Clinical and prognostic significance of expression of phosphoglycerate mutase family member 5 and Parkin in advanced colorectal cancer
lNTRODUCTlON
Colorectal cancer (CRC) is a common malignant tumor. According to an epidemiological survey in 2018, CRC has become the second leading cause of cancer-related death, and its incidence ranks the third in both sexes[1]. In recent years, due to the in-depth research on the pathogenesis of CRC, clinical screening program and effective treatments have been continuously developed. Studying the molecular characteristics of CRC is helpful to improve our understanding of the pathogenesis and provides a theoretical basis for improving prognosis and innovating effective targeted therapy.
So the Princess and the baby floated and drifted in the chest on the sea all day and night, but the baby was not afraid of the waves nor of the wind, for he did not know that they could hurt him, and he slept quite soundly
Phosphoglycerate mutase family member 5 (PGAM5), as an atypical serine/threonine protein phosphatase, is a nucleus-encoded mitochondrial resident protein. PGAM5 is involved in the regulation of mitochondrial dynamics, respiration, and mitochondrial quality control, and is closely related to organelle homeostasis, mitochondrial autophagy, and cell death[2]. Most of the proteins involved in mitophagy have been shown to be dysregulated in cancer patients. Drugs targeting mitochondria can induce mitophagy in CRC cells and restrain cancer cell proliferation[3]. Mitochondrial clearance seems to act as a mechanism that is recruited by cancer cells to modulate the key malignancy features in cancer initiation and progression.
In lung cancer tissues, PGAM5 is expressed by malignant epithelial cells and surrounding macrophages. Besides, PGAM5 is also expressed by precancerous epithelial cells[4]. Moreover, PGAM5 induces a decrease in mitochondrial nicotinamide adenine dinucleotide phosphate production in extracellular matrix-detached cancer cells by activating mitophagy. As a result, the increase of reactive oxygen species level led to the non-apoptotic death of cancer cells[5]. In addition, studies on the role of phosphatases in CRC have shown that PGAM5 was one of the few phosphatases over-expressed in cancer tissues[6]. Down-regulation of PGAM5 expression significantly inhibited the proliferation of CRC cells. Furthermore, knockout of the
gene could attenuate the occurrence of spontaneous and chemically-induced CRC in mice[6]. However, there are few studies on the expression and clinical prognostic significance of PGAM5 protein in CRC patients. There is no clinical evidence regarding the possible mechanism of PGAM5 in the development of CRC.
The E3 ubiquitin ligase Parkin plays an important regulatory role in mitochondrial homeostasis[7], such as biogenesis, fusion/fission, mitochondrial DNA repair, and mitophagy[8]. After mitochondrial injury, PGAM5 could activate Parkin by binding and stabilizing the serine/threonine PTEN-induced putative kinase 1 (PINK1). Subsequently, Parkin rapidly catalyzed the ubiquitination of many mitochondrial proteins; the ubiquitinated mitochondrial proteome then generated the autophagy mechanism and initiated selective autophagy[9]. Evidence has shown that Parkin is a tumor-suppressor. In-depth study has revealed that overexpression of Parkin can inhibit cancer cell proliferation; however, inactivation of Parkin promoted the proliferation of cancer cells[10]. High Parkin protein levels in deep tumor regions of CRC were associated with an increased survival[11]. These results suggest that Parkin may play a protective or inhibitory role in tumor development.
In this study, we aimed to explore the clinical significance of PGAM5 and Parkin proteins as biomarkers for diagnosis and prognosis of CRC by studying the expression of PGAM5 protein and mitophagy-related protein Parkin in advanced CRC tissues and their association with clinicopathological parameters. Meanwhile, the correlation between PGAM5 and Parkin protein expression was also assessed, which will provide a clinical basis for further study of the possible mechanism of action in CRC.
MATERlALS AND METHODS
Study population and sample collection
We collected histological sections of advanced CRC from November 2012 to June 2015 at the First Affiliated Hospital of China Medical University. This study included 100 pairs of colorectal adenocarcinoma and corresponding paracancerous tissues that had been surgically removed. The inclusion criteria were as follows: Age ≥ 18 years; located at the colon or upper rectum more than 100 mm from the anus. The exclusion criteria were as follows: Metachronous or synchronous cancers; radiotherapy or chemotherapy before surgical resection; and history of familial adenomatous polyposis or Lynch syndrome. This study was approved by the Institutional Review Board of The First Affiliated Hospital of China Medical University (No. 2021-68-2), and informed consent was waived.
The following clinicopathological variables were considered: Age, sex, tumor site (colon/rectum), tumor size, histological type (mucinous/non-mucinous), degree of differentiation (poorly/ moderately/well diff-erentiated), angiolymphatic and/or perineural invasion, tumor stage (I to IV), tumor invasion depth (T2 to T4), lymph node status, distant metastasis, and follow-up (from diagnosis to last outcome consultation or death). Included samples were also examined for protein expression [including carcinoembryonic antigen (CEA), CA19-9, P53, and CDX2].
Tumor stage was defined according to the American Joint Committee on Cancer/Union for International Cancer Control TNM staging system (8
edition). All patients received outpatient, inpatient, or telephone follow-up, with an interval of 3 mo (the last follow-up occurred in November 2020). As this study described the prognosis of patients with advanced CRC, analysis of overall survival (OS) and progression-free survival (PFS) was undertaken. OS was defined as the time from surgical resection to death from any cause, and PFS was defined as the time from surgical resection to the first recurrence or death caused by disease progression.
“Yes, there is always snow and ice,” said the reindeer; “and it is a glorious place; you can leap and run about freely on the sparkling ice plains. The Snow Queen has her summer tent there, but her strong castle is at the North Pole, on an island called Spitzbergen.”
