Familial gastrointestinal stromal tumors with KIT germline mutation in a Chinese family: A case report
INTRODUCTION
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors occurring in the gastroin (GI) tract and originate from the interstitial cells of Cajal (ICCs)[1]. Previous research has demonstrated that the constitutive activation of tyrosine kinase, the main pathogenic event in most GISTs, is caused by mutation within the
gene[2]. Although GISTs are mostly sporadic, families with multiple inherited GISTs have been found[3,4]. Familial GISTs are mostly associated with mutations in
(most commonly),
[5],
, and
[6,7] genes.
‘My boy, my dear boy!’ cried the king, ‘who is it you want to marry? We will give her to you for a bride, even if she is the humblest of our slaves. What is there in the whole world that we would not do for you ?’
My fascination3 with the ocean escalated4 as the family spent the summer on the eastern end of Long Island, on the shore of the Atlantic Ocean. I was an early riser, and by age ten I was permitted to go down to the beach in the morning to collect shells on my own. Every day I would dress quickly, grab my bucket and head for the beach. I would climb the sand dunes5 that hid the ocean from view and sit quietly at the top and watch the waves roll into shore as I ate my breakfast roll.
Reported kindreds of familial GISTs (germline
mutation) have represented variable phenotypic features characterized by early presentation, multiple lesions, involvement of some related family members, cutaneous hyperpigmentation and dysphagia[8]. That is because
gene (as a protooncogene) is important in the development of various cell lines, including ICCs and melanocytes[8,9].The rarity of familial GISTs means that their correct diagnosis and therapeutic strategy deserve more attention.
In this paper, we describe two patients (father and daughter) in a Chinese family, for the first time,with germline
-mutated GISTs, who presented to our center with multiple GISTs and cutaneous hyperpigmentation. We also followed their response to imatinib therapy for GISTs and hyperpigmentation.
CASE PRESENTATION
Chief complaints
A 25-year-old Chinese woman (III: 1, Figure 1) presented to the hospital with abdominal pain.
History of present illness
The female patient and her father were eventually diagnosed with familial GIST with a novel
germline mutation (
).
History of past illness
The patient had no previous medical history.
Personal and family history
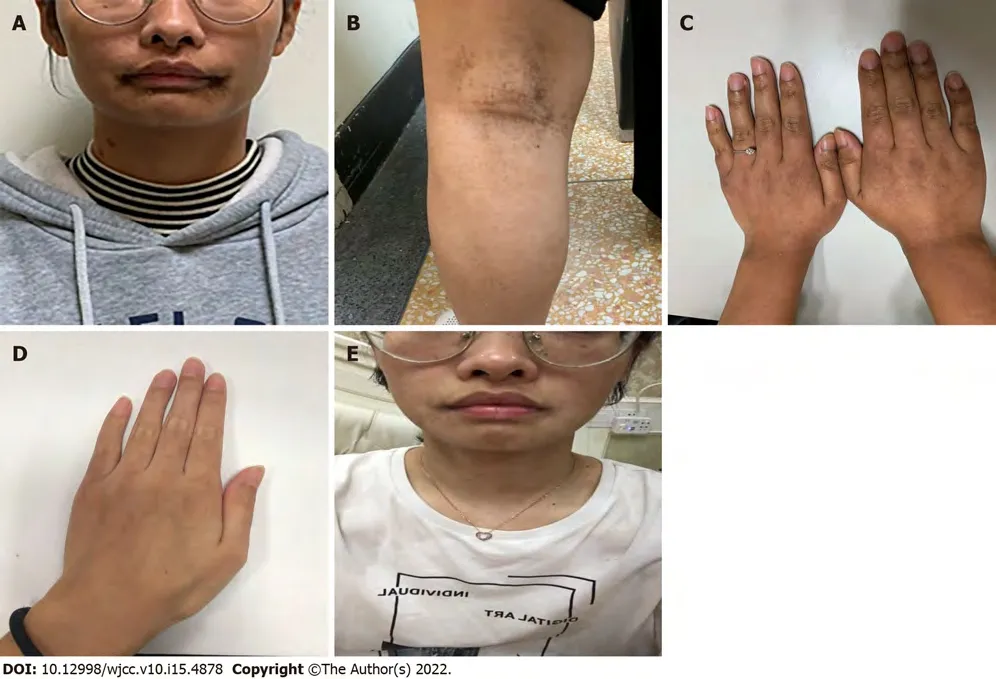
The patient reported a history of progressive cutaneous hyperpigmentation that developed a few months after birth (Figure 2A). Many new dark lentiginous macules, similar to
