Plexiform neurofibroma of the cauda equina with follow-up of 10 years: A case report
lNTRODUCTlON
Neurofibromas are benign nerve sheath tumors formed by a combined proliferation of different histological elements of a peripheral nerve. Two types of neurofibromas have been described according to their extent of tissue involvement[1]. Localized form, which can grow on the skin or as a peripheral nerve lesion, is the most common type seen and represents about 90% of all neurofibromas. Localized neurofibromas have minimal malignant potential, in the majority of cases are sporadic, and present as a solitary mass, but individuals with neurofibromatosis type 1 (NF-1) tend to develop multiple lesions distributed in a diffuse fashion throughout the neural and cutaneous tissues[2]. The second type is plexiform neurofibroma—these tumors grow within the bundles of nervous tissues such as trunks and plexuses. This morphological aspect of neurofibromas makes the surgery difficult and radical tumor excision without neurological compromise virtually impossible. Plexiform lesions are said to have about 10% life-time risk to transform into malignant peripheral nerve sheath tumor and is significantly more likely in NF-1 population[3,4].
The cauda equina region is an extremely uncommon location for plexiform neurofibromas. Only seven cases of plexiform neurofibromas of the cauda equina (CENF) have been reported in the literature up-to-date[5-11]. Here we present a case of cauda equina plexiform neurofibroma with a follow-up period of 10 years.
CASE PRESENTATlON
Chief complaints
A 55-year-old Lithuanian man presented with a progressive weakness in his lower extremities, fasciculations, difficulty urinating, and sexual dysfunction.
History of present illness
Weakness and fasciculations started about 10 years ago and progressed insidiously. Difficulties in urinating and sexual dysfunction started a few years ago and were only revealed after careful history taking.
History of past illness
The patient was previously diagnosed with idiopathic sensorimotor polyneuropathy. There was no history of other comorbidities, surgeries, allergies, or use of medications.
Then she crept to the stove, and began to sob29 and cry and to pour out her poor little heart, and said: Here I sit, deserted30 by all the world, I who am a king s daughter, and a false waiting- maid has forced me to take off my own clothes, and has taken my place with my bridegroom, while I have to fulfil the lowly office of goose-girl
Personal and family history
Patient denied the history of smoking, alcohol or substance abuse. The family history was unremarkable.
Physical examination
During the initial examination, patient vitals were normal (temperature 36.6 °C, respiratory rate 13 times/min, heart rate 69 bpm, and blood pressure 124/81 mmHg). Patient was slightly overweight (body mass index 26 kg/m
). Neurological examination revealed an absence of deep tendon reflexes in the lower extremities with diminished muscle strength, calf muscles atrophy, and mild impairment in proprioceptive and vibratory sensation bilaterally. Further neurological and general examination was unrevealing.
Laboratory examinations
The topic regarding an optimal management strategy and the extent of the surgery remains somewhat controversial, as various operative options could be supported by reasonable arguments. On the more conservative end of the spectrum, the practice of open biopsy together with targeted decompression and duraplasty could be sufficient in selected cases. Anyway, we decided to pursue a complete resection of the most enlarged neural root due to the fact that intraoperative monitoring showed it as silent and achieving adequate decompression was highly unlikely without the removal of the mass. The combination of Th12-L3 Laminectomy and duraplasty together with a resection of prevailing pathological nerve root allowed us to attain a satisfactory degree of decompression, but this came at the expense of permanent urinary retention as a complication. However, lack of primary spinal fixation resulted in some degree of post-operative instability one segment below the most caudal laminectomy site. Over the prolonged period of time (in our case 8 years), this facilitated the evolution of compensatory hypertrophy of facet joints and ligamenta flava, culminating in the spinal canal stenosis at L3-L4 Level. Combination of instability-induced stenosis and slowly but steadily growing CENF creates a recipe for disaster and will eventually result in the demand for an additional neurosurgical input. Therefore, we would recommend leaning right towards the more aggressive pole in terms of operative management - performing wide posterior decompression, adequate duraplasty, and primary spinal fixation in our opinion poses the best possibility to yield sustained symptomatic relief in the long-term and will likely decrease the necessity for additional interventions in the future. However, the sacrifice of neural elements should be discouraged regardless of intra-operatively recorded evoked potentials data, as the risk of complications will likely exceed potential benefits.