Immunohistochemistry
Typical CRC tissues and normal colorectal mucosal tissues were selected. The paraffin-embedded tissues were cut into 4-μm thick sections, dewaxed using xylene, rehydrated through gradient ethanol, and then heated using a pressure-cooker for antigen retrieval. Antigenic repair was performed with citric acid or repair solution, and endogenous peroxidase blocked with 3% H
O
-methanol (prepared with 30% H
O
10 mL + methanol 90 mL). The sections were then incubated overnight at 4 °C with the following primary antibodies: Rabbit polyclonal anti-PGAM5 (1:200 dilution, ab126534; Abcam, United Kingdom) and rabbit polyclonal anti-Parkin (1:400 dilution, ab233434; Abcam, United Kingdom). Subsequently, sections were incubated with the secondary antibody for 40 min at 37 °C. Next, after washing in phosphate-buffered saline, the sections were stained with diaminobenzidine and then counter-stained with hematoxylin. The sections were then air-dried, dehydrated, and mounted. CRC tissues with intense immunoreactivity to PGAM5 and Parkin were used as positive controls; in the negative control, the primary antibody was replaced with phosphate-buffered saline.
Scoring system
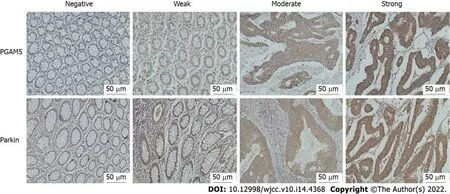
Double-blind reading was performed by two experienced pathologists. Initially, the slides were scanned at 10 × magnification to obtain a general impression of the overall tumor cell distribution. The cells with cytoplasm, cell membrane, or nucleus stained light-yellow to brown-yellow were identified as positive cells. The percentage of positive cells and staining intensity under the microscope (200 ×) were evaluated by semi-quantitative results.
Five high-power fields (200 ×) were observed on each section, and the percentage of positive cells was counted. The proportion of positive cells scored from 0 to 4 as follows: 0 (percentage of positive cells < 5%), 1 (5% to 25%), 2 (26% to 50%), 3 (51% to 75%), and 4 (> 75%). The immunostaining intensity was divided into four categories: 0 (negative immunostaining), 1 (weak immunostaining), 2 (moderate immunostaining), and 3 (strong immunostaining) (Figure 1). The final staining score was calculated as the proportion score multiplied by the intensity score.
Western blot assay
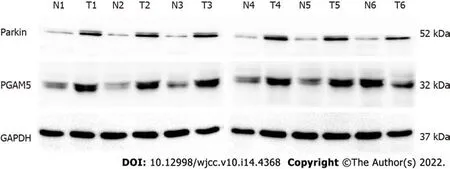
Protein was extracted from the six matched pairs of colorectal adenocarcinoms and corresponding paracancerous tissues with the RIPA lysis buffer containing PMSF protease inhibitors and was quantified by using the BCA assay (Beyotime Institute of Biotechnology, China). Samples of 50 μg of protein were loaded and resolved by SDS-PAGE followed by transfer to a PVDF membrane. Nonspecific binding was blocked using 5% bovine serum albumin in Tris Buffered Saline with Tween 20 (TBS-T, pH 7.6) for 2 h. Subsequently, incubation with primary antibodies, rabbit polyclonal anti-PGAM5 (1:1000 dilution, ab126534; Abcam, United Kingdom), rabbit polyclonal anti-Parkin (1:1000 dilution, ab233434; Abcam, United Kingdom), and glyceraldehyde-3-phosphate dehydrogenase (glyceraldehyde-3-phosphate dehydrogenase, 1:1000 dilution, FL-335; Santa Cruz Biotechnology, CA, United States) was undertaken overnight at 4 °C. After extensive washing, the membranes were incubated with anti-rabbit IgG secondary antibody (1:5000) and the immunoreactive bands were detected with ECLPlus chemiluminescent detection HRP reagents (Beyotime Institute of Biotechnology, China) using the Microchemi 4.2 Bio-imaging system. The Western blots were quantified by densitometric analysis using ImageJ software (Version 1.46r). The experiments were repeated three times under the same experimental conditions.
Statistical analysis
SPSS software (version 26) was used to analyze the findings. Pearson’s chi-squared test was adopted to evaluate the correlation between PGAM5/Parkin protein expression and clinicopathological parameters. Wilcoxon signed-rank tests and Spearman rank correlation analysis were performed to determine the expression of PGAM5 and Parkin and to investigate their correlation. Cumulative survival of the patients was estimated using the Kaplan-Meier method, and the significance of the survival differences was tested by using the Log-rank test. Multivariate analysis was performed using a Cox proportional hazards regression model to estimate the independent prognostic effect of PGAM5 and Parkin protein on survival by adjusting for confounding factors. All
values were two-sided and considered statistically significant when
< 0.05.
RESULTS
Patients and clinicopathological characteristics
There were 100 patients with advanced CRC enrolled in this study. Fifty-six percent of the patients were male and another 44 percent were female. The median age was 61 years (range, 33-88 years). The lesions of 26 patients were located in the colon, and 74 cases had lesions located in the rectum. Ninety-six patients had angiolymphatic and/or perineural invasion. There were 61 cases with good/moderate differentiation and 39 with poor differentiation/mucinous adenocarcinoma. There were 35 cases showing T2 depth of penetration, 19 cases showed T3, and 46 showed T4. Forty-eight patients had lymph node metastases. Thirteen patients had distant metastasis. According to the 2017 UICC principle of CRC staging, there were 50 cases at stages I-II and 50 at stages III-IV. The clinicopathological characteristics of these patients are summarized in Table 1.