macules, had subsequently appeared over her face, body and limbs (Figure 2B), and also showed on her popliteal fossa and hands (Figure 2C). We carefully inquired into the family history of this patient, and obtained the information that her father (II: 1, Figure 1) had a history of multiple GISTs in the small intestine in 2002 and similar cutaneous hyperpigmentation on his body. He had been admitted to the hospital in 2002 for abdominal pain and dark stools, and underwent resection of GISTs (measuring from 3 cm to 20 cm, with a maximum diameter of 20 cm). He did not receive imatinib therapy and had no postoperative follow-up after first surgery. In 2019, he was again referred to the hospital for abdominal pain and tumor recurrence within the abdomen could be seen. He underwent a second operation to remove multiple tumors in the small intestine (with a maximum diameter of 4 cm), which were diagnosed as recurrent GISTs in July 2019. Some small tumors (> 10, diameter 0.5–1 cm) located in specific locations were not surgically removed. The grandmother (I: 2, Figure 1) of the female patient died of spaceoccupying lesions in the abdominal cavity, but the diagnosis was unclear. In addition, she was thought to have the same cutaneous hyperpigmentation.
When she says to the goat in the field, Little goat, bleat, Little table, appear, a table stands before her, spread with the best food, much better than we have; and when she has had enough, she says, Little goat, bleat, Little table, away, and everything disappears again
Physical examination
As the years passed, other occasions--birthdays, recitals13, awards, graduations--were marked with Dad s flowers. My emotions continued to seesaw14 between pleasure and embarrassment.
But all the arguments of father and mother were wasted, for her only answer was: O my father! I have sworn to myself that I will not marry, even if a thousand years go by, unless someone answers my riddle, and that I will give myself to that man only who does answer it
The patient came to our hospital for a pathological consultation, and no laboratory examination was performed.
Laboratory examinations
The peasant and his wife began to lament1 bitterly that Peter had run away in this fashion just when they were to have so much joy of him, and after they had spent so much on his education
Imaging examinations
Abdominal computed tomography (CT) showed multiple tumors (measuring 0.5 cm–4 cm in diameter)in the small intestine with irregular low density. No calcification and necrosis were found.
Finally, one morning, our commanding officer called me into his office. He demanded to know why I was receiving so many parcels. Knowing that the officer was Jewish and would understand my motivation, I decided21 to simply tell him the truth. I admitted that I had misused22 the military mail to help survivors and perform a mitzvah so desperately23 needed. I did not expect this simple gesture to turn into this. He admonished24 me sternly and then smiled. We ll let it go this time, he said, dismissing me.