And it had a lot of people that didn t always see eye to eye(), like a lot of other cities. And it had a rich man, like almost every other town. And this rich man was a pillar in the Baptist Church; and people didn t see eye to eye about him, either.
Imaging examinations
Plexiform neurofibromas of the cauda equina region are extremely rare tumors. To our knowledge, only seven cases have been reported to the date[5-11]. The main clinical features of previous cases are summarized in the table (Table 1). These tumors originate from various cellular elements comprising the nerve roots (
, axons, fibroblasts, Schwann cells)[5]. About 10% of plexiform neurofibromas eventually become malignant, and lesions in locations other than cauda equina are typically associated with NF-1[2,4]. Only in one case of CENF a diagnosis of NF was made, but surprisingly NF-2 was the genetic syndrome reported[8]. Our case demonstrated no association with NF.
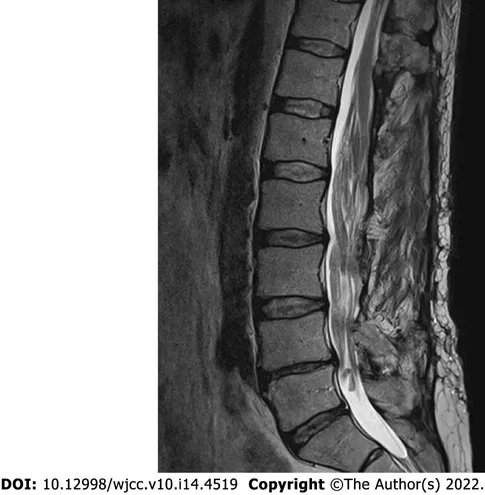
Given the long history of the disease with a progressive course and inconclusive neurophysiological study results, a gadolinium-enhanced lumbar spine magnetic resonance imaging (MRI) was performed. Imaging modality revealed a non-enhancing intradural tumor stretching out through the levels of Th12-L4 vertebrae. Diffusely enlarged, tortuous cauda equina roots were squeezed inside congenitally narrow spinal canal (Figure 1).

FlNAL DlAGNOSlS
Given the marked progression of symptoms and a radiological evidence of segmental stenosis, an operative treatment was proposed for the patient. Initially, patient decided to delay the intervention and wait, but neurological condition continued to worsen and 2 mo later he agreed to comply with the surgical management option. Patient underwent a L4-L5 Laminectomy with a caudal extension of previous duraplasty and L2-L5 transpedicular fixation (Figure 6). Hypertrophied ligamenta flava, facet joints, and visible adhesions were maximally removed from the spinal canal to accommodate the thecal sac. The post-operative period was uneventful, and patient’s pre-operative symptoms in terms of ambulation and pain improved dramatically following the operation. However, the problem of urinary retention remained unaffected, without the significant changes observed between the pre-operative and post-operative status.
TREATMENT
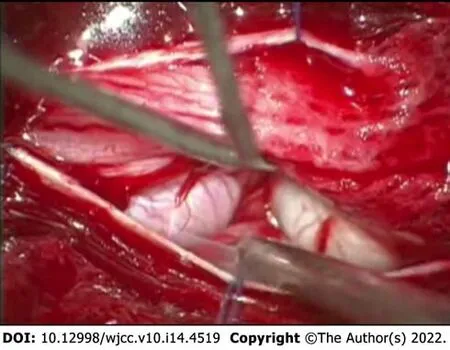
Given the likelihood of symptomatic progression and unclear pathological diagnosis, the surgical treatment was pursued - patient underwent Th12-L3 Laminectomy with duraplasty and biopsy of the lesion. During the operation, a few diffusely enlarged nerve roots were identified, with one nerve root being noticeably bulkier than the rest (Figure 2). The remaining nerve roots and conus medullaris were dislocated to the right. After careful intraoperative neuromonitoring, the decision to resect the most enlarged nerve root was made. The following surgical intervention was limited to the separation of adhesions between damaged roots and subsequent duraplasty with fascia lata graft.
