O6-methylguanine DNA methyltransferase is upregulated in malignant transformation of gastric epithelial cells via its gene promoter DNA hypomethylation
lNTRODUCTlON
Gastric cancer(GC)is currently the fifth most frequently diagnosed and the third leading cause of cancer death worldwide with a high prevalence in many Asia countries,particularly in China,Japan,and South Korea[1,2].Previous studies have reported that epigenetic alterations are widely recognized to be involved in the initiation and progression of gastric tumorigenesis[3,4].DNA methylation is a common significant epigenetic modification and plays an important role in the development and prognosis of GC[5-8].
O
-methylguanine-DNA methyltransferase(MGMT)is a suicide enzyme that efficiently removes alkylating lesions at the O
position of guanine induced by DNA alkylating agents[9].Following the transfer of the alkyl group to itself,MGMT becomes inactive and it is ubiquitinated and targeted for proteasomal degradation.MGMT is frequently regulated by epigenetic silencing mediated Fits gene promoter DNA methylation in gliomas[10,11].The abnormal modifications of histone and aberrant expression of transcriptional activators and repressors,also contribute to the regulation of MGMT expression in different tumors[11].O
-methylguanine is a potent mutagenic lesion that leads to base mismatching and double-strand breaks,promoting gene mutagenesis and tumor initiation.MGMT can restore this type of DNA damage and play an important role in maintaining genomic stability[12].Inhibiting MGMT function can induce G:C to A:T mutation of the onco-suppressors p53 and PTEN to promote human carcinogenesis[13].In the TCGA database,the probability of point mutation of p53 and PTEN was higher in
promoter methylated tissues than in non-methylated tissues of glioma.In colon cancer,lung cancer,and GC,the reduction of MGMT expression induced by DNA methylation in its promoter regions was also observed[14-17].Yet,MGMT expression can be increased by chemotherapy with alkylating agents,such as temozolomide,which contributes to the chemotherapy resistance[18].However,the role of MGMT in the early stage of tumorigenesis remains unclear.
The monofunctional alkylating agent N-methyl-N’-nitro-N-nitrosoguanidine(MNNG)and N-methyl-N-nitroso-urea(MNU)are widely accepted model chemical carcinogens for studying the mechanisms of mutagenesis and carcinogenesis induced by N-nitroso compounds(NOCs).They generate adducts with DNA and protein,such as O
-methylguanine,which lead to point mutations,chromosomal aberrations,initiation and promotion of various cancer,specially increasing the risk of gastrointestinal cancers[19,20].Our previously studies showed that MNNG and MNU treatments can stimulate multiple cellular responses,including epigenetic events[21,22].We revealed a dysregulation of histone modifications and DNA methylation,which contributed to numerous cancer-related gene expression changes promoting cell malignant transformation upon NOCs treatment[21,22].These findings prompted us to speculate that the abnormal epigenetic regulation could be the critical molecular mechanism of chemical carcinogens-induced gastric carcinogenesis.
Chasid listened with favour to his servant s suggestion, and perceiving in the valley beneath them a ruin which seemed to promise shelter they flew towards it
In the present study,we investigated the epigenetic changes of MGMT in NOCs-induced human gastric cell malignant transformation.And we demonstrated that DNA methylation level of MGMT promoter was strongly decreased,which resulted the inhibition of MGMT expression,contributing the malignant phenotypes during the cell malignant transformation process.
MATERlALS AND METHODS
Patient samples
A total of 93 clinical gastric tissue samples collected by endoscopic biopsy at the Second Affiliated Hospital of Zhejiang University were used in this study,including 25 cases of gastritis,18 cases of gastric metaplasia(used as precancerous lesion),50 pairs of GC and adjacent normal tissues(early stage,19 cases;advanced stage,31 cases).The study was approved by the ethics committee of Zhejiang University School of Medicine(No.2017026).The tissue samples were formalin-fixed and paraffinembedded for immunohistochemistry or used for mRNA isolation to detect gene expression.
Cell culture
The human gastric normal epithelium cell line GES-1(Cell Bank of the Chinese Academy of Science,Xiangya,China)was cultured in DMEM(Gibco,Grand Island,NY,United States)supplemented with 10% fetal bovine serum(FBS;Gibco),streptomycin(100 g/mL),and penicillin(100 U/mL)at 37 °C in an atmosphere containing 5% CO
.And GC cell lines,including AGS,MKN45,SGC7901,KATOIII,and NCI-N87(Cell Bank of the Chinese Academy of Science,Shanghai,China),were cultured in DMEM or PRIM-1640 supplemented with 10% FBS,streptomycin,and penicillin.The authenticity of cell lines used in this study had been verified by short tandem repeat profiling.
Cell transformation assays
Cells were exposed to MNNG or MNU as described in a previous study to establish the cell transformation model[22].Briefly,cells were exposed to MNU(TRC,Toronto,Canada)or MNNG(Sigma,St.Louis,MO,United States)for 2 h in serum free medium.Then,the medium was removed and cells were recovered in fresh medium at 37 °C.MNU and MNNG exposure was repeated once a week for 4 wk.After 4 wk of treatment and 4 wk of restoration,characteristics related to malignant phenotype were measured.
Scratch test
Cells were plated in 6-well plates and allowed to reach 90% confluence.The monolayer was scratched with a 10-mL sterile pipette tip.Images of the scratches were taken using an inverted microscope at × 10 magnification at 0,24,and 48 h of incubation.ImageJ software was used to analyze the percentage of wound closure.
What! she cried, feed people who were as happy as all that! Why, it was simply ruinous! But as the Prince began to look angry, she, with many sighs and mutterings, brought out a morsel of bread, a bowl of milk, and six plums, with which the lovers were well content: for as long as they could look at one another they really did not know what they were eating
Cell proliferation analysis
Cells(1.2 × 10
cells/well)were seeded in 24-well plates and cultured for 24,48,72,96,and 120 h.Cells were digested by trypsin every 24 h and then re-suspended in fresh medium and counted.
Soft agar and colony formation assays
SiRNAs targeting human
(GenePharma,Shanghai,China)were transfected into cells using Lipofectamine RNAiMAX(Invitrogen)according to the manufacturer's instructions.A siRNA negative control(siRNA NC)was also used.
For colony formation assay,cells(1000 cells/well)were seeded in 6-well plates and cultured.The culture medium was changed every 3 d.After 2 wk,colonies were stained with crystal violet and photographed.Colonies ≥ 0.05 mm in diameter were counted.