FINAL DIAGNOSIS
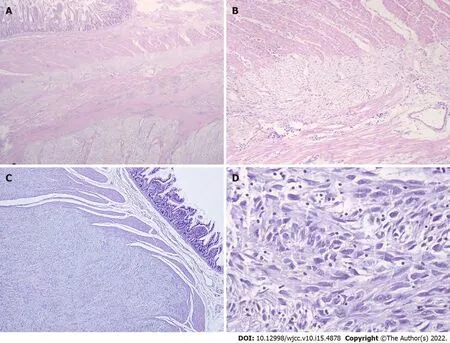
Partial resection of the small intestine for the removal of multiple tumors (0.5–4 cm) was performed in the original hospital and this patient was diagnosed with GIST in November 2019. We retrospectively reviewed the pathological sections, and the results of histomorphology showed that the tumors consisted of spindle cells in a fascicular and whorl-like pattern (Figure 3) with high cellularity, mild to medium polymorphism but almost no mitotic figures (0 mitosis/50 HPF). Diffuse hyperplasia of the ICCs was observed within the myenteric plexus of the small intestine. Immunohistochemistry showed that the majority of tumor cells were positive for CD117 (KIT receptor) and DOG-1, and the Ki67 index was < 1%. For the multiple lesions, diagnosis of low-risk GIST without mitotic activity was confirmed.
DNA was extracted from paraffin-embedded tumor tissue and patient’s saliva. Exons 9, 11, 13 and 17 of
and exons 12 and 18 of
were amplified by polymerase chain reaction and used for direct sequencing. The results of two test samples showed a point mutation at codon 560 in Patient 1, which resulted in the exchange of leucine by proline (
), indicating a
germline mutation.
Suddenly the man who followed fell over a stone. He hurt his foot badly and called: Hey, Bill, I ve hurt my foot. Bill continued straight on without looking back.
We re-examined the biopsy specimen of the tumors resected at the first operation in 2002 in the patient’s father, and the tumors were also composed of spindle cells and showed focally high cellularity and two mitotic figures/50 HPF. These tumors were finally classified into the high-risk group according to NIH criteria. Histopathology of his recurrent tumors (resection in 2019) was similar to the previous ones. DNA sequencing analysis was conducted from the saliva sample and paraffin-embedded specimens, and the results showed the same
germline mutation in exon 11 (
) as the specimens from his daughter showed.
Considering the uncertainty of the implications and prognosis of a potential positive bearer status for germline
, the female patient made a decision to have genetic counseling for her 3-year-old daughter(IV: 1, Figure 1). This child had no phenotypic features (such as cutaneous pigmented lesions). A saliva sample was obtained from this child, and sequence analysis showed no
gene mutation in exon 11(The pedigree of this family is shown in Figure 1).
The patient experienced abdominal pain for 10 d (with symptom aggravation for the last 48 h).
TREATMENT
Two months after tumor resection (January 2020), the female patient was followed up with adjuvant imatinib therapy, 400 mg/d. Besides, adjuvant treatment with imatinib 400 mg/d was administered to her father.
OUTCOME AND FOLLOW-UP
Three months after treatment initiation (April 2020), the female patient noticed a decrease in the number of pigmented lesions around her mouth (Figure 2E); the skin on her hands became lighter in tone(Figure 2D); and the color of her hair, pubic hair, and body hair changed to white. In August 2020 (8 mo after imatinib therapy), she underwent CT scan, and there was no clinical evidence of progression. Her father’s pigmentations diminished within 3 mo (started from October 2019) of imatinib treatment. At their last follow-up visit in April 2021 (about 16 mo after treatment), CT showed long-lasting disease stability in both patients.
DISCUSSION
Familial GIST was first reported by Nishida in 1998[10]. It is a rare inherited disease and has been reported in 35 different families and eight individuals with germline
mutations[11]. Most affected families, including the members described in our study, have been shown to harbor a germline
mutation in exon 11 (encoding the juxtamembrane domain)[6,12]. Some GIST families have been confirmed to have a germline
mutation[1,13]. In the family described by Zsebo
[14] in which one of two consecutive Val residues (codons 559 and 560, GTT–GTT) of the
JM structural domain was missing, two family members showed skin pigmentation in the perineum. By histological and immunohistochemical examinations, as well as molecular genetic analysis, we uncovered a single germline mutation in codon 560 (
> G,
) at
exon 11 in a family with GISTs and hyperpigmentation.