OUTCOME AND FOLLOW-UP
Following the surgery, a slight improvement in sensorimotor function was concealed by development of permanent urinary retention. The histopathological study of biopsy material revealed a hypocellular tumor consisting of Schwann cells with wavy contours and elongated nuclei with fine dense chromatin, fibroblasts, and mast cells, a diagnosis consistent with plexiform neurofibroma (Figure 3). No deletions, duplications and translocations of NF1 gene were detected during fluorescence in situ hybridization (using specific markers for 17q11.2 area). Subsequent molecular genetic testing methods showed no point mutations characteristic for NF-1 or NF-2.
Informed written consent was obtained from the patient for publication of this report and any accompanying images.

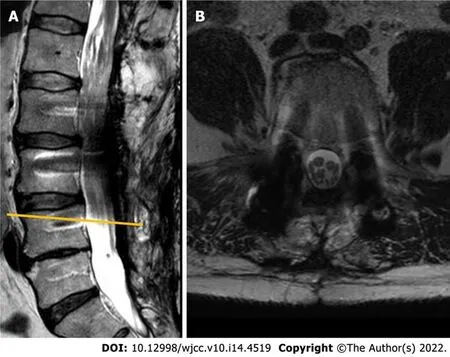
During the follow-up visit 5 years later, the patient reported that he had to intermittently catheterize himself 3-4 times a day and is experiencing some problems with ambulation, faring slightly worse than shortly after the decompression. Subsequent MRI revealed a slowly growing tumorous masses at the region of cauda equina (Figure 4). No compression was noted in the conus region due to extensive duraplasty, and there were no radiological signs of malignant transformation observed.
Cauda equina is an extremely rare location for plexiform neurofibromas to manifest. This case report illustrates complicated diagnostic process as clinical presentation may mimic other polyneuropathies, and challenges associated with management of such individuals. We would strongly recommend a generous posterior decompression with duraplasty and primary spinal fixation in order to achieve the optimal long-term results. Tumor resection, even in the electroneurographically silent roots, should be discouraged.

The final diagnosis of the presented case is plexiform neurofibroma of cauda equina. It was proven after the histopathological examination following the surgical treatment.
During the most recent follow-up 18 mo after the second operation, the patient reported an improved quality of life, minor pain in the projection of lumbar spine, and persistence of minor lower extremities weakness with a similar degree of urinary dysfunction as prior the operation.
It took a long time to find myself, and I had to live alone to do it. But I am not lonely. I am free for the first time in my life. I am Tandaleah, the Fire Goddess of the Volcano, spelled with two Ds and I m living happily ever after.
DlSCUSSlON
An initial instrumental investigation with electroneurography showed sensorimotor axonopathy of the left leg, whereas electromyography demonstrated an ongoing chronic denervation/reinnervation pattern in the muscles of the both legs.
Previously published cases showed various combinations of progressive symptoms, the most common being the lumbar and lower extremities pain for prolonged periods of time before a clinical condition worsened and advanced to polyneuropathy. Although the probability of neoplastic process in such patients remains low, we would advocate for a more comprehensive investigation in a form of neuroimaging, especially if patient reports a prolonged history of gradually worsening complaints. Given the complex differential diagnosis process surrounding polyneuropathies, a low threshold for radiological assessment would minimize the likelihood of delayed diagnosis and treatment not only in cases of CENF, but also other slowly growing lesions.
She went a little further and again she heard the sound of a horse s hoofs and there came another man on horseback galloping21 past her. He was dressed all in red, and the horse under him was blood-red and its harness was red, and just as he passed her the sun rose.
