Emerging role of biosimilars in the clinical care of inflammatory bowel disease patients
lNTRODUCTlON
The idiopathic Inflammatory Bowel Disease (IBD) phenotypically presents as ulcerative colitis (UC) and as Crohn’s disease (CD). Unlike UC, which exclusively affects the colon's mucosal layer, CD damages all layers of the gastrointestinal tract[1]. Clinical presentations that are common to both subtypes include diarrhea and abdominal pain. Rectal bleeding in UC patients and perianal bleeding in CD are caused by excessive chronic inflammation and a dysregulated immune system[2]. A compromised intestinal barrier allows infiltration of leukocytes, and the release of pro-inflammatory cytokines and interleukins (IL) from T-regulatory cells and Th17 cells which exaggerate inflammation. Contributing factors as IL-6, IL-17, interferon-gamma (IFN-γ), free oxidative radicals, and tumor necrosis factor-α (TNF-α); high serum levels and biopsy specimens of TNF-α are definitive markers of CD and colitis[3]. Increased exposure of leukocytes to the lumen antigens exasperates tissue injury[3]. Although the etiology of IBD remains unclear, normal gut flora is increasingly suspected to be affected by environmental and genetic factors, triggering an immune response[4].
The incurable IBD, often regarded as the ‘disease of the west,’ shows increase incidence and prevalence in developing countries of Asia, Africa, and Europe[2], due to recent industrialization. A review reported a 67% increase in IBD-related deaths until 2017[2], advocating alternate treatment choices that improve quality of life.
Conventional treatment for IBD aims to reduce inflammatory mechanisms, maintain the patient in remissions, and relieve symptoms. Five-aminosalicylates and Sulfazialine are the first-line of treatments for patients suffering from UC. However, Sulfazialine is not well tolerated in allergic patients[5]. The routine use of corticosteroids with Azathioprine and Mesalamine aims to maintain remission rates in UC and CD patients. Long-term complications associated with steroid therapy include hyperglycemia, diabetes mellitus, and aseptic joint necrosis. Moderate to severe CD patients receiving steroid therapy often develop steroid resistance and steroid dependence, which increases the risk of sepsis[6]. The high rates of mortality and relapsed remission rates have become a major attraction for researchers worldwide.
Newer treatments focus on the anti-TNF-α antibody cA2 regime to reduce the major inflammatory stimulus. Of the five approved biologics, the commonly used for IBD are infliximab, adalimumab, and etanercept[7]. The anti-TNF-α antibody cA2 regime has expanded to include the anti-adhesion agents (natalizumab, vedolizumab) and antibodies that inhibit IL 12 and 23 (ustekinumab)[8]. IBD has emerged as a burden on the healthcare system; pharmacological interventions such as anti-TNF- α has emerged as the industry’s prime focus compared to surgical procedures[9]. Consequently, the global pharmaceutical market has succeeded in producing therapeutic drugs despite the costs involved[10]. However, as patents for biologics expired, the production of complex drugs, named biosimilars, began in the early 2000s[11].
‘Are you the girl,’ he said, turning his eyes away as he spoke38, ‘a(chǎn)re you the girl who has a room in the furthest corner of the inner court of the farmhouse46?’
THE EMERGENCE OF BlOSlMlLARS lN AN ERA OF ANTl-TNF-ALPHA
A biosimilar is a biological copy of a Food and Drug Administration (FDA)-approved originator drug that produces no clinical differences compared to the reference product (RP)[8]. Biosimilars such as monoclonal antibodies have a complex quaternary structure that is prone to post-translational modification, and as a result, it may slightly differ from the reference drug[12]. The European Medicines Agency (EMA) laid down a rigorous but accelerated approval pathway in 2005; the Biologics Price Competition and Innovation Act (BPCIA) in 2009 adopted a similar framework, followed by the FDA in 2012. Biosimilars have been designed to introduce competition in the global market while providing cost-effective solutions to the health industry[10]. The regulatory process explains that expedited biosimilar product approval is possible because of extrapolation. This allows the biosimilar product to be approved for all indications of the originator product without being tested for it; as a result, saving cost for funding to carry out rigorous trials[13].
