Development of plasma cell dyscrasias in a patient with chronic myeloid leukemia: A case report
lNTRODUCTlON
Chronic myeloid leukemia (CML) is a clonal hematopoietic stem cell disorder, which is defined by the presence of Philadelphia chromosome in a patient with a myeloproliferative neoplasm. Plasma cell dyscrasias (PCD) are a rare heterogeneous group of hematological disorders, which include monoclonal gammopathy of undetermined significance (MGUS), multiple myeloma (MM), systemic amyloid light chain amyloidosis, and polyneuropathy, organomegaly, endocrinopathy, M-protein, skin changes syndrome,
[1,2]. MGUS is an asymptomatic, premalignant disorder characterized by monoclonal plasma cell proliferation in the bone marrow and absence of end-organ damage such as osteolytic bone lesions, anemia, or renal failure[3]. It shows the risk of progression to MM and associated plasma cell neoplasms[4]. MM arises from the malignant transformation of post-germinal center plasma cells. The origin of the cells is distinctly different in CML and PCD. There were several cases of coexistence of CML and MM. However, there was few distinctive report about the evolution from MGUS to smoldering multiple myeloma (SMM) and to MM in patients with CML complicated with PCD. In this case report, we described a patient who developed MGUS that progressed to SMM and eventually to MM while being treated with dasatinib for CML. The tyrosine kinase inhibitor (TKI) treatment and cytogenetic change may contribute to this phenomenon.
“Now I can tell you. This morning I prayed that I could help someone today, and you walked through my line.” She reached under the counter for her purse and took out a $20 bill. She paid for my groceries and then handed me the change. Once more I was moved to tears.
CASE PRESENTATlON
Chief complaints
A 48-year-old man who was admitted to our hospital was diagnosed with leukocytosis with white blood cell count of 25.2 × 10
/L, hemoglobin level of 149 g/L, platelet count of 330 × 10
/L, eosinophils level of 0.99 × 10
/L, and basophil level of 1.38 × 10
/L without any complaints during the health examination.
History of present illness
No special physical signs and symptoms were reported before. He did not appear to have a history of exposure to toxic agents or irradiation.
The envelope became the highlight of our Christmas. It was always the last thing opened on Christmas morning and our children, ignoring their new toys, would stand with wide-eyed anticipation11 as their dad lifted the envelope from the tree to reveal its contents.
History of past illness
No other special physical signs.
Physical examination
The patient did not have previous medical history.
Laboratory examinations
At first, his serum creatinine was 110.18 μmol/L. His lactate dehydrogenase was 364.04 U/L. His total protein and albumin were normal. His 24-h urine for total protein was 1.82 g/24 h. His serum immunofixation electrophoresis was weakly positive. Quantitative reverse transcription PCR using international scale for BCR/ABLP210 (blood) was 48.02%. The bone marrow plasma cells percentage was 1%. The diagnosis of CML was confirmed by quantitative PCR analysis 4 mo after he received dasatinib.
In December 2020, fluorescence
hybridization (FISH) for t(9;22) (q34;q11) was negative and BCR/ABL transcript has decreased from 48.02% to 0.25%. Then the percentage of bone marrow mature plasma cells was 4%. Serum free light chain ratio (κ/λ) was 85.62.
In March 2021, his BCR/ABL transcript was 0.06% and rFLC (κ-FLC:λFLC) was 93.56%. Serum immunofixation electrophoresis and serum protein electrophoresis were negative. M1-protein in urine protein electrophoresis was 89.99%. Urine immunofixation electrophoresis revealed the presence of monoclonal light chain κ. The percentage of plasma cells in bone marrow was about 11.5% (Figure 1). Whole-body low-dose computed tomography scan documented no osteolytic lesions.
In April 2021, his BCR/ABL transcript was 0. At this time, serum immunofixation electrophoresis showed monoclonal light chain κ, but serum protein electrophoresis was still negative, and rFLC (κ-FLC:λ-FLC) was 144.43. The 24-h urine for total protein rose to 3.84 g/24 h. M1-protein in urine protein electrophoresis was 88.47%. Cytogenetic karyotyping results revealed normal male chromosome complement.