Complete blood count, biochemical analysis (including electrolytes, hepatic/renal and inflammatory markers), and coagulation testing were all within the normal values.
But the King was never able to enjoy his treasure, for he might watch and guard them as he liked, as soon as they began to get ripe they were always stolen
This can still be seen in many rich farms on the west coast ofJutland: plenty to eat and drink, clean, prettily decorated rooms,active minds, cheerful tempers, and hospitality can be found there, asin an Arab s tent
Redundant nerve root syndrome is another perplexing condition which requires pathological assessment in order to differentiate from CENF. The syndrome has analogous clinical and radiological picture as it manifests with thickened, large, tortuous, and elongated nerve roots in the area of cauda equina. Although it is believed that mechanical trapping at the level of lumbar spinal stenosis is the mechanism of pathogenesis, the clinical relevance of the syndrome remains a matter of debate[12,13]. Some authors suggest that at least a part of the previously described cases of redundant nerve root syndrome could actually be misdiagnosed plexiform neurofibromas and vice versa[14]. Given the similar clinical, radiological, and gross visual appearance, emphasis should be placed on the importance of histopathological evaluation in every case of suspected redundant nerve root syndrome, as at least part of these lesions could turn out neoplastic in origin.
CONCLUSlON
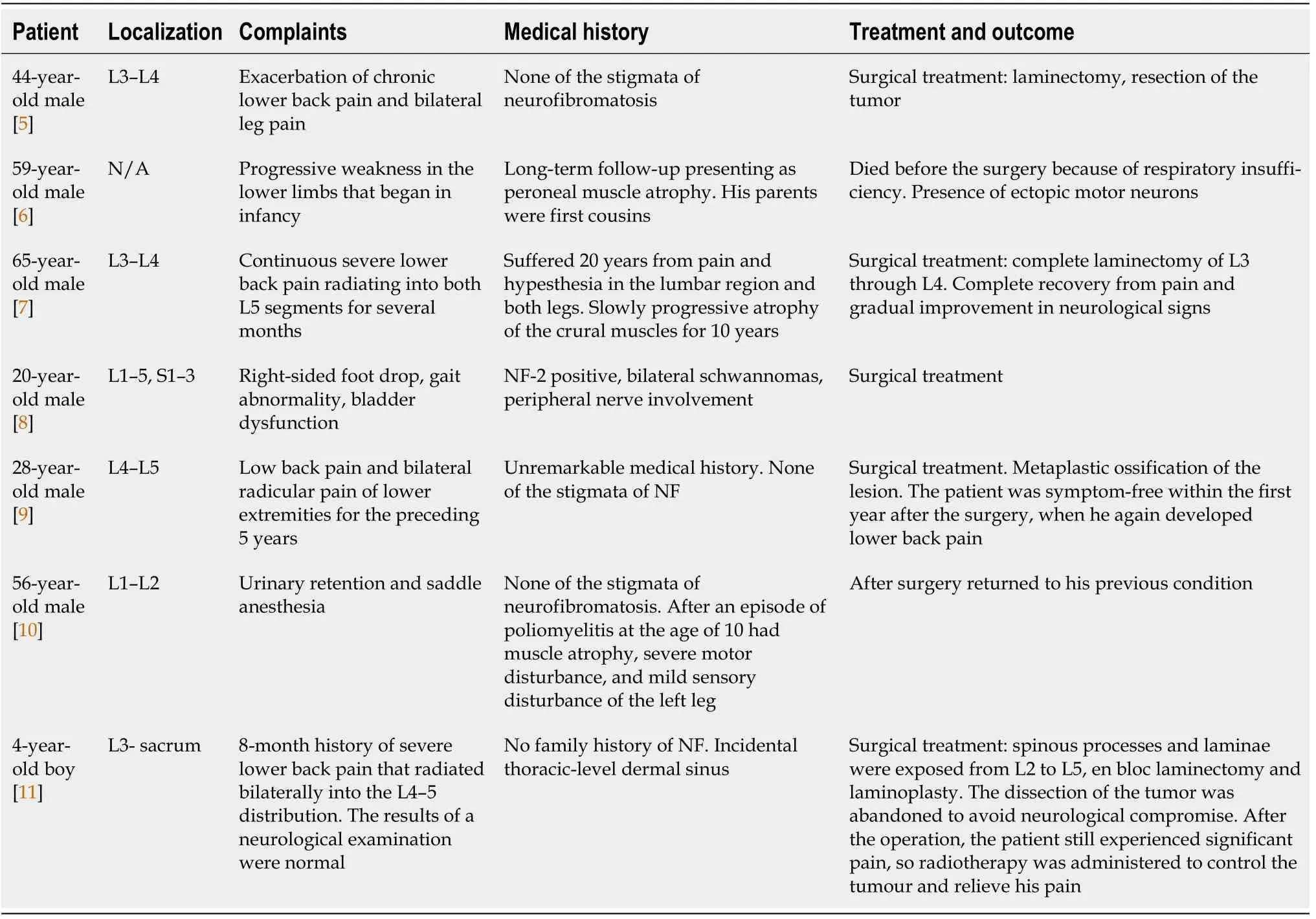
About 3 years later, the patient presented again for the follow-up with complaints of a significant worsening in motor function and pain in lower back which sometimes radiates to lower extremities, especially during physical activity. Neurological examination was significant for neurogenic claudication with a claudicating distance of 50 meters. Consequent MRI revealed a markedly enlarged tumorous masses occupying the majority of the vertebral canal with a central canal stenosis at L3-4 Level, mainly due to hypertrophy of facet joints and ligamenta flava (Figure 5).
