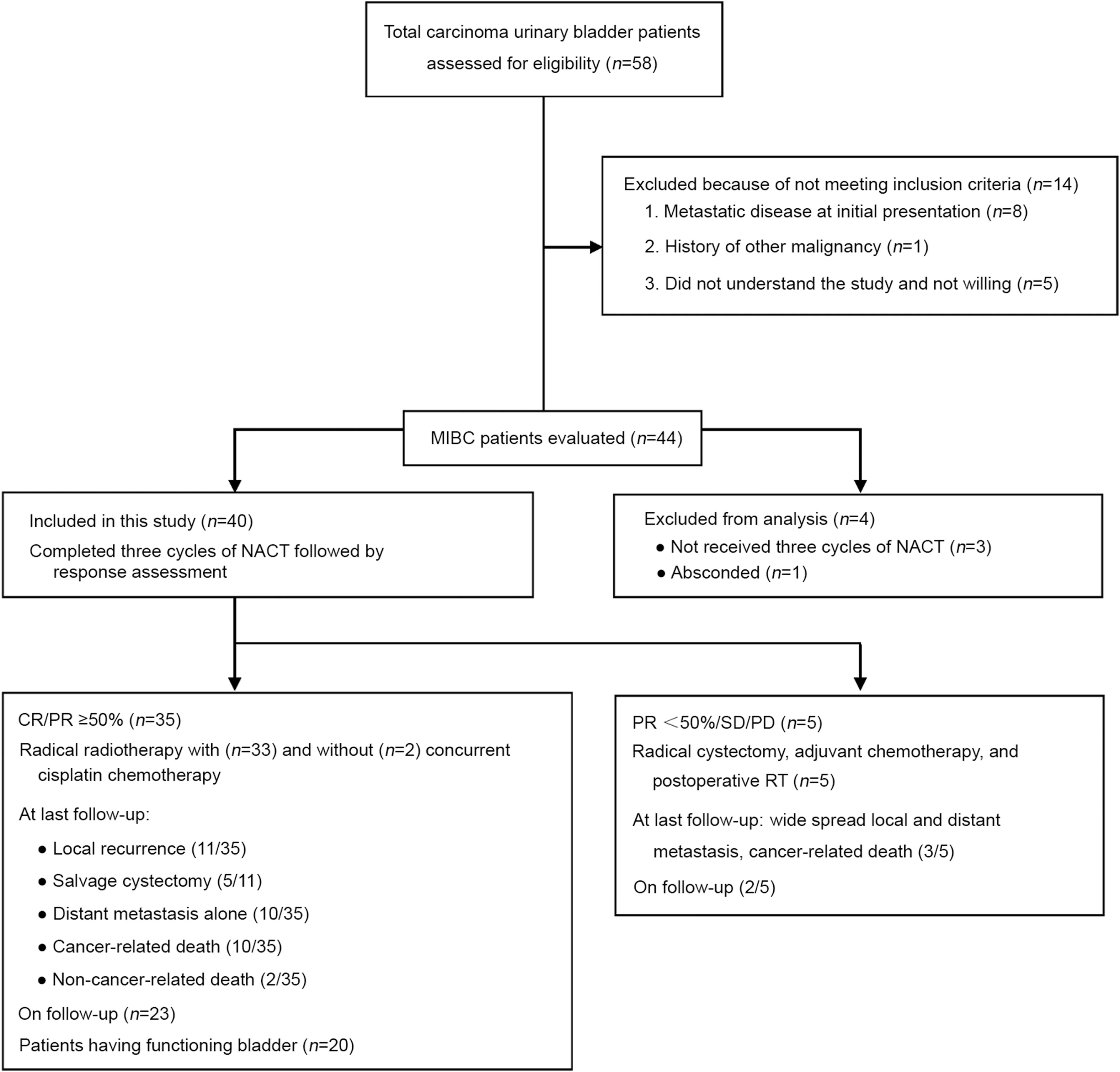
Figure 1 Study flowchart with a summary of methods and results.CR,complete response;NACT,neoadjuvant chemotherapy;PD,progressive disease;PR,partial response;SD,stable disease;MIBC,muscle-invasive bladder cancer;RT,radiotherapy.
Patients were evaluated with a full medical history and physical examination.Staging maximal TURBT,complete blood count,liver and kidney function tests,chest X-ray,cystoscopy,contrast-enhanced computed tomography(CECT)or magnetic resonance imaging(MRI)of pelvis and bone scan were done for all patients.
2.1.NACT
Patients received three cycles of NACT with gemcitabine 1 g/m2on Day 1 and 15 and cisplatin 70 mg/m2on Day 1 every 28 days.Response to NACT was assessed with Response Evaluation Criteria in Solid Tumors(RECIST)criteria after 2 weeks of last chemotherapy by CECT chest,abdomen,and pelvis and cystoscopy.Cystoscopy was done to confirm downstaging in PR patients and biopsies were taken from any visible lesions or multiple random biopsies were mandatory in case of absence of visible lesions to declare CR.Patients who had a CR or PR of more than 50%(i.e.downstaged to T1-T2)underwent radical RT to pelvis(started after 3-4 weeks of completion of NACT)with orwithout concurrent chemotherapy with weekly cisplatin(40 mg/m2).
2.2.RT planning
2.2.1.Simulation
Patients were kept fasting for 6 h prior to planning CT scan.Oral and rectal contrast was used for delineating critical structures.Oral contrast was prepared with 20 mL of urograffin dissolved in 1 L water and given over 1 h before planning CT.Rectal contrast was constituted with the same urograffin in 50 mL water and injected into rectum with a syringe.Patients were asked to void urine 30 min prior to planning CT.Similar water intake and voiding methods were followed while treating the patients,to ensure consistent bladder filling and reproducibility.After preparation,thermoplastic pelvic Orfit-cast was made as immobilization device in the supine position with arms over the chest in headfirst position(phase-I).Phase-II was planned with prone position and arms below the head with an empty bladder.Two laterals and one anterior fiducial tattoos were marked with radio-opaque material aligned with lasers to maintain position accuracy and reproducibility.In intravenous contrast,100 mL of Omnipaque was administered according to the cross method.Planning CT was taken using a CT simulator(GE healthcare technologies,Wankesha,WI,USA)with slice thickness of 2.5 mm from L1 vertebra to mid-thigh.The CT images were transferred to the Eclipse?treatment planning system(v.8.6,Varian Associates,Palo,Alto,CA,USA).