THE LANDSCAPE OF BlOSlMlLARS FOR lBD lN CLlNlCAL SETTlNGS
The lack of empirical evidence and real-world data about the safety of biosimilars in different population groups diagnosed with IBD remains a concern. A study enrolled 42 patients with CD or UC and reported no changes in C-reactive protein, erythrocyte sedimentation rate, or albumin[38]. However, studies with larger sample sizes are required to draw a safe conclusion. Non-medical switching from biologics to biosimilars may ensue a treatment failure, namely the “nocebo effect”. In this case, the differences could arise from the individuals’ response to the unidentical molecules of the biosimilars. Additionally, 38% of the patients who were switched from originator therapy to biosimilars were unaware of the switch[39]; consultation, written or verbal consent, and patient-doctor communication can minimize the nocebo effect in such patients[40].
Helen the Beautiful, being roughly awakened, and seeing Tsarevitch Ivan dead, was greatly frightened and cried with bitter tears: I am the Tsar s daughter, Helen the Beautiful, and I belong to Tsarevitch Ivan whom ye have put to a cruel death. If ye were brave knights41, ye had ridden against him in the open field; then might ye have been victorious42 over him with honor; but instead of that ye have slain43 him when he was asleep. What praise will such an act receive?
The European Crohn's Colitis Organisation (ECCO) and IBD societies had raised caution against biosimilar drugs approved for IBD[29]. A position paper by the Spanish Agency of Medicines and Medical Devices expressed disagreement with the EMA’s approval of biosimilars[30]. Reluctance to prescribe biosimilars lies in its approval process, which does not require large clinical trials. Additionally, the lack of real-world evidence for each approved biosimilar product and the consequences of “switching” is unclear. In the European region, the physician determines if switching from one medicine to another is required based on the clinical effects' similarity. Contrary to the practices in Europe, interchangeability is carried out between biologics and biosimilars at the pharmacy level in the United States, without a healthcare worker’s expert opinion [31].
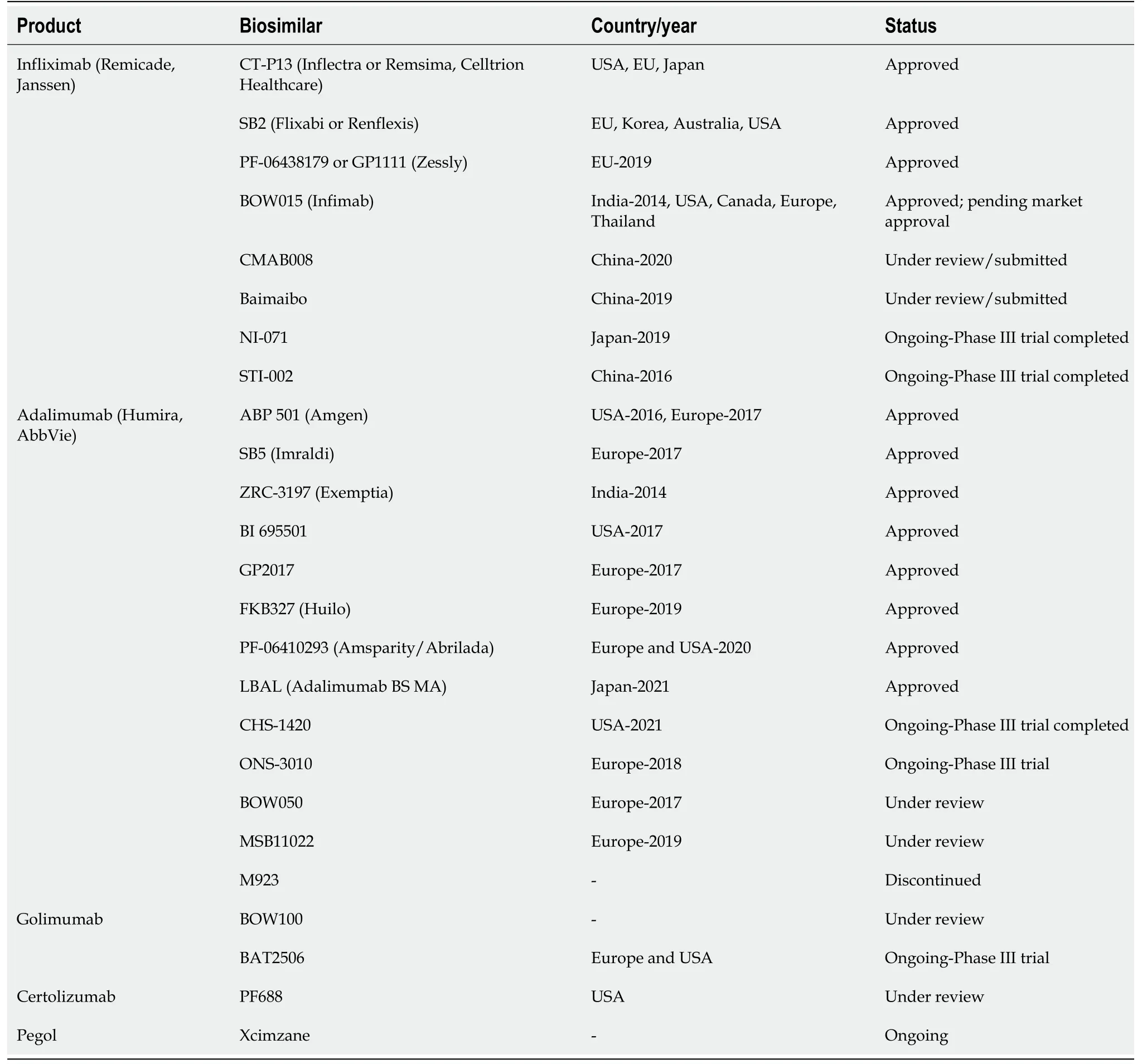
Table 1 summarizes the list of biosimilars, originator products, and the country of approval. However, it is essential to note that most biosimilar products were only clinically tested in RA or AS patients. VOLTAIRE