The origin of mesenchymal tumors in the GI tract has long been a focus of debate. Previous studies[15] have demonstrated that abnormalities of ICC, the “pacemaker cells of the gut”, may cause various motility disorders of the GI tract. Miettinen
[16] argued that GISTs were derived from transformed neoplastic precursors of the ICCs. A GIST model (harboring a germline
mutation) with ICC hyperplasia was produced in mice transfected with the knock-in mutant
(
) [17]. In this study, diffuse ICC hyperplasia that presented in the muscularis propria in the nontumorous wall of the small bowel was found in the female patient and her father. Immunohistochemical examination showed that most tumor cells were positive for DOG-1 and CD117 (KIT protein). ICCs were confirmed to express CD117 and DOG-1. Therefore, data from a transgenic mouse model[18], together with histological findings in previous studies[19] and ours, indicated that activation of germline
results in hyperplasia of the ICCs from which GISTs derive.
Shanghai Municipal Key 306 Clinical Specialty, No. shslczdzk01302.
Although the effectiveness of imatinib, a tyrosine kinase inhibitor, in the treatment of familial GISTs has not been established, many individuals with this disease have accepted therapy with imatinib,which might result in resolution of the hyperpigmentation and stable disease[23,24]. However, not all patients experience pigmentary changes and tumor regression in response to imatinib, nor did all patients experience the same degree of changes[21]. Conca
[25] detected GIST in two family members with the
mutation (in exon 11) who had a poor response to imatinib, since the tumors persisted microscopically. Gupta
[6] reported two siblings with hereditary GISTs with the missense mutation
. Both patients were treated with imatinib, but only one patient’s condition changed to stable after such treatment. After a stable period of > 1 year, disease progression was observed in the other. The different responses to imatinib may have been due to secondary drug resistance, and the type of mutation and its location. Both patients in our study were treated with adjuvant imatinib (400 mg/d) after surgery (after the second operation in the father). Within several months of imatinib treatment, hyperpigmentation diminished and the skin tone became lighter.Previous studies have demonstrated that
and its ligand stem cell factor (SCF) play significant roles in the development of four cell lineages: hematopoietic cells, melanocytes, germ cells, and mast cells[24,26]. Imatinib has been identified as a
inhibitor, which may lead to downstream inhibition of tyrosinase gene promoter and subsequent melanin synthesis inhibition.
studies [21,27], the number of melanocytes decreased significantly after imatinib therapy. This indicates that SCF-KIT interaction can regulate the development and survival of melanocytes in the context of GISTs[28].
The patient came to our hospital for a pathological consultation, and no physical examination was performed.
In conclusion, although GIST associated with germline
mutations is rare, it should be taken into consideration when we encounter patients (especially young) with multiple lesions and other typical manifestations, including cutaneous hyperpigmentation, multiple lentigines and macrocytosis. This case highlights the importance of inquiring about a detailed family history in individuals with GI lesions and abnormal pigmentation. The detection of
germline mutation is the key to diagnosis. Imatinib therapy in both our patients led to stable disease and hypopigmentation.
CONCLUSION
Yuan W participated in the design of this study; Huang W drafted this manuscript; Ren L and Xu C performed literature research and data acquisition; Luan LJ and Huang J provided help for the methodology used; Xue AW, Fang Y and Gao XD analyzed the CT findings; Lv JH collected the background information and contributed to manuscript drafting; Hou YY contributed to funding acquisition; Shen KT and Hou YY reviewed and edited this manuscript; all authors issued final approval for the version to be submitted.
FOOTNOTES
Familial GISTs with
mutations are closely correlated with various symptoms. The diagnosis should be based on comprehensive information including clinical manifestations, detailed family history,pathological examination and molecular determination. Imatinib treatment should be considered in these patients. Additionally, the possibility of genetic testing or counseling is of practical value to the individuals at high risk.