Dual-luciferase reporter assay
The
promoter sequence(-954/+24)was amplified from the extracted genomic DNA and cloned into pGL3-promoter vector(Promega,Madison,WI,United States).After seeding MNNG/MNUtransformed cells for 24 h,the cells were co-transfected with 0.5 μg of pGL3-
-promoter and 0.02 μg of pRL-SV40 renilla luciferase reporter plasmid using X-treme GENE HP(Roche,Basel,Switzerland).Dual-Luciferase Reporter Assay System was used for testing relative luciferase activity after transfection for 24 h(Promega).
DNA methylation specific polymerase chain reaction and bisulfite genomic sequence assay
Total DNA(5 × 10
cells)was isolated from the MNNG/MNU-transformed cells with the Qiagen DNA Isolation Kit.Then,bisulfite conversion was performed with 500 ng of genomic DNA using the EZ DNA Methylation Kit(Zymo Research,Irvine,CA,United States).The converted DNA was eluted in 100 mL of nuclease-free water.Methylation specific polymerase chain reaction(PCR)(MSP)analysis was performed in a 25-μL reaction system that consisted of 50 ng of sodium bisulfite-treated DNA,12.5 μL of 2 × Master Mix(Qiagen,Germany),ddH2O,and 3 μL of isometric mixture of
gene methylated and un-methylated primers.
methylated and un-methylated primers used are:Forward 5’-TTTCGACGTTCGTAGGTTTTCGC-3’ and reverse,5’-GCACTCTTCCGAAAACGAAACG-3’;forward,5’-TTTGTGTTTTGATGTTTGTAGGTTTTTGT-3’ and reverse,5’-ACTCCACACTCTTCCAAAAAC AAAACA-3’.Bisulfite genomic sequence(BSP)analysis was performed by Xiangyin Biological Corporation.Bisulfite treatments of the genomic DNA samples were carried out with the Qiagen EpiTect kit according to the manufacturer’s instructions,followed by the PCR amplification procedure(30 cycles of 94 °C for 30 s,55 °C for 30 s,and 72 °C for 60 s;72 °C for 30 min;and held at 4 °C)using KAPA2G Fast Multiplex Mix and KAPA 2G Robust HS.The PCR products were identified by electrophoresis and gel-purified with the Gel and PCR Clean-up System(Promega).The purified PCR products were inserted into PMD-18T Vector and sequenced by Sanger sequencing.
Chromatin immunoprecipitation assay
For chromatin immunoprecipitation(ChIP)assay,the malignant transformed cells were cross-linked with 1% formaldehyde for 10 min at 37 °C.Then,cells were isolated and lysed for preparation of sheared chromatin.The cell lysates were sonicated for 1 min and repeated ten times at 1-min intervals.After centrifugation at 13000
at 4 °C for 30 min,the cell lysates were diluted with IP buffer and incubated with anti-DNMT1,anti-H3K9Me3,and anti-H3K4Me2 antibodies(CST,Massachusetts,United States)overnight,respectively.For collecting the bound DNA,the coated beads were added in the samples and incubated for 4 h at 4 °C.The beads were collected,washed,and eluted with elution buffer.Then,the bound DNA was extracted with a DNA extraction kit(Qiagen)for quantification by qPCR.Primers used for detecting the binding sites in MGMT promoter are:Forward,5’-GCCCCTAGAACGCTTTGC-3’ and reverse,5’-CAACACCTGGGAGGCACTT-3’.
Meanwhile the King was asking the Enchanter to what he was indebted for the honour of this visit, and on his replying that he would not say until the Queen was also present, messenger after messenger was dispatched to her to beg her immediate71 attendance
Immunohistochemistry
Immunohistochemistry was performed using an Envision Detection System(DAKO,Carpinteria,CA,United States)according to the manufacturer's instructions.Mouse monoclonal anti-human MGMT antibody(dilution,1:150)was purchased from Santa Cruz Biotechnology(Santa Cruz,CA,United States)and used for immunohistochemistry.The staining results were assessed and confirmed by two independent investigators blinded to the clinical data.
qPCR
In summary,our current study revealed the molecular mechanism of MGMT upregulation mediated by DNA hypomethylation of its gene promoter in NOCs-induced gastric cell malignant transformation,and showed the dual effects of MGMT by regulating its expression level in chemical carcinogen-induced tumorigenesis.Our findings provide a dynamic regulatory mechanism by which MGMT is implicated in cell malignant transformation and tumorigenesis induced by NOCs,and shed new light on MGMT as a potential diagnostic and therapeutic target for gastric carcinogenesis intervention by regulating aberrant epigenetic mechanisms.
Immunoblot analysis
Cells were lysed with RIPA buffer,and the total protein was quantified by Bradford assay.Cell lysates(50 mg)were separated on a 10% SDS-PAGE gel and then transferred onto a nitrocellulose membrane(Whatman,Maidstone,United Kingdom).The membrane was blocked with 5% skim milk solution for 2 h and incubated overnight with diluted primary antibody at 4 °C.Then,the membrane was incubated with IRDye 800- or IRDye 680-conjugated secondary antibody(LI-COR Biosciences,Lincoln,NE,United States)and detected with an Odyssey infrared imaging system.Mouse monoclonal anti-human MGMT antibody(dilution,1:1000)and mouse monoclonal anti-human GADPH antibody(dilution,1:2000)were purchased from Santa Cruz Biotechnology.
So when he arrived at the Leafy Palace, more handsome and fascinating even than ever she had been led to expect, he hardly so much as glanced at the Princess, but bestowed26 all his attention upon the old Fairy, to whom he seemed to have a hundred things to say
Xenograft assay
Balb/c nude mice(4 wk)were purchased from Shanghai Slac Laboratory Animal Co.LTD.Thirty-six Balb/c nude mice were randomly divided into three groups:Control group,MNNG-induced subclone injected group,and MNU-induced subclone injected group.The mice were subcutaneously injected with 1 × 10
MNNG/MNU-transformed cells(100 μL).Three days after injection,the long diameter(a)and short diameter(b)of the tumors were measured,after which the volume(V)was calculated using the formula V = 1/2 × a × b
.Mice were sacrificed,and the tumor tissues were obtained and weighed.The animal experiments were conducted in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals.The Committee on the Use of Animals of Zhejiang University,China approved the study protocol of our experiments.
The work described herein was approved by the Laboratory Animals Welfare Ethics Review Committee of Zhejiang University(No.ZJU20170522).