2.2.2.Contouring,planning,and dose prescription
Target volume and organ at risk delineation was done on planning CT images;and the clinical target volume(CTV)was defined as the gross disease,whole bladder,and pelvic lymph nodes[22-24].In patients with tumors at the bladder base,the proximal urethra,and in men,the prostatic urethra and prostate,were included in CTV.External iliac,internal iliac(hypogastric and obturator),and pre-sacral lymph nodes were included in nodal CTV.Sequential boost planning was done in phase-II and CTV was defined as gross disease with extravesical spread and an empty bladder.Planning target volume was generated by 1 cm margin to CTV.Three-dimensional conformal RT plans were made and appropriate wedges were used to produce homogenous dose distribution.Plans were optimized to deliver prescribed dose to planning target volume and achieved optimal doses to organ at risks.External beam RT was delivered using linear accelerator with 6 megavoltage or 15 megavoltage photons,and energy was decided at the discretion of treating physician and physicist.Electronic portal imaging detector verification system was used for image guidance while execution.Total radiation was delivered to a dose of 66 Gy in 33 fractions over 6.5 weeks(2 Gy per fraction,5 days a week),46 Gy in phase-I,and 20 Gy in phase-II.Patients who had PR of<50%(i.e.downstaged to T3),stable disease(SD),and progressive disease(PD)were treated by RC followed by three cycles of adjuvant chemotherapy with the same regimen.Postoperative radiation was delivered to patients with high-risk histopathological features such as margin positivity,T3 and T4a disease,and RT dose of 50.4 Gy in 28 fractions(1.8 Gy per fraction,5 days a week)was used.
2.2.3.Follow-up and assessment of toxicity
First follow-up was done after 6 weeks of RT completion.Cystoscopy and CECT of chest,abdomen,and pelvis were used for response assessment.Cystoscopy and CECT were repeated every 3 months for first 2 years and then sixmonthly for 5 years.Salvage cystectomy was offered for local recurrence after RT.Acute and late treatment-related adverse events were noted and graded by common terminology criteria for adverse events(CTCAE-v3.0).All the parameters were entered in Microsoft excel sheet(Microsoft?Office Professional Plus 2010 version[14.0.7155.5001]SP2 MSO[14.0.7153.5000]developed for Microsoft Corporation by Impressa Systems,Santa Rosa,CA,USA).
2.3.Statistical analysis
Statistical analysis was done using SPSSv.23(IBM Corp,Chicago,IL,USA).The primary endpoint was response rate to NACT and secondary endpoints were survival endpoints such as local control(LC),disease-free survival(DFS),OS,and bladder-intact DFS(BIDFS).Numerical data were condensed and reported using medians and range.Categorical data were summed up as percentages.The LC,OS,DFS,BIDFS,and metastasis-free survival(MFS)were analyzed based on intension to treat basis and computed with Kaplan-Meier method.The impact of various prognostic variables(age,comorbidity,T-stage,composite stage,and NACT response)on survival endpoints was analyzed using the Post Hoc analysis(Bonferroni test).Coxproportional hazard regression model was used as a multivariate analysis.The variables with p-value<0.20 in univariate analysis were entered into multivariate analysis.OS was calculated from the last date of treatment to the last date of follow-up or date of death.DFS was calculated from the last day of treatment to the first date of recurrence or death.LC was calculated from the last day of treatment to the first date of local recurrence or last follow-up.MFS was defined as time period from last date of treatment to first date of metastasis or last follow-up.Qualitative parameters measured as grades of toxicity were analyzed using the Chi-square test.Statistical significance for hypothesis testing was assumed at the 0.05 level.
3.Results
A total of 44 patients were enrolled but only forty patients were evaluable for assessment of response and received three cycles of full-dose NACT without dose reduction.Nonevaluable patients included three patients referred to surgery after completion of first cycle because of severe hematuria and one patient lost to follow-up after second cycle chemotherapy.The median age at presentation was 62 years(range:50-74 years).All weremale patients.The median KPS was 80(range:70-90).Histologically,all patients had transitional cell carcinoma.Pre-treatment patient and disease parameters are depicted in Table 1.The summary of results was depicted in study flowchart(Fig.1).The overall responserate to NACT was 87.5%(35/40 patients)with a CR rate of 22.5%(9/40 patients),and PR≥50%of 65%(26/40 patients)confirmed by radiology,cystoscopy and multiple random biopsies.Five(12.5%)patients showing PR<50%,SD,or PD underwent RC followed by postoperative radiation.The median follow-up was 43 months(range:10-66 months).The results were analyzed in March 2019 and the pattern of failure was analyzed on the basis of disease status at the last followup.Out of 35 patients who received RT,local recurrences occurred in 11(31.4%)patients and distant metastases in 10(28.6%)patients.Five patients underwent salvage cystectomy for local recurrence alone,and later two of them developed widespread metastasis.Twenty(50%)patients had their functional bladders at last follow-up.Two patients died of non-cancer-related disease after their 2nd follow-up(duration:8-10 months).Out of five patients who underwent RC followed by adjuvant therapy,three patients developed local and distant site metastases.Common sites for metastasis were the lungs and bones.Palliative secondline chemotherapy was given with paclitaxel/carboplatin,and palliative radiation was delivered to bony metastatic sites.At last follow-up out of 40 patients,25(62.5%)patients were alive.Median time to local recurrence was 20 months and median DFS was 16 months.Three-year OS,DFS,and LC were 70.1%(95% confidence interval[CI]:60.2%-88.2%),50.6%(95% CI:41.8%-68.5%),and 60.9%(95% CI:45.6%-78.5%),respectively(Fig.2).Two-year and 3-year BIDFS rates were 73.7%and 66.7%,respectively.
