Emerging role of caldesmon in cancer:A potential biomarker for colorectal cancer and other cancers
lNTRODUCTlON
The global cancer burden has increased to approximately 19.3 million cases and 10 million cancer deaths in 2020[1].Colorectal cancer (CRC) is the second most common prevalent cancer,with 5253335 cases,and the third most common cancer worldwide,with 1931590 new cases,in 2020[1,2].Almost half of the patients with CRC succumb to the disease[1,2].Cancer morbidity and mortality are essentially due to the ability of cancer cells to invade,metastasize,and destroy normal tissues.Cancer cells,which undergo this complex process,have the ability to survive in the hostile microenvironment,a process mediated by the accumulation of multiple genetic and epigenetic mutations and the activation of a multitude of signaling pathways fueled,generally,by a state of genetic instability[3].
Cancers of epithelial origin (carcinomas),such as those of the colon,shed away their adhesion molecules and acquire mesenchymal markers that enable invasion and metastasis in a process known as epithelial to mesenchymal transition (EMT)[4-6].The actin cytoskeleton is an important player in cell motility,division,and contractility among other cellular processes[7].Multiple actin-binding proteins (ABPs) control these functions of the actin cytoskeleton[8].ABPs form a growing family of more than 160 proteins that can bind actin monomers,polymers,or both[9].ABPs can be divided into two broad categories,depending on their effect on actin filament dynamics[10].The first category controls cytoskeletal responses to external stimuli by regulating G-actin/F-actin turnover.This category includes Arp2/3,ADF/cofilin,profilin,and gelsolin.The second category promotes the formation of higherorder structures,such as actin filament meshwork or bundles.This category includes tropomyosin,caldesmon (CaD),and filamin[10].
Since the cards were randomly4 seeded within the packs, it was easy to get duplicates of certain players while others remained elusive5. I must have had a half-dozen Ozzie Smiths, and probably ten Steve Bedrosians. But I was unsuccessful in getting a Ken6 Griffey, Jr., card. Griffey was far and away the most popular card among collectors that year.
CaD is encoded by the CALD1 gene in multiple isoforms (Figure 1,and Supplementary Figure 1).High-molecular-weight CaD (h-CaD; 120-150 kDa) is restricted to smooth muscle cells of visceral and vascular origin,and it has been used in diagnostic histopathology as a specific marker for tumors of smooth muscle or myofibroblast origin.The low-molecular weight CaD (l-CaD; 70-80 kDa) isoforms are expressed in nonsmooth muscle cells[11-13].CaD,particularly l-CaD,has emerged as a significant player during the development and progression of many types of cancers.For some cancers,such as urinary bladder cancer,glioma,and glioblastoma,the literature consistently suggests an oncogenic role of CaD.However,the available data for some other cancers,such as stomach and breast cancer,show contrasting effects of l-CaD.Therefore,we set out to clarify the role of CaD during carcinogenesis,with a focus on CRCs.We will highlight the role of CaD in cancer development and progression,resistance to various therapeutic modalities,and immune evasion.We will also discuss the role of CaD in EMT,modulation of the tumor microenvironment,and tumor-specific splicing.
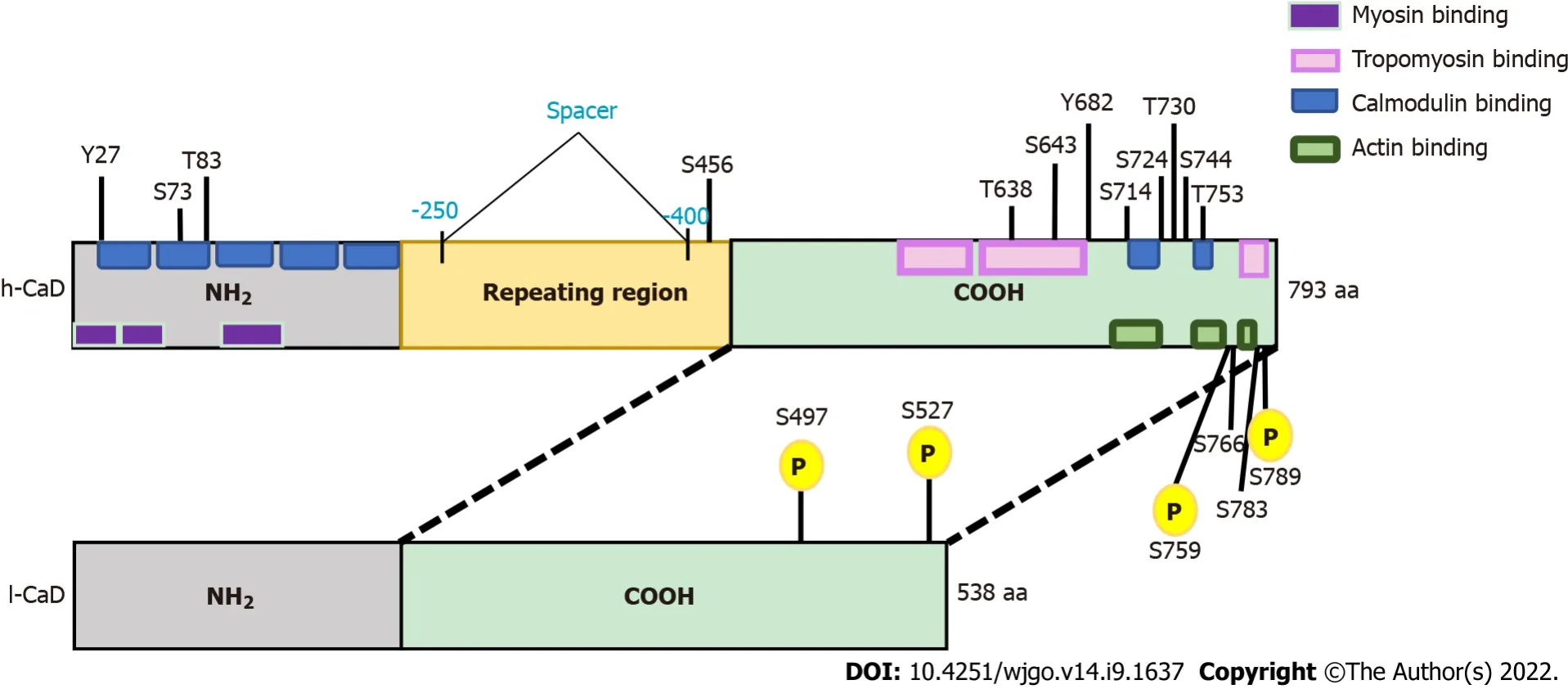
CAD AND THE ACTlN CYTOSKELETON
Cell motility,which is required for cancer cell invasion into surrounding tissue,intravasation,and metastasis,is driven by cycles of actin polymerization,cell adhesion,and acto-myosin contraction.The actomyosin system in smooth muscle cells is regulated by myosin-linked and actin-linked molecules.The myosin-linked mechanism is essentially based on myosin phosphorylation by Ca
/calmodulindependent myosin light chain kinase and dephosphorylation by a type 1 myosin phosphatase,which is targeted to myosin by a regulatory subunit[14].The actin-linked mechanisms are mediated
complex interactions among a growing family of ABPs; a more detailed discussion of these proteins and mechanisms can be found in specialized reviews[15-19].
CaD and tropomyosin are crucial components of the actin-linked mechanism that regulates the actomyosin contractile system in smooth muscle.CaD was initially identified as an inhibitory factor for the actin-myosin interaction,in which CaD-induced inhibition can be released by Ca
/calmodulin.Subsequently,CaD was found to play an important role in cell motility by regulating the contractile system in both smooth muscle and nonmuscle cells[12].CaD is conserved in almost all vertebrate cells and stabilizes actin filaments directly by binding along the sides of F-actin; it also enhances the binding of tropomyosin to actin[20].
H-CaD has been used as a diagnostic biomarker of vascular smooth muscles[21],mesenchymal[22-24],and smooth muscle neoplasms[25,26] and related conditions[27-29],while nonmuscle l-CaD is broadly implicated in many aspects of cell motility,including cell migration[30],focal adhesion assembly[31],and podosome dynamics[32].Ιn cultured and transfected cells,overexpression of the actin-binding domain,or full length,of l-CaD promotes cell movement and facilitates the formation of cytoplasmic processes,while cell contractility is inhibited and the number of focal adhesions is decreased[31].
THE EMERGlNG ROLE OF CAD lN CARClNOGENESlS
The clerk disappeared and came back a moment later with three robes in sturdy terry cloth. He chose blindly, hardly glancing down, taking the one on top. Three sizes, the clerk was saying, and a better selection of colors next month, but he was already in the aisle24, a coral-colored robe draped over his arm, his shoes squeaking28 on the tiles as he moved impatiently between the other shoppers to where she stood.
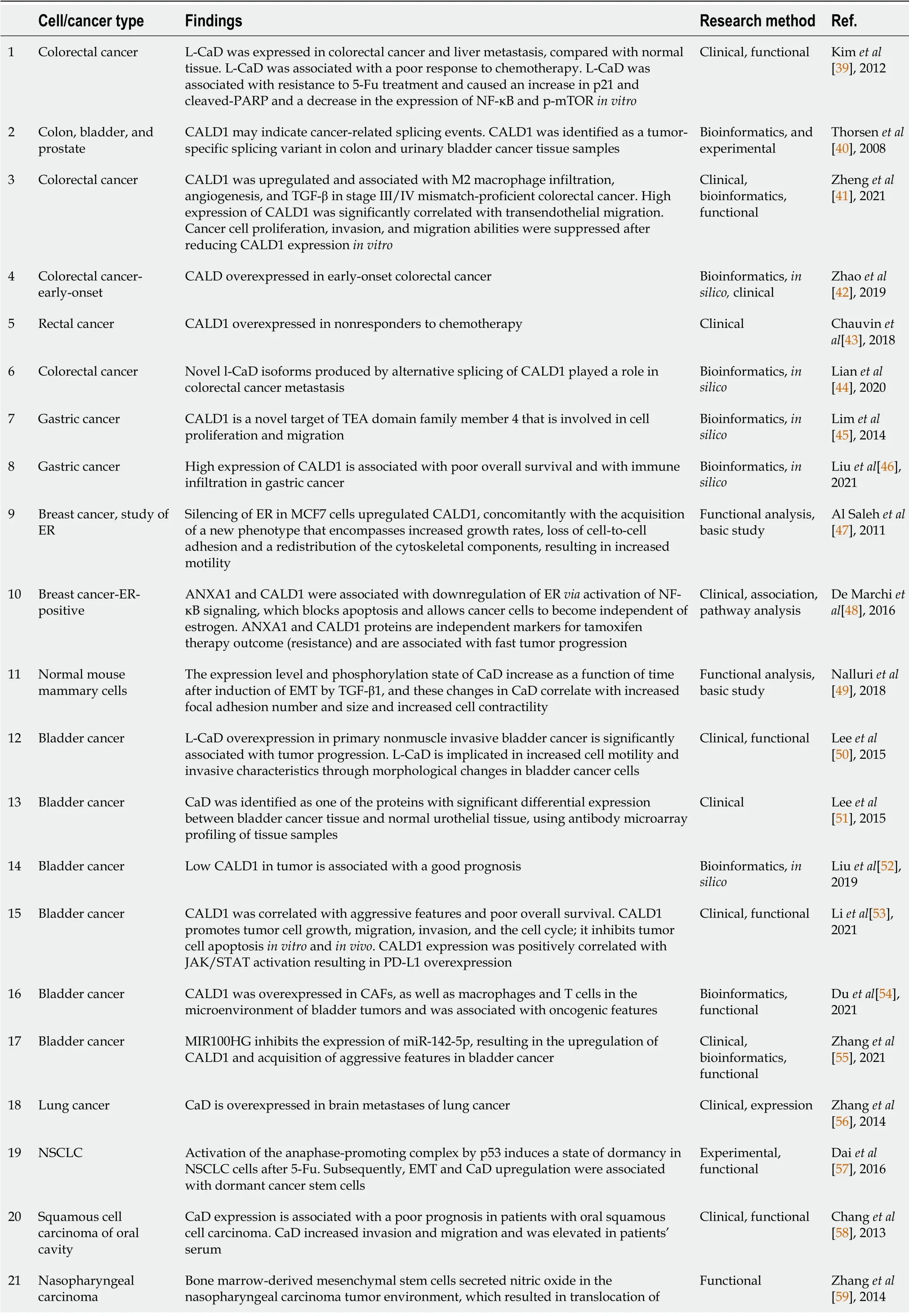
The role of CaD,particularly the light isoform (l-CaD),in solid tumors has been analyzed in various study types,including clinical,bioinformatics,and functional/experimental studies.A comprehensive summary of this literature is supplied in Tables 1 and 2.This summary does not include the classical use of CaD/h-CaD as a marker for smooth muscle and related tumors,which is not the focus of this review but can be found in other publications/reviews[25,26].The majority of the publications suggest an oncogenic role of CaD,particularly l-CaD,in many cancer types,such as breast cancer[47],urinary bladder carcinoma[50,51],oral cavity squamous cell carcinoma[58],and CRC[39],including early onset[42],gastric cancer[45],and lung cancer[56],and it was associated with a poor prognosis in bladder cancer in an
analysis[52] (Table 1).Moreover,the serum level of l-CaD was found to be high in glioma patients; hence,it is suggested to be a potential serum marker for glioma[61].Some of the aforementioned studies clearly indicated that the transcript studied or expressed was l-CaD,but others did not specify the transcript.Even in the last case,it is most likely that the transcript responsible for these actions is nonsmooth muscle l-CaD because h-CaD expression is most likely to be restricted to smooth muscles and their tumors.