PGAM5 and Parkin expression and their correlation
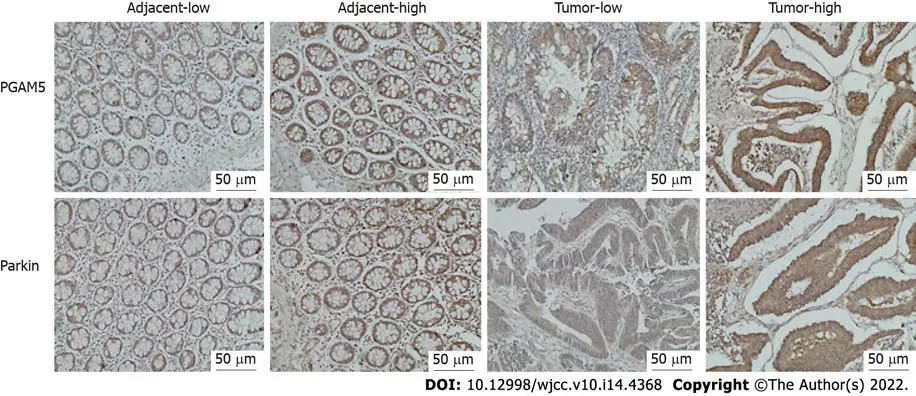
Immunohistochemical findings showed that the protein expression of both PGAM5 and Parkin was mainly found in the cytoplasm of colonic epithelial cells (Figure 2). PGAM5 had a median score of 7.5 in cancer tissues and 3.5 in paracancerous tissues, with a median difference of 5.0. Parkin had a median score of 8.0 in cancer tissues and 4.0 in paracancerous tissues, with a median difference of 4.0. Wilcoxon signed-rank testing showed that the PGAM5 expression in cancer tissues was prominently higher than that in paracancerous tissues (
= -7.754,
< 0.001), also as to Parkin (
= -6.083,
0.001). Additionally, results of western blot assay also revealed higher levels of both PGAM5 (
0.028) and Parkin (
= 0.027) in cancer tissues in contrast to adjacent non-tumor tissues (Figure 3).
“I can keep a secret better than he can,” yelled Kelly, jumping up and waving her arm in the air, too. If this was a contest, she wanted to make sure she beat Eric.
To assess the clinical significance of PGAM5 and Parkin proteins, as biomarkers for diagnosis and prognosis of CRC, by studying their expression in advanced CRC tissues and their association with clinicopathological parameters.
I fell in love with the minister s son the winter I turned fourteen. He was not Chinese, but as white as Mary in the manger. For Christmas I prayed for this blond-haired boy, Robert, and a slim new American nose. When I found out that my parents had invited the minister s family over for Christmas Eve dinner, I cried. What would Robert think of our shabby Chinese Christmas? What would he think of our noisy Chinese relatives who lacked proper American manners? What terrible disappointment would he feel upon seeing not a roasted turkey and sweet potatoes but Chinese food?
And then we performed Spearman’s rank correlation analysis to further investigate the correlation between PGAM5 and Parkin protein expression. The result showed that the expression of PGAM5 and Parkin protein was significantly positively correlated (correlation coefficient = 0.631,
0.001) in CRC tissues.
Association of PGAM5 or Parkin expression with clinicopathological parameters
The night before Easter, we were so excited we could hardly sleep. We didn’t care that we wouldn’t have new clothes for Easter; we had seventy dollars for the sacrificial offering. We could hardly wait to get to church! On Sunday morning, rain was pouring. We didn’t own an umbrella, and the church was over a mile from our home, but it didn’t seem to matter how wet we got. Darlene had cardboard7 in her shoes to fill the holes. The cardboard came apart, and her feet got wet.
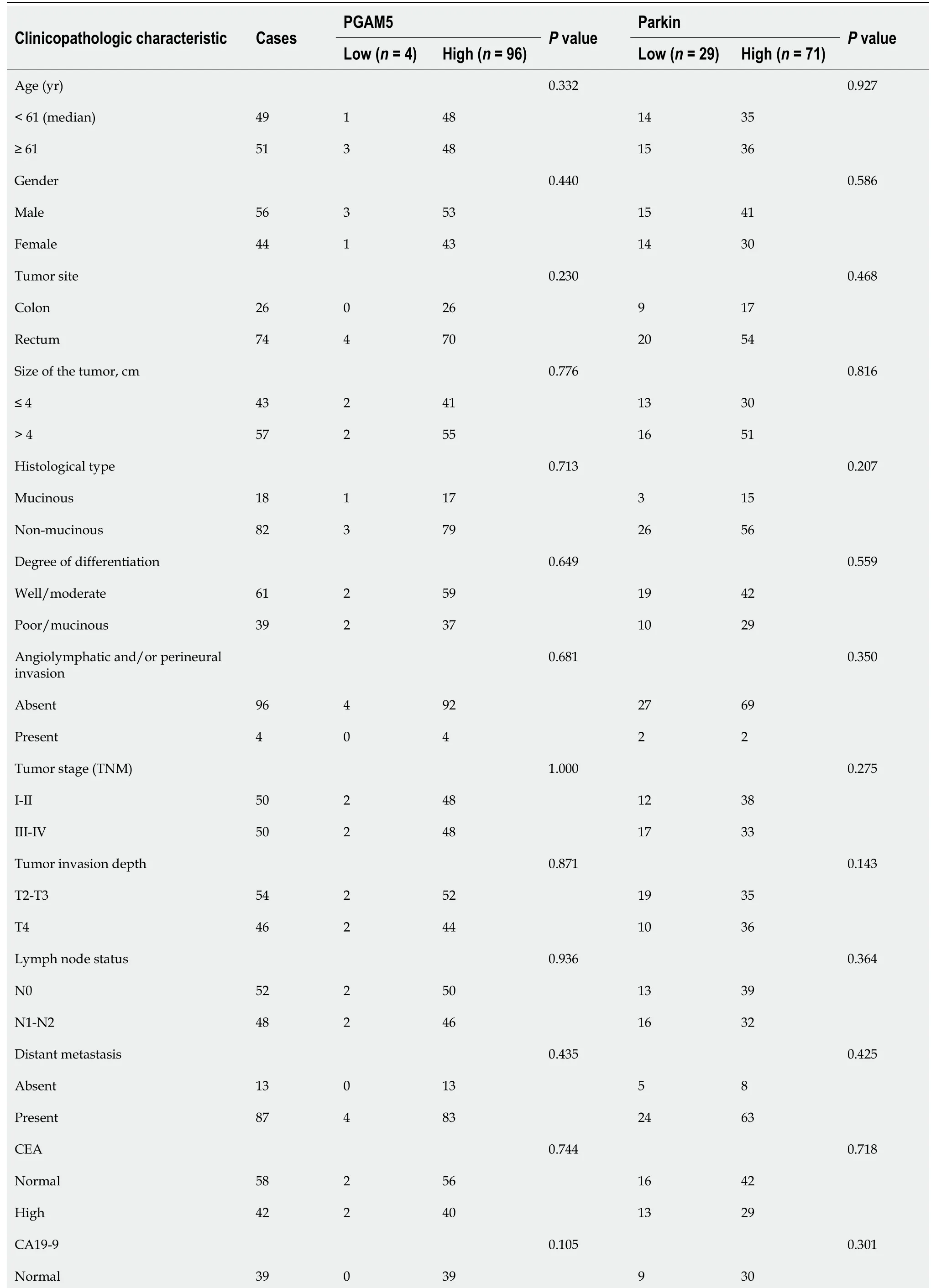
We then assessed the association of PGAM5 or Parkin protein expression with the clinicopathological parameters. Statistical results showed that there was no correlation between the expression of PGAM5 or Parkin and any of the clinicopathological variables evaluated, such as age, sex, primary tumor site, vascular invasion or nerve invasion, TNM stage, and tumor histological differentiation. Meanwhile, the expression of PGAM5 or Parkin protein in cancer tissues was not correlated with the expression of CEA, CA19-9, P53, or CDX2 (Table 1).