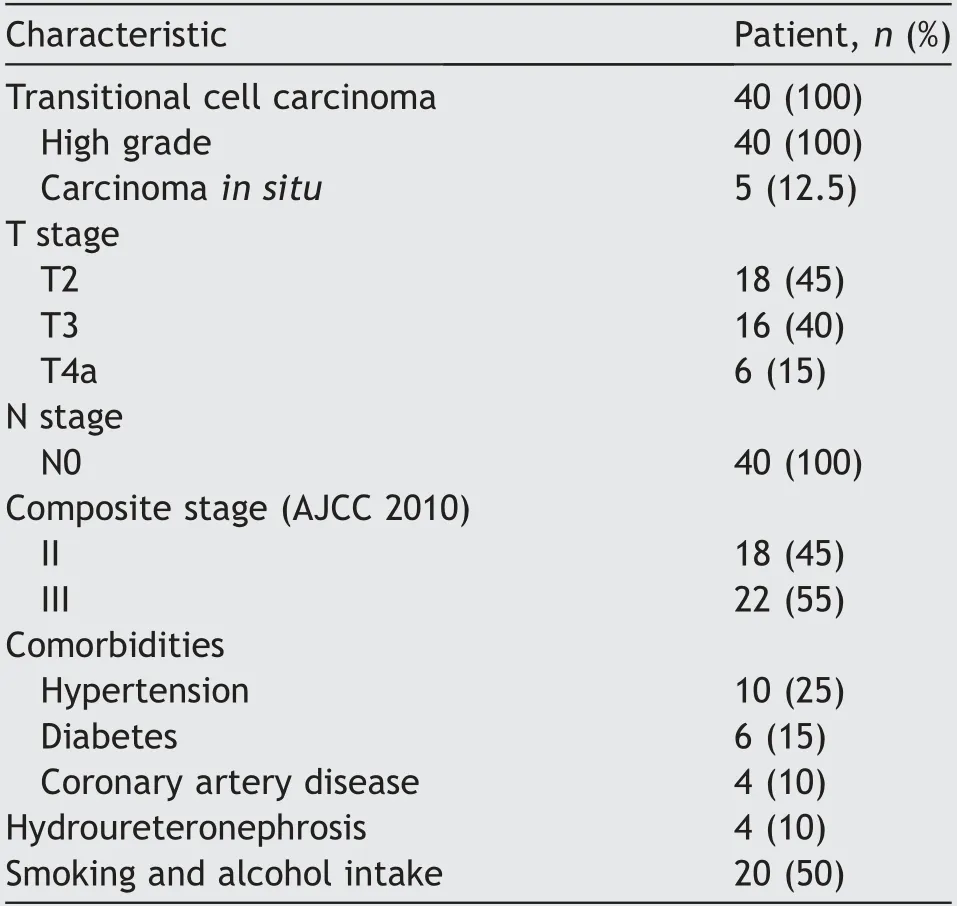
Table 1 Disease and patient characteristics.
Exploratory analysis showed that the 3-year LC rate for patients of T2 stage(76.2%)was higher than T3(60.0%)and T4a(16.7%)stage(Fig.3A),and 3-year LC rate was higher for stage-II(74.0%)than stage-III(42.1%).Three-year LC was significantly better in patients who achieved CR and PR≥50% to NACT compared to those who achieved PR<50%/SD/PD(85.7% vs.57.9% vs.40.0%)(Fig.3B).Interestingly,a similar result was observed with DFS and OS.Three-year DFS was favorable for CR(85.7%)than PR≥50%(40.4%)and PR<50%(26.7%)(Fig.4A).Three-year OS rates were 88.9% for those who achieved CR but dropped down to 73.1%in those with PR≥50%and 40.0%in those with either PR<50%/SD/PD(Fig.4B)(Table 2).MFS was significantly higher for CR patients compared to PR≥50% patients and PR<50% patients(3-year MFS:87.9% vs.51.3% vs.20.3%).
On univariate analysis,advanced tumor(T)stage and poor responders(PR<50%/SD/PD)to NACT had significantly worse LC,DFS,and OS outcomes compared to those with early T-stage and with either CR or PR≥50%to NACT.The patients who were age<60 years and absence of comorbidity did not showed significant difference with their counter groups in terms of LC,DFS,and OS(Table 3).On multivariate analysis,only response to NACT showed statistical significance for LC,DFS,and OS outcomes(Table 4).