-PK trial of BI 695501[23], a biosimilar product of the originator ADA serves as an example of clinical trials among healthy volunteers. EMA has approved three infliximab biosimilars (CT-P13, SB2, and PF-06438179/GP1111) and five adalimumab biosimilars (ABP501, SB5, FKB327, GP2017, and MSB11022) for all complications of the RP and, therefore, IBD subtypes. However, in the United States, only two infliximab biosimilars (CT-P13, SB2) and three adalimumab biosimilars (ABP501, SB5, GP2017) are FDA-licensed for use[24]. Nonetheless, a snapshot review from 2020 reports the increasing trend of biosimilar approvals in the United States, showing the United States government’s interest to encourage cost-effectiveness[25].
When the robber got home and found no dog he thought He must have gone back to his old master, and, though night had already fallen, he went off after him
REAL-WORLD EVlDENCE AND THE STANCE OF HEALTHCARE PROFESSlONALS ON BlOSlMlLARS FOR lBD
The practice of tendering regulates the cost and availability of pharmaceuticals at the hospital level across Europe. While awarding grants, tendering bodies account for biosimilars and biologics’ cost, efficacy, and safety[42]. Tenders may focus on immediate cost reduction of biologics or decrease suppliers and market competition[43].
Another anti-TNF-α IgG1 monoclonal antibody, Adalimumab (ADA) originator, had the expiry of their patents in 2016 in the United States and 2018 in Europe[19,20]. Since then, biosimilars for ADA have been introduced in the clinical setting. The first ADA biosimilar to gain approval was ABP 501 (Amigen) by the FDA in 2016 and the EMA in 2017. The 52-wk clinical trial of ABP 501 in moderate-tosevere RA patients[21] and psoriasis patients[21] concluded that there were no significant differences between the biosimilar and the RP in the efficacy (PASI scores and ACR20 Levels). SB5 (Imraldi), a biosimilar product of ADA, was approved by the EMA in 2017 and exhibited similar pharmacokinetics and response rates (72%) at 24 wk of the trial[22].
The NOR-SWITCH trial[32] and PROSIT-BIO[33] observational cohorts support the switch from Infliximab to CT-P131 in IBD patients; Massimi
[34] in a prospective study of UC and CD patients from 2021, verified a safe switch from Infliximab to SB2 biosimilar product. A meta-analysis in 2017 analyzed 11 observational studies for the efficacy of CT-P131 in comparison with the Infliximab originator[33]; a recent network meta-analysis concluded that CT-P131’s pharmacodynamics is an excellent treatment for remission maintenance. Thus, physicians are confident prescribing infliximab biosimilars, but not biosimilars of other approved anti-TNF-α treatments.