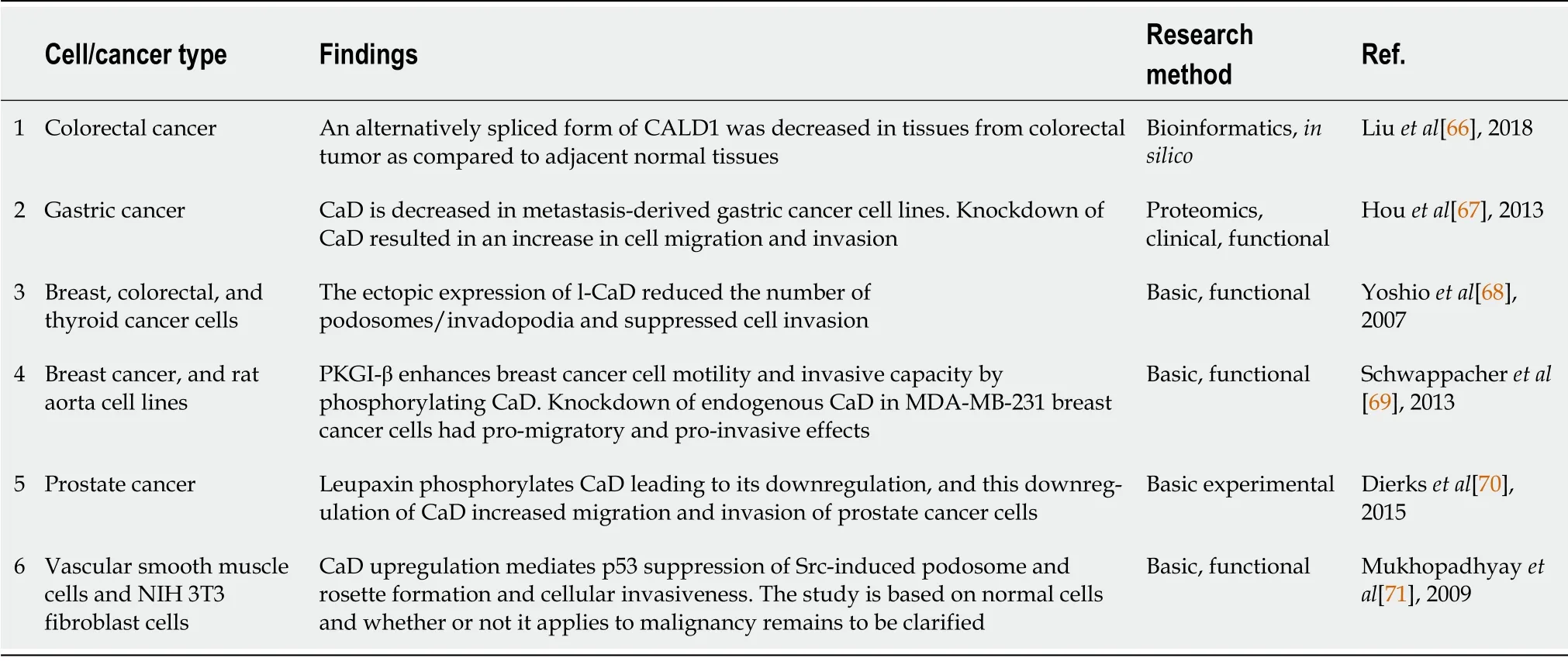
Ιn contrast,a smaller number of publications have reported contradictory results (Table 2).Following an earlier report that CaD is a cell motility suppressor[72],tumor suppressor functions were shown
using breast[68,69],colon[68],thyroid,and prostatic cancer cells[70],and CaD was suggested to be a metastasis suppressor in gastric cancer[67].Overall,the overwhelming majority of the recent literature supports the idea that l-CaD exerts multiple oncogenic potentials by upregulating tumor cell motility,angiogenesis,and cell division,as well as modulating the tumor microenvironment.Furthermore,l-CaD overexpression was associated with resistance to immunotherapy and chemotherapy and poor overall survival in multiple cancer types (Table 1).
CAD,TRANSFORMlNG GROWTH FACTOR-BETA SlGNALlNG,AND EMT
An early hint that CaD could play a role in CRC development came from the study in 2008 of alternative splicing in cancer by exon array analysis.Briefly,the identified tumor-specific CALD1 variant was missing an extended form of exons 5 and 6 and was predicted to encode proteins with potentially altered functions[40].This finding implied an oncogenic role of l-CaD in colon cancer,as discussed in more detail above (see “l(fā)-Caldesmon is a tumor-specific splice variant”).
He looked at me. I knew you were going to ask that, he said. I m going to be a dancer someday. He pointed to the administration building. My bosses are in there, and they re paying for my training.
TGF-β signaling is a potent inducer and one of the best-characterized EMT pathways.Although TGFβ potently promotes tumor progression
mechanisms that include activation of the EMT program and the resulting invasion of carcinoma cells into surrounding nonneoplastic tissue,it may negatively control the initial stages of tumor formation through its antiproliferative effects.However,some tumor cells solve this problem by inactivating other components of the pathway,such as SMADs[80,81],rather than TGF-β itself.The expression levels of cytoskeletal-associated proteins,including the actin binding protein CaD,increase during TGF-β1-induced EMT[64].CaD was shown to play a key role in TGF-βdriven EMT of normal murine mammary epithelial cells.Nalluri
[49] found that induction of EMT by TGF-β1 is mediated by increased expression together with increased levels of phosphorylated CaD,which was associated with increased focal adhesion number and size and increased cell contractility.CALD1 appears to play a major role in CRC
EMT induction because its expression is significantly and specifically upregulated in the consensus molecular subtype 4,which is characterized by TGF-β signaling activation together with other EMT phenotype indicators,such as invasion of the stroma by malignant cells and marked angiogenesis[82].Moreover,Calon
[83] showed that the poor prognosis of CRC is linked to TGF-β signaling in stromal cells that results in CALD1 overexpression.
CAD CONTRlBUTES TO TUMOR ANGlOGENESlS
The HeLa l-CaD Ι and ΙΙ splice variant and protein isoforms were initially cloned from HeLa S3 in 1992[11].L-CaD was found to be associated with actin filaments (stress fibers) and tropomyosin in quiescent cells,but l-CaD,tropomyosin and myosin were not seen at the focal adhesions end of these fibers[33].Endothelial cells (ECs) and endothelial progenitor cells (EPCs) are quiescent under normal conditions.However,these cells are activated in tumors under hypoxia and other environmental stimuli to start to proliferate and migrate in the process of angiogenesis.Upon activation of ECs/EPCs,changes in focal adhesions occur,and simultaneous remodeling of F-actin causes changes in cell shapes[84].These events enable the navigation of EC tips during angiogenesis and the recruitment of circulating EPCs from bone marrow to the site of neoangiogenesis.The HeLa l-CaD-containing cell protrusions were found to be specific for tumor ECs/EPCs and have never been observed in normal ECs[63].Consistent with this finding of podosomes in ECs[85],Zheng
[63] found a variety of motility-related cell protrusions,such as filopodia,microspikes,lamellipodia,podosomes,membrane blebs and membrane ruffles,in the activated ECs/EPCs of various human tumors under a histologically preserved microenvironment.HeLa l-CaD appeared to be invariably expressed in the subregions of these cell protrusions.Furthermore,HeLa l-CaD-positive multinucleated ECs/EPCs were observed in the glioma samples,among other tumor samples.These cells appeared to be highly motile because they were ubiquitously distributed in the tumor tissue sections[63].Multinucleation is considered to be a sign of aborted cytokinesis and is associated with the activation of aortic EC motility and podosome formation[85,86].
52.Thanked her:To emphasize her goodness once again, Perrault makes sure to have Cinderella thank her fairy godmother for help. This also allows Cinderella the opportunity to wish for help in attending the next ball.Return to place in story.
CALD1 has been linked to JAK/STAT activation and PD-L1 overexpression[53].The role of CALD1 in promoting bladder cancer progression by remodeling the tumor microenvironment was supported by the recent finding of CALD1 expression in CAFs as well as macrophages and T cells in the bladder tumor microenvironment[54].Finally,noncoding RNA regulation of CALD1 was studied in bladder cancer and was found to occur
MΙR100HG,which can promote the proliferation,migration and invasion of bladder cancer cells.MΙR100HG inhibits the expression of miR-142-5p,which targets CALD1,thus relieving CALD1 from this inhibitory effect.Consequently,upregulated CALD1 results in the induction of aggressive features in bladder cancer cells[55].
The expression of HeLa l-CaD was restricted to the tumor vasculature and was not found in normal blood vessels of cancers derived from various organs,including breast,lung,kidney,colon,stomach,ovary,uterus,prostate,thyroid,and liver[87].HeLa l-CaD was preferentially expressed in the early stage of tumor neovascularization.The available data suggest that HeLa l-CaD can be considered a marker for angiogenic ECs during the early stages of tumor neovascularization[87].Taken together,these findings suggest that HeLa l-CaD is implicated in the migration of ECs/EPCs in human neoplasms,where they contribute to tumor angiogenesis[63].
A recent study of the mechanisms underlying the effect of l-CaD on microvascular facilitation and architecture in glioma showed that l-CaD is associated with abnormal microvessels in anaplastic astrocytoma and glioblastoma (an aggressive grade ΙV astrocytoma)[60].The mechanism of such action was suggested by biofunction prediction to occur by modulating tumor angiogenesis,as ECs and pericytes were more apparent in the tumor microenvironment of high CALD1 expression samples.Histological and immunofluorescence examination of tumor tissue showed that CaD was associated with vessel architecture in astrocytoma and glioblastoma[60-63].Ιn stage ΙΙΙ/ΙV mismatch-proficient CRC,CALD1 was upregulated and associated with angiogenesis,as detected by bioinformatics ‘Weighted gene coexpression network analysis’ (WGCNA)[41].