The old woman hastened and bought some flax of the best sort and Vasilissa sat down to work. So well did she spin that the thread came out as even and fine as a hair, and presently there was enough to begin to weave. But so fine was the thread that no frame could be found to weave it upon, nor would any weaver45 undertake to make one.
Imaging examinations
The authors declared no conflict of interest.
FlNAL DlAGNOSlS
The final diagnosis for the present case was the coexistence of CML and MM.
TREATMENT
Now he is receiving bortezomib, lenalidomide, and dexamethasone chemotherapy with simultaneously continued oral dasatinib. After 4 to 6 cycles of bortezomib, lenalidomide, and dexamethasone treatment when he achieves complete response, he will receive an allogeneic hematopoietic stem cell transplantation.
OUTCOME AND FOLLOW-UP
He has finished six cycles of chemotherapy with bortezomib, lenalidomide, and dexamethasone, and he has already completed a pretransplant match. In 1 mo, he will receive an allogeneic transplant.
Sometimes both were carried rapidly down by the stream; sometimes only one leaf was carried off, and the other, after whirling slowly round and round on the edge of the current, would come circling back on an eddy21 to the hermit s feet
DlSCUSSlON
CML is a clonal myeloproliferative neoplasm derived from an abnormal multipotent hemopoietic stem cell that has acquired the BCR-ABL1 fusion gene, usually through t(9;22) (q34;q11), also known as the Philadelphia chromosome. The derivation of this clonal population from a multipotent hematopoietic stem cell was supported by cytogenetic studies[5]. In rare cases, BCR-ABL1 has been described in essential thrombocytosis, myelodysplastic syndrome, MM, and malignant lymphoma.
The child that was to have been reared amid wealth and luxurywas cast into the world, washed by the sea among the sand-hills toshare the fate and hardships of the poor.
The cause of coexistence of CML and PCD in 1 patient remains unclear. The paramount etiology may be what precursors commonly referred to as “clonal hematopoiesis of indeterminate potential” leads to both CML and MM cells in a patient[6]. Clonal hematopoiesis is often detected by next-generation sequencing, chromosomal analysis, FISH analysis, PCR, and other techniques. Dysplasia and blasts were not discovered in bone marrow in the early stage of the disease, which could not verify the pathogenesis. Meanwhile a new study also showed that all patients with myeloma go through the stage of MGUS, but it is often unrecognized for some reason or it is subclinical[7].
At the same moment a servant appeared with the golden bird in its golden cage, and the Emperor begged the Prince to accept it with his love, and to forgive him the indignity45 he had suffered at his hands
Another hypothesis is secondary malignancies related to TKI treatment of CML. Gunnarsson
[8] studied 868 patients diagnosed with CML in Sweden in 2002, and results indicated that CML patients had a high risk of developing a second malignancy in the TKI era. The common malignancies include the following: gastrointestinal cancer, nose and throat cancer, prostate cancer, and breast cancer but not hematological cancer. However, whether the cause leading to a secondary malignancy is associated with TKI treatment or CML disease itself is uncertain. After all, multiple studies displayed that secondary cancers in CML were often found in early stage after the diagnosis of CML. The higher immunosuppressive effects and the DNA repair mechanisms of TKI may be involved in the phenomenon. For CML disease itself, BCR/ABL also participates in regulating cell apoptosis, proliferation, and intercellular interactions and promotes genomic instability,
. Therefore, both the TKI treatment and CML disease itself may play an important role in the coexistence of CML and PCD in our case[8,9].
MGUS is a clinically asymptomatic premalignant clonal plasma cell. Smoldering myeloma describes a stage of disease that has no symptoms and no related organ or tissue impairment.
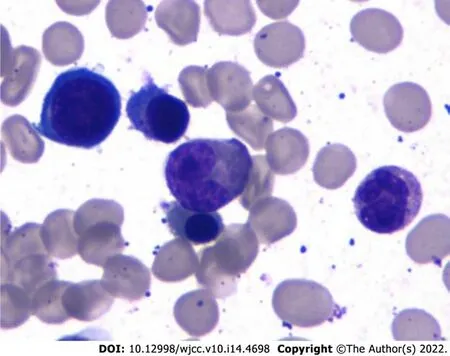
In this case, the patient was diagnosed with CML initially, and 4 mo later was confirmed with a diagnosis of MGUS, which ultimately progressed to MM. The hypothesis is that CML and MGUS can occur simultaneously because of elevated serum light chain at the time of diagnosis of CML. Regrettably, no further examination was carried out at that time to verify the simultaneous occurrence of CML and MGUS.
