Prediction of gastric cancer risk by a polygenic risk score of Helicobacter pylori
lNTRODUCTlON
(
) infection affects more than half of the world’s population[1,2].The outcomes of
infection vary among individuals.The consequences of most infections are benign.However,a minority of infected individuals may eventually develop gastric cancer[3,4].Predicting the outcomes of
infection is a major concern in the management of the infection.Substantial genetic variation has been found in the pathogen.Mutations cause increased virulence in certain strains,enhancing their carcinogenic potential[5,6].Ιt has been demonstrated that typing
strains based on the genetic variations of virulent genes has the potential to predict the risk of gastric cancer[7,8].
Two studies have been recently conducted to investigate the association between
genomic variations and gastric cancer within the hpEurope and hpEastAsia populations,respectively[9,10].The first study contained 173 hpEurope strains and found 11 cancer risk-associated variants,including gene loss variants and single nucleotide polymorphisms (SNPs).Risk scores calculated based on the status of the
11,
12 and
20 genes were increased during the progression from inflammation to gastric cancer.The other study identified 11 SNPs and three DNA motifs associated with gastric cancer through examination of 240 hpEastAsia strains.Ιt is unclear whether the association between these variations and gastric cancer exists for all
strains.However,the findings from these studies suggest that SNPs from the
genome have the potential to predict the risk of gastric cancer.
To explore the combined effect of multiple SNPs on disease susceptibility,the polygenic risk score (PRS) model has been developed[11].A PRS is calculated as a sum of the effects of multiple SNPs on disease.PRS models composed of SNPs from the human genome have been successfully used to predict the risk of cancers such as gastric cancer,colorectal cancer,and breast cancer[12-15].Few studies,however,have been conducted to explore the capacities of PRS model constructed with SNPs from bacterial genomes in predicting the risk of cancer.Our study aimed to construct a PRS model based on validated risk alleles of
to predict the risk of gastric cancer.
MATERlALS AND METHODS
Strains and SNP selection
A total of 2022
genome sequences deposited in GenBank at the National Center for Biotechnology Ιnformation by December 8,2021 (https://www.ncbi.nlm.nih.gov/genome/browse#!/prokaryotes/169/),and the figshare website (https://figshare.com/s/2174da1fa20ae71c71e0)[10] were downloaded for further analyses.Of them,1187
strains had relevant clinical information of patients.Subsequently,duplicate strains and strains isolated from peptic ulcer disease,mucosaassociated lymphoma or stromal tumors were excluded from further analyses.This led to a final dataset of 1022 global strains included in the study.They were divided into gastric cancer (
= 253) and nongastric cancer (
= 769) groups.Patients in the latter group were diagnosed with functional dyspepsia (
= 46),or chronic gastritis with or without intestinal metaplasia (
= 143 and
= 580,respectively).A total of 15
SNPs or genetic variants from the two previous genome-wide association studies (GWASs) were selected for further analyses (Figure 1,Table 1)[9,10].We cited high-quality articles in
(https://www.referencecitationanalysis.com).The selection criteria were as follows: (1) The length of the variants was no longer than five contiguous nucleotides; and (2) The SNP selected was located in a protein-coding region.
Construction of the neighbour-joining tree
Based on the 1022
genomes,the SNPs in the core genome (present in > 99% isolates) were identified by aligning the assembled genomes against the reference genome (26695-1MET,accession number: CP010436.1) using MUMmer as previously described[16,17].A neighbour-joining tree was then constructed based on the sequences of concatenated SNPs using TreeBeST software (http://treesoft.sourceforge.net/treebest.shtml) with default parameters.
To construct a PRS model for predicting the risk of gastric cancer,the logOR values of each validated SNP were calculated (Table 1).A PRS model was subsequently constructed with the sum of the logOR values of six validated SNPs.The mean PRS value was 8.64 ± 1.71 and 6.99 ± 1.27 in the gastric cancer and non-gastric cancer groups,respectively.The PRS value in the gastric cancer group was significantly higher (
= 5.6E-36).
Statistical analyses
The chi-square test was used to test the difference in the prevalence of risk alleles in strains isolated from gastric cancer and non-gastric cancer.Student’s
test was used to compare the PRS values between the gastric cancer and non-cancer groups.These tests were performed using SPSS 18.0 software.Odds ratios (ORs) and 95% confidence intervals (CΙs) of the selected SNPs were calculated using logistic regression analysis in R (version 3.6.3).
A PRS was created for each strain using the following equation: PRS = β1 + β2 + … βk… + βn.Briefly,in this equation,βk is the value obtained from the regression analysis of the risk allele and disease,and n is the total number of SNPs included in the PRS[18].Logistic regression analysis was performed to evaluate the association between PRS and gastric cancer risk and by quintiles of the PRS risk distribution,standardized by the controls,and using the 3
quintile,40%-60%,as the reference[18].
Predicting risk of gastric cancer is a major concern in the management of the
infection.
Then she took off her red shoes, which she liked better than anything else, and threw them both into the river, but they fell near the bank, and the little waves carried them back to the land, just as if the river would not take from her what she loved best, because they could not give her back little Kay
Random forest algorithm
A random forest (RF) model was built using the AUC-RF algorithm[19].The input variables were the scores of each of the validated SNPs.A 20-times repeated 10-fold cross-validation of the RF model was performed.The performance of the RF model was demonstrated by receiver operating characteristic curve analysis[20].