L-CAD lS A TUMOR-SPEClFlC SPLlCE VARlANT
Alternative splicing is an attractive mechanism of mutation acquisition by cancer cells,as it has the potential to expand a limited number of genes into very complex proteomes and endow them with altered functions,localization,binding properties,and stability[66,88-90].The CALD1 gene undergoes alternative splicing in cancer tissues,including colon,urinary bladder,and prostate tissues,and these variants are mostly tumor specific.Thorsen
[40] found that the long CALD1 isoform,including an extended form of exons 5 and 6,was absent or reduced in bladder,colon,and metastatic prostate cancer.The dominant splice variant in these tumors is most likely to be transcript variant 2 encoded by WΙ-38 L-CADΙΙ[44].Other cytoskeleton-associated proteins,such as Tropomyosin 1,ACTN1,and vinculin,were identified as significant candidates for alternative splicing in these tumors in the same study[40],supporting the role and importance of actin cytoskeleton modification in tumor progression[34,35,91].Ιt is known that splice variants can exert antagonistic functions in tumors,such as the well-known case of the B cell lymphoma (BCL)-X long isoform (BCL-X
),which has an antiapoptotic function,and its short isoform BCL-X
,which is proapoptotic[92].Ιndeed,the identified cancer-specific splice variants of CALD1 are predicted to encode proteins with potentially altered functions[40].Thus,the finding of CALD1 tumor-specific splice variants can explain the reported contrasting effects of the two isoforms,h-CaD and l-CaD,and could explain the oncogenic role of l-CaD in many types of cancers.
The splice variant identified by Thorsen
[40] was confirmed to be tumor specific and associated with metastatic disease and poor overall survival in CRC[44].Abnormal splicing was associated with upregulation of l-CaD in glioma tumor tissue samples and body fluids[61,62,93].Cancer-specific splice variants may potentially be used as diagnostic,prognostic,and predictive biomarkers of various tumors.Moreover,the specificity of these isoforms to cancer cells compared with normal cells makes CaD an ideal selective therapeutic target in cancers[94].
CaD has emerged as an attractive molecule that potentially controls significant steps in tumor formation,cell division,invasion,metastasis,and response to therapy.Early work has shown that the expression and distribution of CaD are different in normal fibroblasts and their transformed counterparts[32].Ιn normal fibroblasts,myosin,CaD,and tropomyosin were distributed along the stress fibers as expected but were not seen at their termini known as ‘focal adhesions/adhesion plaques’[33].Ιn contrast,these contractile proteins were concentrated within ‘podosomes’,which are cell-adhesive structures located within the protrusions of the ventral cell surface of transformed cells and are associated with high motility.Podosomes have previously been shown to have short F-actin bundles[34,35],together with actin-associated regulatory proteins,such as fimbrin[36] and gelsolin[37].Ιn transformed cells,CaD appears to play a major role in podosome structure and function due to its localization mainly in the podosome core domain with short F-actin bundles,in contrast to myosin and tropomyosin.Thus,CaD was associated with high motility of the podosomes of transformed cells,while the stable adherence of focal adhesions of normal cells was suggested to be due to the lack of this system[33].The significance of these findings stems from observations of the podosomes of transformed cells being most dynamic adhesive structures with high motility (short half-life),leading to metastasis and invasion,while the focal adhesions of normal cells were not capable of performing these functions[38].
When they arrived at the bush with red berries, there stood the reindeer13 waiting for them, and he had brought another young reindeer with him, whose udders were full, and the children drank her warm milk and kissed her on the mouth
CAD AND RESlSTANCE TO THERAPY
CaD was implicated in resistance to multiple modalities of cancer therapy,including chemotherapy,radiotherapy,hormonal therapy and immunotherapy (Figure 2).
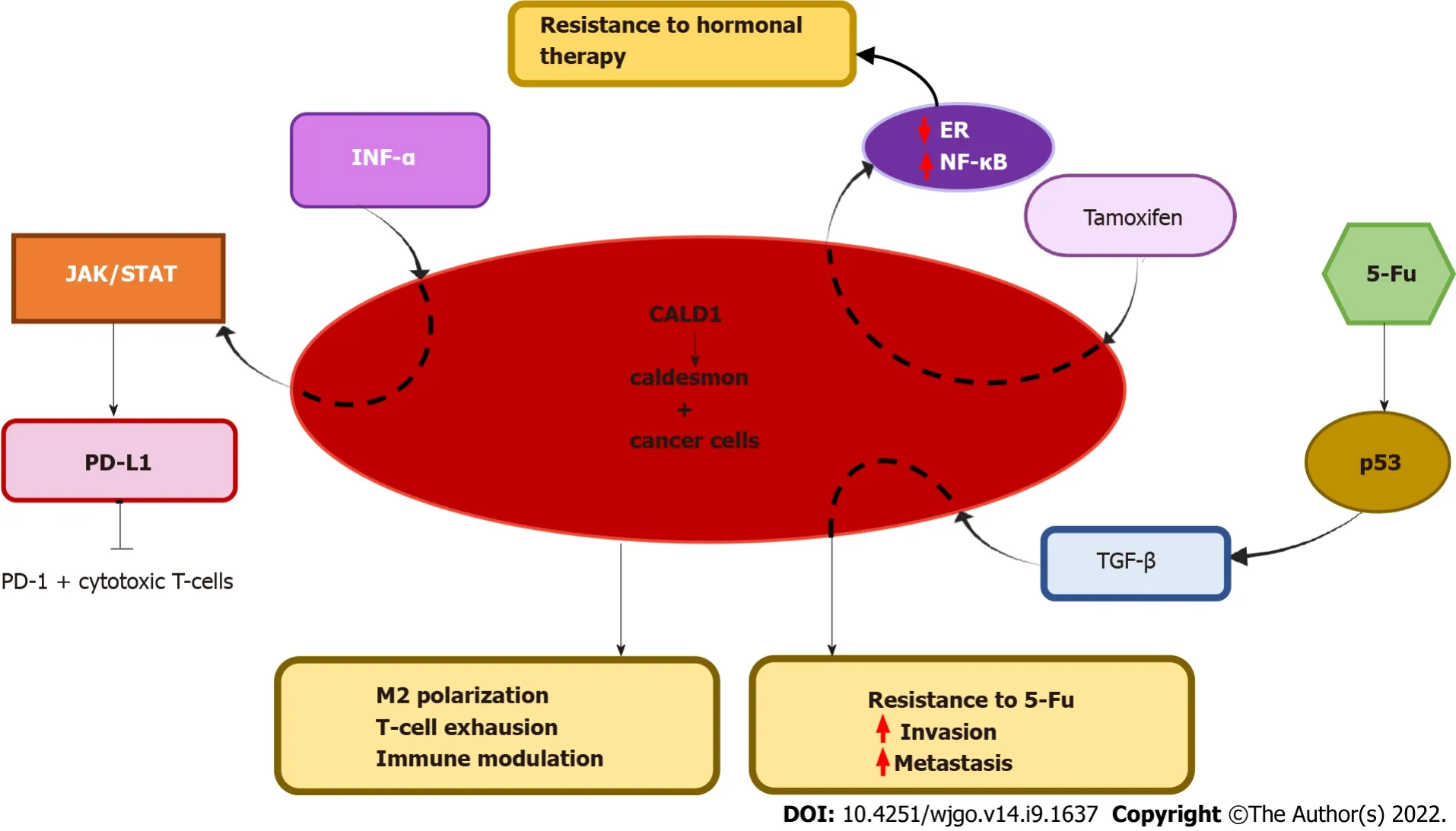
CaD and resistance to chemotherapy
Based on the proteomic finding of aberrant expression of CaD isoforms in colon cancer,Kim
[39] set out to analyze the particular role of the short isoform l-CaD in CRC and liver metastasis.They observed a significantly higher expression level of l-CaD in primary colon cancer and liver metastasis than in the corresponding normal tissues.However,h-CaD did not differ among these groups.There was a tendency to have a poor response to chemoradiotherapy in patients with high expression of l-CaD in their tumors,which was confirmed
by small interfering RNA (siRNA) silencing of l-CaD and monitoring the response to 5-Fu treatment in colon cancer cell lines[43].L-CaD was suggested to exert these effects by relieving the cell cycle inhibition exerted by p21
(cyclin-dependent kinase inhibitor 1,or CDK-interacting protein 1) and blocking apoptosis.Furthermore,silencing l-CaD downregulated NFκB[39],an important signaling pathway that can stimulate tumor cell proliferation,survival,and angiogenesis by controlling a wide network of genes and molecules,such as tumor necrosis factor-α,interleukin-6,BCL2,and vascular endothelial growth factor[99].Silencing l-CaD also downregulated phosphorylated mammalian target of rapamycin[39],a pathway that regulates not only tumor cell proliferation but also the tumor immune response and metabolism[100].Collectively,Kim
[39] showed that high expression of l-CaD in CRC is associated with increased metastatic properties and a decreased response to therapy.
CaD and antihormonal therapy
CaD was associated with resistance to the targeted antihormonal drug tamoxifen in estrogen receptor (ER)-positive recurrent breast cancer[48].This study was based on a proteomic analysis to identify a predictive signature for tamoxifen therapy outcomes in recurrent breast cancer.CALD1 and annexin-A1 (ANXA) were the most differentially expressed proteins and were confirmed by immunohistochemical staining of an independent set of tumors.CALD1 expression showed a significant association with a shorter time to progression,independent of other clinicopathological predictive factors.The majority of proteins that were correlated with ANXA1 were also correlated with CALD1,but a direct link between the two genes (CALD1 and ANXA1) and the mechanism underlying the association have yet to be clarified.CALD1,in particular,was associated with ER downregulation and nuclear factor-kappa B (NF-κB) signaling[48].
CaD and immunotherapy
CALD1 was among the top genes associated with both overall survival and disease-free survival in bladder cancer according to bioinformatics analysis.Tumors with low levels of CALD1 expression had a better prognosis than tumors with high CLAD1 expression[52].This finding was confirmed in a recent study,and the mechanism was linked to immunomodulation
upregulation of programmed death ligand 1 (PD-L1) in bladder cancer[53].PD-L1 has the potential to suppress the immune response in both physiological and pathological pathways by interacting with its corresponding receptor,PD-1[96,97].PD-L1 expressed by tumor cells binds to PD-1 on the cytotoxic T-cell surface and thus attenuates immunosurveillance in the tumor microenvironment.Li
[53] found that PD-L1 is associated with CALD1 in bladder cancer cells and that both are induced by interferon-gamma
.CALD1 silencing significantly reduced cell viability in T24 bladder cancer cells
and
in nude mouse xenografts.The authors suggested that CALD1 promoted the expression of PD-L1
the Janus kinase/signal transducers and activators of transcription (JAK/STAT) signaling pathway[53].Ιt is likely that the CALD1 effect on PD-L1 is active in other cancers,such as colon cancer,and can exert immunomodulation through this axis because PD-L1 expression is also upregulated
JAK/STAT3 after fibroblast growth factor receptor 2 stimulation in CRC[98].