Clinically, symptoms such as multiple primary neoplasms (with several affected relatives), dysphagia and abnormal pigmentation[1,12,20] can be observed in familial GISTs. Median age at diagnosis of sporadic GISTs is around 60 years, while tumors of familial GISTs may present at earlier ages than that of sporadic GISTs[20]. The kindred in our study demonstrated some of these traits. The two patients in this pedigree who were diagnosed with multiple GISTs in their 20s (female patient) and 30s (father)were also noted to have cutaneous pigmentation, which developed a few months after birth and increased in size and number with age. Prior study of familial GISTs has illustrated an association with cutaneous hyperpigmentation[21]. This association results from the role of
in development of melanocytes, as well as ICCs from which GISTs derive[22].
Informed consent was obtained from this patient prior to inclusion in the study.
The authors declare that they have no competing interests.
The King s daughter was now a kitchen-maid,29 and had to be at the cook s beck and call, and do the dirtiest work. In both her pockets she fastened a little jar, in which she took home her share of the leavings, and upon this they lived.
The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
China
Wei Yuan 0000-0001-6255-4605; Wen Huang 0000-0001-9109-6009; Lei Ren 0000-0003-0982-3874; Chen Xu 0000-0002-0863-6199; Li-Juan Luan 0000-0002-8275-6404; Jie Huang 0000-0001-5366-0852; An-Wei Xue 0000-0003-3910-8935; Yong Fang 0000-0002-4374-1509; Xiao-Dong Gao 0000-0003-0362-8699; Kun-Tang Shen 0000-0001-5784-6558;Jing-Huan Lv 0000-0002-4233-7926; Ying-Yong Hou 0000-0002-6282-9717.
Xing YX
Kerr C
Xing YX
1 Li FP, Fletcher JA, Heinrich MC, Garber JE, Sallan SE, Curiel-Lewandrowski C, Duensing A, van de Rijn M, Schnipper LE, Demetri GD. Familial gastrointestinal stromal tumor syndrome: phenotypic and molecular features in a kindred.
2005; 23: 2735-2743 [PMID: 15837988 DOI: 10.1200/JCO.2005.06.009]
2 Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M,Muhammad Tunio G, Matsuzawa Y, Kanakura Y, Shinomura Y, Kitamura Y. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors.
1998; 279: 577-580 [PMID: 9438854 DOI: 10.1126/science.279.5350.577]
3 Sekido Y, Ohigashi S, Takahashi T, Hayashi N, Suzuki K, Hirota S. Familial Gastrointestinal Stromal Tumor with Germline
Mutations Accompanying Hereditary Breast and Ovarian Cancer Syndrome.
2017; 37: 1425-1431 [PMID: 28314314 DOI: 10.21873/anticanres.11466]
4 Robson ME, Glogowski E, Sommer G, Antonescu CR, Nafa K, Maki RG, Ellis N, Besmer P, Brennan M, Offit K.Pleomorphic characteristics of a germ-line KIT mutation in a large kindred with gastrointestinal stromal tumors,hyperpigmentation, and dysphagia.
2004; 10: 1250-1254 [PMID: 14977822 DOI:10.1158/1078-0432.ccr-03-0110]
5 Manley PN, Abu-Abed S, Kirsch R, Hawrysh A, Perrier N, Feilotter H, Pollett A, Riddell RH, Hookey L, Walia JS.Familial PDGFRA-mutation syndrome: somatic and gastrointestinal phenotype.
2018; 76: 52-57 [PMID:29486293 DOI: 10.1016/j.humpath.2018.02.014]
6 Gupta D, Chandrashekar L, Larizza L, Colombo EA, Fontana L, Gervasini C, Thappa DM, Rajappa M, Rajendiran KS,Sreenath GS, Kate V. Familial gastrointestinal stromal tumors, lentigines, and café-au-lait macules associated with germline c-kit mutation treated with imatinib.
2017; 56: 195-201 [PMID: 28074523 DOI: 10.1111/ijd.13516]
7 Postow MA, Robson ME. Inherited gastrointestinal stromal tumor syndromes: mutations, clinical features, and therapeutic implications.