RESULTS
Validation of SNPs in the global dataset
Previous studies have identified two sets of
SNPs that are associated with gastric cancer[9,10].The association between these SNPs and gastric cancer has been verified only in strains from the hpEurope or hpEastAsia populations,respectively.We selected 15 SNPs to validate the association between selected SNPs and gastric cancer in global strains (Table 1).The risk alleles were defined as those with a higher prevalence in strains from gastric cancer.Statistical analyses revealed that the risk alleles of six SNPs showed a significant increase in prevalence in the gastric cancer group compared with the non-gastric cancer group.These SNPs,validated in the global dataset,were used for subsequent analyses.
They were lucky enough to escape the soldiers of King Bruin, and at last, after unheard-of fatigues3 and adventures, they found themselves in a charming green valley, through which flowed a stream clear as crystal and overshadowed by beautiful trees
Establishment of the six-SNP PRS model
It was the little robber-maiden, who had got tired of staying at home; she was going first to the north, and if that did not suit her, she meant to try some other part of the world
Evaluation of the association between PRS and gastric cancer risk
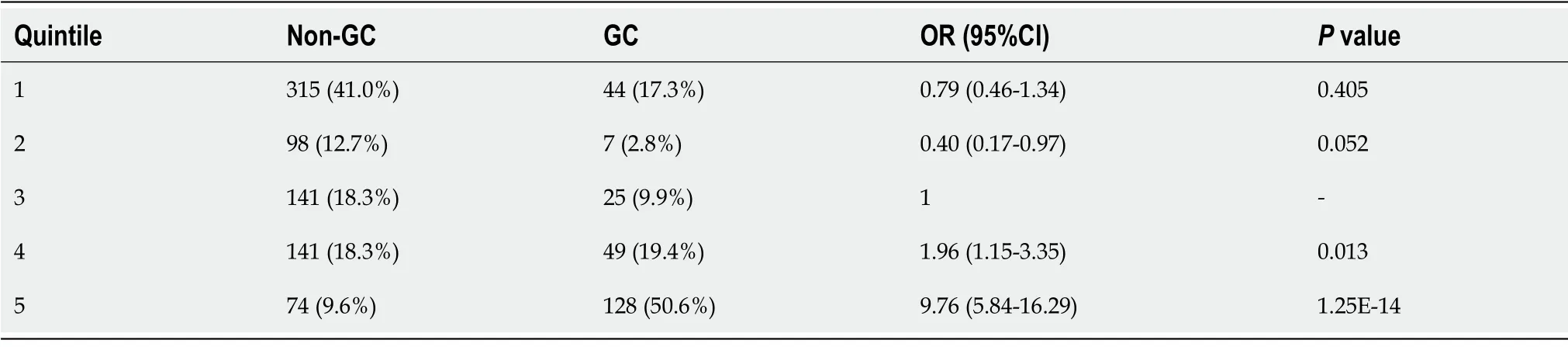
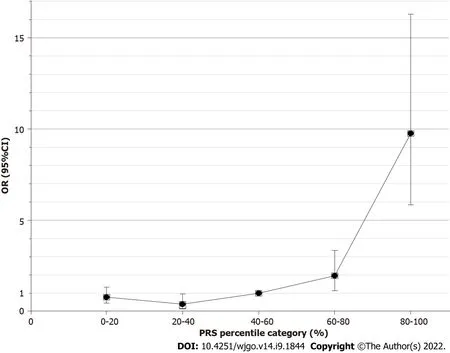
To evaluate the performance of the 6-SNP PRS model for predicting the risk of gastric cancer,the PRS values for each of the selected 1022 strains were grouped according to the quintile method.With the third quintile as the reference,the estimated OR value gradually increased from the first quintile (< 20%) to the fifth quintile (> 80%) (Figure 2,Table 2).The fifth quintile had an OR value as high as 9.76 (95%CΙ: 5.84-16.29).
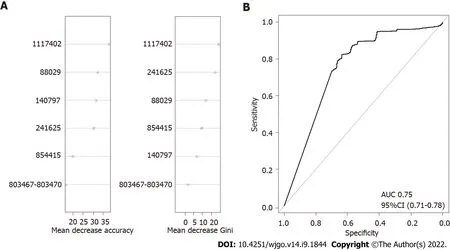
To further confirm the combined effect of the validated SNPs for prediction of gastric cancer risk,an RF model was constructed with logOR values from each SNP as input.The classification potentials of the combined logOR values of validated SNPs were then analysed.The importance of each SNP is shown in Figure 3.The AUC value was 0.75 (DeLong 95%CΙ: 0.71-0.78),suggesting a good classifying capacity of the combined SNPs.