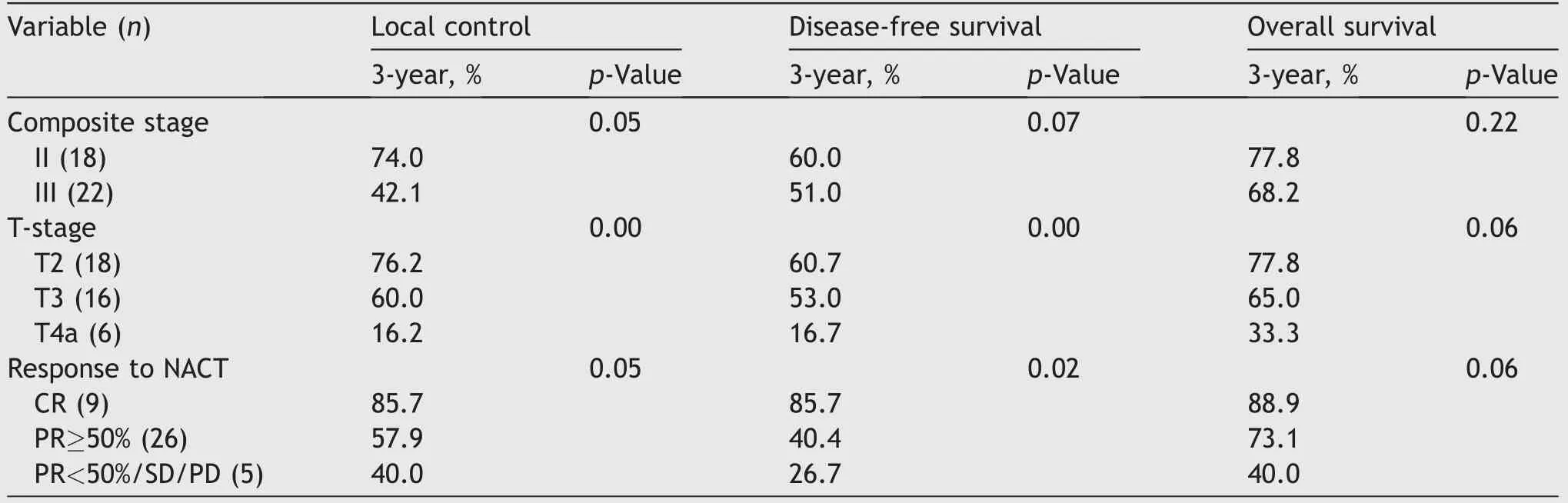
Table 2 Local control,disease-free survival,and overall survival,analyses with Kaplan-Meier method using log-rank test.
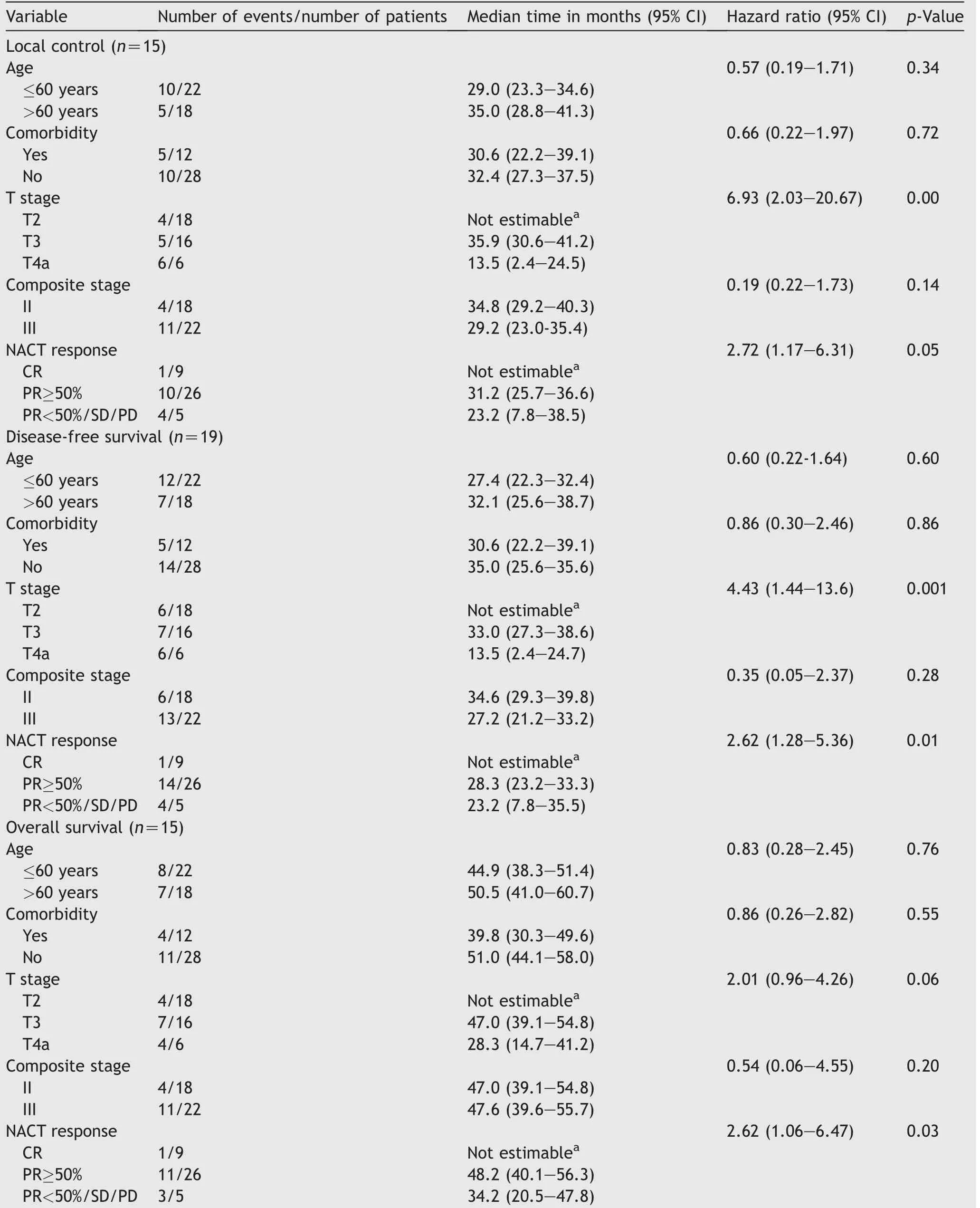
Table 3 Univariate analyses for survival end points(local control,disease-free survival,and overall survival).