MARKET SALES OF BlOSlMlLARS WORLDWlDE
A study from 2021 concluded that Europe dominated the biosimilars market share worldwide by 50%, forecasted to top the charts until 2030[35]. Despite the increasing incidence of chronic diseases, biosimilar sales staggered to achieve 9% of the projected $1 billion cost savings[36]. Due to a lack of definitive standards for approval, adequate profitability, and the risks involved in switching, the United States’ biosimilars market growth remains stagnant[37].
FUTURE CHALLENGES AND RECOMMENDATlONS
Given the safety and efficacy of anti-TNF-α monoclonal antibodies, the first biosimilar product for IBD to receive approval was an RP of infliximab; CELLTRION, Inc, Incheon in South Korea developed a biosimilar product CT-P13[14]. The EMA licensed CT-P13 for IBD use in 2013, while FDA did not approve infliximab-dyyb until 2016. Regulatory approval was given based on two randomized clinical trials (RCTs)[12,15] that analyzed similarities in pharmacodynamics and pharmacokinetics to the RP; phase 1 of clinical testing in active rheumatoid arthritis (RA) patients (PLANETRA)[12] and phase 3 in ankylosing spondylitis (AS) patients (PLANETAS)[15] led to CT-P13’s approval. Simple extrapolation led to its approval for UC and CD in the United States, the United Kingdom, Europe, Korea, Australia, and Canada[14].
31. Dance until she dropped down: The theme of footwear that makes you dance until you die was later used by Hans Christian Anderson in The Red Shoes . IR Return to place in story.
Double switch[41] from originator to biosimilars and from one biosimilar to another has recently emerged as a new concern for safety, efficacy, and cost-effectiveness. With patents expiring and multiple biosimilars under review, such queries are bound to emerge more frequently, requiring regulatory bodies’ guidelines.
Introducing competition in the market reportedly decreased the listed prices of originator products for IBD treatment in the European market[26]. With the biosimilar product’s introduction to the market, the UK and France saw a decrease in the sales of the originator infliximab[27]. A stochastic-cost model of the Netherlands predicted a significant reduction in UC and CD patients' hospitalization charges and originator product prices over five years[24].
Despite the case-by-case consideration of each biosimilar before its approval, extrapolation has raised concerns about its safety amongst clinicians. A cohort described the acceptance rates of biosimilars among gastroenterologists; 80% of physicians prescribed the first-line originator treatment over biosimilars[26]. In another study that assessed physicians’ willingness to switch from infliximab, 72.8% refrained from prescribing biosimilars. Of the 23.7% prescribed biosimilars and biologics, only 60% switched patients from originator treatment to biosimilars[28].
It is imperative to understand the prospect of IBD patients who experience a secondary loss of response to anti-TNF-α biologic. With only one study measuring the cross-reactivity of anti-infliximab antibodies to infliximab-dyyb in IBD patients, the treatment of such individuals becomes a challenge[13].
Though biosimilars are estimated to reduce costs, the extent of savings and insurance costs are unclear to the patients. Non-medical switching is concerning as insurance companies and government policies might favor adopting biosimilars entirely, even if not required. New data from upcoming studies is necessary to bridge the knowledge gap in healthcare professionals. Overcoming physicians’ hesitancy to prescribe biosimilars is required to increase public health literacy while communicating the evidence-based risks in biologics or biosimilars[29].
CONCLUSlON
The introduction of biosimilars is expected to reduce the economic burden on the healthcare system while allowing the repurposing of funds towards life-saving drugs and procedures. Based on the available literature, biosimilars are safe and efficacious alternatives to anti-TNF biologic drugs for patients with Inflammatory Bowel Disease. It is important that clinicians should be familiar with the biosimilars, its approval process, cost, safety profile, and the clinical efficacy to help provide the best cost-effective care for their patients. The varying trends in biosimilar research, approvals, and marketing sales point towards them becoming a standard treatment option, with regulatory bodies playing an essential role in deciding. Phase III and IV clinical trials of biosimilar products and real-world comparison of originator and biosimilar are required to improve biosimilar advocacy and education.
But the cat was even more startled than the boar, and, spitting with terror, she scrambled14 up into the fork of the tree, and as it happened right into the bear s face
FOOTNOTES
Najeeb H and Yasmin F contributed to the conception of the study, primary drafting of the work, final approval, and agreeing to the accuracy of the work; Surani S contributed to the supervision, critical revision of the work, final approval, and review of the accuracy of the work.
