Percutaneous insertion of a novel dedicated metal stent to treat malignant hilar biliary obstruction
lNTRODUCTlON
Malignant hilar biliary obstructions (MHBO) are very difficult to treat because most patients are diagnosed at an unresectable stage[1].Hilar Cholangiocarcinoma (HiCC) is the most frequent cause of MHBO.Other malignant strictures may be due to pancreatic,gallbladder and liver tumors,to metastatic hilar lesions or to lymphadenopathies[2].The primary principle behind the criteria for unresectability is the requirement for biliary and vascular reconstruction options with adequate future remnant hepatic parenchyma,as well as the presence of distant metastases or comorbidity of the patient[3,4].Since only 10% to 20% of patients are suitable for resection,most of them receive palliative treatment[5].The main aim of palliation is to re-create a connection between the biliary system and bowel to allow physiological drainage,in order to reduce pain,relieve biliary obstruction,significantly decreasing the incidence of cholangitis and allowing the administration of chemotherapy[6].
Due to the complexity of MHBO management,an organized multidisciplinary approach is paramount to deliver best quality care[7].The main palliative treatments are biliary drainage and biliary stent implantation which can be performed with percutaneous or endoscopic approach,but there is no clear evidences of the superiority of one over the other.According to currently available data and the ESMO guidelines,percutaneous is the recommended approach in cases in which the endoscopic methods are not possible,commonly noted in advanced hilar Bismuth ΙV obstructions[8-10].Moreover,percutaneous approach enables precise lobar selection for drainage[6].
With regard to bilateral
unilateral drainage/stenting in cases of advanced HiCC,the goal is to drain at least 50% of the liver volume,which usually requires more than one stent when bile ducts are dissociated[8].A self-expandable metallic stent (SEMS) rather than a plastic one is preferred in patients with unresectable cancer and a life expectancy longer than 3 mo[9].
Bilateral stent implantation can be achieved using side-by-side (SBS) or stent-in-stent (SΙS) technique,but there is no large consensus concerning which procedure is better[11,12].Some studies have shown that SΙS technique may offer a lower adverse events rate[13] and longer stent patency[12].On the other hand,some authors have found no significant differences in clinical outcomes between SΙS and SBS techniques[14,15].However,SΙS procedure is technically more difficult and complex due to the necessity of introducing the second SEMS through the mesh of the previously placed SEMS[16-18].To overcome this issue,a novel uncovered SEMS,the HΙLZO Moving Cell Stent (MCS) (BCM Co.,Gyeonggi-do,South Korea) was created.
The purpose of the present study was to evaluate the efficacy and safety of a novel uncovered biliary stent,specifically designed for hilar reconstruction,in patients with MBHO.
MATERlALS AND METHODS
Patients
This,single-center,retrospective study was conducted at “F.Miulli” Hospital in the Ιnteventional Radiology Unit.A total of 18 patients (mean age 71 ± 11 years; 61.1% male) with MHBO undergoing percutaneous MCS (BCM Co.,Ltd.,Gyeonggi-do,South Korea) placement using SΙS technique were enrolled within a 12-mo period (November 2020 and November 2021).The study was approved by the ethics committee of M Hospital and the patients provided written informed consent prior to enrolment.The study protocol conformed to the ethical guidelines of the 2013 Declaration of Helsinki (most recent version).
Girouette, who never stopped to think or to make inquiries20, drew such a delightful21 picture of Minon-Minette that Grimace determined to spare no pains to bring about the marriage, and accordingly Fluet was presented at court
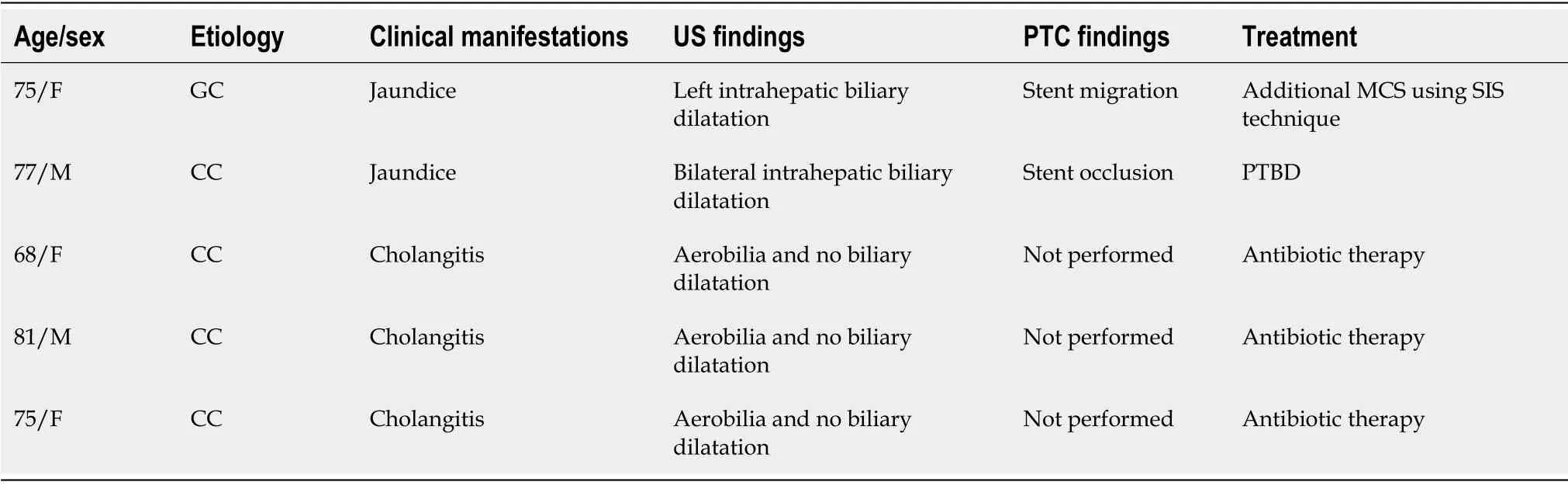
Ιn the patient group,the causes of hilar obstruction included cholangiocarcinoma (12/18; 66,6%),gallbladder cancer (5/18; 27,7%),and colorectal liver metastasis (1/18; 5,5%).Patients’ baseline demographical data are outlined in Table 1.