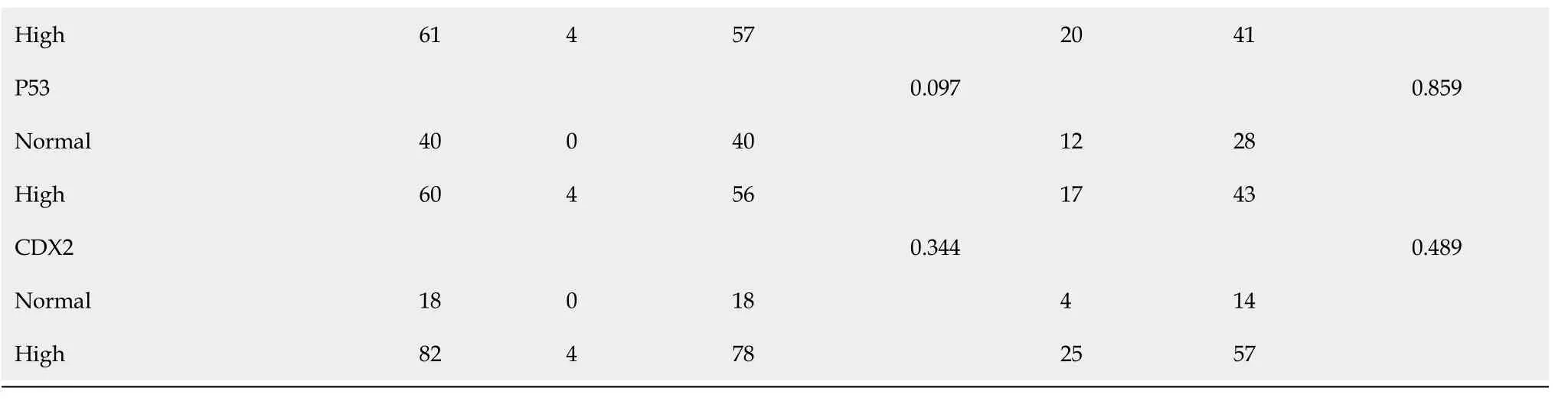
OS and PFS analysis in advanced CRC patients
OS and PFS differed significantly according to the immunohistochemical expression of Parkin protein. Kaplan-Meier survival analysis and Log-rank testing showed a higher survival rate in the group with high Parkin levels compared with the group with low Parkin levels (OS:
= 0.038, PFS:
= 0.030). The Kaplan-Meier survival curves for advanced CRC patients with high and low expression levels of Parkin are shown in Figure 4. However, there was no statistical significance according to the immunohistochemical expression of PGAM5 protein (Figure 4).
Predictive value of PGAM5 and Parkin protein expression for survival
A Cox proportional-hazards model was used to estimate the effects of PGAM5 and Parkin protein expression on OS in advanced CRC patients. Multivariate analysis was performed to assess the independent prognostic effects of PGAM5 and Parkin protein on OS by adjusting for confounding factors.
Several studies have shown that Parkin exhibits tumor-suppressor activity, for example, mice with
gene knockout were more susceptible to cancer. We then studied the expression of Parkin protein in CRC patients. The Parkin protein level in tumor tissue was significantly higher than that in adjacent non-tumor tissue and had diagnostic significance for CRC.