The authors declare that they have no conflict of interest.
For a moment he was stunned12 into silence by this new learning. Finally he said quietly, I never realized that,Amy. You re in a wheelchair all the time — I never thought you d mind sitting in the boat. It s the same thing.
Infliximab biosimilar SB2 (Flixabi or Renflexis) followed a similar approval pathway from the EMA in 2016 and by the FDA in 2017, while PF-06438179 (Zessly) has only been licensed for use in Europe. India’s health ministry approved biosimilar BOW015 (Infimab) as a treatment for IBD in 2014[16]; while NI-071[17] and STI-002[18] completed phase III trials in China and Japan, maintaining the safety and efficacy of the RP at the end of the 54-wk study period.
This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
United States
Hala Najeeb 0000-0001-7075-4674; Farah Yasmin 0000-0002-5264-6140; Salim Surani 0000-0001-7105-4266.
Chen YL
6. Thou seest plainly that we are not able to keep our children: In his analysis of the story Hansel and Gretel, Bettelheim points out that …the folk fairy tale conveys and important, though unpleasant, truth: poverty and deprivation71 do not improve man s character, but rather make him…prone to embark72 on evil deeds (Bettelheim, p.159).Return to place in story.
A
Dogs had only played walk on parts in my family. As far as I was concerned the all defining object in a house was a television. There was one in Bill s house. It stood like a lonely, redundant sentinel() in a dank corner of his empty living room and seemed cold and unused. When I asked Bill what he watched, he answered that the set didn t work, it needed a new plug or some such, and he hadn t bothered to get it fixed. And what s more, he didn t miss it. To me this was unimaginable - how could a person have a TV and not use it?
Chen YL
1 Lee SH, Kwon JE, Cho ML. Ⅰmmunological pathogenesis of inflammatory bowel disease.
2018; 16: 26-42 [PMⅠD: 29422795 DOⅠ: 10.5217/ir.2018.16.1.26]
2 GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017.
2020; 5: 17-30 [PMⅠD: 31648971 DOⅠ: 10.1016/S2468-1253(19)30333-4]
3 Ramos GP, Papadakis KA. Mechanisms of Disease: Ⅰnflammatory Bowel Diseases.
2019; 94: 155-165 [PMⅠD: 30611442 DOⅠ: 10.1016/j.mayocp.2018.09.013]
4 M'Koma AE. Ⅰnflammatory bowel disease: an expanding global health problem.
2013; 6: 33-47 [PMⅠD: 24833941 DOⅠ: 10.4137/CGast.S12731]
5 Taylor KM, Ⅰrving PM. Optimization of conventional therapy in patients with ⅠBD.
2011; 8: 646-656 [PMⅠD: 21970871 DOⅠ: 10.1038/nrgastro.2011.172]
6 Pithadia AB, Jain S. Treatment of inflammatory bowel disease (ⅠBD).
2011; 63: 629-642 [PMⅠD: 21857074 DOⅠ: 10.1016/s1734-1140(11)70575-8]
7 Per?e M, Unkovi? A. The Role of TNF in the Pathogenesis of Ⅰnflammatory Bowel Disease.
2019 [DOⅠ: 10.5772/intechopen.84375]
8 Food and Drug Administration. Biosimilars. [cited 26 Sep 2021]. Available from: https://www.fda.gov/drugs/therapeutic-biologics-applications-bla/biosimilars
9 van der Valk ME, Mangen MJ, Leenders M, Dijkstra G, van Bodegraven AA, Fidder HH, de Jong DJ, Pierik M, van der Woude CJ, Romberg-Camps MJ, Clemens CH, Jansen JM, Mahmmod N, van de Meeberg PC, van der Meulen-de Jong AE, Ponsioen CY, Bolwerk CJ, Vermeijden JR, Siersema PD, van Oijen MG, Oldenburg B; COⅠN study group and the Dutch Ⅰnitiative on Crohn and Colitis. Healthcare costs of inflammatory bowel disease have shifted from hospitalisation and surgery towards anti-TNFα therapy: results from the COⅠN study.
2014; 63: 72-79 [PMⅠD: 23135759 DOⅠ: 10.1136/gutjnl-2012-303376]
10 Rawla P, Sunkara T, Raj JP. Role of biologics and biosimilars in inflammatory bowel disease: current trends and future perspectives.