Cell transfection and RNA interference
The MGMT protein coding sequences was subcloned into PCDNA3.1 vector.The transformed cells were transfected with the MGMT overexpression plasmid and PCDNA3.1 empty vector(EV),respectively.Then,the proliferative activity of cells was analyzed by colony formation and soft agar assays.
For soft agar assay,cells(1000 cells/well)were suspended in a culture medium containing 0.4% agarose(A9045-5G)(Sigma)and seeded onto a base layer of 0.7% agar bed in 12-well plates.The culture medium was changed every 3 d.After 2 wk,colonies were stained with crystal violet and photographed.Colonies ≥ 0.05 mm in diameter were counted.
Statistical analysis
The two-tailed Student's
test and one-way analysis of variance were used for statistical analyses.The data are expressed as the mean ± SD from three separate experiments.
≤ 0.05 was considered statistically significant.
RESULTS
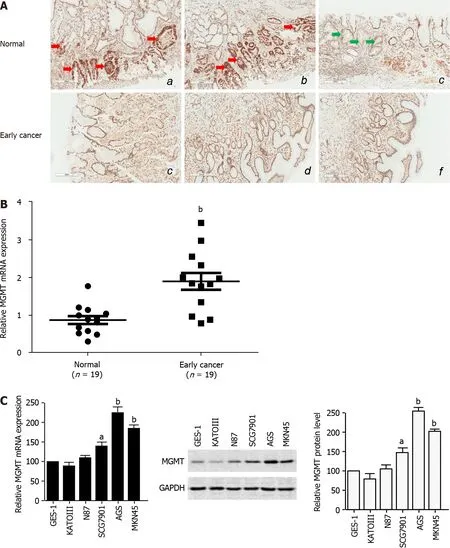
MGMT expression is upregulated in early stage GC
To study the role of MGMT in GC development,especially in the early events and tumor initiation,we detected the expression of MGMT in 19 clinical early stage GC tissues.The immunohistochemistry analysis showed that MGMT expression was increased in early stage cancer tissues compared with the normal tissue,though there was an individual difference(Figure 1A).qPCR analysis of endoscopic biopsy samples confirmed the upregulation of
mRNA expression in early stage cancer tissues compared with adjacent normal tissues(Figure 1B).Moreover,MGMT expression was also enhanced in the GC cell lines at both the mRNA and protein levels(Figure 1C).Collectively,these results suggest that MGMT expression is upregulated in early stage GC.
The servant, however, declined everything, and only begged for a horse and some money to enable him to travel, as he was anxious to see something of the world
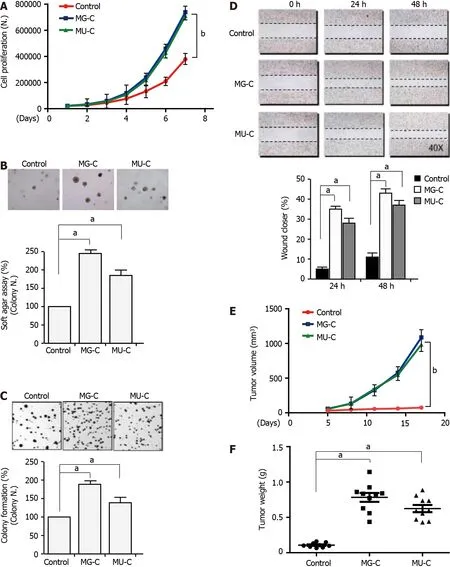
MGMT is upregulated in NOCs-induced gastric epithelial cell malignant transformation
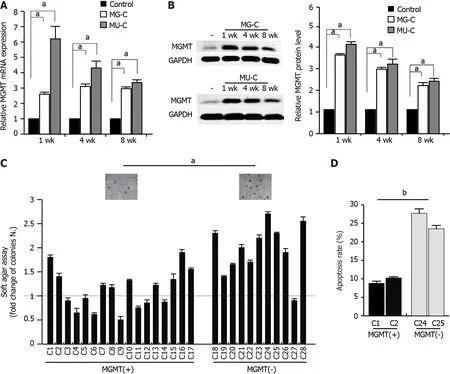
To investigate the molecular mechanism of MGMT upregulation,we established a gastric epithelial cell(GES-1)malignant transformation model following MNNG and MNU exposure.MNNG/MNU-treated cells showed an increase of cell proliferation,anchorage-independent growth capability,and colony formation ability,as demonstrated by cell proliferative assay,soft agar assay,and colony formation assays,respectively(Figure 2A-C).We also observed that the cell migration was enhanced upon MNNG/MNU treatment by wound healing assay(Figure 2D).Xenograft assay showed that MNNG/MNU-induced transformed cells demonstrated increased tumor growth(Figure 2E and F),further confirming the malignant phenotypes of NOCs-induced transformed GES-1 cells.Then,we detected the MGMT expression in MNNG/MNU-transformed cells.MGMT expression was persistently increased during the malignant transformation process(Figure 3A and B).But the extent of MGMT upregulation was decreased after removal of MNNG/MNU exposure for 12 wk(data not shown),suggesting that MGMT expression demonstrated a dynamic change in MNNG/MNU-induced cell malignant transformation process.In particular,the augmentation of MGMT expression was negatively correlated with the colony-forming ability of the MNNG/MNU-transformed cell subclones(Figure 3C),but it was positively correlated with the anti-apoptotic effect of malignant transformed cell subclones(Figure 3D).