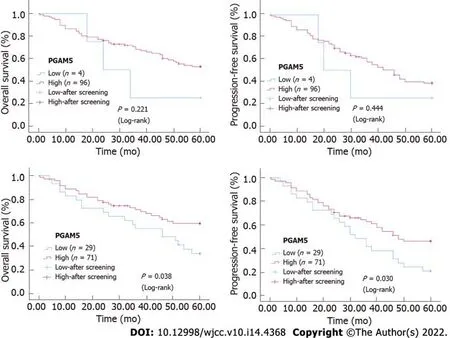
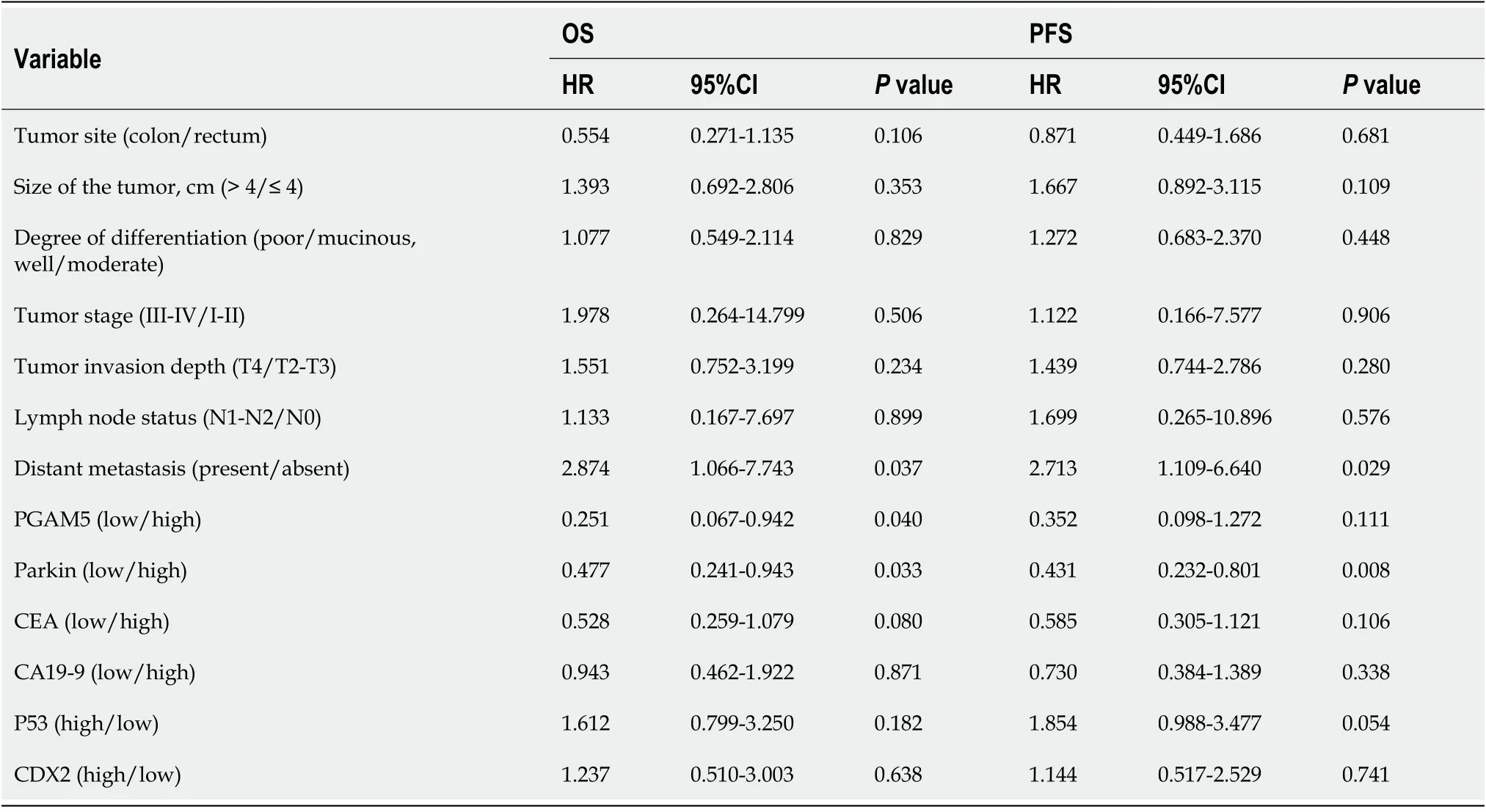
Univariate analysis showed that the following factors were significantly related to PFS: Tumor differentiation (
0.011), tumor stage (
0.001), tumor invasion depth (
0.009), lymph node status (
0.001), distant metastasis (
0.001), and Parkin protein expression level (
0.034) (Table 2). Multivariate analysis found that distal metastasis (
0.029) and Parkin protein expression level (
0.008) were independent prognostic factors for PFS. Patients with distal metastasis had a significantly higher risk of progression-free death than those without (HR = 2.713, 95%CI: 1.109-6.640). Patients with a low Parkin protein level demonstrated a significantly higher risk of progression-free death than those with a high Parkin protein level (HR = 0.431, 95%CI: 0.232-0.801) (Table 3).
DlSCUSSlON
PGAM5 as a unique protein phosphatase existing in the mitochondria is localized to the mitochondria by the N-terminal TM domain[12]. In the case of mitochondrial dysfunction, PGAM5 is cleaved in the transmembrane domain and releases, as a signal mediator to regulate mitochondrial stress response[13]. PGAM5 can activate PINK1/Parkin pathway-mediated mitophagy[12], which is involved in the regulation of apoptosis/necrosis pathways and mitochondrial turnover. Most proteins involved in the process of mitophagy were found to be dysregulated in cancer patients, but whether they act as tumorpromoters or tumor-suppressors seems highly dependent on the cancer subtype and environment[14].
Our data provide a promising foundation for increasing the accuracy of clinical judgment and improving the continued development of cancer treatment and personalized treatment strategies for CRC.
Univariate analysis showed that the following factors were significantly related to OS: Tumor stage (
0.001), tumor invasion depth (
0.014), lymph node status (
0.001), distant metastasis (
0.002), and Parkin protein expression level (
0.042) (Table 2). Multivariate analysis proved that distal metastasis (
0.037), PGAM5 protein expression level (
0.040), and Parkin protein expression level (
0.033) were independent prognostic factors for OS. Patients with distal metastasis exhibited a significantly higher risk of death than those without [hazard ratio (HR) = 2.874, 95% confidence interval (CI): 1.066-7.743]. Patients with a low PGAM5 protein level had a significantly higher risk of death than those with a high PGAM5 protein level (HR = 0.251, 95%CI: 0.067-0.942). Patients with a low Parkin protein level indicated a significantly higher risk of death than those with a high Parkin protein level (HR = 0.477, 95%CI: 0.241-0.943) (Table 3).
We then studied the correlation between PGAM5 and Parkin protein expression in advanced CRC patients. Spearman rank correlation analysis showed that there was a significant positive correlation between PGAM5 and Parkin protein levels in advanced CRC tissues. Knockout of
prevented the elevated expression of PINK1/Parkin pathway in carbonyl cyanide m-chlorophenylhydrazone (CCCP)-induced mitochondrial fragments; PGAM5 regulated PINK1/Parkin-mediated mitophagy, playing a neuroprotective role in CCCP-induced apoptosis[15]. Meanwhile, we found that both proteins were mainly expressed in the cytoplasm of colonic epithelial cells. It is suggested that PGAM5 and Parkin protein may have some function in the cytoplasm in CRC, such as by acting as a regulator of mitochondrial homeostasis and/or mitophagy. We suspect that PGAM5 may be involved in the development of CRC by participating in the Parkin-mediated mechanism.