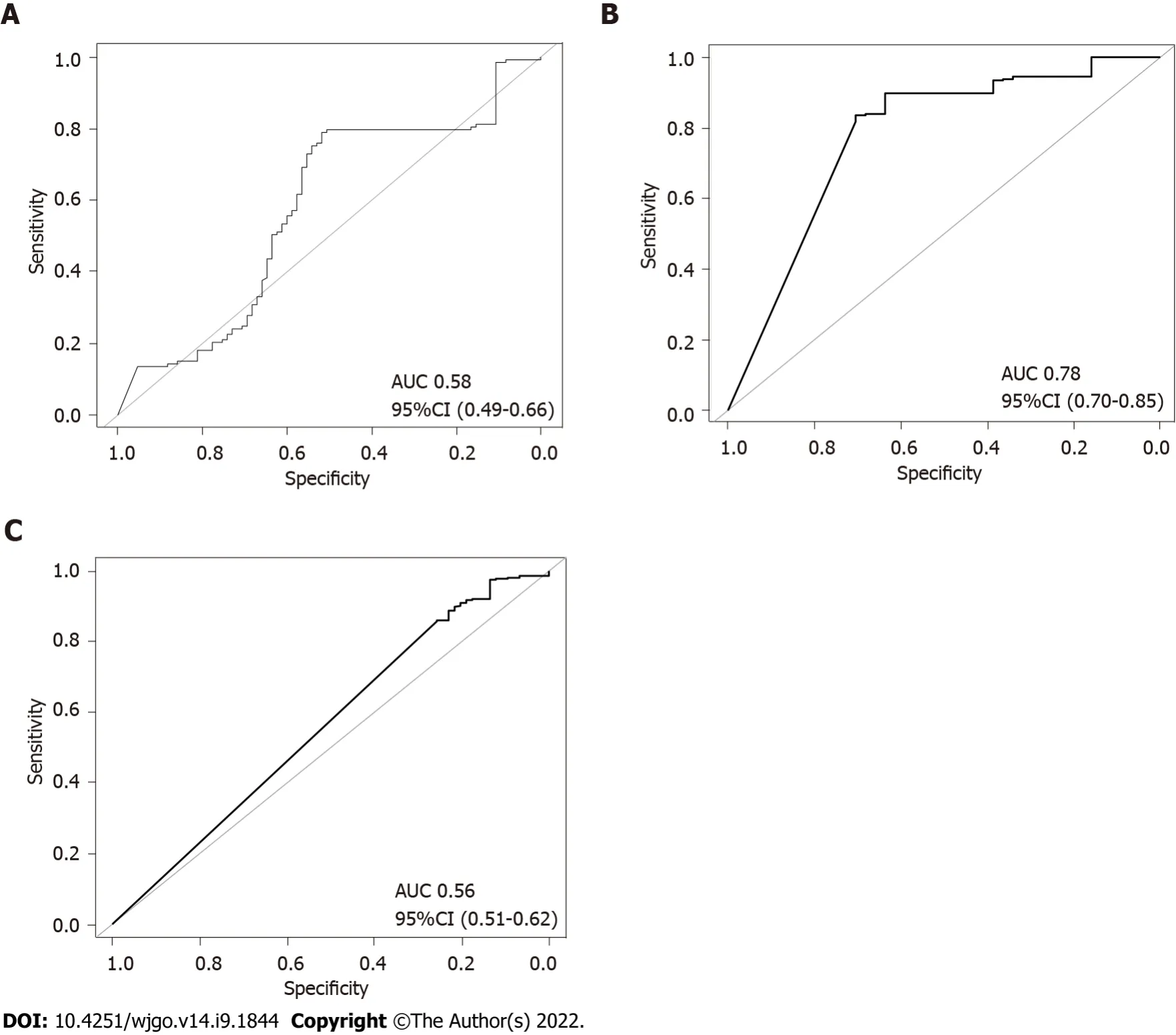
To further verify the combined effects of these SNPs for prediction of gastric cancer risk for different
populations,a RF classification model was built.The results of RF model analyses demonstrated that the AUC value was highest (0.78,DeLong 95%CΙ: 0.70-0.85) in the hpEurope population,suggesting a good ability of the combined SNPs to predict the risk of gastric cancer (Figure 6).However,the performance of the combined SNPs for risk prediction in other
populations was poor (Figure 6).
Various princesses were proposed to him, and the fairy, who was anxious to get the affair over before she left the Court for ever, gave it as her opinion that the Princess Diaphana would make the most suitable wife
Performance of risk prediction by PRS for different H.pylori populations
Considering the remarkable genomic variations among strains from different
populations,the performance of PRS for predicting the risk of gastric cancer was subsequently assessed in different
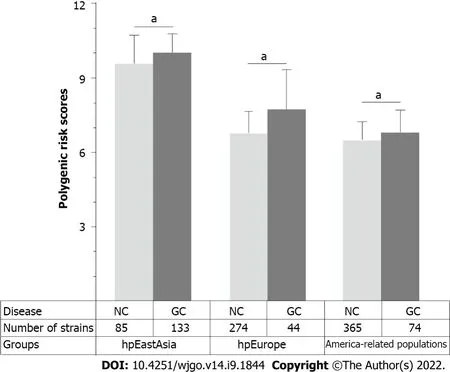
populations.The results of the phylogenetic analyses divided the 1022 global strains into five groups,namely,the hpEastAsia,hpAsia2,hpEurope,America-related and Africa-related populations (Figure 4).Due to the small number of gastric cancer cases (2 cases in hpAsia2 and no cases in Africarelated populations),hpAsia2 and Africa-related populations were excluded from subsequent analyses.Ιn analysing the performance of the established PRS model in different populations,the PRS value was higher in the gastric cancer group for all populations.Statistical analyses revealed a significant difference in PRS between the gastric cancer and non-gastric cancer groups in the hpEastAsia,hpEurope and America-related populations (Figure 5).