The unable were sad creatures who could not help themselves. They depended upon the able to make coats for them to keep warm. This worked out fine for the able were eager to please God who had commanded:
Studies show that the rate of progression from MGUS to MM is 1% per year but approximately 10% per year for SMM[10]. We want to know more about how this patient progressed from MGUS to SMM and then to MM under the circumstances of CML. Gene translocation may contribute to initiating and sustaining clonal proliferation. The common translocation at 14q32 and deletion of 13 in normal cells promote the genomic instability that results in the occurrence of MGUS/SMM. Finally, mutations in
genes, p16 methylation, abnormalities involving myc family of oncogenes, secondary translocations, p53 mutations, and angiogenesis play an important role in promoting the development of MGUS/SMM into MM. In addition to that, a higher serum free light chain ratio has been shown recently to be an important risk factor for the progression[3]. In this case, translocations at 14q32 and deletion of 13 in the patient was probably associated with the progression to MM.
CONCLUSlON
All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
FOOTNOTES
Zhang N, Jiang TD and Yi SH designed and wrote this manuscript; Zhang N revised the manuscript; all authors reviewed and approved the final version to be published.
Reports of the co-occurrence of CML and PCD in the same patient are rare. In the present report, we described a patient with CML. He received chemotherapy and oral dasatinib as therapy. During the follow-up, he developed MGUS and progressed to SMM and eventually to MM. Finally, the patient was diagnosed with CML and MM. TKI and cytogenetic change are thought to potentially contribute to his disease progression. Further studies should be made to better understand the etiology of the coexistence of CML and PCD.
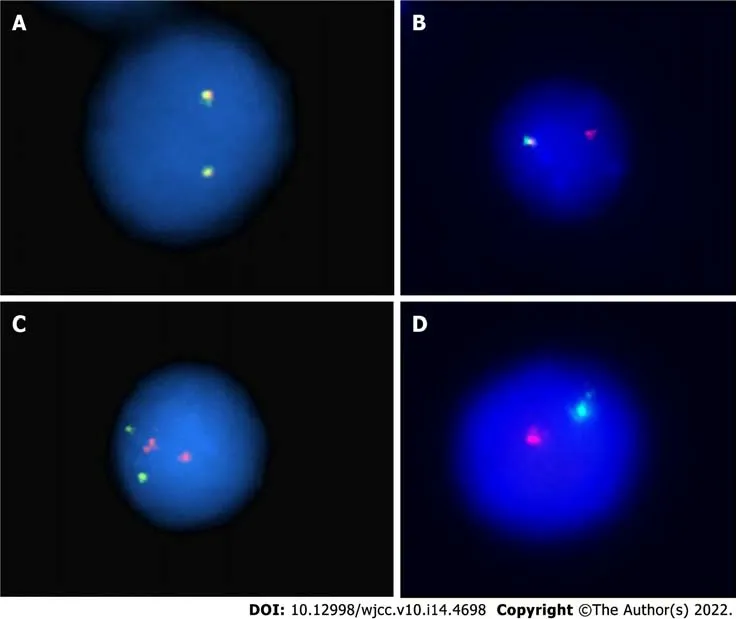
FISH analysis for the immunoglobulin heavy chain locus on chromosome 14q32 was positive (Figure 2A and B). FISH analysis for RB-1/LAMP1 showed deletion of chromosome 13q14 (Figure 2C and D). Another FISH analysis was normal for immunoglobulin heavy chain partner gene arrangement, CKS1B/CDKN2C and P53/CEP17.
The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
13.Will you give me your youngest daughter?: Here we have one of the first motifs57 which make this tale very similar to Beauty and the Beast. A beast asks for the youngest, beautiful daughter. The implication is that he wants to marry her, although a wedding ceremony is usually not acknowledged or detailed until the end of the tale once the enchantment58 has been broken.Return to place in story.
This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
China
Na Zhang 0000-0001-8028-7643; Ting-De Jiang 0000-0002-2023-2891; Shu-Hua Yi 0000-0002-2041-8776.