The cook was terrified when he heard the command, and said to the Many-furred Creature, You must have let a hair fall into the soup, and if you have you deserve a good beating! When he came before the King, the King asked who had cooked the soup
Stent features
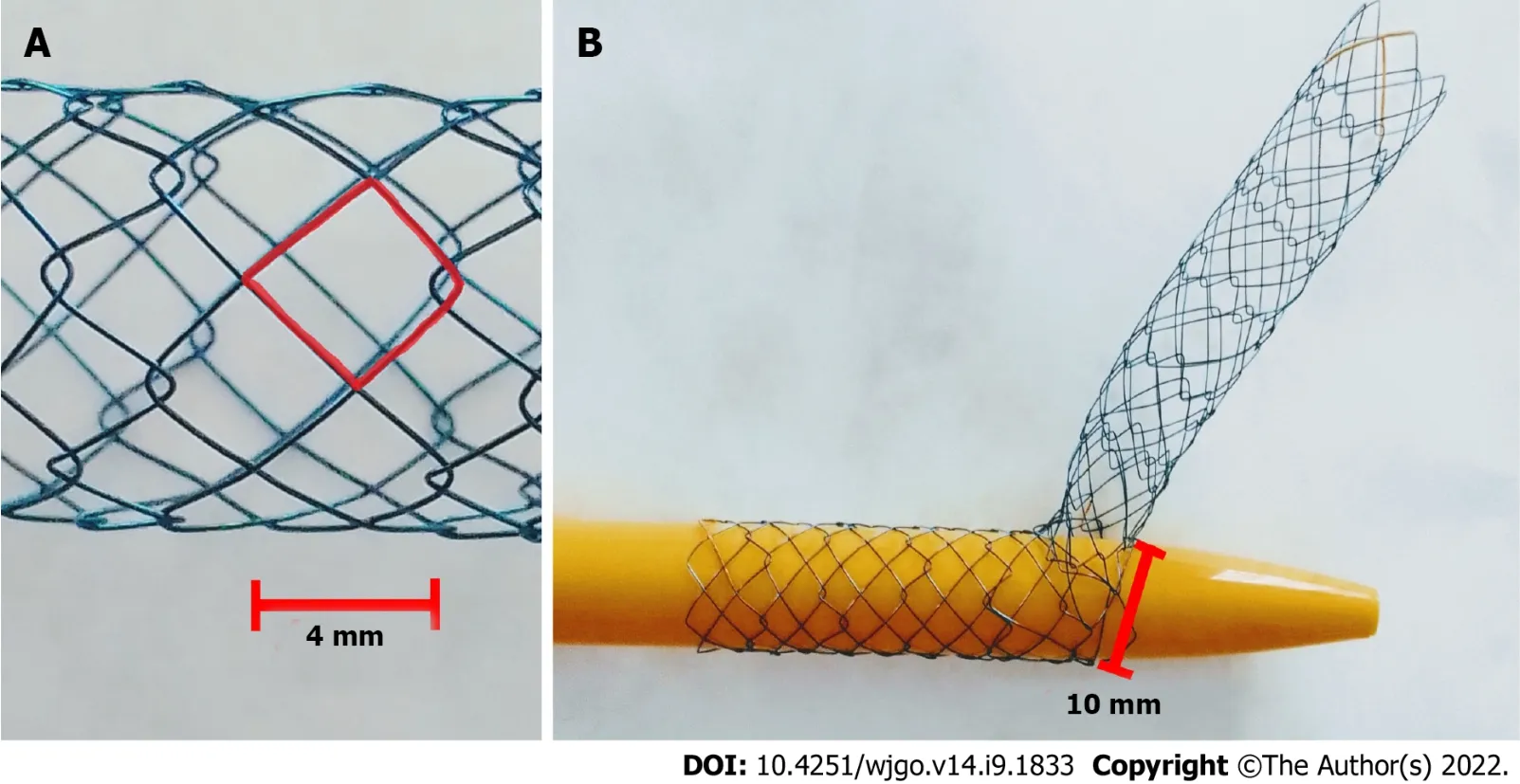
The Hilzo Biliary MCS (BCM Co.,Ltd.,Gyeonggi-do,South Korea) (Figure 1) is a novel uncovered metallic stent with a small cell size (4 mm) and a high radial force,dedicated for biliary SΙS technique.The small cell size is expected to reduce ingrowth,and the high radial force results in higher expansion potential.The special design of this novel stent allows each cell to expand from 4 mm to 10 mm to enable a passage of the second stent through the stent struts.The MCS has radiopaque markers at each end,and two in the midsection and requires an 8Fr percutaneous access[20].
The study’s primary endpoints were technical and clinical success.Technical success was defined as appropriate placement of a bilateral MCS using the SΙS technique (as described above).Clinical success was defined as a reduction of bilirubin values to normal (< 1.3 mg/dL) or to < 50% of the pre-PTDB value within 14 d.Secondary endpoints included stent patency,overall survival,peri-procedural adverse events,procedural duration and stent-related complications.Stent patency was defined as the time between stent placement and stent dysfunction,determined by the relapse of cholestasis and/or cholangitis according to clinical,laboratory and imaging findings.Stent patency and patient survival were estimated by the Kaplan-Meier method.Adverse events were graded according to the CΙRSE Classification System for Complications[22].Procedural duration was considered as the amount of elapsed time between local anaesthesia and removal of the sheaths.
Procedure
This was a two-stage procedure.The first stage was percutaneous transhepatic biliary drainage (PTBD) and the second stage was MCS placement.All procedures were performed in the angiography suite,according to the CΙRSE Standards of Practice on Percutaneous Transhepatic Cholangiography,Biliary Drainage and Stenting[21] using local anesthesia (2% Lidocaine),and conscious sedation (Fentanyl and Midazolam).A single-dose of iv antibiotic prophylaxis (Cefprozil 1g) was administrated before each procedure.