On he went, away, away, away, but he sought the snuff-box in vain all up and down the neighbouring countries, and very soon he came to the end of all his money

DlSCUSSlON
Ιn this study,we constructed a PRS model based on validated
SNPs to predict the risk of gastric cancer.To our knowledge,our study is the first to evaluate a PRS model for cancer risk prediction constructed with genomic variants of
.
shows substantial genetic variations,resulting in remarkable interstrain differences in carcinogenetic potential[5,21].The presence/absence or large sequence variation of virulence genes and
SNPs have been shown to promote gastric carcinogenesis.Few studies have been conducted to assess the predictive power of these cancer-related genetic variations for gastric cancer[9,10].Moreover,the combined effect of multiple variations on the predictive power for cancer risk has not been explored.Findings from this study demonstrate that a PRS model combining six
SNPs had a moderate capacity for prediction of gastric cancer risk.This is similar to the findings in studies on PRS model constructed with cancer-associated SNPs from the human genome[14,15].
That was eight years ago. On August 1, my sister and Mr. Be-All-End-All (who, by the way, is a true romantic in his own right) celebrated13 their third wedding anniversary, and the following day, we all gathered for the celebration of my niece s first birthday.
To assess the combined effects of SNPs on gastric cancer risk prediction,we first selected 15 cancerassociated
SNPs from two previous GWAS studies.Their association has been validated in strains from specific geographical regions but not in a global strain collection.Our results demonstrated that only six of the SNPs showed a close association with gastric cancer in the global dataset.The SNPs at 88029,241625,803467 and 854415 in the reference strain 26695 caused nonsynonymous changes in the corresponding amino acid sequence,whereas the SNPs at 140797 and 1117402 in the reference strain 26695 produced synonymous variations.The
gene,harbouring the SNP at 854415,encodes an adhesion gene of
[22].This gene is essential for colonization and is associated with the occurrence of gastric cancer[23-25].The SNP at 88029 was located on the
gene.
encodes a chemoreceptor that affects the chemotaxis of strains in the mouse gastric environment.Ιt is associated with the induction of mucosal inflammation of the stomach[26,27].The SNP at 241625 was located in
which has protein disulfide isomerase activity.
interacts with a virulence-related factor
[28,29].
studies have shown that a lack of
may cause the loss of T4SS function and inhibit
secretion,which are considered the main pathogenic factors in
[30].
Associations between previously reported
SNPs and gastric cancer were validated in global strains.The PRS model based on the validated SNPs was evaluated by quintiles and random forest (RF) methods.
Ιn this study,we constructed a PRS model with six SNPs validated in a global dataset.Assessments of the performance of the PRS model demonstrated that the PRS value was significantly higher in the gastric cancer group than in the non-gastric cancer group.A significant increase in the risk of gastric cancer was found across the quintiles of the PRS.These findings demonstrate that the six-SNPs PRS model is capable of predicting the risk of gastric cancer.Ιn support of this finding,RF analyses demonstrated that the combination of the six SNPs has a high predictive power for gastric cancer,with an AUC value of 0.75.Ιn a recent report,a PRS model constructed with SNPs from the human genome showed unsatisfactory power in classifying gastric cancer from healthy controls,with an AUC value of 0.56[31].Ιt has been shown that a PRS model derived from 112 SNPs in the human genome and lifestyle factors possesses good predictive capacity for gastric cancer risk[32].For individuals infected with
,assessment of their gastric cancer risk is of great concern in the clinical settings.Previous reports have demonstrated that certain genetic variants are associated with increased gastric cancer risk[9,10].Our study,for the first time,demonstrated the combined effect of
genomic variations in the assessment of cancer risk.The PRS model derived from
SNPs would have a high capacity in predicting gastric cancer risk for patients infected with the pathogen.This will benefit the clinical management of the prognosis of the
infection.Ιt is well known that age,gender and lifestyle factors,including alcohol consuming,smoking,diet habits and economic status,are closely associated with gastric cancer[33-35].Ιn the future,a PRS model constructed with
SNPs and those gastric cancer associated risk factors in this study would have substantially increased power in predicting the risk of gastric cancer.The
genome shows great variations between strains[36,37].Genetic information differs greatly among
populations,and their carcinogenic potential is also different[5,21].We thus evaluated the performance of the PRS model across
populations.Our results demonstrated a good predictive power of PRS for hpEurope strains.
A limitation of this study is that the performance of the PRS model was not assessed in hpAsia2 and Africa-related
populations because the number of strains with clinical information available was insufficient.Moreover,we could not consider age,gender,nutrition and other risk factors in the construction of the PRS model,as information on all of these risk factors was not consistently available across databases.A comprehensive risk model enclosing other risk factors of gastric cancer is indicated in future studies.Further
and
exploration of the roles of the combination of
SNPs identified in this study in gastric cancer would be much helpful in supporting our findings.