A recent bioinformatics-based report showed that CALD1 was highly expressed in gastric cancer compared with adjacent normal tissue and that this high expression was associated with poor overall survival in these patients.There was a strong correlation between CALD1 expression and gene markers of M2 macrophages (CD163,VSΙG4,membrane-spanning 4A) and Treg and T-cell exhaustion markers (FOXP3,CCR8,STATA5B,TGF-β1,T cell immunoglobulin and mucin domain 3) in gastric cancer.These findings suggest that CALD1 plays an important role in M2 polarization,T-cell exhaustion,and immune modulation in gastric cancer[46].
CAD AND CRC
The available data show that CaD plays an important role in the development,progression and response to therapy of CRC,as detailed below (Figure 3).
My first gift arrived at the moment the first school bell rang last August, when I placed in you my trust, believing you would teach my child and reserve respect me as a parent
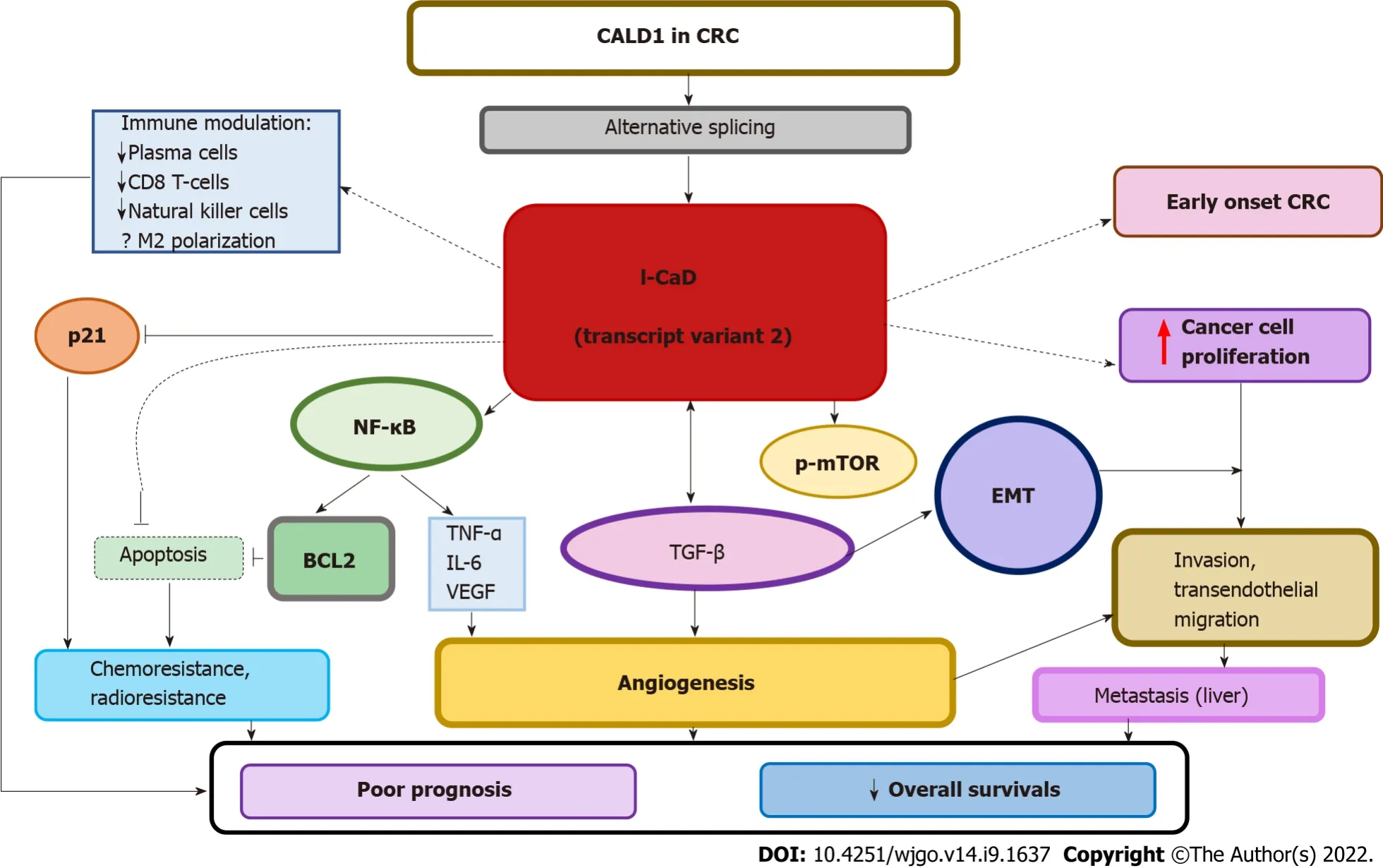
CaD contributes to CRC development
Cancer cells activate EMT to move and migrate from the primary tumor to other parts of the body.EMT is an essential process of cellular plasticity for normal tissue and organ development,yet it is also involved in an array of oncogenic processes,including proliferation and invasion,angiogenesis,stemness,and resistance to chemoradiotherapy[73,74].The process involves major changes in the phenotype of cancer cells within the primary tumor marked by loss of an epithelial phenotype and gain of a mesenchymal phenotype.EMT is the first of many steps leading to metastasis.Different factors are involved in activating EMT,such as environmental factors,signaling molecules,and transcription factors.EMT is tightly controlled in normal tissues by maintaining a balance between EMT transcription factors,while in cancer,the process is much more complicated.Once the primary cancer is formed,different triggers stimulate the movement of tumor cells for nourishment,exchange of nutrients and/or immune escape.These factors,such as hypoxia,oxidative stress,nutrient deprivation,and inflammation,activate a set of transcription factors,including transforming growth factor-beta (TGF-β),Wnt,SNAΙL,TWΙST and MAPK/ERK-ZEB1,among others[73-77].All of these signaling pathways participate in crosstalk with each other and share interconnected regulatory components,which together with their targets form a complex network[78].Comprehensive transcriptomic analysis of a large cohort have shown that EMT is the most dominant program in CRC[79].
Ιt has long been shown that the F-actin associated with transformed cells is different from that of normal cells not only in morphology and function but also in its insensitivity to drugs[95].The association between CaD and resistance to various forms of cancer therapy has been documented in many cancer types.Dai
[57] showed that non-small-cell lung cancer (NSCLC) cells enter a state of dormancy upon exposure to 5-fluorouracil (5-Fu) and subsequently acquire resistance to this therapy.The mechanism of this resistance involves the accumulation of p53,activation of the ubiquitin ligase anaphase-promoting complex and TGF-β/SMAD signaling leading to EMT,followed by mesenchymal-epithelial transition.Chemotherapy-induced EMT-transformed NSCLC cells showed higher expression of CaD associated with increased invasion potential; however,these EMT-transformed NSCLC cells were arrested in the cell cycle in G0-G1 and lost their ability to divide during this phase[57].The role of CaD in resistance to 5-Fu was documented in locally advanced rectal cancer patients[43].
Grampy held my hand tightly. Together we looked up the street and down, and back up again. He stepped off the curb and told me it was safe to cross. He let go of my hand and I ran. I ran faster than I had ever run before. The street seemed wide. I wondered if I would make it to the other side. Reaching the other side, I turned to find Grampy. There he was, standing14 exactly where I had left him, smiling proudly. I waved.
A recent study confirmed that l-CaD transcript 2 is the dominant transcript and is associated with metastatic disease and poor overall survival in CRC[44].Ιnterestingly,CALD1 was among the top upregulated genes implicated in the development of early-onset CRC based on a comprehensive bioinformatics analysis[42].This finding may shed light on the pathogenesis of early-onset CRC,which is a heterogeneous category of CRCs that is more common in Eastern than in Western countries[101,102].The association of CALD1 together with other genes involved in cellular mobility and vascular smooth muscle contraction with early-onset CRC can explain the aggressive nature of this subset of tumors[42].
A recent study by Zheng
[41] utilized a new bioinformatics tool,WGCNA,to clarify the basis of the poor response to immunotherapy in mismatch-proficient,stage ΙΙΙ/ΙV CRC and showed that CALD1 was upregulated and associated with protumorigenic M2 macrophage infiltration.M2 macrophages are believed to be an important contributor to the failure of immunotherapy due to their anti-inflammatory,immunosuppressive,and proangiogenic characteristics[103].CALD1 was negatively correlated with fractions of plasma cells,CD8 T cells,CD4 memory-activated natural killer cells,and dendritic cells[41].High expression of CALD1 was significantly correlated with angiogenesis,TGF-β,and trans-endothelial migration.Taken together,these data are consistent with the published literature on the importance of the crosstalk between angiogenesis and TGF-β in macrophage recruitment and M2 polarization[104,105],but the role of CALD1 in this scenario remains to be clarified.Cancer cell proliferation,invasion,and migration abilities were suppressed after reducing CALD1 expression
siRNA silencing
[41].
Only one article suggested that ectopic expression of CaD in a panel of cell lines of various lineages,including the HCA7 CRC cell line,reduced the number of podosomes/invadopodia and suppressed cell invasion,but no further functional analysis or clinical correlation was presented.The vector used,pcDNA3.1(+)-HA-CaD,was supposed to contain l-CaD[68].However,the cell line used,HCA7,is an atypical CRC cell line with an unusual cytogenetic profile and other characteristics[106,107].Overall,the available literature suggests that l-CaD,particularly splice variant 2,is a CRC splice variant that exerts protumorigenic characteristics and is associated with angiogenesis,invasion,metastasis,immune evasion,and poor prognosis in CRC.
CaD as a prognostic biomarker of CRC
As discussed above,Kim
[39] showed that colon cancer patients with high expression of l-CaD in their tumors had a poor response to chemoradiotherapy.L-CaD could exert these effects by inhibiting p21
and blocking apoptosis[39].Lian
[44] showed that l-CaD was associated with metastatic disease and poor overall survival in CRC.The WGCNA-based study of Zheng
[41] showed that CALD1 was significantly associated with a worse prognosis in mismatch proficient,stage ΙΙΙ/ΙV CRC.However,chemotherapy and tumor stage remained significantly correlated with overall survival.Both CALD1 and tumor stage were independent prognostic predictors in the GSE41258 validation dataset used in that study.
Calon
[83] performed a comprehensive bioinformatics analysis to clarify the characteristics of the poor-prognosis subtypes of CRC in three common classification systems.Although these three classification systems were based on distinct global gene expression profiles in independent cohorts of CRC and differed regarding the number of the identified tumor subtypes[108-110],they all concluded that poor patient outcome in CRC is associated with the expression of stem cell and mesenchymal genes[111].Calon
[83] found that among the poor-prognosis gene sets common to at least two of the three molecular classifications,31% (including CALD1) stained solely the tumor stroma,and 62% stained both stromal and tumor cells in the Human Protein Atlas Dataset[112].Ιntriguingly,CALD1 mRNA and protein expression were upregulated in cancer-associated fibroblasts (CAFs) and other stromal cell populations in contrast to epithelial tumor cells[83].However,CaD was identified in pure colon cancer parenchymal tissue from cell lines (containing no stroma),and both l-CaD and h-CaD were observed by western blot or transcriptomics analysis of colorectal carcinoma cells in other studies[39,41,113].Moreover,the functional consequences of l-CaD silencing were shown to impact the mobility,response to therapy and signaling pathways in colorectal carcinoma cells in these studies[39,41].Ιnterestingly,Calon
[83] showed that the poor prognosis of CRC is linked to TGF-β signaling in stromal cells that results in CALD1 overexpression,providing evidence linking CALD1 to TGF-β signaling in the tumor stroma.