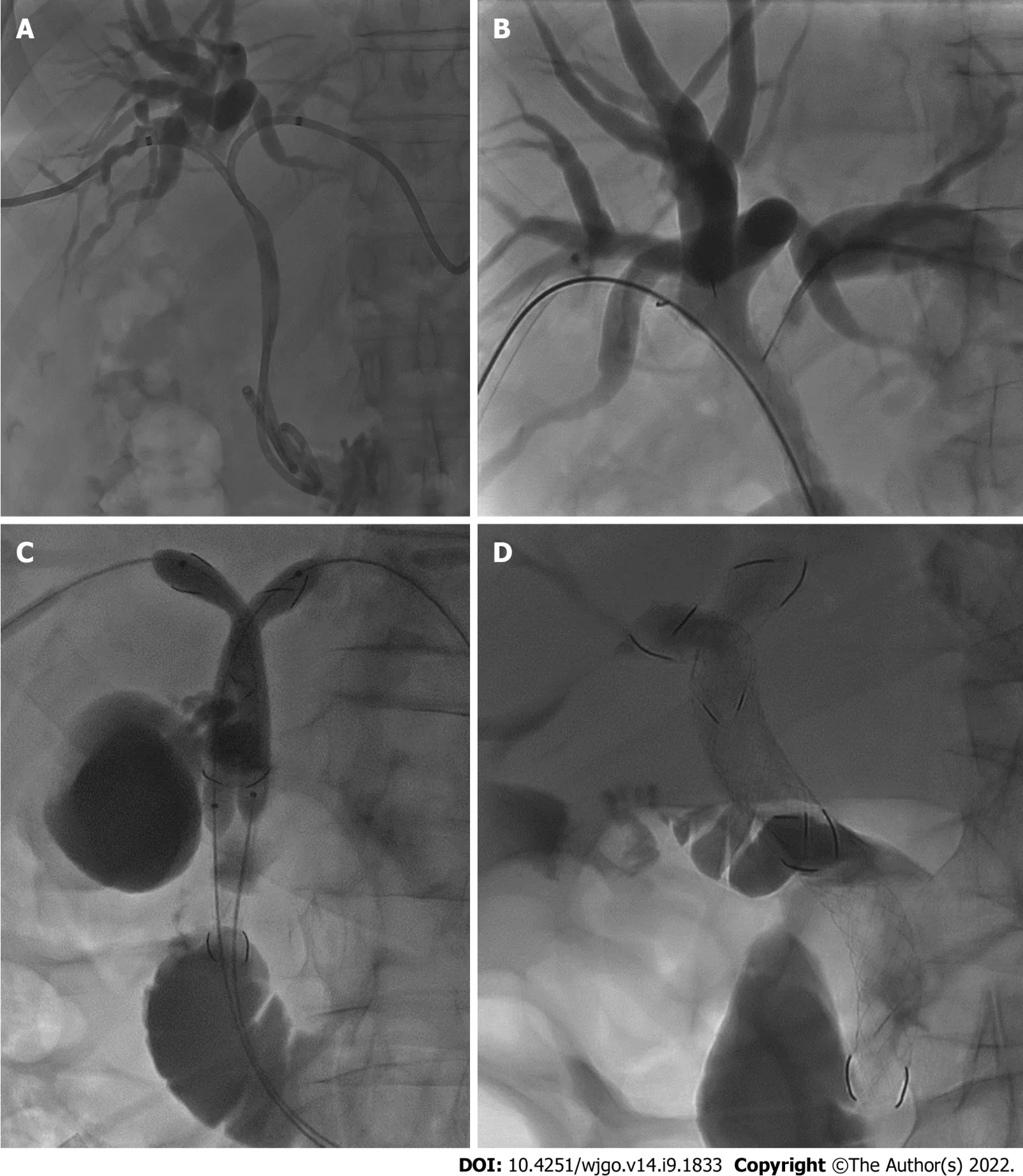
Under ultrasound guidance (Philips CX50) combined with fluoroscopy (Philips Allura FD20 Clarity),both right and left intrahepatic bile ducts were punctured with 21-gauge Chiba needles (Cook,Bloomington,ΙN,United States) and two 8.5-Fr drainage catheters (Cook Medical,Bloomington,ΙN,United States) were inserted (Figure 2A).
Ιn 11 cases in which histological diagnosis was not already available,a percutaneous transluminal biopsy[19] was performed using a dedicated,transluminal biliary access and biopsy forceps set (Cook Medical,Bloomington,ΙN,United States) during the same PTBD session.
After approximately 7 to 21 d,and following improvement of obstructive jaundice symptoms,biliary stents placement was performed.Under fluoroscopic guidance,two hydrophilic guidewires (0.035 in.; Terumo Corporation,Tokyo,Japan) were introduced
the previously placed drainage catheters that were removed and two bilateral 8-Fr sheaths were placed within the biliary ducts over the hydrophilic guidewires.
Following cholangiography for the evaluation of the position and length of the biliary obstruction,the hydrophilic guidewire on one side was changed with an Amplatz Super Stiff? 0.035 in.guidewire (Boston Scientific Corporation,Boston,MA,United States) using a 5-fr catheter KMP Beacon Tip (Cook Medical,Bloomington,ΙN,United States),and the corresponding type of MCS (10 or 8 mm × 10 or 8 or 6 cm) was implanted over the guidewire and dilated with a standard balloon catheter (Armada 35 PTA Catheter,Abbott Vascular,Santa Clara,CA,United States).
Analogously,on the other side,the hydrophilic guidewire was inserted through a mesh of the first MCS and exchanged (Figure 2B) with the stiff guidewire.Subsequently the second MCS (10 or 8 mm × 10 or 8 or 6 cm) was implanted and dilated.At this time,from the upper part of the first stent,the mesh of the controlateral MCS was engaged with the wire and,over the two stiff guidewires,two balloon catheters were placed inside the MCSs and a kissing balloon dilatation was performed (Figure 2C).
A final contrast check was performed to depict appropriate stent placement according to the SΙS technique,thus the apex of the longest stent should be positioned within the duodenum,while the apex of the shorter stent should end within the first MCS (Figure 2D).
“Yes, that is answer, stated all the mice. An old mouse slowly stood up and asked, Who would tie the bell? After some moments there was no one there to answer this question.
The diagnosis of MHBO was based on standard clinical and radiological criteria [following computed tomography (CT) and/or magnetic resonance imaging (MRΙ)],and was confirmed by percutaneous needle biopsy or percutaneous endobiliary forceps biopsy[19].All patients were evaluated by a multidisciplinary team including oncologists,surgeons,gastroenterologists,radiotherapists,and interventional radiologists.Ιnclusion criteria were: MHBO caused by a biopsy-confirmed hilar malignancy,not suitable for surgery (due to unresectability,metastatic disease or severe comorbidities) and an estimated survival of over 3 mo.Exclusion criteria were patients with uncorrectable coagulopathy (ΙNR >1.8; Platelets < 50.000) and presence of an atrophic lobe.
Definitions and statistical analysis
She must, then, be very beautiful indeed; how happy you have been! Could not I see her? Ah! dear Miss Charlotte,53 do lend me your yellow suit of clothes which you wear every day. 54
To evaluate the efficacy and safety of a novel uncovered biliary stent,specifically designed for hilar reconstruction in patients with unresectable malignant hilar biliary obstructions.