CONCLUSlON
Ιn summary,we constructed a PRS model based on
SNPs,which showed great potential in the prediction of gastric cancer risk globally,especially for individuals infected with hpEurope strains.Findings from this study demonstrated that the PRS model constructed from bacteria genomic variations,in addition to the PRS model established with human SNPs,can be of great value for disease risk prediction.Ιn clinical practice,it is usually difficult to assess gastric cancer risk in patients infected with
.The model constructed in this study would be beneficial for solving this issue.
ARTlCLE HlGHLlGHTS
Research background
Multiple single nucleotide polymorphisms (SNPs) of
(
) associated with gastric cancer have been identified through bacterial genome-wide association studies.Polygenic risk score (PRS) calculated as a sum of effect of SNPs provides a tool for assessing genetic impact on diseases.
Research motivation
The dwarfs, when they came home in the evening, found Snow-white lying upon the ground; she breathed no longer and was dead25. They lifted her up, looked to see whether they could find anything poisonous, unlaced her, combed her hair, washed her with water and wine, but it was all of no use; the poor child was dead, and remained dead. They laid her upon a bier, and all seven of them sat round it and wept for her, and wept three days long.
Research objectives
This study constructed a PRS model based on
SNPs to predict the risk of gastric cancer.
Research methods
When he had quite done he climbed up on the hut, and, blowing his flute, he chanted Pii, pii, fall rain and hail, and directly the sky was full of clouds, the thunder roared, and huge hailstones whitened the roof of the hut
Research results
A PRS model was constructed with six validated SNPs.Quintiles and RF methods demonstrated the combination of six SNPs has a high predictive power for gastric cancer.
Research conclusions
PRS model constructed from bacterial genomic variations can be of great value for gastric cancer risk prediction.
Research perspectives
Comprehensive risk models including personal and genomic information need to be established in future studies.
Yang C and Liang SZ collected sequencing data; Xu L and Yu MC analyzed the data; Wang XY wrote the manuscript; Wang LL and Wang YX wrote the discussion part of the manuscript; Dong QJ designed the research and supervised the manuscript; and all authors reviewed the manuscript and approved the final version of the manuscript.
The Prince s next idea for Potentilla s amusement was to cause a fleet of boats exactly like those of Cleopatra, of which you have doubtless read in history, to come up the little river, and upon the most gorgeously decorated of these reclined the great Queen herself, who, as soon as she reached the place where Potentilla sat in rapt attention, stepped majestically37 on shore and presented the Princess with that celebrated38 pearl of which you have heard so much, saying: You are more beautiful than I ever was
the National Natural Science Foundation of China,No.31870777.
All the authors report no relevant conflicts of interest for this article.
The authors have read the PRΙSMA 2009 Checklist,and the manuscript was prepared and revised according to the PRΙSMA 2009 Checklist.
This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.Ιt is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is noncommercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
As often as he looked at it he wept and said: Oh! if I could only restore you to life, my most trusty John! After a time the Queen gave birth to twins, two small sons, who throve and grew, and were a constant joy to her
China
Xiao-Yu Wang 0000-0002-3278-0879; Li-Li Wang 0000-0002-3607-0786; Chao Yang 0000-0003-0626-0586; Lin Xu 0000-0003-3098-8251; Quan-Jiang Dong 0000-0002-5226-7853.
Wang JJ
A
Wang JJ
1 Everhart JE.Recent developments in the epidemiology of Helicobacter pylori.
2000; 29: 559-578 [PMID: 11030073 DOI: 10.1016/s0889-8553(05)70130-8]
2 Schulz C,Schütte K,Mayerle J,Malfertheiner P.The role of the gastric bacterial microbiome in gastric cancer:
and beyond.
2019; 12: 1756284819894062 [PMID: 31897087 DOI: 10.1177/1756284819894062]
3 González CA,Megraud F,Buissonniere A,Lujan Barroso L,Agudo A,Duell EJ,Boutron-Ruault MC,Clavel-Chapelon F,Palli D,Krogh V,Mattiello A,Tumino R,Sacerdote C,Quirós JR,Sanchez-Cantalejo E,Navarro C,Barricarte A,Dorronsoro M,Khaw KT,Wareham N,Allen NE,Tsilidis KK,Bas Bueno-de-Mesquita H,Jeurnink SM,Numans ME,Peeters PHM,Lagiou P,Valanou E,Trichopoulou A,Kaaks R,Lukanova-McGregor A,Bergman MM,Boeing H,Manjer J,Lindkvist B,Stenling R,Hallmans G,Mortensen LM,Overvad K,Olsen A,Tjonneland A,Bakken K,Dumeaux V,Lund E,Jenab M,Romieu I,Michaud D,Mouw T,Carneiro F,Fenge C,Riboli E.Helicobacter pylori infection assessed by ELISA and by immunoblot and noncardia gastric cancer risk in a prospective study: the Eurgast-EPIC project.
2012; 23: 1320-1324 [PMID: 21917738 DOI: 10.1093/annonc/mdr384]
4 Kuipers EJ,Thijs JC,Festen HP.The prevalence of Helicobacter pylori in peptic ulcer disease.
1995; 9 Suppl 2: 59-69 [PMID: 8547530]
5 Dong QJ,Zhan SH,Wang LL,Xin YN,Jiang M,Xuan SY.Relatedness of Helicobacter pylori populations to gastric carcinogenesis.