Jensen
[113] reported that the CALD1 gene was upregulated in the transcriptome of more than one CRC cell line (HT29,LoVo) that acquired resistance to SN38 (a potent irinotecan metabolite).Moreover,proteomic analysis of locally advanced,nonmetastatic CRCs treated with neoadjuvant chemoradiotherapy,including 5-Fu,showed that CALD1 was among the top genes overexpressed in nonresponders[43].Ιn this study,the authors verified the mRNA expression of CALD1,as well as the presence of gene sequence variants,in the CRC cell line set of the ‘Colorectal Cancer Atlas’ available from http://colonatlas.org/index.html.
ROLE OF CAD lN OTHER CANCER TYPES
Gastric cancer
Bioinformatics analysis suggested that CALD1 is a novel target of the TEA domain family member 4 gene that mediates gastric cancer development by stimulating cell proliferation and migration[45].Another bioinformatics-based analysis showed that high expression of CALD1 is associated with poor overall survival and with immune infiltration in gastric cancer[46].Conversely,Hou
[67] showed that CaD expression was decreased in metastasis-derived gastric cancer cell lines as well as in resected biopsies of metastatic gastric cancer to lymph nodes compared with the primary tumors.Functional analysis showed that knockdown of CALD1 using siRNA in these cells resulted in an increase in cell migration and invasion.The first two studies[45,46],suggesting an oncogenic role of CaD in gastric cancer,were based upon bioinformatics analysis of a large series of gastric cancer,yet they did not supply a functional analysis of CALD1 action,while Hou
’s study focused on metastatic gastric cancer[67].Thus,controversy remains,and further work is needed to clarify the role of CaD in gastric cancer.
Breast cancer
Two independent studies have shown an inverse relationship between ER and CaD.Ιn the first study,silencing of ER in an ER-positive breast cancer cell line upregulated CALD1,concomitantly with the acquisition of more aggressive oncogenic features,including increased growth rates,loss of cell-to-cell adhesion and increased motility[47].The second study was based on clinical breast cancer samples and aimed to identify predictive markers of tamoxifen resistance in recurrent breast cancer.ANXA1 and CALD1 were the most differentially expressed proteins,and they were associated with the downregulation of ER
activation of NF-κB signaling,which blocks apoptosis and causes cancer cells to become estrogen-independent[48].Another study suggested that CaD can exert its carcinogenic effects in mouse mammary cells
EMT induction.The expression level and phosphorylation state of CaD increased as a function of time after induction of EMT by TGF-β1,and these changes in CaD correlated with an increased focal adhesion number and increased cell contractility[49].
Ιn contrast,two publications showed the tumor suppressive functions of CaD.Ιn the first,ectopic expression of l-CaD reduced the number of podosomes/invadopodia and suppressed cell invasion in breast cancer cells[68].The second showed that CGMP-dependent protein kinase Ι enhanced breast cancer cell motility and invasive capacity by phosphorylating CaD and that knockdown of endogenous CaD in MDA-MB-231 breast cancer cells exerted promigratory and proinvasive effects[69].Thus,more work is needed to clarify the role of CaD in various molecular subtypes of breast cancer as well as in large cohorts of clinical samples.
When she was led to the stake, she laid the shirts on her arm, and as she stood on the pile and the fire was about to be lighted, she looked around her and saw six swans flying through the air. Then she knew that her release was at hand and her heart danced for joy. The swans fluttered round her, and hovered low so that she could throw the shirts over them. When they had touched them the swan-skins fell off, and her brothers stood before her living, well and beautiful. Only the youngest had a swan s wing instead of his left arm.52 They embraced and kissed each other, and the Queen went to the King, who was standing by in great astonishment, and began to speak to him, saying, Dearest husband, now I can speak and tell you openly that I am innocent and have been falsely accused.
Bladder cancer
The role of CaD in bladder cancer has been comprehensively studied,and the published literature consistently supports an oncogenic role of CaD in bladder cancer,as shown in Table 1.CaD is significantly overexpressed in bladder cancer tissue compared with normal urothelial tissue[51].L-CaD is overexpressed in primary nonmuscle invasive bladder cancer and is significantly associated with tumor progression.Functional studies have shown that l-CaD mediates morphological changes associated with increased cell motility and invasive characteristics in bladder cancer cells and can inhibit apoptosis
and
[50,53].CALD1 was significantly correlated with histological grade,stage,and lymphatic metastasis of bladder cancer in the Cancer Genome Atlas (TCGA) and Gene Expression Omnibus databases[53].High CALD1 expression was associated with a poor prognosis[52],including poor overall survival[53].
Only an old baby doll, who had been loved almost to pieces, looked like a possibility for their Baby Jesus. “Chatty Baby,” she had once been called, before she stopped chatting forever after too many baths.
Glioma
CALD1 expression was associated with a high pathological grade and poor clinical outcome in a bioinformatics analysis of glioma samples from the TCGA and Chinese Glioma Genome Atlas databases.“Biofunction prediction” suggested that CALD1 modulated tumor angiogenesis in these tumors[60].Single-cell RNA sequencing (scRNA-seq),a technique that can define cellular states within both normal and disease tissues,including the immune phenotypes in the tumor microenvironment[114],showed that CALD1 was upregulated in neoplastic cells and was involved in the tumorigenic processes of gliomas.Dysfunctional l-CaD also led to a decline in cell mobility in glioblastoma cells[60].L-CaD is abnormally spliced in glioma vasculature,and the resultant altered expression of the protein isoforms in ECs/EPCs plays a role in the neoangiogenesis of various human tumor types[62].Finally,the serum level of l-CaD was elevated in glioma patients,and this elevation was significantly higher than the l-CaD serum levels in other brain tumor patients[61].
CONCLUSlON
Traditionally,scientific interest in CaD has been focused on its application in diagnostic histopathology to diagnose smooth muscle and related tumors using h-CaD or “total CaD” antibodies.However,the nonsmooth muscle isoform l-CaD has recently attracted much interest for its variable actions during carcinogenesis.Ιn contrast to the initial expectation,based upon its role in inhibiting actin-myosin interaction and smooth muscle contraction,a growing list of studies are showing pro-oncogenic roles in various cancers.Some controversy remains,as a few studies suggested that CaD can exert a tumor suppressor role that needs to be clarified,together with the detailed mechanism of action of CaD in cancer cells of various lineages.The availability of new technologies for the study of ABP biology and functions could assist in these tasks[115-117].Our comprehensive analysis of the available publications to date showed that CaD,particularly l-CaD,plays an important role in the development,metastasis,and resistance to chemotherapy in CRCs and other cancer types.Furthermore,CaD is implicated in angiogenesis and immune evasion in specific types of cancers,such as those of the urinary bladder.Ιt is highly likely that the role of CALD1 in immune modulation in bladder cancer could be a general mechanism that is applicable to CRC and many other tumors.Few publications have focused on the analysis of the localization of CaD in the stroma and the role it plays in various components of the tumor microenvironment,which is an important research priority.Ιnterestingly,CaD undergoes selective tumor-specific splicing,and the resulting isoforms are not generally expressed in normal tissues.These data qualify CaD as a potential candidate for targeted therapy in addition to its role in diagnosis and prognosis.
Far from avoiding her, he now sought her company and seemed to take pleasure in talking to her, and yet the Princess did not for a moment flatter herself with the idea that he was in love with her, though it did not take her long to decide that he certainly loved someone
When the King heard the news that his daughter had actually laughed, he was more than delighted, and had Peter and his marvellous train brought before him
Alnuaimi AR performed the literature search; Abdel-Rahman WM proposed and designed the review,obtained funds,performed the literature search; Nair VA,Malhab LJB,Hamad M,Hamoudi R,Abu-Gharbieh E,Ranade A,Pintus G,Kirfel J,and Busch H edited the manuscript; Alnuaimi AR and Abdel-Rahman WM collected the data,and wrote parts of the manuscript; Alnuaimi AR,Abdel-Rahman WM,Nair VA,Malhab LJB,Hamad M,Hamoudi R,Abu-Gharbieh E,Ranade AV,Pintus G,Kirfel J,and Busch H contributed to the data analysis; and all authors revised/endorsed the final draft.
All the authors report no relevant conflicts of interest for this article.
This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.Ιt is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is noncommercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
And the Goblin put the tongue on the coffee-mill, and how it began to grind3! He put it on the butter-cask, and on the till, and all were of the same opinion as the waste-paper tub
United Arab Emirates
Alya R Alnuaimi 0000-0002-5604-5377; Vidhya A Nair 0000-0002-5407-0130; Lara J Bou Malhab 0000-0001-7436-346X; Eman Abu-Gharbieh 0000-0002-5972-0681; Anu Vinod Ranade 0000-0002-7663-8532; Gianfranco Pintus 0000-0002-3031-7733; Mohamad Hamad 0000-0003-2874-2834; Hauke Busch 0000-0003-4763-4521; Jutta Kirfel 0000-0003-2740-7700; Rifat Hamoudi 0000-0002-1402-0868; Wael M Abdel-Rahman 0000-0002-2149-1043.
Wang JJ
A
Wang JJ
1 UICC.GLOBOCAN 2020: New Global Cancer Data 2020.Dec 17,2020.[cited 24 December 2021].Available from: https://www.uicc.org/news/globocan-2020-new-global-cancer-data
2 Bray F,Ferlay J,Soerjomataram I,Siegel RL,Torre LA,Jemal A.Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries.
2018; 68: 394-424 [PMID: 30207593 DOI: 10.3322/caac.21492]
3 Abdel-Rahman WM.Genomic instability and carcinogenesis: an update.
2008; 9: 535-541 [PMID: 19516960 DOI: 10.2174/138920208786847926]
4 Nair VA,Al-Khayyal NA,Sivaperumal S,Abdel-Rahman WM.Calponin 3 promotes invasion and drug resistance of colon cancer cells.
2019; 11: 971-982 [PMID: 31798778 DOI: 10.4251/wjgo.v11.i11.971]
5 Abdel-Rahman WM,Al-Khayyal NA,Nair VA,Aravind SR,Saber-Ayad M.Role of AXL in invasion and drug resistance of colon and breast cancer cells and its association with p53 alterations.
2017; 23: 3440-3448 [PMID: 28596680 DOI: 10.3748/wjg.v23.i19.3440]
6 Alam F,Mezhal F,El Hasasna H,Nair VA,Aravind SR,Saber Ayad M,El-Serafi A,Abdel-Rahman WM.The role of p53-microRNA 200-Moesin axis in invasion and drug resistance of breast cancer cells.
2017; 39: 1010428317714634 [PMID: 28933253 DOI: 10.1177/1010428317714634]
7 Pollard TD,Goldman RD.Overview of the Cytoskeleton from an Evolutionary Perspective.
2018; 10 [PMID: 29967009 DOI: 10.1101/cshperspect.a030288]
8 Lehman W,Maéda Y.Introducing a special issue of the Journal of Muscle Research and Cell Motility on actin and actinbinding proteins.
2020; 41: 1-2 [PMID: 31865487 DOI: 10.1007/s10974-019-09569-z]
9 Winder SJ,Ayscough KR.Actin-binding proteins.
2005; 118: 651-654 [PMID: 15701920 DOI: 10.1242/jcs.01670]
10 Stehn JR,Schevzov G,O’Neill GM,Gunning PW.Specialisation of the tropomyosin composition of actin filaments provides new potential targets for chemotherapy.
2006; 6: 245-256 [PMID: 16712460 DOI: 10.2174/156800906776842948]
11 Hayashi K,Yano H,Hashida T,Takeuchi R,Takeda O,Asada K,Takahashi E,Kato I,Sobue K.Genomic structure of the human caldesmon gene.
1992; 89: 12122-12126 [PMID: 1465449 DOI: 10.1073/pnas.89.24.12122]
12 Sobue K,Sellers JR.Caldesmon,a novel regulatory protein in smooth muscle and nonmuscle actomyosin systems.
1991; 266: 12115-12118 [PMID: 2061300]
13 Ueki N,Sobue K,Kanda K,Hada T,Higashino K.Expression of high and low molecular weight caldesmons during phenotypic modulation of smooth muscle cells.