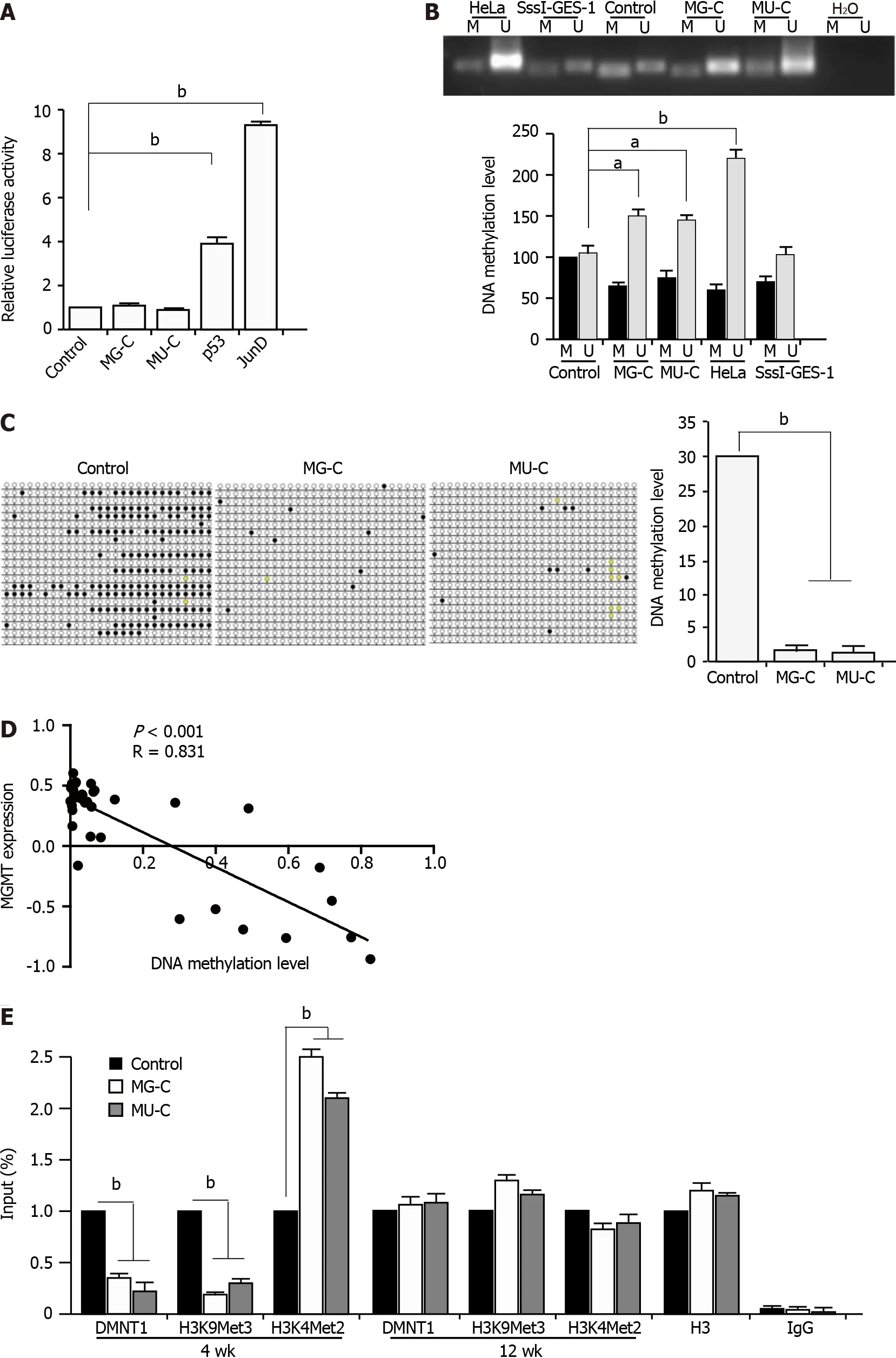
DNA hypomethylation is responsible for MGMT upregulation in cell malignant transformation
To further investigate the regulatory mechanism underlying the MGMT upregulation upon MNNG/MNU treatment,we constructed a
gene promoter luciferase reporter.We did not find an increase of the
gene promoter in MNNG/MNU-induced cells by dual-luciferase reporter assay.We used p53 and JunD as the positive controls,since it was reported that they are the transcriptional activators of the
promoter(Figure 4A).It is known that MGMT expression was closely related with its promoter DNA methylation in different cancers.We preformed MSP to detect the DNA methylation level in the
promoter,which showed that in MNNG/MNU-transformed subcolones,unmethylated DNA was accumulated,indicating a reduction of DNA methylation level in the
gene promoter(Figure 4B).Since it is known that HeLa cells exhibit high expression of MGMT with a low DNA methylation level in the promoter region,they were used as a positive control.M.SssI(CpG methyltransferase)treated cell was used as a negative control.As shown by BSP analysis,MNNG/MNU-transformed subcolones showed few DNA methylation sites compared with the controls,confirming the reduction of DNA methylation level in the
gene promoter(Figure 4C).Furthermore,we used 5-aza,a DNMT specific inhibitor,to treat CES-1 cells.After 48 and 72 h,MGMT expression was increased upon 5-aza treatment(Supplementary Figure 1).Moreover,based on the CCLE database,we found that MGMT expression was negatively related with DNA methylation levels(Figure 4D),indicating that the DNA methylation level is involved in the upregulation of MGMT.
Next,we preformed ChIP-PCR with anti-DNMT1 and anti-H3K9Met3 and anti-H3K4Met2(against specific methylation sites)antibodies.H3K9Met3 was known as a transcriptional inhibition signal and H3K4Met2 was reported as a transcriptional activation signal.The results showed that the DMNT1 recruitment was significantly decreased to the promoter region of
.We also detected the reduction of H3K9Met3 and the augment of H3K4Met2 located in the promoter region of
,as well as a reduction of DNMT1 binding to the
promoter(Figure 4E).The results suggested that the upregulation of MGMT expression was dependent on the DNA hypomethylation in its promoter.Interestingly,after removal of NOCs exposure for 12 wk,the binding of DNMT1 to the
gene promoter returned to the baseline level.The same changes of H3K9Met3 and H3K4Met2 levels in the
gene promoter were also observed.These data suggest that the binding of DNMT1 to the
gene promoter changes dynamically,which could help us to understand the dynamic changes of MGMT expression regulated by DNA methylation in NOCs-induced malignant transformation.