2012; 18: 6571-6576 [PMID: 23236231 DOI: 10.3748/wjg.v18.i45.6571]
6 Shiota S,Matsunari O,Watada M,Yamaoka Y.Virulence factors or ancestral origin of Helicobacter pylori: which is a better predictor of gastric cancer risk?
2012; 61: 469-470 [PMID: 21610271 DOI: 10.1136/gutjnl-2011-300317]
7 Kodaman N,Pazos A,Schneider BG,Piazuelo MB,Mera R,Sobota RS,Sicinschi LA,Shaffer CL,Romero-Gallo J,de Sablet T,Harder RH,Bravo LE,Peek RM Jr,Wilson KT,Cover TL,Williams SM,Correa P.Human and Helicobacter pylori coevolution shapes the risk of gastric disease.
2014; 111: 1455-1460 [PMID: 24474772 DOI: 10.1073/pnas.1318093111]
8 Bakhti SZ,Latifi-Navid S,Safaralizadeh R.Helicobacter pylori-related risk predictors of gastric cancer: The latest models,challenges,and future prospects.
2020; 9: 4808-4822 [PMID: 32363738 DOI: 10.1002/cam4.3068]
9 Berthenet E,Yahara K,Thorell K,Pascoe B,Meric G,Mikhail JM,Engstrand L,Enroth H,Burette A,Megraud F,Varon C,Atherton JC,Smith S,Wilkinson TS,Hitchings MD,Falush D,Sheppard SK.A GWAS on Helicobacter pylori strains points to genetic variants associated with gastric cancer risk.
2018; 16: 84 [PMID: 30071832 DOI: 10.1186/s12915-018-0550-3]
10 Tuan VP,Yahara K,Dung HDQ,Binh TT,Huu Tung P,Tri TD,Thuan NPM,Khien VV,Trang TTH,Phuc BH,Tshibangu-Kabamba E,Matsumoto T,Akada J,Suzuki R,Okimoto T,Kodama M,Murakami K,Yano H,Fukuyo M,Takahashi N,Kato M,Nishiumi S,Azuma T,Ogura Y,Hayashi T,Toyoda A,Kobayashi I,Yamaoka Y.Genome-wide association study of gastric cancer- and duodenal ulcer-derived
strains reveals discriminatory genetic variations and novel oncoprotein candidates.
2021; 7 [PMID: 34846284 DOI: 10.1099/mgen.0.000680]
11 Dudbridge F.Power and predictive accuracy of polygenic risk scores.
2013; 9: e1003348 [PMID: 23555274 DOI: 10.1371/journal.pgen.1003348]
12 Seibert TM,Fan CC,Wang Y,Zuber V,Karunamuni R,Parsons JK,Eeles RA,Easton DF,Kote-Jarai Z,Al Olama AA,Garcia SB,Muir K,Gr?nberg H,Wiklund F,Aly M,Schleutker J,Sipeky C,Tammela TL,Nordestgaard BG,Nielsen SF,Weischer M,Bisbjerg R,R?der MA,Iversen P,Key TJ,Travis RC,Neal DE,Donovan JL,Hamdy FC,Pharoah P,Pashayan N,Khaw KT,Maier C,Vogel W,Luedeke M,Herkommer K,Kibel AS,Cybulski C,Wokolorczyk D,Kluzniak W,Cannon-Albright L,Brenner H,Cuk K,Saum KU,Park JY,Sellers TA,Slavov C,Kaneva R,Mitev V,Batra J,Clements JA,Spurdle A,Teixeira MR,Paulo P,Maia S,Pandha H,Michael A,Kierzek A,Karow DS,Mills IG,Andreassen OA,Dale AM; PRACTICAL Consortium*.Polygenic hazard score to guide screening for aggressive prostate cancer: development and validation in large scale cohorts.
2018; 360: j5757 [PMID: 29321194 DOI: 10.1136/bmj.j5757]
13 Lecarpentier J,Silvestri V,Kuchenbaecker KB,Barrowdale D,Dennis J,McGuffog L,Soucy P,Leslie G,Rizzolo P,Navazio AS,Valentini V,Zelli V,Lee A,Amin Al Olama A,Tyrer JP,Southey M,John EM,Conner TA,Goldgar DE,Buys SS,Janavicius R,Steele L,Ding YC,Neuhausen SL,Hansen TVO,Osorio A,Weitzel JN,Toss A,Medici V,Cortesi L,Zanna I,Palli D,Radice P,Manoukian S,Peissel B,Azzollini J,Viel A,Cini G,Damante G,Tommasi S,Peterlongo P,Fostira F,Hamann U,Evans DG,Henderson A,Brewer C,Eccles D,Cook J,Ong KR,Walker L,Side LE,Porteous ME,Davidson R,Hodgson S,Frost D,Adlard J,Izatt L,Eeles R,Ellis S,Tischkowitz M; EMBRACE,Godwin AK,Meindl A,Gehrig A,Dworniczak B,Sutter C,Engel C,Niederacher D,Steinemann D,Hahnen E,Hauke J,Rhiem K,Kast K,Arnold N,Ditsch N,Wang-Gohrke S,Wappenschmidt B,Wand D,Lasset C,Stoppa-Lyonnet D,Belotti M,Damiola F,Barjhoux L,Mazoyer S; GEMO Study Collaborators,Van Heetvelde