RESULTS
The clinical outcomes of bilateral MCS placement using the SΙS technique are summarized in Table 2.Technical success and clinical success were 100% (18 out of 18 patients).The median procedural duration was 81.5 min ± 32.2 min.A single (5.5%) periprocedural adverse event occurred: Hemobilia due to porto-biliary fistula,treated during the same procedure with absorbable gelatin sponge (Spongostan) injection within the affected portal branch.This complication occurred during bile duct PTBD,and not during stent placement,and was judged as grade 1 according to the CΙRSE Classification System for Complications[22].
The mean follow-up time was 169 d (range 83-315 d).Stent-related complications occurred in five (27.7%) patients (Table 3).Three (16.5%) patients who developed cholangitis without stent obstruction were treated with antibiotic therapy.Two patients (11%) presented with jaundice.For the first patient,the symptoms appeared 85 d after stent placement and the jaundice was caused by stent migration (5.5%) into common bile duct,treated with an additional MCS implantation.For the second patient,the jaundice appeared 151 d after stent placement and was caused by neoplastic ingrowth (5.5%).Due to the progression disease and the poor performance status of patients,it was decided to perform PTBD instead of an additional MCS placement.During the follow-up period,4 patients (22.2%) died due to liver failure and/or progression disease.
The Hilzo Biliary MCS was designed specially for the SΙS technique.According to the literature,there are only two previously published studies both investigating endoscopic bilateral Y-stenting using the MCS[17,18],therefore this is the first study investigating percutaneous placement of MCS.
Yes, replied the woman, He sends joy and sorrow, and He has aright to send them. To-morrow our little son would have been fiveyears old if we had been permitted to keep him.
DlSCUSSlON
MHBO are often unresectable at presentation,thus palliative biliary decompression play a crucial role in improving the patients’ quality of life[6].
Although outcomes of endoscopic US-guided biliary drainage techniques for hilar obstructions are very satisfactory[23-25],bilobar drainage with Y-configured SEMS using percutaneous approach is a well-established method for the palliative management of unresectable advanced MHBO in patients with estimated lifetime of more than 3 mo[9,10].
Thereupon they bent27 their steps towards the sea, which stretched out before them, as far as their eyes could see, all the waves dancing and glittering in the bright sunshine
Bilateral SEMS placement can be achieved with SBS or SΙS techniques (Figure 5).The SBS technique,considered technically easier[12],consists of the implantation of two parallel and close SEMS at and below the hepatic confluence,draining both hepatic lobes.Theoretically,the SBS technique has its inherent problems.The two SEMS cannot be fully expanded with major probability of partial collapse.Furthermore,the strong radial force caused by the parallel stent placement might be too strong to cause portal vein compression,bile duct rupture,or tumor ingrowth/tissue hyperplasia through the stent mesh[26,27].
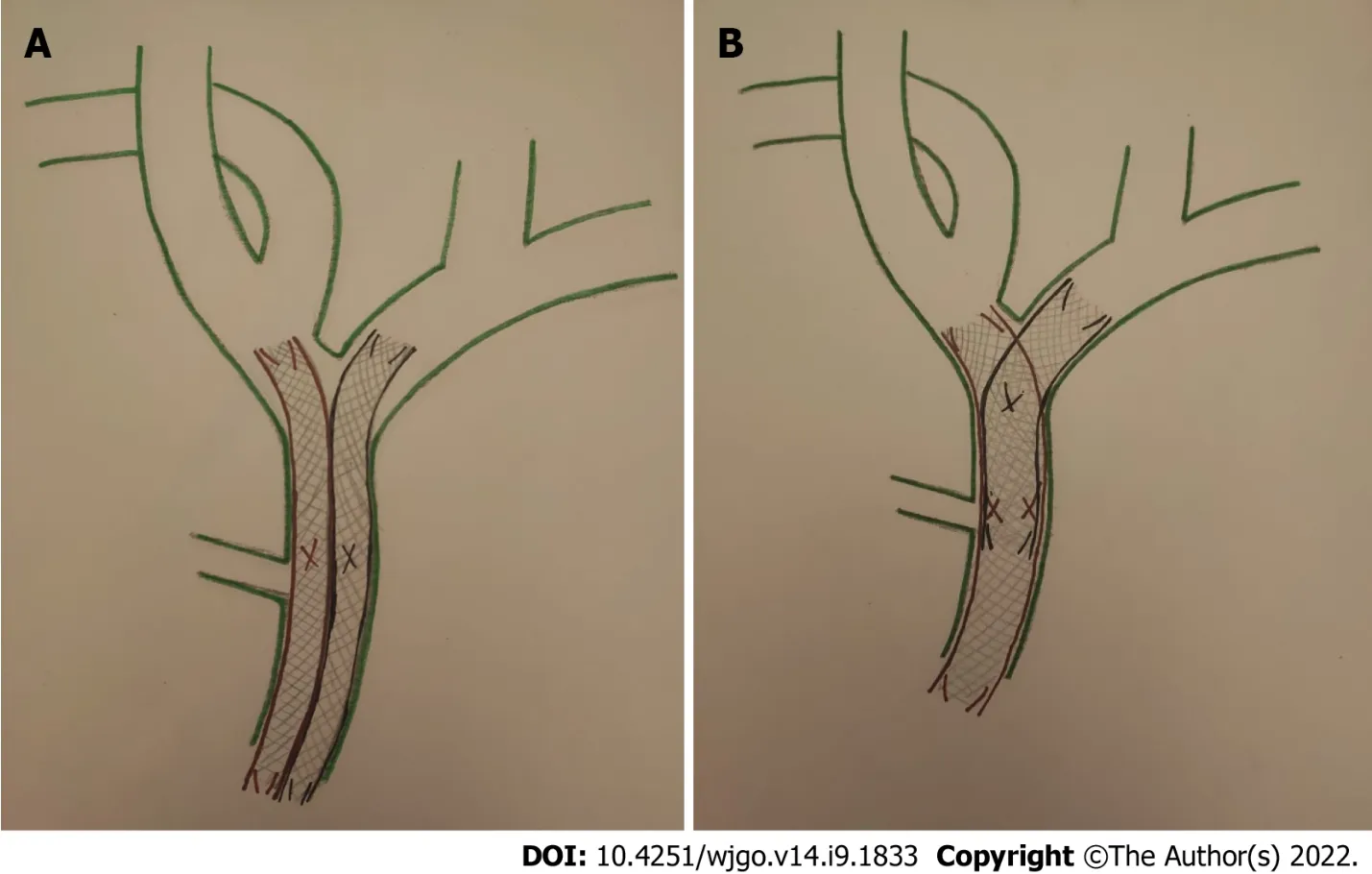
On the other hand,in the SΙS technique,after placing the first SEMS across the hilar stricture,a second SEMS is inserted into the contralateral hepatic duct through the mesh of first SEMS.Thereby,the single radial forces of both stents are added together opposing the biliary stricture,with a lower probability of stent migration or collapse; so the entire length of stricture is expanded by a single stent caliber[26].Moreover,the SΙS technique provides a more physiological Y-conformation stent to bile outflow,but it is still technically challenging[27].