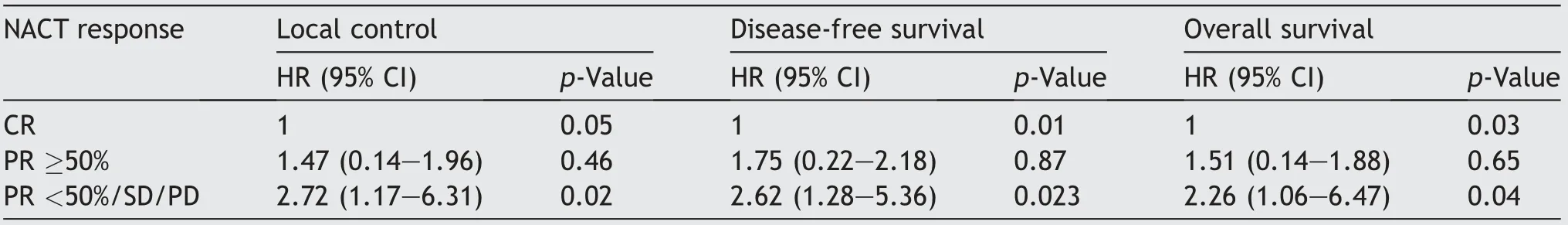
Table 4 Multivariate analyses for survival end points with Cox-proportional hazard regression model.
Majority of the treatment-related toxicities were gastrointestinal.Grade-II nausea was observed in 28 patients;grade-II vomiting was seen in 16 patients;and grade-III vomiting in two patients.Similarly,grade-I and grade-II diarrhea were seen in 10 and 20 patients,respectively.Urinary frequencies of grade-III,-II,and-I were reported in two,14,and 12 patients,respectively.Acute hematological toxicities in form of anemia grade-I and-II were seen in eight and eight patients,respectively.Two patients who developed grade-III anemia were corrected by blood transfusion.Neutropenia grade-II was seen in six patients.None of the patients experienced acute thrombocytopenia.None of the patients had grade-III or higher hepatic/renal/small bowel/skin toxicity.
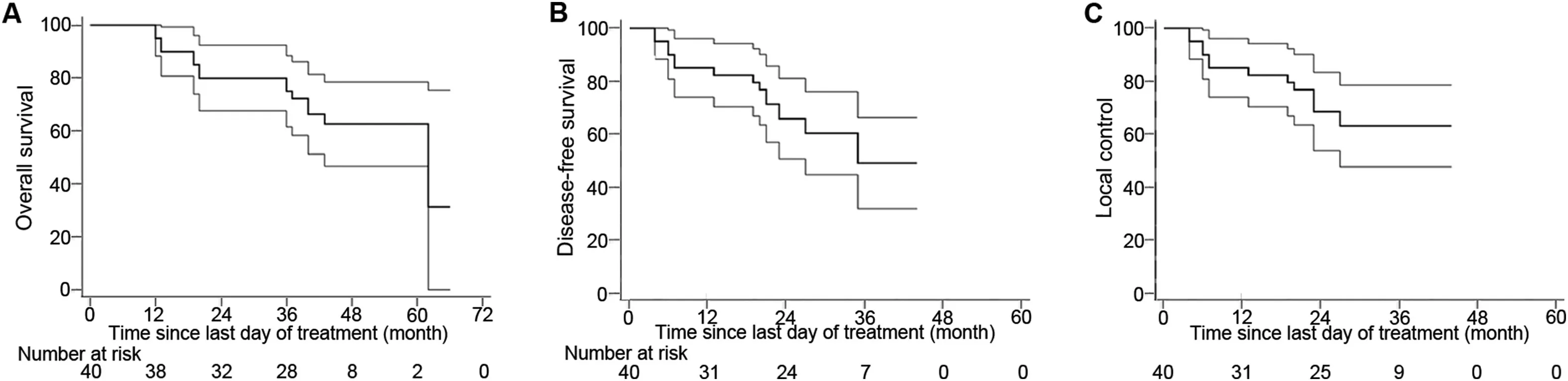