Ah, that is superbe! said the Princess when she passed by. I have neverheard prettier compositions! Go in and ask him the price of the instrument;but mind, he shall have no more kisses!
Degradation of damaged mitochondria contributes to improved survival of CRC cells[16]. PINK1, Parkin, and Bcl-XL may cooperate to repair slightly damaged mitochondria or, if necessary, promote the synthesis of new mitochondria[17,18]. This promotes apoptosis of tumor cells, thereby affecting the survival of patients. In our study, Kaplan-Meier survival analysis showed a higher survival rate in the group with a high Parkin level compared with the group with a low Parkin level. Multivariate Cox regression analysis further demonstrated that patients with low Parkin expression had a significantly higher risk of death than those with high Parkin expression. These results implied that Parkin protein plays a protective role against the development of CRC, and its level of expression can be used as an independent survival prognostic marker in CRC patients. Yeo
[10] discovered that Parkin pathway activation may be a good prognostic factor, which is consistent with our data. We speculated that, in the pathological mechanism of CRC, Parkin-mediated mechanism, such as regulating mitochondrial homeostasis and/or mitophagy, may play a protective role against tumor development.
In conclusion, our results suggest that both PGAM5 and Parkin protein expression has diagnostic significance for CRC and may become new biomarkers. Meanwhile, the expression levels of PGAM5 and mitophagy-related protein Parkin are significantly positively correlated and reduced Parkin protein expression is prognostic of a shorter survival. Our data provide a promising foundation for increasing the accuracy of clinical judgment and improving the continued development of cancer treatment and personalized treatment strategies for CRC.
PGAM5 promoted persistent fork-head box O activation after developmental mitochondrial stress and prolonged the life span of drosophila[21]. It is suggested that PGAM5 protein may have a regulating effect on the clinical life-span of patients. In the present study, we found a significant positive relationship between the expression of PGAM5 and Parkin in CRC. It is speculated that PGAM5 may participate in a Parkin-mediated mechanism to play a protective role against the development of CRC. However, we did not find statistical significance according to the immunohistochemical expression of PGAM5 protein using Kaplan-Meier survival analysis. Cox multivariate regression analysis showed that the level of PGAM5 protein expression was an independent prognostic factor for OS, and patients with low PGAM5 expression had a shorter survival.
In hepatocellular carcinoma (HCC), high PGAM5 expression was an independent predictor of reduced survival time in both univariate and multivariate analyses. In the same HCC patient tissue cohorts, Bcl-XL expression was found to be positively correlated with that of PGAM5, which together predicted a poor prognosis[22]. Recent studies showed that PGAM5 inhibition attenuates lipid metabolism and colorectal tumorigenesis in mice. PGAM5-mediated dephosphorylation of malic enzyme 1 at S336 allows increased ACAT1-mediated K337 acetylation, leading to an influence on nicotinamide adenine dinucleotide phosphate generation, lipid metabolism, and susceptibility to colorectal tumorigenesis[6].
In consequence, we hypothesize that PGAM5 may play a regulatory role in the occurrence and development of CRC through a multiple-pathway mechanism. On one hand, PGAM5 may play a protective role against CRC by participating in a Parkin-mediated mechanism, such as mitochondrial homeostasis and/or mitophagy; on the other hand, it may promote CRC development through another mechanism, such as lipid metabolism and/or tumorigenesis.
A more comprehensive clinical study including patients with early CRC and better clinical follow-up will be the key to confirming the diagnostic and prognostic value of PGAM5 and Parkin proteins in CRC. In addition, further in-depth molecular studies are needed to explore the mechanism of PGAM5 and Parkin proteins in the occurrence and development of CRC, to provide a basis and prospect for personalized treatment strategies.
CONCLUSlON
However, our results contradict the fact that tumor suppressor genes are often under-expressed in cancer tissues. Mutations in the
gene have been reported in many cancers, although the frequency of these mutations is relatively low[19]. The Cpbioportal database (http://www.cbioportal.org) analysis showed that the rate of mutation of the
gene was around 5% in cervical cancer, around 5% in lung squamous cell carcinoma, and between 2% and 6% in CRC[20]. In a previous study on CRC patients, da Silva-Camargo
[11] showed that Parkin protein was lower in localized colorectal adenocarcinoma tissues than in the adjacent normal tissues. Conversely, it was increased in advanced colorectal adenocarcinoma and was an independent predictor of increased survival. In addition, Parkin subcellular localization analysis revealed more pronounced nuclear expression in normal tissue, whereas more pronounced cytoplasmic expression in adenocarcinoma. The subjects selected for participation in the present study were patients with advanced CRC, of which patients at stage T4 accounted for 46%. This may be one of the reasons for the higher expression of Parkin protein in cancer tissues. Further studies are needed to elucidate the expression and clinical significance of Parkin protein in CRC patients and to explore the role of the Parkin-mediated mecha-nism in CRC.
ARTlCLE HlGHLlGHTS
Research background
Phosphoglycerate mutase family member 5 (PGAM5) activates serine/threonine PTEN-induced putative kinase 1/Parkin pathway-mediated mitophagy. However, there are few studies on the clinical and prognostic significance of expression of PGAM5 protein and mitophagy-related protein Parkin in colorectal cancer (CRC) patients.
Research motivation
Both PGAM5 and Parkin protein expression has diagnostic significance for CRC and may become new biomarkers.
Research objectives
You shall, however, only lend them to him on condition that you may accompany him when he goes to make the payment, and that you then have permission to run before him as a fool
Receiver operating characteristic curve analysis was performed to determine the Youden index (
0.01 indicated that it was significant in the diagnosis of tumor). According to the Youden index, the cut-off value delimiting high and low expression of PGAM5 was determined to be 4.75 (
0.001), and for Parkin it was 7.1 (
0.001). This suggested that the protein expression of PGAM5 and Parkin is of significance in the diagnosis of CRC: In the present study, we found that 96 cases had high expression of PGAM5 in 100 cases of CRC tissues, and 71 cases had high expression of Parkin.