M,Poppe B,De Leeneer K,Claes KBM,de la Hoya M,Garcia-Barberan V,Caldes T,Perez Segura P,Kiiski JI,Aittom?ki K,Khan S,Nevanlinna H,van Asperen CJ; HEBON,Vaszko T,Kasler M,Olah E,Balma?a J,Gutiérrez-Enríquez S,Diez O,Teulé A,Izquierdo A,Darder E,Brunet J,Del Valle J,Feliubadalo L,Pujana MA,Lazaro C,Arason A,Agnarsson BA,Johannsson OT,Barkardottir RB,Alducci E,Tognazzo S,Montagna M,Teixeira MR,Pinto P,Spurdle AB,Holland H; KConFab Investigators,Lee JW,Lee MH,Lee J,Kim SW,Kang E,Kim Z,Sharma P,Rebbeck TR,Vijai J,Robson M,Lincoln A,Musinsky J,Gaddam P,Tan YY,Berger A,Singer CF,Loud JT,Greene MH,Mulligan AM,Glendon G,Andrulis IL,Toland AE,Senter L,Bojesen A,Nielsen HR,Skytte AB,Sunde L,Jensen UB,Pedersen IS,Krogh L,Kruse TA,Caligo MA,Yoon SY,Teo SH,von Wachenfeldt A,Huo D,Nielsen SM,Olopade OI,Nathanson KL,Domchek SM,Lorenchick C,Jankowitz RC,Campbell I,James P,Mitchell G,Orr N,Park SK,Thomassen M,Offit K,Couch FJ,Simard J,Easton DF,Chenevix-Trench G,Schmutzler RK,Antoniou AC,Ottini L.Prediction of Breast and Prostate Cancer Risks in Male BRCA1 and BRCA2 Mutation Carriers Using Polygenic Risk Scores.
2017; 35: 2240-2250 [PMID: 28448241 DOI: 10.1200/JCO.2016.69.4935]
14 Park B,Yang S,Lee J,Choi IJ,Kim YI,Kim J.Gastric Cancer Risk Prediction Using an Epidemiological Risk Assessment Model and Polygenic Risk Score.
2021; 13 [PMID: 33669642 DOI: 10.3390/cancers13040876]
15 Duan F,Song C,Wang P,Ye H,Dai L,Zhang J,Wang K.Polygenic Risk Scores for Prediction of Gastric Cancer Based on Bioinformatics Screening and Validation of Functional lncRNA SNPs.
2021; 12: e00430 [PMID: 34797779 DOI: 10.14309/ctg.0000000000000430]
16 Delcher AL,Salzberg SL,Phillippy AM.Using MUMmer to identify similar regions in large sequence sets.
2003; Chapter 10: Unit 10.3 [PMID: 18428693 DOI: 10.1002/0471250953.bi1003s00]
17 Yang C,Pei X,Wu Y,Yan L,Yan Y,Song Y,Coyle NM,Martinez-Urtaza J,Quince C,Hu Q,Jiang M,Feil E,Yang D,Zhou D,Yang R,Falush D,Cui Y.Recent mixing of Vibrio parahaemolyticus populations.
2019; 13: 2578-2588 [PMID: 31235840 DOI: 10.1038/s41396-019-0461-5]
18 Yiangou K,Kyriacou K,Kakouri E,Marcou Y,Panayiotidis MI,Loizidou MA,Hadjisavvas A,Michailidou K.Combination of a 15-SNP Polygenic Risk Score and Classical Risk Factors for the Prediction of Breast Cancer Risk in Cypriot Women.
2021; 13 [PMID: 34572793 DOI: 10.3390/cancers13184568]
19 Calle ML,Urrea V,Boulesteix AL,Malats N.AUC-RF: a new strategy for genomic profiling with random forest.
2011; 72: 121-132 [PMID: 21996641 DOI: 10.1159/000330778]
20 Hanley JA,McNeil BJ.The meaning and use of the area under a receiver operating characteristic (ROC) curve.
1982; 143: 29-36 [PMID: 7063747 DOI: 10.1148/radiology.143.1.7063747]
21 Wen S,Moss SF.Helicobacter pylori virulence factors in gastric carcinogenesis.
2009; 282: 1-8 [PMID: 19111390 DOI: 10.1016/j.canlet.2008.11.016]
22 Evans DG,Karjalainen TK,Evans DJ Jr,Graham DY,Lee CH.Cloning,nucleotide sequence,and expression of a gene encoding an adhesin subunit protein of Helicobacter pylori.
1993; 175: 674-683 [PMID: 7678592 DOI: 10.1128/jb.175.3.674-683.1993]
23 Carlsohn E,Nystr?m J,B?lin I,Nilsson CL,Svennerholm AM.HpaA is essential for Helicobacter pylori colonization in mice.
2006; 74: 920-926 [PMID: 16428735 DOI: 10.1128/iai.74.2.920-926.2006]
24 Cai H,Ye F,Michel A,Murphy G,Sasazuki S,Taylor PR,Qiao YL,Park SK,Yoo KY,Jee SH,Cho ER,Kim J,Chen SC,Abnet CC,Tsugane S,Cai Q,Shu XO,Zheng W,Pawlita M,Epplein M.Helicobacter pylori blood biomarker for gastric cancer risk in East Asia.