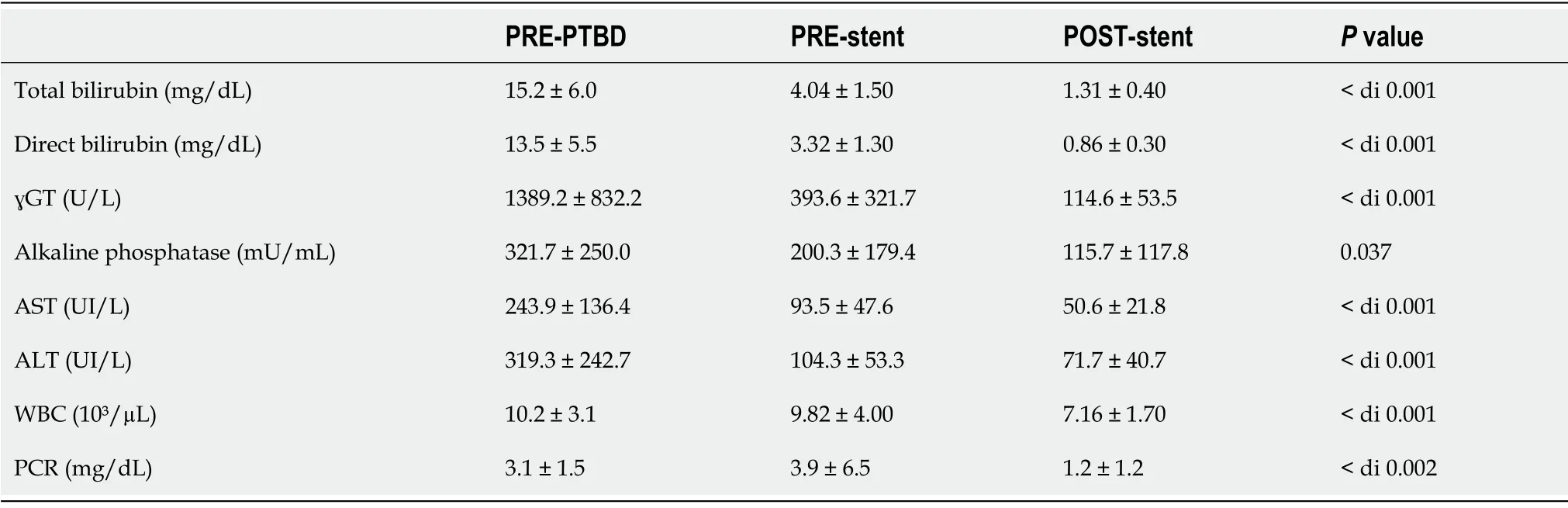
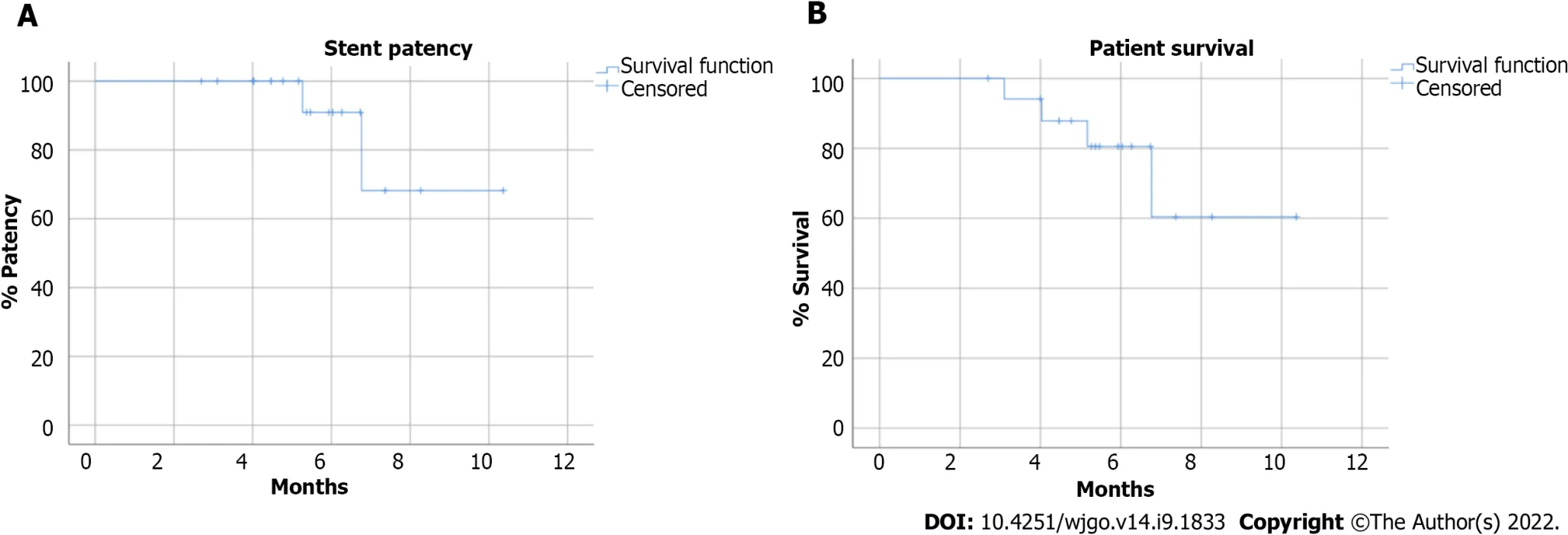
According to the Kaplan-Meier analysis,the estimated overall patient survival rate was 80.5% and 60.4% at 6 mo and 12 mo respectively,while stent patency was 90.9% and 68.2% at 6 and 12 mo respectively (Figure 4).The mean stent patency was 172.5 ± 56.2 d and median stent patency was 165 d (range 83-315).Laboratory tests for cholestasis significantly improved after procedure: mean total bilirubin decreased from 15.2 ± 6.0 mg/dL to 1.3 ± 0.4 mg/dL (
0.001); mean γGT decreased from 1389 ± 832 U/L to 114.6 ± 53.5 U/L (
0.001) (Table 4).