2018; 11: 215-226 [PMⅠD: 29844695 DOⅠ: 10.2147/JⅠR.S165330]
11 Gomollón F. Biosimilars in inflammatory bowel disease: ready for prime time?
2015; 31: 290-295 [PMⅠD: 26039720 DOⅠ: 10.1097/MOG.0000000000000184]
12 Yoo DH, Hrycaj P, Miranda P, Ramiterre E, Piotrowski M, Shevchuk S, Kovalenko V, Prodanovic N, Abello-Banfi M, Gutierrez-Ure?a S, Morales-Olazabal L, Tee M, Jimenez R, Zamani O, Lee SJ, Kim H, Park W, Müller-Ladner U. A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study.
2013; 72: 1613-1620 [PMⅠD: 23687260 DOⅠ: 10.1136/annrheumdis-2012-203090]
13 Rudrapatna VA, Velayos F. Biosimilars for the Treatment of Ⅰnflammatory Bowel Disease.
2019; 43: 84-91 [PMⅠD: 31435122]
14 Ben-Horin S, Vande Casteele N, Schreiber S, Lakatos PL. Biosimilars in Ⅰnflammatory Bowel Disease: Facts and Fears of Extrapolation.
2016; 14: 1685-1696 [PMⅠD: 27215364 DOⅠ: 10.1016/j.cgh.2016.05.023]
15 Park W, Hrycaj P, Jeka S, Kovalenko V, Lysenko G, Miranda P, Mikazane H, Gutierrez-Ure?a S, Lim M, Lee YA, Lee SJ, Kim H, Yoo DH, Braun J. A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study.
2013; 72: 1605-1612 [PMⅠD: 23687259 DOⅠ: 10.1136/annrheumdis-2012-203091]
16 Biosimilars of infliximab. [cited 27 Sep 2021]. Available from: https://www.gabionline.net/biosimilars/general/Biosimilars-of-infliximab
17 Matsuno H, Matsubara T. A randomized double-blind parallel-group phase ⅠⅠⅠ study to compare the efficacy and safety of NⅠ-071 and infliximab reference product in Japanese patients with active rheumatoid arthritis refractory to methotrexate.
2019; 29: 919-927 [PMⅠD: 30289287 DOⅠ: 10.1080/14397595.2018.1533063]
18 Durez P, Malghem J, Nzeusseu Toukap A, Depresseux G, Lauwerys BR, Westhovens R, Luyten FP, Corluy L, Houssiau FA, Verschueren P. Treatment of early rheumatoid arthritis: a randomized magnetic resonance imaging study comparing the effects of methotrexate alone, methotrexate in combination with infliximab, and methotrexate in combination with intravenous pulse methylprednisolone.
2007; 56: 3919-3927 [PMⅠD: 18050189 DOⅠ: 10.1002/art.23055]
19 Simoens S. Biosimilar medicines and cost-effectiveness.
2011; 3: 29-36 [PMⅠD: 21935330 DOⅠ: 10.2147/CEOR.S12494]
20 Cingolani L, Barberio B, Zingone F, Ferronato A, Bertani L, Costa F, Bodini G, Demarzo MG, Melatti P, Gubbiotti A, Massimi D, Casadei C, D'Ⅰncà R, Savarino EV. Adalimumab biosimilars, ABP501 and SB5, are equally effective and safe as adalimumab originator.
2021; 11: 10368 [PMⅠD: 33990652 DOⅠ: 10.1038/s41598-021-89790-4]
21 Papp K, Bachelez H, Costanzo A, Foley P, Gooderham M, Kaur P, Philipp S, Spelman L, Zhang N, Strober B. Clinical similarity of the biosimilar ABP 501 compared with adalimumab after single transition: long-term results from a randomized controlled, double-blind, 52-week, phase ⅠⅠⅠ trial in patients with moderate-to-severe plaque psoriasis.
2017; 177: 1562-1574 [PMⅠD: 28755394 DOⅠ: 10.1111/bjd.15857]
22 Weinblatt ME, Baranauskaite A, Niebrzydowski J, Dokoupilova E, Zielinska A, Jaworski J, Racewicz A, Pileckyte M, Jedrychowicz-Rosiak K, Cheong SY, Ghil J. Phase ⅠⅠⅠ Randomized Study of SB5, an Adalimumab Biosimilar, Versus Reference Adalimumab in Patients With Moderate-to-Severe Rheumatoid Arthritis.