2012; 2: 16 [PMID: 23036227 DOI: 10.1186/2045-3329-2-16]
8 Carballo M, Roig I, Aguilar F, Pol MA, Gamundi MJ, Hernan I, Martinez-Gimeno M. Novel c-KIT germline mutation in a family with gastrointestinal stromal tumors and cutaneous hyperpigmentation.
2005; 132A: 361-364[PMID: 15742474 DOI: 10.1002/ajmg.a.30388]
9 Vilain RE, Dudding T, Braye SG, Groombridge C, Meldrum C, Spigelman AD, Ackland S, Ashman L, Scott RJ. Can a familial gastrointestinal tumour syndrome be allelic with Waardenburg syndrome?
2011; 79: 554-560 [PMID:20636395 DOI: 10.1111/j.1399-0004.2010.01489.x]
10 Nishida T, Hirota S, Taniguchi M, Hashimoto K, Isozaki K, Nakamura H, Kanakura Y, Tanaka T, Takabayashi A,Matsuda H, Kitamura Y. Familial gastrointestinal stromal tumours with germline mutation of the KIT gene.
1998; 19: 323-324 [PMID: 9697690 DOI: 10.1038/1209]
11 Hasegawa M, Shimizu A, Ieta K, Shibusawa K, Ishikawa O, Ishida-Yamamoto A, Tamura A. Generalized lentigines associated with familial gastrointestinal stromal tumors dramatically improved by imatinib treatment.
2020; 47:e241-e242 [PMID: 32220019 DOI: 10.1111/1346-8138.15321]
12 Jones DH, Caracciolo JT, Hodul PJ, Strosberg JR, Coppola D, Bui MM. Familial gastrointestinal stromal tumor syndrome:report of 2 cases with KIT exon 11 mutation.
2015; 22: 102-108 [PMID: 25504284 DOI:10.1177/107327481502200113]
13 Chompret A, Kannengiesser C, Barrois M, Terrier P, Dahan P, Tursz T, Lenoir GM, Bressac-De Paillerets B. PDGFRA germline mutation in a family with multiple cases of gastrointestinal stromal tumor.
2004; 126: 318-321[PMID: 14699510 DOI: 10.1053/j.gastro.2003.10.079]
14 Zsebo KM, Williams DA, Geissler EN, Broudy VC, Martin FH, Atkins HL, Hsu RY, Birkett NC, Okino KH, Murdock DC. Stem cell factor is encoded at the Sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor.
1990; 63: 213-224 [PMID: 1698556 DOI: 10.1016/0092-8674(90)90302-u]
15 Hirota S, Okazaki T, Kitamura Y, O'Brien P, Kapusta L, Dardick I. Cause of familial and multiple gastrointestinal autonomic nerve tumors with hyperplasia of interstitial cells of Cajal is germline mutation of the c-kit gene.
2000; 24: 326-327 [PMID: 10680913 DOI: 10.1097/00000478-200002000-00045]
16 Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis.
2001; 438: 1-12 [PMID: 11213830 DOI:10.1007/s004280000338]
17 Gromova P, Ralea S, Lefort A, Libert F, Rubin BP, Erneux C, Vanderwinden JM. Kit K641E oncogene up-regulates Sprouty homolog 4 and trophoblast glycoprotein in interstitial cells of Cajal in a murine model of gastrointestinal stromal tumours.
2009; 13: 1536-1548 [PMID: 19453770 DOI: 10.1111/j.1582-4934.2009.00768.x]
18 Maeyama H, Hidaka E, Ota H, Minami S, Kajiyama M, Kuraishi A, Mori H, Matsuda Y, Wada S, Sodeyama H, Nakata S,Kawamura N, Hata S, Watanabe M, Iijima Y, Katsuyama T. Familial gastrointestinal stromal tumor with hyperpigmentation: association with a germline mutation of the c-kit gene.