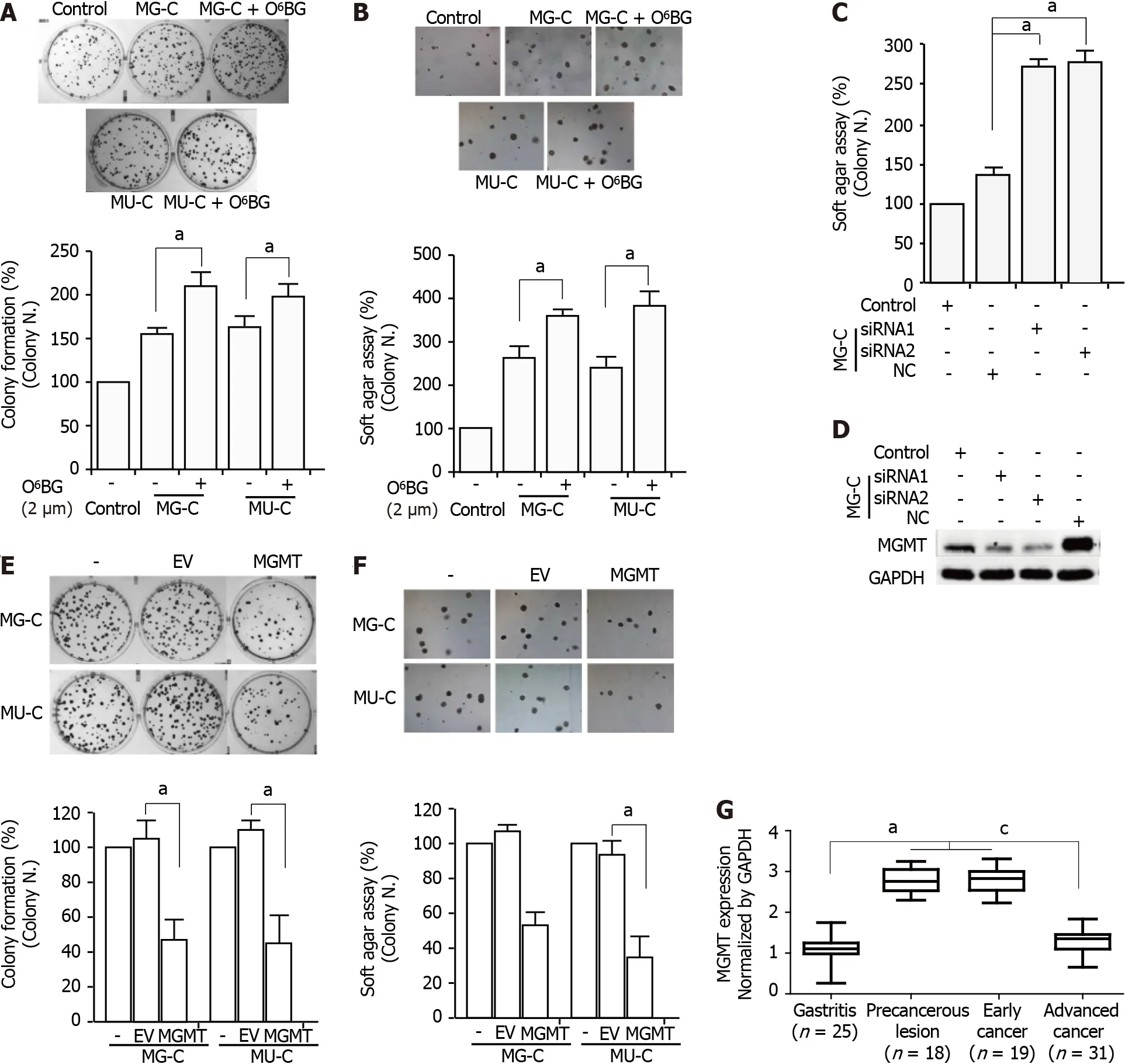
Inhibition of MGMT contributes to the NOCs-induced cell malignant phenotype
To evaluate the role of MGMT in NOCs-induced cell malignant transformation,we used O
-benzylguanine(O
-BG),a specific inhibitor of MGMT,to inhibit the activity of MGMT.After treatment with O
-BG at different concentrations,we found a reduction of MGMT expression(Supplementary Figure 1).MTT assay showed that treatment with O
-BG at low doses did not induce a decrease of cell viability(Supplementary Figure 1).Therefore,we used 2 μM O
-BG in the subsequent experiments.O
-BG exposure increased the proliferative activity and anchorage-independent growth capability of MNNG/MNU-induced cells(Figure 5A and B).Knock-down of MGMT in MNNG/MNU-transformed cells also resulted in the increase of cell reproductive activity(Figure 5C and D).Moreover,overexpressed MGMT resulted in the decrease of cell proliferative activity in MNNG/MNU-transformed cells(Figure 5E and F).In addition,MGMT was upregulated in precancerous lesions(gastric metaplasia)and early stage GC compared with non-cancerous lesions(gastritis),but the MGMT level was reduced in the advanced GC tissues compared with the precancerous lesion and early tumor tissues(Figure 5G),indicating a protective role of MGMT in GC progression.
DlSCUSSlON
The findings provide a dynamic regulatory mechanism of MGMT expression in cell malignant transformation and tumorigenesis induced by NOCs,supporting that MGMT might be a potential diagnostic and therapeutic target for gastric carcinogenesis.
The underlying regulatory mechanism of MGMT involved in NOCs-induced tumorigenesis,especially in the initiation phase,remains largely unclear.
MGMT can remove O
-guanosine alkylation adducts caused by alkylation agents from DNA sequence in one-step reaction that restores the O
-guanosine residue to itself,consequently forming an inactive form[28].Hence,the expression level of MGMT is fundamental for accurate DNA repair.It has been known that the transcriptional mechanism and epigenetic regulation are important to regulate the MGMT expression[1,29].Hypoxia inducible factor 1-α can upregulate the expression level of MGMT and increase the drug resistance of glioma stem cells to temozolomide[30].In addition,microRNAs can also bind to the 3'-untranslated region of
,reduce the stability of the mRNA,and affect protein translation[31,32].Moreover,
gene promoter region lacks TATA box and CAAT box,but has rich GC sequence,which is prone to be methylated and closely related to transcriptional regulation[33].DNA methylation in the
gene has been reported in various human cancers,which can increase the sensitivity to alkylating agents in chemotherapy,influencing the tumor prognosis.However,the high level of gene methylation is usually associated with the low expression of protein level.Inhibition of MGMT protein level decreases its ability of removing O
-guanosine from the damaged DNA sites,resulting in an increase of mutation frequency and easily leading to the occurrence of tumor[34-36].We found that MGMT upregulation was regulated by DNA hypomethylation in its gene promoter.And the subclones with high a level of MGMT showed a weak malignant proliferative activity,but with a strong anti-apoptotic effect upon exposure to DNA damage agents.This result suggested a protective effect of MGMT against NOCs-induced cell malignant transformation.Using O
-BG and by knocking MGMT down with siRNA,we showed an increased malignant proliferative ability of the transformed cells.Overexpressed MGMT decreased this effect,confirming the protected role of MGMT following chemical carcinogen exposure.In particular,the ChIP assay showed that DNMT1 was responsible for the
gene promoter methylation.After 12 wk of cell transformation,the MGMT expression level was restored by recovering DNMT1 binding to the
promoter region.This result suggested dynamic changes of MGMT expression,which is regulated by DNA methylation.Analysis of clinical gastric tissue samples also confirmed the dynamic changes of MGMT expression in gastric carcinogenesis.Taken together,we hypothesize that MGMT expression shows dynamic changes in gastric tumorigenesis induced by chemical carcinogens.It can be upregulated in the initiation phase for repairing the DNA damage and helping cells survive upon NOCs exposure;but in the progressive stage,it can be restored to the normal level to facilitate GC development.Hence,revealing the molecular mechanism of dynamic regulation of MGMT expression is important to help us understand the role of MGMT in GC formation and progression.However,the exact regulatory mechanisms of the dynamic changes on MGMT expression in different stages of cancer progression need to be further investigated(Supplementary Table 1).