Figure 2 Survival function curves estimated by Kaplan-Meier method along with their 95%confidence interval bands(at any time point,there is a 95%chance that the interval band contains true percentage survival).(A)Overall survival;(B)Disease-free survival;(C)Local control.
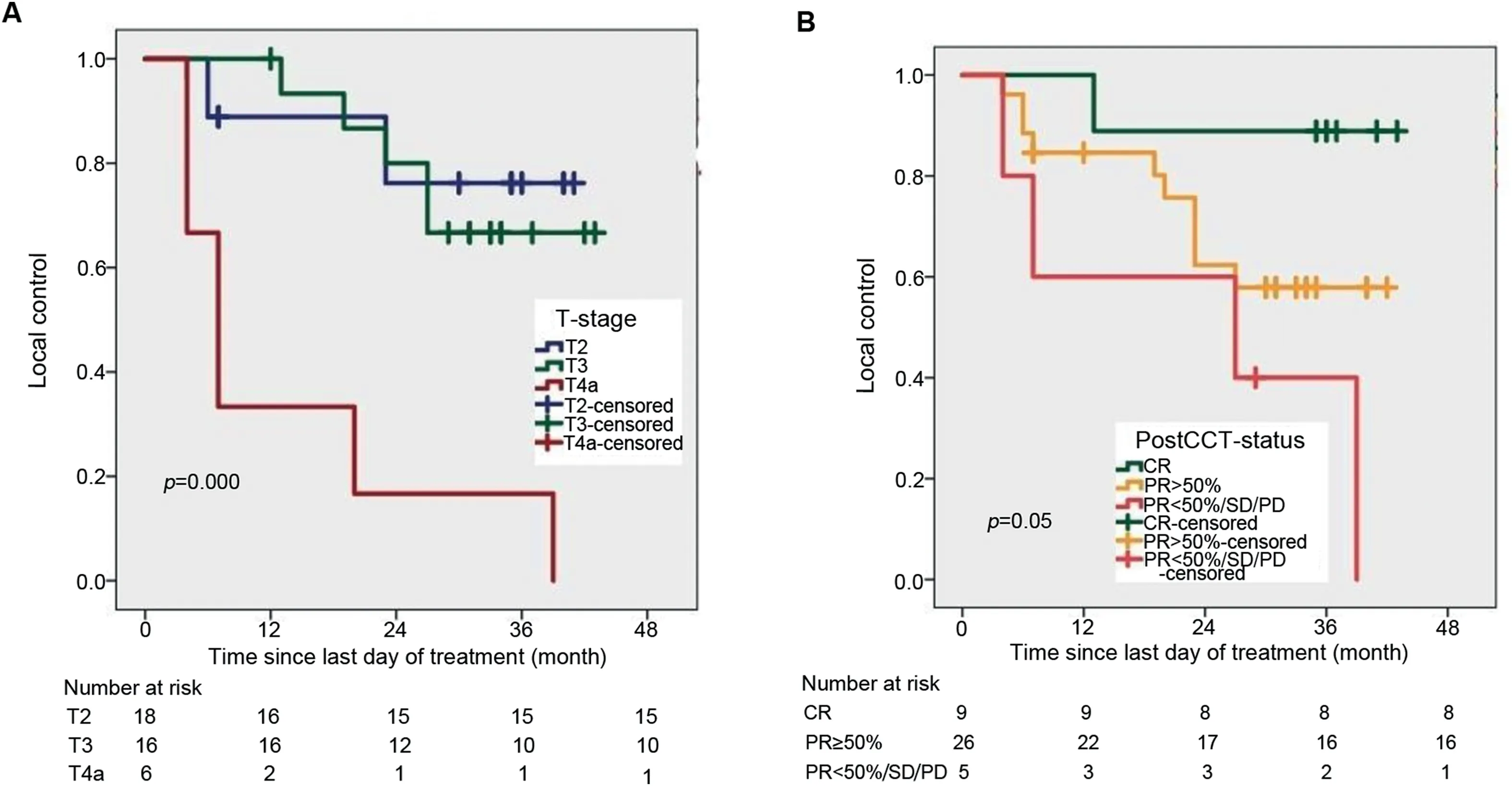
Figure 3 Survival function curve computed by Kaplan-Meier method.(A)For local control with tumor(T)stage;(B)With NACT response.CR,complete response;NACT,neoadjuvant chemotherapy;PD,progressive disease;PR,partial response;SD,stable disease;PostCCT-status,post-chemotherapy(NACT)status.
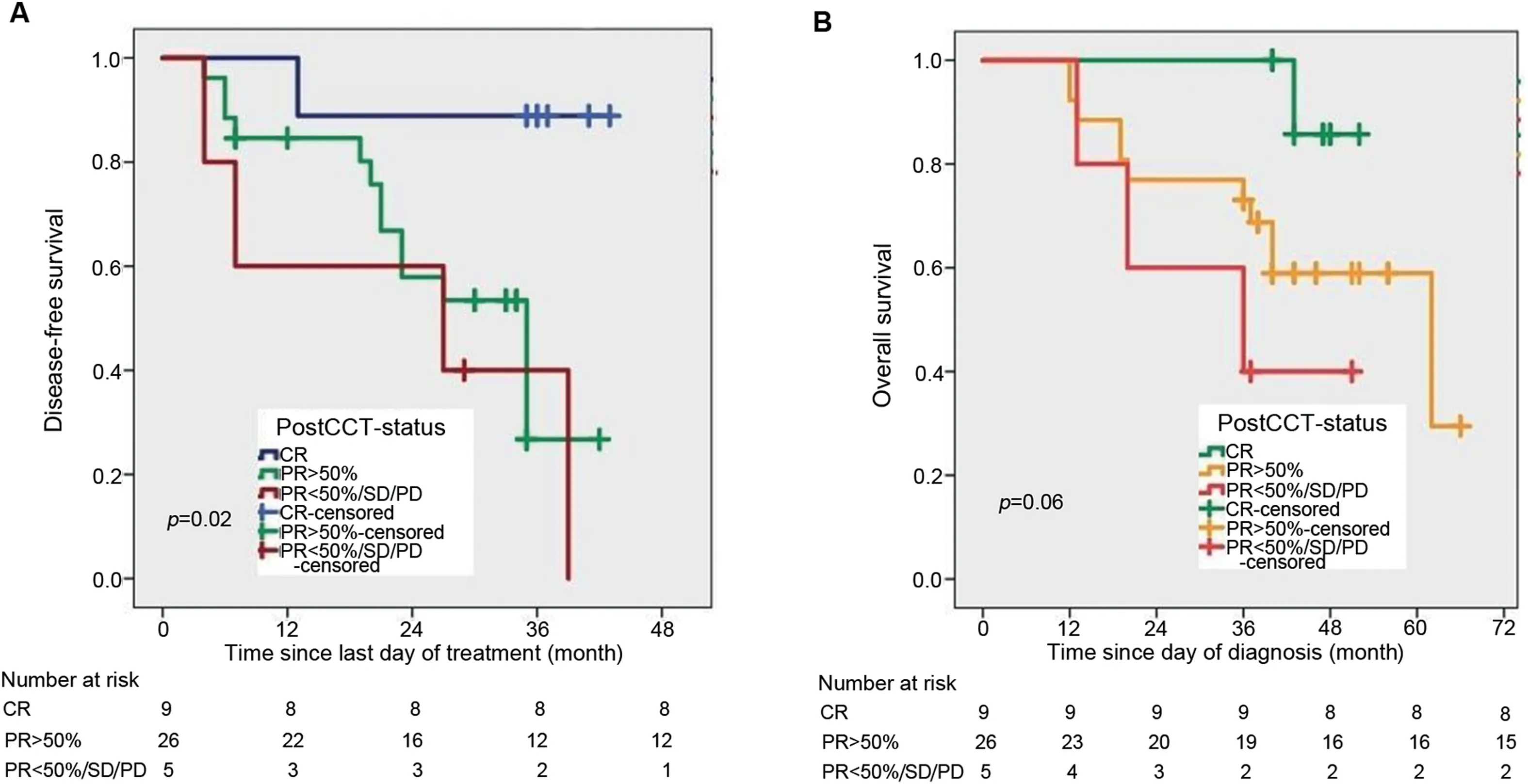
Figure 4 Survival function curve computed by Kaplan-Meier method.(A)For disease-free survival with NACT response;(B)For overall survival with NACT response.CR,complete response;NACT,neoadjuvant chemotherapy;PD,progressive disease;PR,partial response;SD,stable disease;PostCCT-status,post-chemotherapy(NACT)status.