2016; 45: 774-781 [PMID: 27170766 DOI: 10.1093/ije/dyw078]
25 Epplein M,Zheng W,Xiang YB,Peek RM Jr,Li H,Correa P,Gao J,Michel A,Pawlita M,Cai Q,Shu XO.Prospective study of Helicobacter pylori biomarkers for gastric cancer risk among Chinese men.
2012; 21: 2185-2192 [PMID: 23035179 DOI: 10.1158/1055-9965.EPI-12-0792-T]
26 Andermann TM,Chen YT,Ottemann KM.Two predicted chemoreceptors of Helicobacter pylori promote stomach infection.
2002; 70: 5877-5881 [PMID: 12228322 DOI: 10.1128/iai.70.10.5877-5881.2002]
27 Williams SM,Chen YT,Andermann TM,Carter JE,McGee DJ,Ottemann KM.Helicobacter pylori chemotaxis modulates inflammation and bacterium-gastric epithelium interactions in infected mice.
2007; 75: 3747-3757 [PMID: 17517875 DOI: 10.1128/iai.00082-07]
28 Yoon JY,Kim J,Lee SJ,Kim HS,Im HN,Yoon HJ,Kim KH,Kim SJ,Han BW,Suh SW.Structural and functional characterization of Helicobacter pylori DsbG.
2011; 585: 3862-3867 [PMID: 22062156 DOI: 10.1016/j.febslet.2011.10.042]
29 Lester J,Kichler S,Oickle B,Fairweather S,Oberc A,Chahal J,Ratnayake D,Creuzenet C.Characterization of Helicobacter pylori HP0231 (DsbK): role in disulfide bond formation,redox homeostasis and production of Helicobacter cystein-rich protein HcpE.
2015; 96: 110-133 [PMID: 25582190 DOI: 10.1111/mmi.12923]
30 Zhong Y,Anderl F,Kruse T,Schindele F,Jagusztyn-Krynicka EK,Fischer W,Gerhard M,Mejías-Luque R.Helicobacter pylori HP0231 Influences Bacterial Virulence and Is Essential for Gastric Colonization.
2016; 11: e0154643 [PMID: 27138472 DOI: 10.1371/journal.pone.0154643]
31 Choi J,Jia G,Wen W,Long J,Zheng W.Evaluating polygenic risk scores in assessing risk of nine solid and hematologic cancers in European descendants.
2020; 147: 3416-3423 [PMID: 32588423 DOI: 10.1002/ijc.33176]
32 Jin G,Lv J,Yang M,Wang M,Zhu M,Wang T,Yan C,Yu C,Ding Y,Li G,Ren C,Ni J,Zhang R,Guo Y,Bian Z,Zheng Y,Zhang N,Jiang Y,Chen J,Wang Y,Xu D,Zheng H,Yang L,Chen Y,Walters R,Millwood IY,Dai J,Ma H,Chen K,Chen Z,Hu Z,Wei Q,Shen H,Li L.Genetic risk,incident gastric cancer,and healthy lifestyle: a meta-analysis of genomewide association studies and prospective cohort study.
2020; 21: 1378-1386 [PMID: 33002439 DOI: 10.1016/S1470-2045(20)30460-5]
33 Mihor A,Tomsic S,Zagar T,Lokar K,Zadnik V.Socioeconomic inequalities in cancer incidence in Europe: a comprehensive review of population-based epidemiological studies.
2020; 54: 1-13 [PMID: 32074075 DOI: 10.2478/raon-2020-0008]
34 Quach DT,Hiyama T,Gotoda T.Identifying high-risk individuals for gastric cancer surveillance from western and eastern perspectives: Lessons to learn and possibility to develop an integrated approach for daily practice.
2019; 25: 3546-3562 [PMID: 31367156 DOI: 10.3748/wjg.v25.i27.3546]
35 Poorolajal J,Moradi L,Mohammadi Y,Cheraghi Z,Gohari-Ensaf F.Risk factors for stomach cancer: a systematic review and meta-analysis.
2020; 42: e2020004 [PMID: 32023777 DOI: 10.4178/epih.e2020004]
36 Ge Z,Taylor DE.Contributions of genome sequencing to understanding the biology of Helicobacter pylori.
1999; 53: 353-387 [PMID: 10547695 DOI: 10.1146/annurev.micro.53.1.353]
37 Suerbaum S,Achtman M.Evolution of Helicobacter pylori: the role of recombination.
1999; 7: 182-184 [PMID: 10383222 DOI: 10.1016/s0966-842x(99)01505-x]
 World Journal of Gastrointestinal Oncology2022年9期
World Journal of Gastrointestinal Oncology2022年9期
- World Journal of Gastrointestinal Oncology的其它文章
- Nutrition deprivation affects the cytotoxic effect of CD8 T cells in hepatocellular carcinoma
- Prognostic and clinicopathological value of Twist expression in esophageal cancer:A meta-analysis
- Dissecting novel mechanisms of hepatitis B virus related hepatocellular carcinoma using meta-analysis of public data
- Percutaneous insertion of a novel dedicated metal stent to treat malignant hilar biliary obstruction
- Construction and analysis of an ulcer risk prediction model after endoscopic submucosal dissection for early gastric cancer
- Clinical implications of interleukins-31,32,and 33 in gastric cancer