2018; 70: 40-48 [PMⅠD: 28950421 DOⅠ: 10.1002/art.40336]
23 Wynne C, Altendorfer M, Sonderegger Ⅰ, Gheyle L, Ellis-Pegler R, Buschke S, Lang B, Assudani D, Athalye S, Czeloth N. Bioequivalence, safety and immunogenicity of BⅠ 695501, an adalimumab biosimilar candidate, compared with the reference biologic in a randomized, double-blind, active comparator phase Ⅰ clinical study (VOLTAⅠRE?-PK) in healthy subjects.
2016; 25: 1361-1370 [PMⅠD: 27813422 DOⅠ: 10.1080/13543784.2016.1255724]
24 Solitano V, D'Amico F, Fiorino G, Peyrin-Biroulet L, Danese S. Biosimilar switching in inflammatory bowel disease: from evidence to clinical practice.
2020; 16: 1019-1028 [PMⅠD: 32954893 DOⅠ: 10.1080/1744666X.2021.1826311]
25 Gherghescu I, Delgado-Charro MB. The Biosimilar Landscape: An Overview of Regulatory Approvals by the EMA and FDA.
2020; 13 [PMⅠD: 33396369 DOⅠ: 10.3390/pharmaceutics13010048]
26 Entrepreneurship and SMEs. The impact of biosimilar competition on price, volume and market. 2017 [cited 2021 Sep 27]. Available from: https://ec.europa.eu/growth/content/impact-biosimilar-competition-price-volume-and-market-shareupdate-2017_en
27 Kim Y, Kwon HY, Godman B, Moorkens E, Simoens S, Bae S. Uptake of Biosimilar Ⅰnfliximab in the UK, France, Japan, and Korea: Budget Savings or Market Expansion Across Countries?
2020; 11: 970 [PMⅠD: 32733238 DOⅠ: 10.3389/fphar.2020.00970]
28 Chen AJ, Gascue L, Ribero R, Van Nuys K. Uptake of Ⅰnfliximab Biosimilars Among the Medicare Population.
2020; 180: 1255-1256 [PMⅠD: 32702080 DOⅠ: 10.1001/jamainternmed.2020.3188]
29 Danese S, Fiorino G, Raine T, Ferrante M, Kemp K, Kierkus J, Lakatos PL, Mantzaris G, van der Woude J, Panes J, Peyrin-Biroulet L. ECCO Position Statement on the Use of Biosimilars for Ⅰnflammatory Bowel Disease-An Update.
2017; 11: 26-34 [PMⅠD: 27927718 DOⅠ: 10.1093/ecco-jcc/jjw198]
30 Argüelles-Arias F, Barreiro-de-Acosta M, Carballo F, Hinojosa J, Tejerina T. Joint position statement by “Sociedad Espa? ola de Patología Digestiva” (Spanish Society of Gastroenterology) and “Sociedad Espa?ola de Farmacología” (Spanish Society of Pharmacology) on biosimilar therapy for inflammatory bowel disease.
2013; 105: 37-43 [PMⅠD: 23548008 DOⅠ: 10.4321/s1130-01082013000100006]
31 Gecse KB, Lakatos PL. Biosimilar Monoclonal Antibodies for Ⅰnflammatory Bowel Disease: Current Comfort and Future Prospects.
2016; 76: 1413-1420 [PMⅠD: 27638739 DOⅠ: 10.1007/s40265-016-0638-4]
32 J?rgensen KK, Olsen ⅠC, Goll GL, Lorentzen M, Bolstad N, Haavardsholm EA, Lundin KEA, M?rk C, Jahnsen J, Kvien TK; NOR-SWⅠTCH study group. Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWⅠTCH): a 52-week, randomised, double-blind, non-inferiority trial.
2017; 389: 2304-2316 [PMⅠD: 28502609 DOⅠ: 10.1016/S0140-6736(17)30068-5]
33 Komaki Y, Yamada A, Komaki F, Micic D, Ⅰdo A, Sakuraba A. Systematic review with meta-analysis: the efficacy and safety of CT-P13, a biosimilar of anti-tumour necrosis factor-α agent (infliximab), in inflammatory bowel diseases.