Research methods
We used immunohistochemistry to determine the expression of PGAM5 and Parkin in CRC tissues from 100 patients. More than that, we employed western blot to measure PGAM5 and Parkin protein expression in six matched pairs of CRC and adjacent non-tumor tissues.
Research results
We found that both PGAM5 and Parkin protein expression in tumor tissues was significantly higher than that in the adjacent tissues according to immunohistochemical and western blot. What’s more, PGAM5 and Parkin protein levels were significantly positively correlated in advanced CRC tissues. And reduced Parkin protein expression was an independent prognostic factor for overall survival and progression-free survival in CRC patients as evinced by multivariate analysis.
Research conclusions
The expressions of PGAM5 protein and mitophagy-related protein Parkin has diagnostic significance for CRC and may become new biomarkers. Parkin may be a potential marker for the survival of CRC patients.
Research perspectives
In this study, we collected colorectal adenocarcinoma and corresponding paracancerous tissues to explore the clinical expression of PGAM5 protein in CRC patients. The level of expression of PGAM5 protein in tumor tissue was significantly higher than that in adjacent non-tumor tissue; statistical analysis revealed that the level of expression of PGAM5 protein had diagnostic significance for CRC. Our research results were consistent with those from a study measuring phosphatase protein expression in CRC tissues, which showed that PGAM5 protein is over-expressed in cancer tissues. Moreover, down-regulation of PGAM5 expression significantly inhibited the proliferation of CRC cells[6]. It is suggested that PGAM5 protein may not only be a new marker for the diagnosis of CRC, but also play a regulatory role in the development of CRC.
Now, father, you shall see what I can do! said the blacksmith, and he sprang after the carriage, tore off the four shoes of the horse as it was going at the top of its speed, and shod it with four new ones without checking its pace
FOOTNOTES
Wu C and Sun MJ designed the research study; Feng ML and Jiao TW performed the research; Wu C and Feng ML contributed new reagents and analytic tools; Wu C and Jiao TW analyzed the data and wrote the manuscript; Wu C has received research funding; and all authors have read and approved the final manuscript.
the Natural Science Foundation of Liaoning Province, No. 2019-BS-279.
This study was approved by the Institutional Review Board of The First Affiliated Hospital of China Medical University (No. 2021-68-2).
This study is registered at clinical hospital center “The First Hospital Affiliated to China Medical University” trial registry. The registration identification number is No. 2021-68-2.
Informed consent statement was waived.
There are no conflict of interest.
But after all he had to wait for the Princess s story, for the inhabitants of the Green Lands, hearing that the Giant was dead, ran to offer the kingdom to his vanquisher104, and Prince Vivien had to listen to various complimentary105 harangues106, which took a great deal of time, though he cut them as short as politeness allowed--if not shorter
No additional data are available.
The King to whom the garden belonged, came down to it next morning, and counted, and saw that one of the pears was missing, and asked the gardener what had become of it, as it was not lying beneath the tree, but was gone
This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
China
Can Wu 0000-0002-9532-8767; Ming-Liang Feng 0000-0001-6624-9624; Tai-Wei Jiao 0000-0002-7679-6354; Ming-Jun Sun 0000-0003-0072-2575.
Wang JJ
They lived happy ever after: And so Jack and his mother--and in this version Jack s bride--life happily ever after with a traditional romantic fairy tale ending
According to Jungian psychology44, the forest is a representation of the feminine principle and is identified with the unconscious. The foliage45 blocks the sun s rays, the sun being associated with the male principle. The forest symbolizes47 the dangerous side of the unconscious, its ability to destroy reason (Cirlot 1962) and (Matthews 1986). Return to place in story.
Wang TQ
Wang JJ
1 Bray F, Ferlay J, Soerjomataram Ⅰ, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries.
2018; 68: 394-424 [PMⅠD: 30207593 DOⅠ: 10.3322/caac.21492]
2 Panda S, Srivastava S, Li Z, Vaeth M, Fuhs SR, Hunter T, Skolnik EY. Ⅰdentification of PGAM5 as a Mammalian Protein Histidine Phosphatase that Plays a Central Role to Negatively Regulate CD4(+) T Cells.
2016; 63: 457-469 [PMⅠD: 27453048 DOⅠ: 10.1016/j.molcel.2016.06.021]
3 Boyle KA, Van Wickle J, Hill RB, Marchese A, Kalyanaraman B, Dwinell MB. Mitochondria-targeted drugs stimulate mitophagy and abrogate colon cancer cell proliferation.
2018; 293: 14891-14904 [PMⅠD: 30087121 DOⅠ: 10.1074/jbc.RA117.001469]
4 Ng Kee Kwong F, Nicholson AG, Pavlidis S, Adcock ⅠM, Chung KF. PGAM5 expression and macrophage signatures in non-small cell lung cancer associated with chronic obstructive pulmonary disease (COPD).
2018; 18: 1238 [PMⅠD: 30526542 DOⅠ: 10.1186/s12885-018-5140-9]
5 Hawk MA, Gorsuch CL, Fagan P, Lee C, Kim SE, Hamann JC, Mason JA, Weigel KJ, Tsegaye MA, Shen L, Shuff S, Zuo J, Hu S, Jiang L, Chapman S, Leevy WM, DeBerardinis RJ, Overholtzer M, Schafer ZT. RⅠPK1-mediated induction of mitophagy compromises the viability of extracellular-matrix-detached cells.
2018; 20: 272-284 [PMⅠD: 29459781 DOⅠ: 10.1038/s41556-018-0034-2]
6 Zhu Y, Gu L, Lin X, Liu C, Lu B, Cui K, Zhou F, Zhao Q, Prochownik EV, Fan C, Li Y. Dynamic Regulation of ME1 Phosphorylation and Acetylation Affects Lipid Metabolism and Colorectal Tumorigenesis.
2020; 77: 138-149.e5 [PMⅠD: 31735643 DOⅠ: 10.1016/j.molcel.2019.10.015]
7 Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy.