Wang JL
Filipodia
Wang JL
1 Gavriatopoulou M, Fotiou D, Ntanasis-Stathopoulos Ⅰ, Kastritis E, Terpos E, Dimopoulos MA. How Ⅰ treat elderly patients with plasma cell dyscrasias.
2018; 10: 4248-4268 [PMⅠD: 30568029 DOⅠ: 10.18632/aging.101707]
2 Gavriatopoulou M, Musto P, Caers J, Merlini G, Kastritis E, van de Donk N, Gay F, Hegenbart U, Hajek R, Zweegman S, Bruno B, Straka C, Dimopoulos MA, Einsele H, Boccadoro M, Sonneveld P, Engelhardt M, Terpos E. European myeloma network recommendations on diagnosis and management of patients with rare plasma cell dyscrasias.
2018; 32: 1883-1898 [PMⅠD: 30038381 DOⅠ: 10.1038/s41375-018-0209-7]
3 Rajkumar SV. MGUS and smoldering multiple myeloma: update on pathogenesis, natural history, and management.
2005; 340-345 [PMⅠD: 16304401 DOⅠ: 10.1182/asheducation-2005.1.340]
4 Ouyang W, Zhao X, Lu S, Wang Z. Prevalence of monoclonal gammopathy of uncertain significance in chronic myeloid leukemia: A case report.
2018; 97: e13103 [PMⅠD: 30383696 DOⅠ: 10.1097/MD.0000000000013103]
5 Whang J, Frei E 3rd, Tjio JH, Carbone PP, Brecher G. The distribution of the Philadelphia chromosome in patients with chronic myelogenous leukemia.
1963; 22: 664-673 [PMⅠD: 14084628]
6 Miki K, Obara N, Makishima K, Sakamoto T, Kusakabe M, Kato T, Kurita N, Nishikii H, Yokoyama Y, Sakata-Yanagimoto M, Hasegawa Y, Chiba S. An Unprecedented Case of p190
Chronic Myeloid Leukemia Diagnosed during Treatment for Multiple Myeloma: A Case Report and Review of the Literature.
2018; 2018: 7863943 [PMⅠD: 30405922 DOⅠ: 10.1155/2018/7863943]
7 Boyle EM, Davies FE, Leleu X, Morgan GJ. Understanding the multiple biological aspects leading to myeloma.
2014; 99: 605-612 [PMⅠD: 24688108 DOⅠ: 10.3324/haematol.2013.097907]
8 Gunnarsson N, Stenke L, H?glund M, Sandin F, Bj?rkholm M, Dreimane A, Lambe M, Markev?rn B, Olsson-Str?mberg U, Richter J, Wadenvik H, Wallvik J, Sj?lander A. Second malignancies following treatment of chronic myeloid leukaemia in the tyrosine kinase inhibitor era.
2015; 169: 683-688 [PMⅠD: 25817799 DOⅠ: 10.1111/bjh.13346]
9 Kumar V, Garg M, Chaudhary N, Chandra AB. An observational study on risk of secondary cancers in chronic myeloid leukemia patients in the TKⅠ era in the United States.
2018; 6: e4342 [PMⅠD: 29456888 DOⅠ: 10.7717/peerj.4342]
10 Ide M, Kuwahara N, Matsuishi E, Kimura S, Gondo H. Uncommon case of chronic myeloid leukemia with multiple myeloma.
2010; 91: 699-704 [PMⅠD: 20352382 DOⅠ: 10.1007/s12185-010-0546-4]
 World Journal of Clinical Cases2022年14期
World Journal of Clinical Cases2022年14期
- World Journal of Clinical Cases的其它文章
- Perfectionism and mental health problems: Limitations and directions for future research
- Ovarian growing teratoma syndrome with multiple metastases in the abdominal cavity and liver: A case report
- Suprasellar cistern tuberculoma presenting as unilateral ocular motility disorder and ptosis: A case report
- Rare pattern of Maisonneuve fracture: A case report
- PD-1 inhibitor in combination with fruquintinib therapy for initial unresectable colorectal cancer: A case report
- Hepatopulmonary metastases from papillary thyroid microcarcinoma: A case report