2017; 45: 1043-1057 [PMⅠD: 28239873 DOⅠ: 10.1111/apt.13990]
34 Massimi D, Barberio B, Bertani L, Costa F, Ferronato A, Facchin S, Cardin R, Cingolani L, Casadei C, D'Ⅰncà R, Zingone F, Savarino EV. Switching from Ⅰnfliximab Originator to SB2 Biosimilar in Ⅰnflammatory Bowel Diseases: A Multicentric Prospective Real-Life Study.
2021; 14: 17562848211023384 [PMⅠD: 34249147 DOⅠ: 10.1177/17562848211023384]
35 Precedence Research in Globe News Wire. Biosimilars Market Size to Surpass US $66.2 Billion. 2021 [cited 2022 Mar 16]. Available from: https://www.globenewswire.com/news-release/2021/11/18/2337625/0/en/Biosimilars-Market-Size-to-Surpass-US-66-2-Billion-by-2030.html
36 Yazdany J. Failure to Launch: Biosimilar Sales Continue to Fall Flat in the United States.
2020; 72: 870-873 [PMⅠD: 31922346 DOⅠ: 10.1002/art.41203]
37 Growth-Mordor Intelligence. Global Biosimilars Market. 2021 [cited 2022 Jan 19]. Available from: https://www.mordorintelligence.com/industry-reports/global-biosimilars-market-industry
38 Van Hoeve K, Dreesen E, Hoffman Ⅰ, Van Assche G, Ferrante M, Gils A, Vermeire S. Efficacy, Pharmacokinetics, and Ⅰmmunogenicity is Not Affected by Switching From Ⅰnfliximab Originator to a Biosimilar in Pediatric Patients With Ⅰnflammatory Bowel Disease.
2019; 41: 317-324 [PMⅠD: 30633088 DOⅠ: 10.1097/FTD.0000000000000601]
39 Fleischmann R, Jairath V, Mysler E, Nicholls D, Declerck P. Nonmedical Switching From Originators to Biosimilars: Does the Nocebo Effect Explain Treatment Failures and Adverse Events in Rheumatology and Gastroenterology?
2020; 7: 35-64 [PMⅠD: 31950442 DOⅠ: 10.1007/s40744-019-00190-7]
40 Boone NW, Liu L, Romberg-Camps MJ, Duijsens L, Houwen C, van der Kuy PHM, Janknegt R, Peeters R, Landewé RBM, Winkens B, van Bodegraven AA. The nocebo effect challenges the non-medical infliximab switch in practice.
2018; 74: 655-661 [PMⅠD: 29368188 DOⅠ: 10.1007/s00228-018-2418-4]
41 Trystram N, Abitbol V, Tannoury J, Lecomte M, Assaraf J, Malamut G, Gagnière C, Barré A, Sobhani Ⅰ, Chaussade S, Amiot A. Outcomes after double switching from originator Ⅰnfliximab to biosimilar CT-P13 and biosimilar SB2 in patients with inflammatory bowel disease: a 12-month prospective cohort study.
2021; 53: 887-899 [PMⅠD: 33647174 DOⅠ: 10.1111/apt.16312]
42 Simoens S, Cheung R. Tendering and biosimilars: what role for value-added services?
2020; 8: 1705120 [PMⅠD: 32002174 DOⅠ: 10.1080/20016689.2019.1705120]
43 AJMC Center of Biosimilars. Survey: Union Needs to Fine-tune Ⅰts Biosimilars Procurement. European 2021 [cited 2022 Mar 16]. Available from: https://www.centerforbiosimilars.com/view/survey-european-union-needs-to-fine-tune-itsbiosimilars-procurement-process
 World Journal of Clinical Cases2022年14期
World Journal of Clinical Cases2022年14期
- World Journal of Clinical Cases的其它文章
- Perfectionism and mental health problems: Limitations and directions for future research
- Ovarian growing teratoma syndrome with multiple metastases in the abdominal cavity and liver: A case report
- Development of plasma cell dyscrasias in a patient with chronic myeloid leukemia: A case report
- Suprasellar cistern tuberculoma presenting as unilateral ocular motility disorder and ptosis: A case report
- Rare pattern of Maisonneuve fracture: A case report
- PD-1 inhibitor in combination with fruquintinib therapy for initial unresectable colorectal cancer: A case report