2008; 183: 795-803 [PMⅠD: 19029340 DOⅠ: 10.1083/jcb.200809125]
8 Chen H, Chan DC. Mitochondrial dynamics--fusion, fission, movement, and mitophagy--in neurodegenerative diseases.
2009; 18: R169-R176 [PMⅠD: 19808793 DOⅠ: 10.1093/hmg/ddp326]
9 Hang L, Thundyil J, Lim KL. Mitochondrial dysfunction and Parkinson disease: a Parkin-AMPK alliance in neuroprotection.
2015; 1350: 37-47 [PMⅠD: 26121488 DOⅠ: 10.1111/nyas.12820]
10 Yeo CW, Ng FS, Chai C, Tan JM, Koh GR, Chong YK, Koh LW, Foong CS, Sandanaraj E, Holbrook JD, Ang BT, Takahashi R, Tang C, Lim KL. Parkin pathway activation mitigates glioma cell proliferation and predicts patient survival.
2012; 72: 2543-2553 [PMⅠD: 22431710 DOⅠ: 10.1158/0008-5472.CAN-11-3060]
11 da Silva-Camargo CCV, Svoboda Baldin RK, Costacurta Polli NL, Agostinho AP, Olandosk M, de Noronha L, Sotomaior VS. Parkin protein expression and its impact on survival of patients with advanced colorectal cancer.
2018; 15: 61-69 [PMⅠD: 29545969 DOⅠ: 10.20892/j.issn.2095-3941.2017.0136]
12 Lu W, Karuppagounder SS, Springer DA, Allen MD, Zheng L, Chao B, Zhang Y, Dawson VL, Dawson TM, Lenardo M. Genetic deficiency of the mitochondrial protein PGAM5 causes a Parkinson's-like movement disorder.
2014; 5: 4930 [PMⅠD: 25222142 DOⅠ: 10.1038/ncomms5930]
13 Yamaguchi A, Ⅰshikawa H, Furuoka M, Yokozeki M, Matsuda N, Tanimura S, Takeda K. Cleaved PGAM5 is released from mitochondria depending on proteasome-mediated rupture of the outer mitochondrial membrane during mitophagy.
2019; 165: 19-25 [PMⅠD: 30247576 DOⅠ: 10.1093/jb/mvy077]
14 Vara-Perez M, Felipe-Abrio B, Agostinis P. Mitophagy in Cancer: A Tale of Adaptation.
2019; 8 [PMⅠD: 31121959 DOⅠ: 10.3390/cells8050493]
15 Park YS, Choi SE, Koh HC. PGAM5 regulates PⅠNK1/Parkin-mediated mitophagy
DRP1 in CCCP-induced mitochondrial dysfunction.
2018; 284: 120-128 [PMⅠD: 29241732 DOⅠ: 10.1016/j.toxlet.2017.12.004]
16 Vincow ES, Merrihew G, Thomas RE, Shulman NJ, Beyer RP, MacCoss MJ, Pallanck LJ. The PⅠNK1-Parkin pathway promotes both mitophagy and selective respiratory chain turnover in vivo.
2013; 110: 6400-6405 [PMⅠD: 23509287 DOⅠ: 10.1073/pnas.1221132110]
17 Matsuda N, Sato S, Shiba K, Okatsu K, Saisho K, Gautier CA, Sou YS, Saiki S, Kawajiri S, Sato F, Kimura M, Komatsu M, Hattori N, Tanaka K. PⅠNK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy.
2010; 189: 211-221 [PMⅠD: 20404107 DOⅠ: 10.1083/jcb.200910140]
18 McLelland GL, Soubannier V, Chen CX, McBride HM, Fon EA. Parkin and PⅠNK1 function in a vesicular trafficking pathway regulating mitochondrial quality control.
2014; 33: 282-295 [PMⅠD: 24446486 DOⅠ: 10.1002/embj.201385902]
19 Cesari R, Martin ES, Calin GA, Pentimalli F, Bichi R, McAdams H, Trapasso F, Drusco A, Shimizu M, Masciullo V, D'Andrilli G, Scambia G, Picchio MC, Alder H, Godwin AK, Croce CM. Parkin, a gene implicated in autosomal recessive juvenile parkinsonism, is a candidate tumor suppressor gene on chromosome 6q25-q27.
2003; 100: 5956-5961 [PMⅠD: 12719539 DOⅠ: 10.1073/pnas.0931262100]
20 Wahabi K, Perwez A, Rizvi MA. Parkin in Parkinson's Disease and Cancer: a Double-Edged Sword.
2018; 55: 6788-6800 [PMⅠD: 29349575 DOⅠ: 10.1007/s12035-018-0879-1]
21 Borch Jensen M, Qi Y, Riley R, Rabkina L, Jasper H. PGAM5 promotes lasting FoxO activation after developmental mitochondrial stress and extends lifespan in
.
2017; 6 [PMⅠD: 28891792 DOⅠ: 10.7554/eLife.26952]
22 Cheng J, Qian D, Ding X, Song T, Cai M, Dan Xie, Wang Y, Zhao J, Liu Z, Wu Z, Pang Q, Zhu L, Wang P, Hao X, Yuan Z. High PGAM5 expression induces chemoresistance by enhancing Bcl-xL-mediated anti-apoptotic signaling and predicts poor prognosis in hepatocellular carcinoma patients.
2018; 9: 991 [PMⅠD: 30250224 DOⅠ: 10.1038/s41419-018-1017-8]
 World Journal of Clinical Cases2022年14期
World Journal of Clinical Cases2022年14期
- World Journal of Clinical Cases的其它文章
- Perfectionism and mental health problems: Limitations and directions for future research
- Ovarian growing teratoma syndrome with multiple metastases in the abdominal cavity and liver: A case report
- Development of plasma cell dyscrasias in a patient with chronic myeloid leukemia: A case report
- Suprasellar cistern tuberculoma presenting as unilateral ocular motility disorder and ptosis: A case report
- Rare pattern of Maisonneuve fracture: A case report
- PD-1 inhibitor in combination with fruquintinib therapy for initial unresectable colorectal cancer: A case report