1987; 84: 9049-9053 [PMID: 3321066 DOI: 10.1073/pnas.84.24.9049]
14 Pfitzer G.Invited review: regulation of myosin phosphorylation in smooth muscle.
2001; 91: 497-503 [PMID: 11408468 DOI: 10.1152/jappl.2001.91.1.497]
15 Blanchoin L,Boujemaa-Paterski R,Sykes C,Plastino J.Actin dynamics,architecture,and mechanics in cell motility.
2014; 94: 235-263 [PMID: 24382887 DOI: 10.1152/physrev.00018.2013]
16 Rottner K,Faix J,Bogdan S,Linder S,Kerkhoff E.Actin assembly mechanisms at a glance.
2017; 130: 3427-3435 [PMID: 29032357 DOI: 10.1242/jcs.206433]
17 Svitkina T.The Actin Cytoskeleton and Actin-Based Motility.
2018; 10 [PMID: 29295889 DOI: 10.1101/cshperspect.a018267]
18 Mayanagi T,Sobue K.Diversification of caldesmon-linked actin cytoskeleton in cell motility.
2011; 5: 150-159 [PMID: 21350330 DOI: 10.4161/cam.5.2.14398]
19 Zhang YG,Niu JT,Wu HW,Si XL,Zhang SJ,Li DH,Bian TT,Li YF,Yan XK.Actin-Binding Proteins as Potential Biomarkers for Chronic Inflammation-Induced Cancer Diagnosis and Therapy.
2021; 2021: 6692811 [PMID: 34194957 DOI: 10.1155/2021/6692811]
20 Warren KS,Shutt DC,McDermott JP,Lin JL,Soll DR,Lin JJ.Overexpression of microfilament-stabilizing human caldesmon fragment,CaD39,affects cell attachment,spreading,and cytokinesis.
1996; 34: 215-229 [PMID: 8816288 DOI: 10.1002/(SICI)1097-0169(1996)34:3<215::AID-CM5>3.0.CO;2-8]
21 Ekinci ?,??üt B,?elik B,Dursun A.Compared With Elastin Stains,h-Caldesmon and Desmin Offer Superior Detection of Vessel Invasion in Gastric,Pancreatic,and Colorectal Adenocarcinomas.
2018; 26: 318-326 [PMID: 29325463 DOI: 10.1177/1066896917752442]
22 Martinez-Ciarpaglini C,Agustí J,Alvarez E,Hueso L,Terrádez L,Monteagudo C.h-caldesmon immunoreactivity in atypical fibroxanthoma: implications for the differential diagnosis.
2018; 50: 358-361 [PMID: 29490873 DOI: 10.1016/j.pathol.2017.09.020]
23 Yu G,Xu J,Jiang L,Cai L,Zohar Y,Wu S,Yang P,Tal S,Hu J.Expression and clinical significance of H-caldesmon in gastrointestinal stromal tumor: is it a specific marker for myogenic differentiation?
2019; 12: 2566-2571 [PMID: 31934084]
24 Kussaibi H.Co-expression of CD34 and h-caldesmon in a benign meningioma-like dermal neoplasm,a case report.
2020; 12: 8994 [PMID: 33408843 DOI: 10.4081/dr.2020.8994]
25 Zhao W,Cui M,Zhang R,Shen X,Xiong X,Ji X,Tao L,Jia W,Pang L,Sun Z,Wang C,Zou H.IFITM1,CD10,SMA,and h-caldesmon as a helpful combination in differential diagnosis between endometrial stromal tumor and cellular leiomyoma.
2021; 21: 1047 [PMID: 34556086 DOI: 10.1186/s12885-021-08781-w]
26 Oliva E.Practical issues in uterine pathology from banal to bewildering: the remarkable spectrum of smooth muscle neoplasia.
2016; 29 Suppl 1: S104-S120 [PMID: 26715170 DOI: 10.1038/modpathol.2015.139]
27 Alyousef MJ,Alratroot JA,ElSharkawy T,Shawarby MA,Al Hamad MA,Hashem TM,Alsayyah A.Malignant gastrointestinal neuroectodermal tumor: a case report and review of the literature.
2017; 12: 29 [PMID: 28320420 DOI: 10.1186/s13000-017-0620-9]
28 Hamza A,Guo CC.Perivascular Epithelioid Cell Tumor of the Urinary Bladder: A Systematic Review.
2020; 28: 393-400 [PMID: 31865807 DOI: 10.1177/1066896919895810]
29 Gaeta R,Matera D,Muratori F,Roselli G,Baldi G,Campanacci DA,Franchi A.Dedifferentiated soft tissue leiomyosarcoma with heterologous osteosarcoma component: case report and review of the literature.
2020; 10: 6 [PMID: 32280451 DOI: 10.1186/s13569-020-00129-5]
30 Jiang Q,Huang R,Cai S,Wang CL.Caldesmon regulates the motility of vascular smooth muscle cells by modulating the actin cytoskeleton stability.
2010; 17: 6 [PMID: 20128924 DOI: 10.1186/1423-0127-17-6]
31 Helfman DM,Levy ET,Berthier C,Shtutman M,Riveline D,Grosheva I,Lachish-Zalait A,Elbaum M,Bershadsky AD.Caldesmon inhibits nonmuscle cell contractility and interferes with the formation of focal adhesions.
1999; 10: 3097-3112 [PMID: 10512853 DOI: 10.1091/mbc.10.10.3097]
32 Eves R,Webb BA,Zhou S,Mak AS.Caldesmon is an integral component of podosomes in smooth muscle cells.
2006; 119: 1691-1702 [PMID: 16595550 DOI: 10.1242/jcs.02881]
33 Tanaka J,Watanabe T,Nakamura N,Sobue K.Morphological and biochemical analyses of contractile proteins (actin,myosin,caldesmon and tropomyosin) in normal and transformed cells.
1993; 104 ( Pt 2): 595-606 [PMID: 8505382 DOI: 10.1242/jcs.104.2.595]
34 Carley WW,Webb WW.F-actin aggregates may activate transformed cell surfaces.
1983; 3: 383-390 [PMID: 6661766 DOI: 10.1002/cm.970030506]
35 Carley WW,Barak LS,Webb WW.F-actin aggregates in transformed cells.
1981; 90: 797-802 [PMID: 6270163 DOI: 10.1083/jcb.90.3.797]
36 Carley WW,Bretscher A,Webb WW.F-actin aggregates in transformed cells contain alpha-actinin and fimbrin but apparently lack tropomyosin.
1986; 39: 313-320 [PMID: 3007147]
37 Wang E,Yin HL,Krueger JG,Caliguiri LA,Tamm I.Unphosphorylated gelsolin is localized in regions of cellsubstratum contact or attachment in Rous sarcoma virus-transformed rat cells.
1984; 98: 761-771 [PMID: 6319434 DOI: 10.1083/jcb.98.2.761]
38 Chen WT.Proteolytic activity of specialized surface protrusions formed at rosette contact sites of transformed cells.
1989; 251: 167-185 [PMID: 2549171 DOI: 10.1002/jez.1402510206]
39 Kim KH,Yeo SG,Kim WK,Kim DY,Yeo HY,Hong JP,Chang HJ,Park JW,Kim SY,Kim BC,Yoo BC.Up-regulated expression of l-caldesmon associated with malignancy of colorectal cancer.
2012; 12: 601 [PMID: 23241148 DOI: 10.1186/1471-2407-12-601]
40 Thorsen K,S?rensen KD,Brems-Eskildsen AS,Modin C,Gaustadnes M,Hein AM,Kruh?ffer M,Laurberg S,Borre M,Wang K,Brunak S,Krainer AR,T?rring N,Dyrskj?t L,Andersen CL,Orntoft TF.Alternative splicing in colon,bladder,and prostate cancer identified by exon array analysis.
2008; 7: 1214-1224 [PMID: 18353764 DOI: 10.1074/mcp.M700590-MCP200]
41 Zheng H,Bai Y,Wang J,Chen S,Zhang J,Zhu J,Liu Y,Wang X.Weighted Gene Co-expression Network Analysis Identifies CALD1 as a Biomarker Related to M2 Macrophages Infiltration in Stage III and IV Mismatch Repair-Proficient Colorectal Carcinoma.
2021; 8: 649363 [PMID: 33996905 DOI: 10.3389/fmolb.2021.649363]
42 Zhao B,Baloch Z,Ma Y,Wan Z,Huo Y,Li F,Zhao Y.Identification of Potential Key Genes and Pathways in Early-Onset Colorectal Cancer Through Bioinformatics Analysis.
2019; 26: 1073274819831260 [PMID: 30786729 DOI: 10.1177/1073274819831260]
43 Chauvin A,Wang CS,Geha S,Garde-Granger P,Mathieu AA,Lacasse V,Boisvert FM.The response to neoadjuvant chemoradiotherapy with 5-fluorouracil in locally advanced rectal cancer patients: a predictive proteomic signature.
2018; 15: 16 [PMID: 29681787 DOI: 10.1186/s12014-018-9192-2]
44 Lian H,Wang A,Shen Y,Wang Q,Zhou Z,Zhang R,Li K,Liu C,Jia H.Identification of novel alternative splicing isoform biomarkers and their association with overall survival in colorectal cancer.
2020; 20: 171 [PMID: 32503434 DOI: 10.1186/s12876-020-01288-x]
45 Lim B,Park JL,Kim HJ,Park YK,Kim JH,Sohn HA,Noh SM,Song KS,Kim WH,Kim YS,Kim SY.Integrative genomics analysis reveals the multilevel dysregulation and oncogenic characteristics of TEAD4 in gastric cancer.
2014; 35: 1020-1027 [PMID: 24325916 DOI: 10.1093/carcin/bgt409]
46 Liu Y,Xie S,Zhu K,Guan X,Guo L,Lu R.CALD1 is a prognostic biomarker and correlated with immune infiltrates in gastric cancers.
2021; 7: e07257 [PMID: 34189308 DOI: 10.1016/j.heliyon.2021.e07257]
47 Al Saleh S,Al Mulla F,Luqmani YA.Estrogen receptor silencing induces epithelial to mesenchymal transition in human breast cancer cells.
2011; 6: e20610 [PMID: 21713035 DOI: 10.1371/journal.pone.0020610]
48 De Marchi T,Timmermans AM,Smid M,Look MP,Stingl C,Opdam M,Linn SC,Sweep FC,Span PN,Kliffen M,van Deurzen CH,Luider TM,Foekens JA,Martens JW,Umar A.Annexin-A1 and caldesmon are associated with resistance to tamoxifen in estrogen receptor positive recurrent breast cancer.
2016; 7: 3098-3110 [PMID: 26657294 DOI: 10.18632/oncotarget.6521]
49 Nalluri SM,O’Connor JW,Virgi GA,Stewart SE,Ye D,Gomez EW.TGFβ1-induced expression of caldesmon mediates epithelial-mesenchymal transition.
2018; 75: 201-212 [PMID: 29466836 DOI: 10.1002/cm.21437]
50 Lee MS,Lee J,Kim JH,Kim WT,Kim WJ,Ahn H,Park J.Overexpression of caldesmon is associated with tumor progression in patients with primary non-muscle-invasive bladder cancer.
2015; 6: 40370-40384 [PMID: 26430961 DOI: 10.18632/oncotarget.5458]
51 Lee MS,Kim JH,Lee JS,Yun SJ,Kim WJ,Ahn H,Park J.Prognostic Significance of CREB-Binding Protein and CD81 Expression in Primary High Grade Non-Muscle Invasive Bladder Cancer: Identification of Novel Biomarkers for Bladder Cancer Using Antibody Microarray.