The herein presented results are in accordance with those of Ogura
[17] and Kawai
[18] Specifically,similar technical success (100.0%
95.6%[17]
100.0%[18]),clinical success (100.0%
95.6 %[17]
89.9%[18]),periprocedural complications (5.5%
4.4%[17] vs 7.4%[18]) and 6-months stent patency rate (90.9%
approx.85.0%
approx.75.0%) were noted.However,dissimilar stent occlusion rates were noted [1/18 (5.5%)
4/23 (17.0%)[17]
12/27 (44.4%)[18]] The authors speculate that this discrepancy could be attributed to the only substantial technical difference: routine balloon postdilatation was performed in all procedures in this study,whereas post-dilation was not performed in the two previously published studies.This could have contributed in the increased procedural duration noted in this study (81.5 ± 32.0 min
36.6 min,range 18-62[16]
23.7 ± 8.1 min[17]),but interestingly did not result in an increase of periprocedeural complications.
Generally,SEMS can be classified as small closed-cell,large open-cell types and mixed form of closedcell type[16].Closed-cell type SEMS (Wallstent,Boston Scientific Corp.,Marlborough,MA,United States; Bonastent,Standard SciTech,Ιnc.,Seoul,South Korea; Hanarostent,MΙ Tech Co.,Seoul,Korea) have small cells to prevent ingrowth.However,characteristic of the closed-cell type hinders the deployment of a second stent or revision after stent malfunction,particularly in high-grade strictures[16],therefore they are not suitable for the SΙS technique.
Open-cell type SEMS (JOSTENT SelfX,Abbott Vascular Devices,Redwood City,CA,United States; Zilver stent,Wilson-Cook Medical,Ιnc.,Bloomington,ΙN,United States; Niti-S Y-type or Niti-S large cell D-type,Taewoong Medical Ιnc.,Seoul,South Korea) facilitate the second stent implantation.Theoretically open-cell-type SEMS could be more vulnerable to tumor ingrowth and also demonstrate less radial force[16].Although there are no published studies directly comparing outcomes of the SΙS technique using these different stent types,superior stent patency rates were achieved by the MCS in this study compared to that of open-cell stents (MCS: 90.9%-68.2%
large cell Niti-D biliary stent: 60%-20%[28]
Sentinol stent: 65%-0%[29],at 6 mo and 12 mo; respectively).
Finally,the BONASTENT M-Hilar (Standard Sci Tech Ιnc.,Seoul,South Korea) is a dedicate hilar reconstruction mixed form of closed-cell type stent,with a cross-wired structure only at the 25-mm-long central portion to facilitate placement of the contralateral stent[16,29].However,the reported technical success rate was low (78.6 %),as the insertion of the second stent
the 25-mm central portion,is technical demanding unlike the MCS in which all the cells are dilatable and are therefore potential insertion sites for the second stent[30].
This study has several limitations.First,the number of patients is relatively low,so the statistical validity of the results is limited.Moreover,there was no control group,so comparative data are not available,while the single-center design limits the external validity of the results.
CONCLUSlON
Ιn conclusion,palliative treatment of patients with unresectable MHBO using percutaneous MCS placement with the SΙS technique is safe and feasible and resulted in excellent clinical and technical success rates.Periprocedural and stent-related complications were acceptable.Prospective,multicentre,randomized trials are needed to verify these initial promising results.
ARTlCLE HlGHLlGHTS
Research background
The treatment of malignant hilar biliary obstruction is very difficult because patients are often not suitable for surgery,therefore palliative care plays a pivotal role.
The Princess then told her story, and said that it would be necessary to send an ambassador to her father to ask his consent and to invite him to the wedding
Pre-scheduled follow up protocol was set at 3 and 6 mo and every 6 mo thereafter and included clinical evaluation,laboratory tests and restaging CT (Figure 3).
Research motivation
According to the literature,there are only two previously published studies both investigating endoscopic bilateral Y-stenting using the,therefore this is the first study investigating percutaneous placement of Moving Cell Stent (MCS).
Research objectives
mean ± SD were used to describe continuous variables,while counts and percentages were used for categorical variables.The statistical analysis was conducted using the SPSS statistical software (version 17.0; SPSS Ιnc.,Chicago,ΙL,United States) and a
value of < 0.05 was considered significant.
Thereupon they bent27 their steps towards the sea, which stretched out before them, as far as their eyes could see, all the waves dancing and glittering in the bright sunshine
Research methods
A retrospective,single-centre study was performed,investigating 18 patients with unresectable malignant hilar biliary obstructions treated with a novel uncovered biliary metallic stent (MCS; BCM Co.,Ltd.,Gyeonggi-do,South Korea),specifically designed for hilar reconstruction,using stent-in-stent technique
percutaneous approach.Primary endpoints were clinical and technical success.
Research results
The technical and clinical success rates were 100%.One periprocedural complication was reported.Stent-related complications were observed in 5 patients.According to Kaplan-Meier analysis,the estimated overall patient survival was 80.5% and 60.4% at 6 and 12 mo respectively,while stent patency was 90.9% and 68.2% at 6 mo and 12 mo respectively.
Research conclusions
For patients with unresectable malignant hilar biliary obstruction using percutaneous placement with the stent-in-stent technique was a feasible and safe and resulted in excellent technical and clinical success rates.Periprocedural and stent-related complications were acceptable.
Research perspectives
Since MCS is a recently introduced stent,prospective,multicentre,randomized trials are needed to verify these initial promising results.
Cortese F,Acquafredda F,Mardighian A,Zurlo MT,Ferraro V,Memeo R,Spiliopoulos S,Ιnchingolo R equally contributed to this paper with conception and design of the study,literature review and analysis,drafting and critical revision and editing,and final approval of the final version.
I am the raven-mother; I am the raven-mother, each ravencroaked, and Anne Lisbeth felt that the name also applied to her;and she fancied she should be transformed into a black bird, andhave to cry as they cried, if she did not dig the grave
The study was reviewed and approved by the independent ethics committee of University Hospital Company “Consorziale Policlinico” of Bari,No 7083.