FOOTNOTES
Chomanskis Z, Cepkus S and Rocka S were the patient’s neurosurgeons, reviewed the literature and contributed to manuscript drafting; Chomanskis Z followed the patient; Juskys R and Dulko J reviewed the literature, contributed to manuscript drafting and edition; Hendrixson V, Ruksenas O, Chomanskis Z were responsible for the revision of the manuscript for important intellectual content; all authors issued final approval for the version to be submitted.
How fragrant11 and beautiful it was! Every flower that could be thought of for every season of the year was here in full bloom; no picture-book could have more beautiful colors
The authors declare that they have no conflict of interest.
Oh! ay, with all my heart, Goody, 14 said this pretty little girl; and rinsing4 immediately the pitcher, she took up some water from the clearest place of the fountain, and gave it to her, holding up the pitcher all the while, that she might drink the easier.
The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Lithuania
Zilvinas Chomanskis 0000-0002-0381-0346; Raimondas Juskys 0000-0003-4303-3542; Saulius Cepkus 0000-0003-2674-4975; Justyna Dulko 0000-0003-3409-3527; Vaiva Hendrixson 0000-0003-2585-3782; Osvaldas Ruksenas 0000-0002-4177-9351; Saulius Rocka 0000-0002-9715-641X.
Be sure that you are kind and civil to everyone you meet, called his mother, running after him; but he was in such a hurry to be off, that he did not wait to answer her, or even to look back
Chang KL
A
Chang KL
1 Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary.
2016; 131: 803-820 [PMⅠD: 27157931 DOⅠ: 10.1007/s00401-016-1545-1]
2 Hirbe AC, Gutmann DH. Neurofibromatosis type 1: a multidisciplinary approach to care.
2014; 13: 834-843 [PMⅠD: 25030515 DOⅠ: 10.1016/S1474-4422(14)70063-8]
3 Prada CE, Rangwala FA, Martin LJ, Lovell AM, Saal HM, Schorry EK, Hopkin RJ. Pediatric plexiform neurofibromas: impact on morbidity and mortality in neurofibromatosis type 1.