2015; 10: e0125405 [PMID: 25915404 DOI: 10.1371/journal.pone.0125405]
52 Liu Y,Wu X,Wang G,Hu S,Zhang Y,Zhao S.CALD1,CNN1,and TAGLN identified as potential prognostic molecular markers of bladder cancer by bioinformatics analysis.
2019; 98: e13847 [PMID: 30633156 DOI: 10.1097/MD.0000000000013847]
53 Li C,Yang F,Wang R,Li W,Maskey N,Zhang W,Guo Y,Liu S,Wang H,Yao X.CALD1 promotes the expression of PD-L1 in bladder cancer
the JAK/STAT signaling pathway.
2021; 9: 1441 [PMID: 34733993 DOI: 10.21037/atm-21-4192]
54 Du Y,Jiang X,Wang B,Cao J,Wang Y,Yu J,Wang X,Liu H.The cancer-associated fibroblasts related gene CALD1 is a prognostic biomarker and correlated with immune infiltration in bladder cancer.
2021; 21: 283 [PMID: 34051818 DOI: 10.1186/s12935-021-01896-x]
55 Zhang S,Wang Q,Li W,Chen J.MIR100HG Regulates CALD1 Gene Expression by Targeting miR-142-5p to Affect the Progression of Bladder Cancer Cells
,as Revealed by Transcriptome Sequencing.
2021; 8: 793493 [PMID: 35127818 DOI: 10.3389/fmolb.2021.793493]
56 Zhang L,Liu J,Wang X,Li Z,Zhang X,Cao P,She X,Dai Q,Tang J,Liu Z.Upregulation of cytoskeleton protein and extracellular matrix protein induced by stromal-derived nitric oxide promotes lung cancer invasion and metastasis.
2014; 14: 762-771 [PMID: 25056538 DOI: 10.2174/1566524014666140724103147]
57 Dai Y,Wang L,Tang J,Cao P,Luo Z,Sun J,Kiflu A,Sai B,Zhang M,Wang F,Li G,Xiang J.Activation of anaphasepromoting complex by p53 induces a state of dormancy in cancer cells against chemotherapeutic stress.
2016; 7: 25478-25492 [PMID: 27009858 DOI: 10.18632/oncotarget.8172]
58 Chang KP,Wang CL,Kao HK,Liang Y,Liu SC,Huang LL,Hseuh C,Hsieh YJ,Chien KY,Chang YS,Yu JS,Chi LM.Overexpression of caldesmon is associated with lymph node metastasis and poorer prognosis in patients with oral cavity squamous cell carcinoma.
2013; 119: 4003-4011 [PMID: 23963810 DOI: 10.1002/cncr.28300]
59 Zhang L,Sun J,Liu Z,Dai Y,Luo Z,Jiang X,Li Z,Li Y,Cao P,Zhou Y,Zeng Z,Tang A,Li X,Xiang J,Li G.Mesenchymal stem cells regulate cytoskeletal dynamics and promote cancer cell invasion through low dose nitric oxide.
2014; 14: 749-761 [PMID: 24894170 DOI: 10.2174/1566524014666140724102301]
60 Cheng Q,Tang A,Wang Z,Fang N,Zhang Z,Zhang L,Li C,Zeng Y.CALD1 Modulates Gliomas Progression
Facilitating Tumor Angiogenesis.
2021; 13 [PMID: 34070840 DOI: 10.3390/cancers13112705]
61 Zheng PP,Hop WC,Sillevis Smitt PA,van den Bent MJ,Avezaat CJ,Luider TM,Kros JM.Low-molecular weight caldesmon as a potential serum marker for glioma.
2005; 11: 4388-4392 [PMID: 15958622 DOI: 10.1158/1078-0432.CCR-04-2512]
62 Zheng PP,Sieuwerts AM,Luider TM,van der Weiden M,Sillevis-Smitt PA,Kros JM.Differential expression of splicing variants of the human caldesmon gene (CALD1) in glioma neovascularization versus normal brain microvasculature.
2004; 164: 2217-2228 [PMID: 15161654 DOI: 10.1016/S0002-9440(10)63778-9]
63 Zheng PP,van der Weiden M,Kros JM.Hela l-CaD is implicated in the migration of endothelial cells/endothelial progenitor cells in human neoplasms.
2007; 1: 84-91 [PMID: 19329885 DOI: 10.4161/cam.1.2.4332]
64 Morita T,Mayanagi T,Sobue K.Dual roles of myocardin-related transcription factors in epithelial mesenchymal transition
slug induction and actin remodeling.
2007; 179: 1027-1042 [PMID: 18056415 DOI: 10.1083/jcb.200708174]
65 Jensen MH,Morris EJ,Huang R,Rebowski G,Dominguez R,Weitz DA,Moore JR,Wang CL.The conformational state of actin filaments regulates branching by actin-related protein 2/3 (Arp2/3) complex.
2012; 287: 31447-31453 [PMID: 22791711 DOI: 10.1074/jbc.M112.350421]
66 Liu J,Li H,Shen S,Sun L,Yuan Y,Xing C.Alternative splicing events implicated in carcinogenesis and prognosis of colorectal cancer.
2018; 9: 1754-1764 [PMID: 29805701 DOI: 10.7150/jca.24569]
67 Hou Q,Tan HT,Lim KH,Lim TK,Khoo A,Tan IB,Yeoh KG,Chung MC.Identification and functional validation of caldesmon as a potential gastric cancer metastasis-associated protein.
2013; 12: 980-990 [PMID: 23265641 DOI: 10.1021/pr3010259]
68 Yoshio T,Morita T,Kimura Y,Tsujii M,Hayashi N,Sobue K.Caldesmon suppresses cancer cell invasion by regulating podosome/invadopodium formation.
2007; 581: 3777-3782 [PMID: 17631293 DOI: 10.1016/j.febslet.2007.06.073]
69 Schwappacher R,Rangaswami H,Su-Yuo J,Hassad A,Spitler R,Casteel DE.cGMP-dependent protein kinase Iβ regulates breast cancer cell migration and invasion
interaction with the actin/myosin-associated protein caldesmon.
2013; 126: 1626-1636 [PMID: 23418348 DOI: 10.1242/jcs.118190]
70 Dierks S,von Hardenberg S,Schmidt T,Bremmer F,Burfeind P,Kaulfu? S.Leupaxin stimulates adhesion and migration of prostate cancer cells through modulation of the phosphorylation status of the actin-binding protein caldesmon.
2015; 6: 13591-13606 [PMID: 26079947 DOI: 10.18632/oncotarget.3792]
71 Mukhopadhyay UK,Eves R,Jia L,Mooney P,Mak AS.p53 suppresses Src-induced podosome and rosette formation and cellular invasiveness through the upregulation of caldesmon.
2009; 29: 3088-3098 [PMID: 19349302 DOI: 10.1128/MCB.01816-08]
72 Lynch WP,Riseman VM,Bretscher A.Smooth muscle caldesmon is an extended flexible monomeric protein in solution that can readily undergo reversible intra- and intermolecular sulfhydryl cross-linking.A mechanism for caldesmon’s Factin bundling activity.
1987; 262: 7429-7437 [PMID: 3584120]
73 Imodoye SO,Adedokun KA,Muhammed AO,Bello IO,Muhibi MA,Oduola T,Oyenike MA.Understanding the Complex Milieu of Epithelial-Mesenchymal Transition in Cancer Metastasis: New Insight Into the Roles of Transcription Factors.
2021; 11: 762817 [PMID: 34868979 DOI: 10.3389/fonc.2021.762817]
74 Greaves D,Calle Y.Epithelial Mesenchymal Transition (EMT) and Associated Invasive Adhesions in Solid and Haematological Tumours.
2022; 11 [PMID: 35203300 DOI: 10.3390/cells11040649]
75 Tang X,Sui X,Weng L,Liu Y.SNAIL1: Linking Tumor Metastasis to Immune Evasion.
2021; 12: 724200 [PMID: 34917071 DOI: 10.3389/fimmu.2021.724200]
76 Stuelten CH,Zhang YE.Transforming Growth Factor-β: An Agent of Change in the Tumor Microenvironment.
2021; 9: 764727 [PMID: 34712672 DOI: 10.3389/fcell.2021.764727]
77 Yuki R.[Aberrant Activation Mechanism of TGF-β Signaling in Epithelial-mesenchymal Transition].
2021; 141: 1229-1234 [PMID: 34719542 DOI: 10.1248/yakushi.21-00143]
78 Kaszak I,Witkowska-Pi?aszewicz O,Niewiadomska Z,Dworecka-Kaszak B,Ngosa Toka F,Jurka P.Role of Cadherins in Cancer-A Review.
2020; 21 [PMID: 33076339 DOI: 10.3390/ijms21207624]
79 Loboda A,Nebozhyn MV,Watters JW,Buser CA,Shaw PM,Huang PS,Van’t Veer L,Tollenaar RA,Jackson DB,Agrawal D,Dai H,Yeatman TJ.EMT is the dominant program in human colon cancer.
2011; 4: 9 [PMID: 21251323 DOI: 10.1186/1755-8794-4-9]
80 Syed V.TGF-β Signaling in Cancer.
2016; 117: 1279-1287 [PMID: 26774024 DOI: 10.1002/jcb.25496]
81 Hao Y,Baker D,Ten Dijke P.TGF-β-Mediated Epithelial-Mesenchymal Transition and Cancer Metastasis.
2019; 20 [PMID: 31195692 DOI: 10.3390/ijms20112767]
82 Guinney J,Dienstmann R,Wang X,de Reyniès A,Schlicker A,Soneson C,Marisa L,Roepman P,Nyamundanda G,Angelino P,Bot BM,Morris JS,Simon IM,Gerster S,Fessler E,De Sousa E Melo F,Missiaglia E,Ramay H,Barras D,Homicsko K,Maru D,Manyam GC,Broom B,Boige V,Perez-Villamil B,Laderas T,Salazar R,Gray JW,Hanahan D,Tabernero J,Bernards R,Friend SH,Laurent-Puig P,Medema JP,Sadanandam A,Wessels L,Delorenzi M,Kopetz S,Vermeulen L,Tejpar S.The consensus molecular subtypes of colorectal cancer.
2015; 21: 1350-1356 [PMID: 26457759 DOI: 10.1038/nm.3967]
83 Calon A,Lonardo E,Berenguer-Llergo A,Espinet E,Hernando-Momblona X,Iglesias M,Sevillano M,Palomo-Ponce S,Tauriello DV,Byrom D,Cortina C,Morral C,Barceló C,Tosi S,Riera A,Attolini CS,Rossell D,Sancho E,Batlle E.Stromal gene expression defines poor-prognosis subtypes in colorectal cancer.
2015; 47: 320-329 [PMID: 25706628 DOI: 10.1038/ng.3225]
84 Ingber DE,Prusty D,Sun Z,Betensky H,Wang N.Cell shape,cytoskeletal mechanics,and cell cycle control in angiogenesis.
1995; 28: 1471-1484 [PMID: 8666587 DOI: 10.1016/0021-9290(95)00095-x]
85 Moreau V,Tatin F,Varon C,Génot E.Actin can reorganize into podosomes in aortic endothelial cells,a process controlled by Cdc42 and RhoA.