2001; 120: 210-215 [PMID:11208730 DOI: 10.1053/gast.2001.20880]
19 Moskaluk CA, Tian Q, Marshall CR, Rumpel CA, Franquemont DW, Frierson HF Jr. Mutations of c-kit JM domain are found in a minority of human gastrointestinal stromal tumors.
1999; 18: 1897-1902 [PMID: 10086344 DOI:10.1038/sj.onc.1202496]
20 Kuroda N, Tanida N, Hirota S, Daum O, Hes O, Michal M, Lee GH. Familial gastrointestinal stromal tumor with germ line mutation of the juxtamembrane domain of the KIT gene observed in relatively young women.
2011; 15:358-361 [PMID: 20952281 DOI: 10.1016/j.anndiagpath.2010.05.003]
21 Coleman E, Panse G, Cowper S, Lacy J, Leventhal J. Disappearing pigmentary mosaicism during imatinib treatment for gastrointestinal stromal tumors.
2019; 5: 170-172 [PMID: 30740499 DOI: 10.1016/j.jdcr.2018.11.021]
22 Shibusawa Y, Tamura A, Mochiki E, Kamisaka K, Kimura H, Ishikawa O. c-kit Mutation in generalized lentigines associated with gastrointestinal stromal tumor.
2004; 208: 217-220 [PMID: 15118370 DOI:10.1159/000077302]
23 Wali GN, Halliday D, Dua J, Ieremia E, McPherson T, Matin RN. Cutaneous hyperpigmentation and familial gastrointestinal stromal tumour associated with KIT mutation.
2019; 44: 418-421 [PMID: 30280421 DOI: 10.1111/ced.13757]
24 Farag S, van der Kolk LE, van Boven HH, van Akkooi ACJ, Beets GL, Wilmink JW, Steeghs N. Remarkable effects of imatinib in a family with young onset gastrointestinal stromal tumors and cutaneous hyperpigmentation associated with a germline KIT-Trp557Arg mutation: case report and literature overview.
2018; 17: 247-253 [PMID: 28710566 DOI: 10.1007/s10689-017-0024-8]
25 Conca E, Negri T, Gronchi A, Fumagalli E, Tamborini E, Pavan GM, Fermeglia M, Pierotti MA, Pricl S, Pilotti S. Activate and resist: L576P-KIT in GIST.
2009; 8: 2491-2495 [PMID: 19723893 DOI:10.1158/1535-7163.MCT-09-0662]
26 Antonescu CR. Gastrointestinal stromal tumor (GIST) pathogenesis, familial GIST, and animal models.
2006; 23: 63-69 [PMID: 17193819 DOI: 10.1053/j.semdp.2006.08.003]
27 Cario-André M, Ardilouze L, Pain C, Gauthier Y, Mahon FX, Taieb A. Imatinib mesilate inhibits melanogenesis in vitro.
2006; 155: 493-494 [PMID: 16882205 DOI: 10.1111/j.1365-2133.2006.07359.x]
28 Leong KW, Lee TC, Goh AS. Imatinib mesylate causes hypopigmentation in the skin.
2004; 100: 2486-7; author reply 2487 [PMID: 15160360 DOI: 10.1002/cncr.20267]
 World Journal of Clinical Cases2022年15期
World Journal of Clinical Cases2022年15期
- World Journal of Clinical Cases的其它文章
- Diet and intestinal bacterial overgrowth: Is there evidence?
- Spontaneous liver rupture following SARS-CoV-2 infection in late pregnancy: A case report
- Metastasis of liver cancer to the thyroid after surgery: A case report
- Solitary primary pulmonary synovial sarcoma: A case report
- Knot impingement after arthroscopic rotator cuff repair mimicking infection: A case report
- Clear aligner treatment for a four-year-old patient with anterior crossbite and facial asymmetry: A case report