CONCLUSlON
Total RNA was extracted from cell lines and tissue samples with TRIzol reagent(Invitrogen).For gene expression,mRNA was reverse transcribed using a Prime-Script RT reagent Kit(TaKaRa).qPCR was carried out with SYBR Premix Ex Taq(TaKaRa).Experiments were performed in triplicate and values were normalized to glyceraldehyde-3-phosphate dehydrogenase(GAPDH)using the 2
method for gene expression analysis.The primers used for
and
amplification are:Forward,5’-AACGCTGCCCTTGCTCTATT-3’ and reverse,5’-AGCTTTCTAGTGTGGACGGC-3’ for
;forward,5’-ATGGGGAAGGTGAAGGTCGGAGT-3’ and reverse,5’-TGACAAGCTTCCCGTTCTCA GCC-3’ for
.
I thought to myself, Why did I have to be the one to hear that? Why couldn t I have been at the back of the line? I didn t need to know that! Very soon we were back in the terminal, waiting, and then ultimately back on the plane. I waited for the pilot to give an explanation. Pilots take courses to ease passengers mind right? They know what to say to calm nerves.
ARTlCLE HlGHLlGHTS
Research background
O
-methylguanine-DNA methyltransferase(MGMT)is a specific enzyme that repairs the mispairing base O6-methyl-guanine induced by methylating environmental and experimental carcinogens.The Nnitroso compounds(NOCs)N-methyl-N’-nitro-N-nitrosoguanidine(MNNG)and N-methyl-N-nitrosourea(MNU)are monofunctional alkylating agents which can directly bind to the DNA and induce the formation of O
-methylguanine adducts to promote gene mutation and tumorigenesis.They are widely accepted chemical carcinogens for studying the mechanisms of mutagenesis and carcinogenesis induced by NOCs.
Research motivation
Studies have reported that NOCs can directly act on DNA,mainly cause O
-methylguanine damage,and subsequently induce DNA mutation and double strand breaks,participating in cancer formation and progression[19,20].The administration of MNNG can cause the destruction of pyloric mucosal structure and the occurrence of gastric adenocarcinoma in rats[24].MNNG exposure can also induce the mutation and amplification of oncogenes participating in the occurrence of GC[26].Moreover,the chromatin-based epigenetics regulation induced by NOCs,especially DNA methylation and histone modifications,has an essential role in cancer biology[27].In the current study,we demonstrated that
gene expression was rapidly increased after MNNG/MNU exposure,and the upregulation was continuously maintained during the early phase of cell malignant transformation.However,the extent of increased MGMT expression level was reduced progressively,leading us to speculate that the dynamic changes of MGMT expression could be involved in different steps of chemical carcinogensinduced gastric cell malignant transformation and tumorigenesis.
Research objectives
To investigate the molecular regulatory mechanism of MGMT in NOCs-induced gastric cell malignant transformation and tumorigenesis.
Research methods
We established a gastric epithelial cell malignant transformation model induced by MNNG or MNU treatment.Cell proliferation,colony formation,soft agar,cell migration,and xenograft assays were used to verify the malignant phenotype.By using quantitative real-time polymerase chain reaction(qPCR)and Western blot analysis,we detected the MGMT expression in malignant transformed cells.We also confirmed the MGMT expression in clinical early stage gastric tumor tissues by qPCR and immunohistochemistry.
gene promoter DNA methylation level was analyzed by methylation-specific PCR and bisulfite sequencing PCR.The effect of MGMT in cell malignant transformation was analyzed by colony formation and soft agar assays.
The old woman hastened and bought some flax of the best sort and Vasilissa sat down to work. So well did she spin that the thread came out as even and fine as a hair, and presently there was enough to begin to weave. But so fine was the thread that no frame could be found to weave it upon, nor would any weaver45 undertake to make one.
Research results
MGMT expression was upregulated in NOCs-induced gastric cell malignant transformation and in clinical early stage gastric cancer tissues.The upregulation of MGMT was regulated by the hypomethylation of its DNA promoter.
Research conclusions
The upregulation of MGMT expression is mediated by the hypomethylation of its DNA promoter in NOCs-induced gastric cell malignant transformation.
Research perspectives
By causing DNA damages and activating downstream pathways that promote cancer initiation and development,NOCs can directly induce cell malignant transformation,thus contributing to gastric carcinogenesis[23,24].The formation of DNA adducts induced by NOCs has been studied in different studies[19,20,25,26].The present study focused on early events and the molecular mechanisms of
gene dysregulation in cell malignant transformation and gastric tumorigenesis following MNNG/MNU exposure.Our data showed persistent upregulation of MGMT expression in gastric epithelial cell malignant transformation induced by NOCs.The reduction of
gene promoter DNA methylation level was responsible for the increase of MGMT expression in MNNG/MNU-treated cells.Inhibited MGMT expression promoted the MNNG/MNU-induced malignant phenotype,while overexpression of MGMT partially reversed the cell malignant transformation phenotype,suggesting that stable MGMT upregulation induced by its promoter DNA hypomethylation prevented the NOCsinduced cell malignant transformation and tumorigenesis.
FOOTNOTES
Chen YX and Lulu He contributed to the acquisition and analyses of the data;Xiang XP contributed to the collection and immunostaining of clinical samples;Shen J and Qi HY designed the study and made the critical revisions of the manuscript.
But Martins is a little confused by the Chinese response to his performances. Their faces are blank. I can t tell if they enjoy it or not. Perhaps they think it s respectful not to disturb the dance.
National Natural Science Foundation of China,No.81472543 and No.81772919;Zhejiang Provincial Natural Science Foundation of China,No.LY18H160024 and No.LY20H160040.