4.Discussion
Radical treatment modalities for MIBC are RC and radical RT.To date,no randomized trial has been performed to compare RC with conservative therapy.However,it should be pointed out that patients in the conservative approach group were in poor performance status,relatively older age,with more advanced clinical stage and significant comorbidities.The most optimal therapeutic conservative approach consists of TMT,including TURBT,RT,and chemotherapy,with cystectomy reserved for salvage.In a recent study from Canada,Kulkarni et al.[17]retrospectivepropensity score-matched analysis in MIBC patients between TMT(n=56)and RC(n=56)showed similar survival outcomes(76.6%vs.73.2%;p=0.49)and only 10.7%of TMT group patients underwent salvage cystectomy for invasive local recurrence.Many clinical trials aimed to achieve a high level of LC and decreased metastatic failure while evaluating neoadjuvant or adjuvant therapies[14].Transitional cell carcinoma is a chemo-sensitive disease,with response rates reported from 10%-70% to different agents[25].Randomized trials of adjuvant chemotherapy have had mixed results on OS improvement while NACT trials have shown modest improvement[25].Standard NACT regimens at present are MVAC,CMV(cisplatin,methotrexate and vinblastine),and GC.The triple combination CMV regimen was widely used but has not been compared with MVAC or GC directly.GC regimen showed better response rate and less toxicity profile in comparison with MVAC,as proved from the phase III study by von der Maase et al.[10].
There are two principal rationales for NACT:first,to improve survival in patients with the micrometastatic disease,and second,to preserve the bladder by shrinking the primary tumor to facilitate RT as an alternative to surgery[9,25,26].However,the potential disadvantage of NACT is a delay in the definitive treatment,because this may lead to local disease progression in non-responding patients who may conceivably become inoperable or unsuitable for radical organ preservation treatment.However,it is equally plausible that undergoing 2-3 cycles of NACT followed by response assessment can identify the nonresponders and they have biologically aggressive disease.Advanced bladder cancer(ABC)meta-analysis reported a 5%absolute benefit in five-year survival with NACT(45% vs.50%),9% improvement in DFS,and 13% scaling down in risk of death.This survival benefit was observed regardless of the type of local treatment modality and did not vary between subgroups of surgery and RT[9].Comparative results were shown by Cochrane database systemic review in 2011,and enrolled 2809 patients in 10 randomized trials.NACT with cisplatin-combination resulted in 4% benefit in fiveyear OS(45% vs.49%)and 11% reduction in the risk of death.This supports our trial for promise role of NACT followed by organ preservation in MIBC.
In the setting of locally advanced and metastatic bladder cancer,the response rates after MVAC chemotherapy were initially reported by Sternberg et al.[26]in 1985.Significant tumor regression was noted in 71%and CR in 50% patients.Southwest Oncology Group study in 2003 showed CR in 38% of patients who received MVAC[27].However,our results are similar to studies that used GC in neoadjuvant setting.Yeshchina et al.[28]conducted a study on 114 patients,and showed pathological CR in 25%patients receiving GC chemotherapy.Yuh et al.[29]reviewed seven studies including 164 patients and reported pathological CR in 25.6% with GC regimen.von der Maase et al.[10]compared GC(n=203)versus MVAC(n=202)in locally advanced and metastatic bladder cancer patients,and showed an overall response rate of 54.3% with GC and 55% with MVAC regimen in both local and metastatic measurable lesions.As compared to this result,current study showed a better response because of isolated local lesions.Mertens et al.[30]reported that in patients(n=441)with cT3-4 disease,the occurrence of occult lymph node metastases was significantly lower in the NACT group than in the non-neoadjuvant group(21.9% vs.40.7%,p=0.002);these data suggest that NACT not only downstages the primary tumor,but also decreases the incidence of occult lymph nodal metastases.A case series(n=35)on conservative approach with maximal TURBT,NACT with GC followed by accelerated RT showed an overall response rate of 78%,CR in 30%,PR in 48%,and SD in 22%after two cycles of NACT[31].Our study results are comparable,with an overall response rate of 87.5% and CR of 22.5% after NACT.
Tolerance to the multidrug chemotherapy regimen in locally ABC was studied in RTOG trials and MD Anderson cancer center trial.RTOG phase I and II trials with CMV regimen reported no treatment-related deaths and less toxicity.However,in the RTOG 8903 randomized control trial that compared TURBT,two cycles of NACT with CMV regimen followed by CCRT versus CCRT alone,the neoadjuvant arm was poorly tolerated.Four treatment-related deaths were reported with CMV regimen due to neutropenia and sepsis[32].Similarly,in the MD Anderson cancer center trial,100 patients were treated with MVAC regimen and three treatment-related deaths were reported,with two ofthem attributed to complications from MVAC chemotherapy[33].Trial results from Memorial hospital,New York,using MVAC regimen in metastatic patients showed a sepsis rate of 20% and four chemotherapy-related deaths[26].In comparison with the above studies,present trial using GC chemotherapy showed good tolerance to the regimen with low-grade neutropenia in 15% of patients,and no treatment-related sepsis or deaths.