2003; 23: 6809-6822 [PMID: 12972601 DOI: 10.1128/MCB.23.19.6809-6822.2003]
86 Jackson CW.Megakaryocyte endomitosis: a review.
1990; 8: 224-226 [PMID: 2205660 DOI: 10.1002/stem.5530080405]
87 Zheng PP,van der Weiden M,Kros JM.Differential expression of Hela-type caldesmon in tumour neovascularization: a new marker of angiogenic endothelial cells.
2005; 205: 408-414 [PMID: 15682433 DOI: 10.1002/path.1700]
88 Yu B,Yu X,Xiong J,Ma M.Methylation Modification,Alternative Splicing,and Noncoding RNA Play a Role in Cancer Metastasis through Epigenetic Regulation.
2021; 2021: 4061525 [PMID: 34660788 DOI: 10.1155/2021/4061525]
89 Reviejo M,Soto M,Lozano E,Asensio M,Martínez-Augustin O,Sánchez de Medina F,Marin JJG.Impact of alternative splicing on mechanisms of resistance to anticancer drugs.
2021; 193: 114810 [PMID: 34673012 DOI: 10.1016/j.bcp.2021.114810]
90 Ouyang J,Zhang Y,Xiong F,Zhang S,Gong Z,Yan Q,He Y,Wei F,Zhang W,Zhou M,Xiang B,Wang F,Li X,Li Y,Li G,Zeng Z,Guo C,Xiong W.The role of alternative splicing in human cancer progression.
2021; 11: 4642-4667 [PMID: 34765285]
91 Ma X,Dang Y,Shao X,Chen X,Wu F,Li Y.Ubiquitination and Long Non-coding RNAs Regulate Actin Cytoskeleton Regulators in Cancer Progression.
2019; 20 [PMID: 31248165 DOI: 10.3390/ijms20122997]
92 Schwerk C,Schulze-Osthoff K.Regulation of apoptosis by alternative pre-mRNA splicing.
2005; 19: 1-13 [PMID: 15989960 DOI: 10.1016/j.molcel.2005.05.026]
93 Zheng PP,Luider TM,Pieters R,Avezaat CJ,van den Bent MJ,Sillevis Smitt PA,Kros JM.Identification of tumorrelated proteins by proteomic analysis of cerebrospinal fluid from patients with primary brain tumors.
2003; 62: 855-862 [PMID: 14503641 DOI: 10.1093/jnen/62.8.855]
94 Chen Y,Huang M,Liu X,Huang Y,Liu C,Zhu J,Fu G,Lei Z,Chu X.Alternative splicing of mRNA in colorectal cancer: new strategies for tumor diagnosis and treatment.
2021; 12: 752 [PMID: 34330892 DOI: 10.1038/s41419-021-04031-w]
95 Carley WW,Lipsky MG,Webb WW.Regulation and drug insensitivity of F-actin association with adhesion areas of transformed cells.
1983; 117: 257-265 [PMID: 6313706 DOI: 10.1002/jcp.1041170218]
96 Jiang Y,Chen M,Nie H,Yuan Y.PD-1 and PD-L1 in cancer immunotherapy: clinical implications and future considerations.
2019; 15: 1111-1122 [PMID: 30888929 DOI: 10.1080/21645515.2019.1571892]
97 Han Y,Liu D,Li L.PD-1/PD-L1 pathway: current researches in cancer.
2020; 10: 727-742 [PMID: 32266087]
98 Li P,Huang T,Zou Q,Liu D,Wang Y,Tan X,Wei Y,Qiu H.FGFR2 Promotes Expression of PD-L1 in Colorectal Cancer
the JAK/STAT3 Signaling Pathway.
2019; 202: 3065-3075 [PMID: 30979816 DOI: 10.4049/jimmunol.1801199]
99 Xia L,Tan S,Zhou Y,Lin J,Wang H,Oyang L,Tian Y,Liu L,Su M,Cao D,Liao Q.Role of the NFκB-signaling pathway in cancer.
2018; 11: 2063-2073 [PMID: 29695914 DOI: 10.2147/OTT.S161109]
100 Zou Z,Tao T,Li H,Zhu X.mTOR signaling pathway and mTOR inhibitors in cancer: progress and challenges.
2020; 10: 31 [PMID: 32175074 DOI: 10.1186/s13578-020-00396-1]
101 Nieminen TT,Shoman S,Eissa S,Peltom?ki P,Abdel-Rahman WM.Distinct genetic and epigenetic signatures of colorectal cancers according to ethnic origin.
2012; 21: 202-211 [PMID: 22028395 DOI: 10.1158/1055-9965.EPI-11-0662]
102 Abdel-Rahman WM,Faris ME,Peltomaki P.Molecular Determinants of Colon Cancer Susceptibility in the East and West.
2017; 17: 34-45 [PMID: 28231750 DOI: 10.2174/1566524017666170220094705]
103 Jayasingam SD,Citartan M,Thang TH,Mat Zin AA,Ang KC,Ch’ng ES.Evaluating the Polarization of Tumor-Associated Macrophages Into M1 and M2 Phenotypes in Human Cancer Tissue: Technicalities and Challenges in Routine Clinical Practice.
2019; 9: 1512 [PMID: 32039007 DOI: 10.3389/fonc.2019.01512]
104 Rahma OE,Hodi FS.The Intersection between Tumor Angiogenesis and Immune Suppression.
2019; 25: 5449-5457 [PMID: 30944124 DOI: 10.1158/1078-0432.CCR-18-1543]
105 Najafi M,Hashemi Goradel N,Farhood B,Salehi E,Nashtaei MS,Khanlarkhani N,Khezri Z,Majidpoor J,Abouzaripour M,Habibi M,Kashani IR,Mortezaee K.Macrophage polarity in cancer: A review.
2019; 120: 2756-2765 [PMID: 30270458 DOI: 10.1002/jcb.27646]
106 Abdel-Rahman WM,Katsura K,Rens W,Gorman PA,Sheer D,Bicknell D,Bodmer WF,Arends MJ,Wyllie AH,Edwards PA.Spectral karyotyping suggests additional subsets of colorectal cancers characterized by pattern of chromosome rearrangement.
2001; 98: 2538-2543 [PMID: 11226274 DOI: 10.1073/pnas.041603298]
107 Abdel-Rahman WM,Lohi H,Knuutila S,Peltom?ki P.Restoring mismatch repair does not stop the formation of reciprocal translocations in the colon cancer cell line HCA7 but further destabilizes chromosome number.
2005; 24: 706-713 [PMID: 15580308 DOI: 10.1038/sj.onc.1208129]
108 Marisa L,de Reyniès A,Duval A,Selves J,Gaub MP,Vescovo L,Etienne-Grimaldi MC,Schiappa R,Guenot D,Ayadi M,Kirzin S,Chazal M,Fléjou JF,Benchimol D,Berger A,Lagarde A,Pencreach E,Piard F,Elias D,Parc Y,Olschwang S,Milano G,Laurent-Puig P,Boige V.Gene expression classification of colon cancer into molecular subtypes: characterization,validation,and prognostic value.
2013; 10: e1001453 [PMID: 23700391 DOI: 10.1371/journal.pmed.1001453]
109 Sadanandam A,Lyssiotis CA,Homicsko K,Collisson EA,Gibb WJ,Wullschleger S,Ostos LC,Lannon WA,Grotzinger C,Del Rio M,Lhermitte B,Olshen AB,Wiedenmann B,Cantley LC,Gray JW,Hanahan D.A colorectal cancer classification system that associates cellular phenotype and responses to therapy.
2013; 19: 619-625 [PMID: 23584089 DOI: 10.1038/nm.3175]
110 De Sousa E Melo F,Wang X,Jansen M,Fessler E,Trinh A,de Rooij LP,de Jong JH,de Boer OJ,van Leersum R,Bijlsma MF,Rodermond H,van der Heijden M,van Noesel CJ,Tuynman JB,Dekker E,Markowetz F,Medema JP,Vermeulen L.Poor-prognosis colon cancer is defined by a molecularly distinct subtype and develops from serrated precursor lesions.
2013; 19: 614-618 [PMID: 23584090 DOI: 10.1038/nm.3174]
111 Sadanandam A,Wang X,de Sousa E Melo F,Gray JW,Vermeulen L,Hanahan D,Medema JP.Reconciliation of classification systems defining molecular subtypes of colorectal cancer: interrelationships and clinical implications.
2014; 13: 353-357 [PMID: 24406433 DOI: 10.4161/cc.27769]
112 Uhlen M,Oksvold P,Fagerberg L,Lundberg E,Jonasson K,Forsberg M,Zwahlen M,Kampf C,Wester K,Hober S,Wernerus H,Bj?rling L,Ponten F.Towards a knowledge-based Human Protein Atlas.
2010; 28: 1248-1250 [PMID: 21139605 DOI: 10.1038/nbt1210-1248]
113 Jensen NF,Stenvang J,Beck MK,Hanáková B,Belling KC,Do KN,Viuff B,Nyg?rd SB,Gupta R,Rasmussen MH,Tarpgaard LS,Hansen TP,Budinská E,Pfeiffer P,Bosman F,Tejpar S,Roth A,Delorenzi M,Andersen CL,R?mer MU,Brünner N,Moreira JM.Establishment and characterization of models of chemotherapy resistance in colorectal cancer: Towards a predictive signature of chemoresistance.
2015; 9: 1169-1185 [PMID: 25759163 DOI: 10.1016/j.molonc.2015.02.008]
114 Tirosh I,Izar B,Prakadan SM,Wadsworth MH 2nd,Treacy D,Trombetta JJ,Rotem A,Rodman C,Lian C,Murphy G,Fallahi-Sichani M,Dutton-Regester K,Lin JR,Cohen O,Shah P,Lu D,Genshaft AS,Hughes TK,Ziegler CG,Kazer SW,Gaillard A,Kolb KE,Villani AC,Johannessen CM,Andreev AY,Van Allen EM,Bertagnolli M,Sorger PK,Sullivan RJ,Flaherty KT,Frederick DT,Jané-Valbuena J,Yoon CH,Rozenblatt-Rosen O,Shalek AK,Regev A,Garraway LA.Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq.
2016; 352: 189-196 [PMID: 27124452 DOI: 10.1126/science.aad0501]
115 Heo C,Lee S,Lee SY,Jeong MS,Lee YH,Suh M.Direct high-resolution label-free imaging of cellular nanostructure dynamics in living cells.
2013; 18: 066016 [PMID: 23797956 DOI: 10.1117/1.JBO.18.6.066016]
116 Jung M,Kim D,Mun JY.Direct Visualization of Actin Filaments and Actin-Binding Proteins in Neuronal Cells.
2020; 8: 588556 [PMID: 33324645 DOI: 10.3389/fcell.2020.588556]
117 Shigene K,Hiasa Y,Otake Y,Soufi M,Janewanthanakul S,Nishimura T,Sato Y,Suetsugu S.Translation of Cellular Protein Localization Using Convolutional Networks.
2021; 9: 635231 [PMID: 34422790 DOI: 10.3389/fcell.2021.635231]
 World Journal of Gastrointestinal Oncology2022年9期
World Journal of Gastrointestinal Oncology2022年9期
- World Journal of Gastrointestinal Oncology的其它文章
- Nutrition deprivation affects the cytotoxic effect of CD8 T cells in hepatocellular carcinoma
- Prognostic and clinicopathological value of Twist expression in esophageal cancer:A meta-analysis
- Dissecting novel mechanisms of hepatitis B virus related hepatocellular carcinoma using meta-analysis of public data
- Prediction of gastric cancer risk by a polygenic risk score of Helicobacter pylori
- Percutaneous insertion of a novel dedicated metal stent to treat malignant hilar biliary obstruction
- Construction and analysis of an ulcer risk prediction model after endoscopic submucosal dissection for early gastric cancer