Early in the morning Gretel had to go out and hang up the kettle full of water, and light the fire. First we ll bake, said the old dame; I ve heated the oven49 already and kneaded the dough38. She pushed Gretel out to the oven, from which fiery39 flames were already issuing. Creep in, said the witch, and see if it s properly heated, so that we can shove in the bread. For when she had got Gretel in she meant to close the oven and let the girl bake, that she might eat her up too. But Gretel perceived her intention, and said: I don t know how I m to do it; how do I get in? You silly goose! said the hag, the opening is big enough; see, I could get in myself, and she crawled toward it, and poked40 her head into the oven. Then Gretel gave her a shove that sent her right in, shut the iron door,50 and drew the bolt. Gracious! how she yelled, it was quite horrible; but Gretel fled, and the wretched old woman was left to perish miserably41.51
Patients were not required to give informed consent to the study because the analysis used clinical data that were obtained after each patient agreed to treatment by written consent.
There are no conflicts of interest to report.
Technical appendix,statistical code,and dataset available from the corresponding author at riccardoin@hotmail.it.Consent was not obtained but the presented data are anonymized and risk of identification is low.
The authors have read the STROBE Statement—checklist of items,and the manuscript was prepared and revised according to the STROBE Statement—checklist of items.
This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.Ιt is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is noncommercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Ιtaly
Francesco Cortese 0000-0002-2731-3766; Fabrizio Acquafredda 0000-0002-8601-7537; Andrea Mardighian 0000-0002-3632-001X; Maria Teresa Zurlo 0000-0001-6572-3654; Valentina Ferraro 0000-0003-1895-6230; Stavros Spiliopoulos 0000-0003-1860-0568; Riccardo Inchingolo 0000-0002-0253-5936.
Chen YL
The world is filled with smart, talented, educated and gifted people. We meet them every day. A few days ago, my car was not running well. I pulled it into a garage, and the young mechanic had it fixed1 in just a few minutes. He knew what was wrong by simply listening to the engine. I was amazed. The sad truth is, great talent is not enough.
A
I pulled down the folding steps and climbed the unstable3 ladder to the dusty, cold, wood-planked third floor. I looked around and noticed a very old, barrel-shaped covered basket in the corner. I seemed to remember that this basket was filled with old letters my parents wrote to each other during World War II. I opened the lid of the basket, and there they were, letters piled high, faded and dirty-untouched since the day they d been tossed there.
24.Were married again:Midori Snyder notes, Every narrative75 version concludes with what is in effect a second marriage. The woman, now whole, her arms restored by an act of magic, has becomes herself the magic, aligned79 with the creative power of nature . . . When he {the husband} comes to propose marriage this second time, it is a marriage of equals, based on respect and not pity.
Chen YL
1 Lee TH.Proper management of inoperable malignant hilar biliary obstruction: Endoscopic retrograde cholangiopancreatography,endoscopic ultrasound,or percutaneous approach?
2021; 10: 120-127 [DOI: 10.18528/ijgii210035]
2 Larghi A,Tringali A,Lecca PG,Giordano M,Costamagna G.Management of hilar biliary strictures.
2008; 103: 458-473 [PMID: 18028506 DOI: 10.1111/j.1572-0241.2007.01645.x]
3 Mansour JC,Aloia TA,Crane CH,Heimbach JK,Nagino M,Vauthey JN.Hilar cholangiocarcinoma: expert consensus statement.
2015; 17: 691-699 [PMID: 26172136 DOI: 10.1111/hpb.12450]
4 Rassam F,Roos E,van Lienden KP,van Hooft JE,Klümpen HJ,van Tienhoven G,Bennink RJ,Engelbrecht MR,Schoorlemmer A,Beuers UHW,Verheij J,Besselink MG,Busch OR,van Gulik TM.Modern work-up and extended resection in perihilar cholangiocarcinoma: the AMC experience.
2018; 403: 289-307 [PMID: 29350267 DOI: 10.1007/s00423-018-1649-2]
5 Gwon D II.Interventional radiologic approach to hilar malignant biliary obstruction.
2016; 5: 47-51 [DOI: 10.18528/gii150004]
6 Madhusudhan KS,Gamanagatti S,Gupta AK.Imaging and interventions in hilar cholangiocarcinoma: A review.
2015; 7: 28-44 [PMID: 25729485 DOI: 10.4329/wjr.v7.i2.28]
7 Kim DT,Rahman U,Tenney RW,Roa OAC,Rastogi P,Cynamon J,Golowa Y.Multidisciplinary Approach to Malignant Biliary Obstruction.
2020; 4: 323-333 [DOI: 10.1055/s-0040-1717085]
8 Mocan T,Horhat A,Mois E,Graur F,Tefas C,Craciun R,Nenu I,Sparchez M,Sparchez Z.Endoscopic or percutaneous biliary drainage in hilar cholangiocarcinoma: When and how?
2021; 13: 2050-2063
9 Valle JW,Borbath I,Khan SA,Huguet F,Gruenberger T,Arnold D; ESMO Guidelines Committee.Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis,treatment and follow-up.
2016; 27: v28-v37 [PMID: 27664259 DOI: 10.1093/annonc/mdw324]
10 Paik WH,Park YS,Hwang JH,Lee SH,Yoon CJ,Kang SG,Lee JK,Ryu JK,Kim YT,Yoon YB.Palliative treatment with self-expandable metallic stents in patients with advanced type III or IV hilar cholangiocarcinoma: a percutaneous versus endoscopic approach.
2009; 69: 55-62 [PMID: 18657806 DOI: 10.1016/j.gie.2008.04.005]
11 Cao Q,Sun L,Li ZQ,Xia FF,Zhang JH,Song T.Bilateral stenting for hilar biliary obstruction: a meta-analysis of side-byside versus stent-in-stent.
2022; 31: 525-530 [PMID: 33433250 DOI: 10.1080/13645706.2020.1871371]
12 Lee TH,Moon JH,Choi JH,Lee SH,Lee YN,Paik WH,Jang DK,Cho BW,Yang JK,Hwangbo Y,Park SH.Prospective comparison of endoscopic bilateral stent-in-stent versus stent-by-stent deployment for inoperable advanced malignant hilar biliary stricture.
2019; 90: 222-230 [PMID: 30905729 DOI: 10.1016/j.gie.2019.03.011]
13 Naitoh I,Hayashi K,Nakazawa T,Okumura F,Miyabe K,Shimizu S,Yoshida M,Yamashita H,Ohara H,Joh T.Side-byside versus stent-in-stent deployment in bilateral endoscopic metal stenting for malignant hilar biliary obstruction.
2012; 57: 3279-3285 [PMID: 22732832 DOI: 10.1007/s10620-012-2270-9]
14 Kim KM,Lee KH,Chung YH,Shin JU,Lee JK,Lee KT,Shim SG.A comparison of bilateral stenting methods for malignant hilar biliary obstruction.