NACT followed by RC improves survival,increases pathological down-staging rate[34],and has become a standard of care in locally ABC;however,NACT followed by TMT studies was limited and employed with different radiation doses,radiation sensitizers,old radiation techniques,and small sample sizes[16,35].All these factors complicated the interpretation of results.Effect of chemotherapy sequencing and its benefit in combination with RT were studied by the RTOG group.In the RTOG 8903 trial,NACT-arm did not show any difference in absolute survival rates,freedom from metastasis,and bladder-intact survival.After induction-phase CCRT,clinical CR was observed in 61% patients from the NACT arm and 55% patients in no-NACT arm.Thirty-eight percent of patients had intact functioning bladders at five-years.In this trial CMV schedule was poorly tolerated,with only 67% patients completed the treatment and observed treatment-related deaths,and expected patient accrual was not reached[32].Jiang et al.[21]administered two cycles of NACT with GC followed by response assessment in 57 patients.In responsive patients,the same chemotherapy was continued till four cycles followed by CCRT.In patients with PD,chemotherapy was discontinued and they were considered for immediate RC.Majority(95%)of patients showed a good response and completed four cycles of NACT followed by CCRT(external beam RT to a total dose of 60-66 Gy with weekly cisplatin[40 mg/m2]).An overall response rate of 70% and 2-year DFS rate of 57.3% were observed in those who received NACT followed by CCRT.Current trial showed similar oncological outcomes with an overall response rate of 80% after three cycles of NACT and 3-year DFS of 50.6%.Unerring assessment of post-NACT response rate makes a paramount yardstick while planning bladder preservation,as poor responders to NACT had very unlikely to spare their bladders.However,our short duration of follow-up and small sample size limit the interpretation,and further studies with a large sample size are required to derive the benefit of NACT followed by organ preservation.
The acute toxicity profile in this study was comparable and similar to what has been observed in other GC chemotherapy based trials.von der Maase et al.[10]reported grade≥III anemia in 27%,grade≥III thrombocytopenia in 57%,grade≥III neutropenia in 71%,and grade≥III vomiting in 22% of patients treated using GC regimen.Present study showed lower hematological and nonhematological toxicity with grade≥II anemia seen in 25%of patients,grade-II neutropenia in 15% of patients,none with acute thrombocytopenia,and grade≥III vomiting in 5%of patients.
Another important concern with conservative bladdersparing therapy is field cancerization.This urothelial field cancerization effect increases the risk of subsequent new bladder tumors and superficial relapses within the retained bladder,which have a negative impact on LC.Among patients who achieved a CR to TMT,20%-30% will develop a recurrent tumor.The Massachusetts General Hospital study reported a 26% rate of superficial recurrence(Tis,Ta,and T1)among 121 complete responders after a median followup of 7 years[33].In current study,31.4%(11/35)of patients developed local relapses.
Late toxicities are those that occur 3 months after the completion of RT and could manifest as painless hematuria,chronic frequency,and in up to 11%of cases as a contracted bladder[33].An important QoL study after TURBT,chemotherapy,and RT for MIBC was reported by Zietman et al.[36].Reduced bladder compliance as a late complication of radiation was reported in 22% of patients and bowel symptoms occurred in 22% of patients.In present study,grade≥II frequency was observed in 16 patients at 3 months follow-up,and all of them responded well to conservative management.
Locoregional recurrences after RC have given less attention because few pelvic recurrences were reported in surgical literature.Most studies underestimated the localregional failures due to many reasons,i.e.,routine CT surveillance has a significant risk of false-negative reporting that results in under-detection of pelvic disease,and many studies did not report pelvic recurrences unless they were first and the only site of failure.Approximately 33% of pelvic recurrences have been reported with≥pT3 disease after RC,either as isolated local-regional recurrences or co-synchronous with distant metastasis[37,38].The National Cancer Institute in Egypt conducted a follow-up randomized trial on adjuvant chemotherapy alone versus sequential chemotherapy followed by RT in locally ABC after RC(≥pT3b,grade-III,and positive nodes).Two-year locoregional failure-free survival was 96% in sequential chemo-RT arm in comparison with 69% in chemo-alone arm(p<0.01)[39].In present study,five patients were treated with postoperative RT because of pT3,pT4a tumor,and margin positivity.
5.Limitations
This is a single institute prospective phase-II study with 40 patients.All patients were males and at relatively young age with median age of 62 years(range:50-74 years).Only node negative patients were included in this study,so the results are applicable to a specific patient cohort.Small sample size with short duration of follow-up restricts the interpretation of results and survival methods are vulnerable to bias.In addition,it’s difficult to compare treatment outcomes in between sub-groups.However,we could determine the response rates of NACT and survival outcomes in node negative MIBC patients because of lack of heterogeneity in the group,and further we have followed specific treatment protocol.
6.Conclusion
The present study evaluated the feasibility of NACT followed by RT for organ preservation in MIBC and resulted in bladder preservation in 50%(20/40)patients.We observed that organ preservation is a valid option for patients who attain CR to NACT and early-stage MIBC.Even though our results showed good survival outcomes and freedom from metastasis,multicenter studies with a larger sample size and longer follow-up are needed to draw clear conclusions.
Author contributions
Study design:Narendra Kumar,Chinna Babu Dracham,Santosh Kumar.
Data acquisition:Chinna Babu Dracham,Arun Elangovan.
Data analysis:Chinna Babu Dracham,Pramod K.Gupta.
Drafting of manuscript:Chinna Babu Dracham,Narendra Kumar,Ravimohan S.Mavuduru.
Critical revision of the manuscript:Narendra Kumar,Budhi Singh Yadav,Anupam Lal,Rakesh Kapoor.
Conflicts of interest
The authors declare no conflict of interest.
 Asian Journal of Urology2022年3期
Asian Journal of Urology2022年3期
- Asian Journal of Urology的其它文章
- Burned-out testicular seminoma with retroperitoneal metastasis and contralateral sertoli cell-only syndrome
- Endoscopic management of adolescent closed Cowper’s gland syringocele with holmium:YAG laser
- Transcutaneous dorsal penile nerve stimulation for the treatment of premature ejaculation:A novel technique
- Bilateral calcified Macroplastique? after 12 years
- Culture-positive urinary tract infection following micturating cystourethrogram in children
- Augmentation cystoplasty in children with stages III and IV chronic kidney disease secondary to neurogenic bladder