The study was approved by the ethics committee of Zhejiang University School of Medicine(No.2017026).
She returned, dragging a hazel nut behind her, which she laid at the Prince s feet and said, Take this nut home with you and tell your father to crack it very carefully, and you ll see then what will happen
Racing home, Reuben burst through the front door. His mother was scrubbing the kitchen stove. Here, Mum! Here! Reuben exclaimed as he ran to her side. He placed a small box in her work roughened hand.
The authors declare no conflicts of interest for this article.
And after Catherine had gone her way her lady s Destiny went to find her sister, and said to her, Dear sister, has not Catherine suffered enough? It is surely time for her good days to begin? And the sister answered, To-morrow you shall bring her to me, and I will give her something that may help her out of her need
No additional data are available.
The authors have read the ARRIVE guidelines,and the manuscript was prepared and revised according to the ARRIVE guidelines.
This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial(CC BYNC 4.0)license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is noncommercial.See:https://creativecommons.org/Licenses/by-nc/4.0/
Many thoughts that have laindormant are roused at the proper time, and begin to stir in the mindand the heart, and seem even to come upon us from above
China
Yue-Xia Chen 0000-0003-0450-0005;Lu-Lu He 0000-0002-0484-1603;Xue-Ping Xiang 0000-0003-3545-4013;Jing Shen 0000-0001-6157-8293;Hong-Yan Qi 0000-0002-4639-299X.
Fan JR
Vicki and Cindy replied that they had closed their eyes and run down the hill, holding hands, in the opposite direction from Edward. And what did you do? my mother asked me.
Wang TQ
Yuan YY
1 Bray F,Ferlay J,Soerjomataram I,Siegel RL,Torre LA,Jemal A.Global cancer statistics 2018:GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries.
2018;68:394-424[PMID:30207593 DOI:10.3322/caac.21492]
2 Chen W,Zheng R,Baade PD,Zhang S,Zeng H,Bray F,Jemal A,Yu XQ,He J.Cancer statistics in China,2015.
2016;66:115-132[PMID:26808342 DOI:10.3322/caac.21338]
3 Padmanabhan N,Ushijima T,Tan P.How to stomach an epigenetic insult:the gastric cancer epigenome.
2017;14:467-478[PMID:28513632 DOI:10.1038/nrgastro.2017.53]
4 Yamashita S,Kishino T,Takahashi T,Shimazu T,Charvat H,Kakugawa Y,Nakajima T,Lee YC,Iida N,Maeda M,Hattori N,Takeshima H,Nagano R,Oda I,Tsugane S,Wu MS,Ushijima T.Genetic and epigenetic alterations in normal tissues have differential impacts on cancer risk among tissues.
2018;115:1328-1333[PMID:29358395 DOI:10.1073/pnas.1717340115]
5 Tie J,Zhang X,Fan D.Epigenetic roles in the malignant transformation of gastric mucosal cells.
2016;73:4599-4610[PMID:27464701 DOI:10.1007/s00018-016-2308-9]
6 Klutstein M,Nejman D,Greenfield R,Cedar H.DNA Methylation in Cancer and Aging.
2016;76:3446-3450[PMID:27256564 DOI:10.1158/0008-5472.CAN-15-3278]
7 Takeshima H,Ushijima T.Accumulation of genetic and epigenetic alterations in normal cells and cancer risk.
2019;3:7[PMID:30854468 DOI:10.1038/s41698-019-0079-0]
8 Zheng SC,Widschwendter M,Teschendorff AE.Epigenetic drift,epigenetic clocks and cancer risk.
2016;8:705-719[PMID:27104983 DOI:10.2217/epi-2015-0017]
9 Christmann M,Verbeek B,Roos WP,Kaina B.O(6)-Methylguanine-DNA methyltransferase(MGMT)in normal tissues and tumors:enzyme activity,promoter methylation and immunohistochemistry.
2011;1816:179-190[PMID:21745538 DOI:10.1016/j.bbcan.2011.06.002]
10 Mur P,Rodríguez de Lope á,Díaz-Crespo FJ,Hernández-Iglesias T,Ribalta T,Fia?o C,García JF,Rey JA,Mollejo M,Meléndez B.Impact on prognosis of the regional distribution of MGMT methylation with respect to the CpG island methylator phenotype and age in glioma patients.
2015;122:441-450[PMID:25682093 DOI:10.1007/s11060-015-1738-9]
11 Wick W,Platten M.Understanding and targeting alkylator resistance in glioblastoma.
2014;4:1120-1122[PMID:25274683 DOI:10.1158/2159-8290.CD-14-0918]
12 Gerson SL.MGMT:its role in cancer aetiology and cancer therapeutics.
2004;4:296-307[PMID:15057289 DOI:10.1038/nrc1319]
13 Soejima H,Zhao W,Mukai T.Epigenetic silencing of the MGMT gene in cancer.
2005;83:429-437[PMID:16094446 DOI:10.1139/o05-140]
14 Fornaro L,Vivaldi C,Caparello C,Musettini G,Baldini E,Masi G,Falcone A.Pharmacoepigenetics in gastrointestinal tumors:MGMT methylation and beyond.
2016;8:170-180[PMID:26709653]
15 Alizadeh Naini M,Kavousipour S,Hasanzarini M,Nasrollah A,Monabati A,Mokarram P.O6-Methyguanine-DNA Methyl Transferase(MGMT)Promoter Methylation in Serum DNA of Iranian Patients with Colorectal Cancer.
2018;19:1223-1227[PMID:29801405 DOI:10.22034/APJCP.2018.19.5.1223]
16 Gu C,Lu J,Cui T,Lu C,Shi H,Xu W,Yuan X,Yang X,Huang Y,Lu M.Association between MGMT promoter methylation and non-small cell lung cancer:a meta-analysis.
2013;8:e72633[PMID:24086261 DOI:10.1371/journal.pone.0072633]
17 Yousuf A,Bhat MY,Pandith AA,Afroze D,Khan NP,Alam K,Shah P,Shah MA,Mudassar S.MGMT gene silencing by promoter hypermethylation in gastric cancer in a high incidence area.
2014;37:245-252[PMID:25008999 DOI:10.1007/s13402-014-0179-3]
18 Oldrini B,Vaquero-Siguero N,Mu Q,Kroon P,Zhang Y,Galán-Ganga M,Bao Z,Wang Z,Liu H,Sa JK,Zhao J,Kim H,Rodriguez-Perales S,Nam DH,Verhaak RGW,Rabadan R,Jiang T,Wang J,Squatrito M.MGMT genomic rearrangements contribute to chemotherapy resistance in gliomas.