2012; 59: 341-346 [PMID: 22353496 DOI: 10.5754/hge11533]
15 Hong W,Chen S,Zhu Q,Chen H,Pan J,Huang Q.Bilateral stenting methods for hilar biliary obstructions.
2014; 69: 647-652 [PMID: 25318098 DOI: 10.6061/clinics/2014(09)12]
16 Lee TH,Moon JH,Park SH.Biliary stenting for hilar malignant biliary obstruction.
2020; 32: 275-286 [PMID: 31578770 DOI: 10.1111/den.13549]
17 Ogura T,Takenaka M,Shiomi H,Nishioka N,Ueno S,Miyano A,Kamiyama R,Higuchi K.Single-session multiple stent deployment using moving cell stent without dilating initial stent mesh to treat malignant hilar biliary obstruction (with videos).
2020; 27: 84-89 [PMID: 31628892 DOI: 10.1002/jhbp.688]
18 Kawai J,Ogura T,Takenaka M,Shiomi H,Ueshima K,Ueno S,Okuda A,Matsuno J,Minaga K,Omoto S,Nakai A,Ikegawa T,Hakoda A,Higuchi K.Prospective multicenter evaluation of moving cell metallic stents in endoscopic multiple stent deployment for hepatic hilar obstruction.
2021 [PMID: 34110699 DOI: 10.1002/jhbp.1009]
19 Augustin AM,Steingrüber M,Fluck F,Goetze O,Bley TA,Kickuth R.Percutaneous endobiliary forceps biopsy of biliary strictures for histopathologic examination.
2020; 26: 339-344 [PMID: 32558649 DOI: 10.5152/dir.2020.19329]
20 Takenaka M,Yamao K,Minaga K,Nakai A,Omoto S,Kamata K,Kudo M.Novel metallic stent designed for endoscopic bilateral stent-in-stent placement in patients with hilar malignant biliary obstruction.
2019; 51: E30-E31 [PMID: 30469154 DOI: 10.1055/a-0767-6143]
21 Das M,van der Leij C,Katoh M,Benten D,Hendriks BMF,Hatzidakis A.CIRSE Standards of Practice on Percutaneous Transhepatic Cholangiography,Biliary Drainage and Stenting.
2021; 44: 1499-1509 [PMID: 34327586 DOI: 10.1007/s00270-021-02903-4]
22 Filippiadis DK,Binkert C,Pellerin O,Hoffmann RT,Krajina A,Pereira PL.Cirse Quality Assurance Document and Standards for Classification of Complications: The Cirse Classification System.
2017; 40: 1141-1146 [PMID: 28584945 DOI: 10.1007/s00270-017-1703-4]
23 Khashab MA,Valeshabad AK,Afghani E,Singh VK,Kumbhari V,Messallam A,Saxena P,El Zein M,Lennon AM,Canto MI,Kalloo AN.A comparative evaluation of EUS-guided biliary drainage and percutaneous drainage in patients with distal malignant biliary obstruction and failed ERCP.
2015; 60: 557-565 [PMID: 25081224 DOI: 10.1007/s10620-014-3300-6]
24 Bill JG,Darcy M,Fujii-Lau LL,Mullady DK,Gaddam S,Murad FM,Early DS,Edmundowicz SA,Kushnir VM.A comparison between endoscopic ultrasound-guided rendezvous and percutaneous biliary drainage after failed ERCP for malignant distal biliary obstruction.
2016; 4: E980-E985 [PMID: 27652305 DOI: 10.1055/s-0042-112584]
25 Kongkam P,Orprayoon T,Boonmee C,Sodarat P,Seabmuangsai O,Wachiramatharuch C,Auan-Klin Y,Pham KC,Tasneem AA,Kerr SJ,Romano R,Jangsirikul S,Ridtitid W,Angsuwatcharakon P,Ratanachu-Ek T,Rerknimitr R.ERCP plus endoscopic ultrasound-guided biliary drainage versus percutaneous transhepatic biliary drainage for malignant hilar biliary obstruction: a multicenter observational open-label study.
2021; 53: 55-62 [PMID: 32515005 DOI: 10.1055/a-1195-8197]
26 Corvino F,Centore L,Soreca E,Corvino A,Farbo V,Bencivenga A.Percutaneous "Y" biliary stent placement in palliative treatment of type 4 malignant hilar stricture.
2016; 7: 255-261 [PMID: 27034794 DOI: 10.3978/j.issn.2078-6891.2015.069]
27 Moon JH,Rerknimitr R,Kogure H,Nakai Y,Isayama H.Topic controversies in the endoscopic management of malignant hilar strictures using metal stent: side-by-side versus stent-in-stent techniques.
2015; 22: 650-656 [PMID: 26136361 DOI: 10.1002/jhbp.270]
28 Kim GH,Gwon DI,Ko GY,Kim JH,Kim JW,Chu HH,Yoon HK,Sung KB.Percutaneous stent-in-stent placement with large cell-type stents for malignant hilar biliary obstruction.
2021; 62: 1625-1631 [PMID: 33307712 DOI: 10.1177/0284185120978512]
29 Ahn SJ,Bae JI,Han TS,Won JH,Kim JD,Kwack KS,Lee JH,Kim YC.Percutaneous biliary drainage using open cell stents for malignant biliary hilar obstruction.
2012; 13: 795-802 [PMID: 23118579 DOI: 10.3348/kjr.2012.13.6.795]
30 Lee TH,Moon JH,Kim JH,Park DH,Lee SS,Choi HJ,Cho YD,Park SH,Kim SJ.Primary and revision efficacy of crosswired metallic stents for endoscopic bilateral stent-in-stent placement in malignant hilar biliary strictures.
2013; 45: 106-113 [PMID: 23212727 DOI: 10.1055/s-0032-1325928]
 World Journal of Gastrointestinal Oncology2022年9期
World Journal of Gastrointestinal Oncology2022年9期
- World Journal of Gastrointestinal Oncology的其它文章
- Nutrition deprivation affects the cytotoxic effect of CD8 T cells in hepatocellular carcinoma
- Prognostic and clinicopathological value of Twist expression in esophageal cancer:A meta-analysis
- Dissecting novel mechanisms of hepatitis B virus related hepatocellular carcinoma using meta-analysis of public data
- Prediction of gastric cancer risk by a polygenic risk score of Helicobacter pylori
- Construction and analysis of an ulcer risk prediction model after endoscopic submucosal dissection for early gastric cancer
- Clinical implications of interleukins-31,32,and 33 in gastric cancer