2012; 160: 461-467 [PMⅠD: 21996156 DOⅠ: 10.1016/j.jpeds.2011.08.051]
4 Nguyen R, Dombi E, Akshintala S, Baldwin A, Widemann BC. Characterization of spinal findings in children and adults with neurofibromatosis type 1 enrolled in a natural history study using magnetic resonance imaging.
2015; 121: 209-215 [PMⅠD: 25293439 DOⅠ: 10.1007/s11060-014-1629-5]
5 Joseffer SS, Babu RP, Kleinman G. Plexiform neurofibroma of the cauda equina: case report.
2005; 63: 182-4; discussion 184 [PMⅠD: 15680670 DOⅠ: 10.1016/j.surneu.2004.02.037]
6 Berciano J, Figols J, Combarros O, Calleja J, Pascual J, Oterino A. Plexiform neurofibroma of the cauda equina presenting as peroneal muscular atrophy.
1996; 19: 250-253 [PMⅠD: 8559180 DOⅠ: 10.1002/(SⅠCⅠ)1097-4598(199602)19:2<250::AⅠD-MUS24>3.0.CO;2-X]
7 Schumacher M, Gilsbach J, Friedrich H, Mennel HD. Plexiform neurofibroma (Rankenneurofibrom) of the cauda equina.
1978; 15: 221-224 [PMⅠD: 692868 DOⅠ: 10.1007/BF00327530]
8 Kara M, Akyüz M, Y?lmaz A, Hatipo?lu C, Oz?akar L. Peripheral nerve involvement in a neurofibromatosis type 2 patient with plexiform neurofibroma of the cauda equina: a sonographic vignette.
2011; 92: 1511-1514 [PMⅠD: 21878222 DOⅠ: 10.1016/j.apmr.2011.04.011]
9 Azarpira N, Derakhshan N, Esfahani MH. Plexiform Neurofibroma of the Cauda Equina With Ectopic Bone Formation.
2015; 25: 97-99 [DOⅠ: 10.1097/WNQ.0b013e3182a2fe01]
10 Simou N, Zioga A, Zygouris A, Pahatouridis D, Charalabopoulos K, Batistatou A. Plexiform neurofibroma of the cauda equina: a case report and review of the literature.
2008; 16: 78-80 [PMⅠD: 18203792 DOⅠ: 10.1177/1066896907304521]
11 Nadkarni TD, Rekate HL, Coons SW. Plexiform neurofibroma of the cauda equina. Case report.
1999; 91: 112-115 [PMⅠD: 10419355 DOⅠ: 10.3171/spi.1999.91.1.0112]
12 Poureisa M, Daghighi MH, Eftekhari P, Bookani KR, Fouladi DF. Redundant nerve roots of the cauda equina in lumbar spinal canal stenosis, an MR study on 500 cases.
2015; 24: 2315-2320 [PMⅠD: 26071946 DOⅠ: 10.1007/s00586-015-4059-y]
13 Nogueira-Barbosa MH, Savarese LG, Herrero CFP da S, et al. Redundant nerve roots of the cauda equina: review of the literature.
2012; 45: 155-159 [DOⅠ: 10.1590/S0100-39842012000300007]
14 Hakan T, Celiko?lu E, Aydoseli A, Demir K. The redundant nerve root syndrome of the Cauda equina.
2008; 18: 204-206 [PMⅠD: 18597240]
 World Journal of Clinical Cases2022年14期
World Journal of Clinical Cases2022年14期
- World Journal of Clinical Cases的其它文章
- Perfectionism and mental health problems: Limitations and directions for future research
- Ovarian growing teratoma syndrome with multiple metastases in the abdominal cavity and liver: A case report
- Development of plasma cell dyscrasias in a patient with chronic myeloid leukemia: A case report
- Suprasellar cistern tuberculoma presenting as unilateral ocular motility disorder and ptosis: A case report
- Rare pattern of Maisonneuve fracture: A case report
- PD-1 inhibitor in combination with fruquintinib therapy for initial unresectable colorectal cancer: A case report