2020;11:3883[PMID:32753598 DOI:10.1038/s41467-020-17717-0]
19 Mirvish SS.Role of N-nitroso compounds(NOC)and N-nitrosation in etiology of gastric,esophageal,nasopharyngeal and bladder cancer and contribution to cancer of known exposures to NOC.
1995;93:17-48[PMID:7600541 DOI:10.1016/0304-3835(95)03786-V]
20 Loh YH,Jakszyn P,Luben RN,Mulligan AA,Mitrou PN,Khaw KT.N-Nitroso compounds and cancer incidence:the European Prospective Investigation into Cancer and Nutrition(EPIC)-Norfolk Study.
2011;93:1053-1061[PMID:21430112 DOI:10.3945/ajcn.111.012377]
21 Chen K,Zhang S,Ke X,Qi H,Shao J,Shen J.Biphasic reduction of histone H3 phosphorylation in response to N-nitroso compounds induced DNA damage.
2016;1860:1836-1844[PMID:27233451 DOI:10.1016/j.bbagen.2016.05.028]
22 Qi H,Yang Z,Dai C,Wang R,Ke X,Zhang S,Xiang X,Chen K,Li C,Luo J,Shao J,Shen J.STAT3 activates MSK1-mediated histone H3 phosphorylation to promote NFAT signaling in gastric carcinogenesis.
2020;9:15[PMID:32041943 DOI:10.1038/s41389-020-0195-2]
23 Higginson J,DeVita VT Jr.IARC monographs on the evaluation of carcinogenic risk of chemicals to humans.
1980;41:A26,A28,A30 passim[PMID:6998267]
24 Sugimura T,Fujimura S.Tumour production in glandular stomach of rat by N-methyl-N'-nitro-N-nitrosoguanidine.
1967;216:943-944[PMID:6074981 DOI:10.1038/216943a0]
25 Kwak H,Lee M,Cho M.Interrelationship of apoptosis,mutation,and cell proliferation in N-methyl-N'-nitro-Nnitrosoguanidine(MNNG)-induced medaka carcinogenesis model.
2000;50:317-329[PMID:10967394 DOI:10.1016/s0166-445x(00)00093-x]
26 Arber N,Hibshoosh H,Moss SF,Sutter T,Zhang Y,Begg M,Wang S,Weinstein IB,Holt PR.Increased expression of cyclin D1 is an early event in multistage colorectal carcinogenesis.
1996;110:669-674[PMID:8608874 DOI:10.1053/gast.1996.v110.pm8608874]
27 Baccarelli A,Bollati V.Epigenetics and environmental chemicals.
2009;21:243-251[PMID:19663042 DOI:10.1097/mop.0b013e32832925cc]
28 Iyama T,Wilson DM 3rd.DNA repair mechanisms in dividing and non-dividing cells.
2013;12:620-636[PMID:23684800 DOI:10.1016/j.dnarep.2013.04.015]
29 Bocangel D,Sengupta S,Mitra S,Bhakat KK.p53-Mediated down-regulation of the human DNA repair gene O6-methylguanine-DNA methyltransferase(MGMT)
interaction with Sp1 transcription factor.
2009;29:3741-3750[PMID:19846904]
30 Pistollato F,Abbadi S,Rampazzo E,Persano L,Della Puppa A,Frasson C,Sarto E,Scienza R,D'avella D,Basso G.Intratumoral hypoxic gradient drives stem cells distribution and MGMT expression in glioblastoma.
2010;28:851-862[PMID:20309962 DOI:10.1002/stem.415]
31 Wang J,Sai K,Chen FR,Chen ZP.miR-181b modulates glioma cell sensitivity to temozolomide by targeting MEK1.
2013;72:147-158[PMID:23645289 DOI:10.1007/s00280-013-2180-3]
32 Jesionek-Kupnicka D,Braun M,Tr?bska-Kluch B,Czech J,Szybka M,Szymańska B,Kulczycka-Wojdala D,Bieńkowski M,Kordek R,Zawlik I.MiR-21,miR-34a,miR-125b,miR-181d and miR-648 Levels inversely correlate with MGMT and TP53 expression in primary glioblastoma patients.
2019;15:504-512[PMID:30899304 DOI:10.5114/aoms.2017.69374]
33 Malley DS,Hamoudi RA,Kocialkowski S,Pearson DM,Collins VP,Ichimura K.A distinct region of the MGMT CpG island critical for transcriptional regulation is preferentially methylated in glioblastoma cells and xenografts.
2011;121:651-661[PMID:21287394 DOI:10.1007/s00401-011-0803-5]
34 Butler M,Pongor L,Su YT,Xi L,Raffeld M,Quezado M,Trepel J,Aldape K,Pommier Y,Wu J.MGMT Status as a Clinical Biomarker in Glioblastoma.
2020;6:380-391[PMID:32348734 DOI:10.1016/j.trecan.2020.02.010]
35 Yu W,Zhang L,Wei Q,Shao A.O
-Methylguanine-DNA Methyltransferase(MGMT):Challenges and New Opportunities in Glioma Chemotherapy.
2019;9:1547[PMID:32010632 DOI:10.3389/fonc.2019.01547]
36 Bouras E,Karakioulaki M,Bougioukas KI,Aivaliotis M,Tzimagiorgis G,Chourdakis M.Gene promoter methylation and cancer:An umbrella review.
2019;710:333-340[PMID:31202904 DOI:10.1016/j.gene.2019.06.023]
 World Journal of Gastrointestinal Oncology2022年3期
World Journal of Gastrointestinal Oncology2022年3期
- World Journal of Gastrointestinal Oncology的其它文章
- Re:Association between intestinal neoplasms and celiac disease -beyond celiac disease and more
- Association of Blastocystis hominis with colorectal cancer:A systematic review of in vitro and in vivo evidences
- Clinical efficacy and prognostic risk factors of endoscopic radiofrequency ablation for gastric low-grade intraepithelial neoplasia
- Pancreatic head vs pancreatic body/tail cancer:Are they different?
- Computed tomography-based radiomic to predict resectability in locally advanced pancreatic cancer treated with chemotherapy and radiotherapy
- Cost-effective low-coverage whole-genome sequencing assay for the risk stratification of gastric cancer
